Abstract
Objective
To (i) determine whether women with polycystic ovary syndrome (PCOS) from a population-based cohort experience elevated depression symptoms and (ii) characterize the trajectory of symptoms over the lifespan.
Design
The association between PCOS and longitudinal depression scores was investigated among 1127 black and white women participating in Coronary Artery Risk Development in Young Adults study. PCOS was ascertained at baseline (ages 20 to 32) by U.S. National Institutes of Health (NIH) criteria, incorporating androgens and symptoms of oligomenorrhea and hirsutism. The Center for Epidemiologic Studies-Depression (CES-D) scale was repeated prospectively in 5-year intervals over 25 years. Mixed-effects models evaluated the association between depression scores and PCOS after adjustment for confounders and characterized the trajectory of scores. The impact of race was explored.
Results
Eighty-three of 1127 (7.4%) participants met NIH PCOS criteria. Of these, 33 (40%) were black and 50 (60%) were white. CES-D scores were higher among women with PCOS [coefficient (coef) 2.51; 95% CI 1.49, 3.54; P < 0.01] across the lifespan. Scores decreased across the lifespan in women with and without PCOS (coef −0.1 point per year; P < 0.001). Black women experienced higher depression burden than white women (coef 1.80; 95% CI 1.20, 2.41; P < 0.001); however, an interaction was not detected between PCOS and race (P = 0.68).
Conclusions
Women with PCOS-NIH from a population-based cohort are at risk for higher depression scores across the lifespan. Depression scores decline over time in women with PCOS in a trajectory similar to that in women without PCOS. Racial differences in depression risk should be acknowledged clinically and further explored.
In a population-based cohort of 1127 women, depression scores were higher across the lifespan in women with polycystic ovary syndrome (PCOS) however declined with aging in a trajectory similar to that in women without PCOS.
Polycystic ovary syndrome (PCOS) is a complex, multisystem disorder affecting reproductive, metabolic, and psychological well-being across the lifespan. PCOS is common among reproductive-age women, affecting between 5%–8% (1, 2) and 15% (3), depending on diagnostic criteria. PCOS is characterized by ovulatory dysfunction, hyperandrogenism, and polycystic ovarian morphology on transvaginal ultrasound (4). Insulin resistance (5) and metabolic dysfunction are common clinical correlates of PCOS; women with PCOS are at increased risk of diabetes, obesity, and metabolic syndrome (6, 7).
Mood disorders also disproportionately afflict women with PCOS (8–11). Cross-sectional studies estimate a three- to eightfold increase in prevalence of depression in PCOS compared with controls (12). However, the majority of studies uses a convenience sample of women with PCOS presenting to clinical care, largely as a result of a PCOS-related complaint, such as irregular menses, hirsutism, or mood issues, which might falsely inflate the estimated burden of depressive symptoms. Data assessment of whether an unselected sample of women with PCOS in the community setting also has increased symptoms of depression is sparse (12, 13).
Another crucial gap in the literature pertains to the longitudinal course of depressive symptoms in aging women with PCOS. To date, two prospective American studies have characterized depression risk in women with PCOS. One followed 60 women for 1.8 years (14), and we reported our experience with a clinical cohort of 163 women followed for an average of 5.5 years (15); both studies suggest a stable chronology of depression symptoms over this relatively limited purview. A longitudinal, population-based study derived from the Northern Finland Birth Cohort 1966 similarly found an unchanged prevalence of depression at ages 31 and 46 years in women reporting oligomenorrhea and hirsutism (16). Meanwhile, the incidence and prevalence of depression have been found to decline with aging in population-based samples, primarily after 45 years of age (17, 18). Although the symptomatic burden of certain PCOS features, such as irregular menses, acne, and hirsutism, have been shown to attenuate with aging (19–21), there is a paucity of data examining depression trends in older women with histories of PCOS. In its 2018 evidence-based position statement, the Androgen Excess–PCOS Society communicated a call to action for additional studies of psychological well-being in longitudinal cohorts of women with PCOS (22). The new international, evidence-based PCOS guidelines also encourage further studies in this field (23).
The objectives of this study were the following: to (i) characterize depressive symptoms in a population-based sample of women with and without PCOS across the lifespan and (ii) compare trajectories of depression symptoms in women with and without PCOS. We examined subjects with and without PCOS from the Coronary Artery Risk Development in Young Adults (CARDIA) population, a population-based sample of black and white women across the United States enrolled in a 30-year prospective, multicenter, observational study. We hypothesized the following: that (i) women with PCOS would have greater burden of depression symptoms compared with women without PCOS across the lifespan and (ii) the trajectory of depression symptoms would decline with aging in women with and without PCOS.
Materials and Methods
Study design and subjects
The study cohort was selected from the CARDIA study. CARDIA is a prospective, multicenter investigation of cardiovascular risk factor development in black and white young adults in the United States (24). The study enrolled 5115 men and women ages 18 to 30 years at an initial visit between 1985 and 1986 and followed them for 30 years. Subject recruitment occurred at four study centers across the United States: Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; and Oakland, California. The study protocol was approved by the Institutional Review Board at each study site, and subjects provided written, informed consent.
The sampling strategy aimed to balance sex (54% women), race (52% black), and education (40% high school graduate or less). After the enrollment examination in 1985 through 1986, subsequent follow-up examinations occurred at years 2, 5, 7, 10, 15, 20, 25, and 30. Age range at the final follow-up examination was 48 to 60 years. Additional details about the study design and recruitment strategies have been previously published (24).
The CARDIA Women’s Study (CWS) is an ancillary study designed to investigate the impact of polycystic ovarian physiology and serum androgen levels on cardiovascular health in women. CWS recruited women at an additional study visit, performed at year 16. Eligibility included participation in the year-15 examination; pregnant women and those lacking ovaries were excluded. Eighty-six percent of eligible women were recruited for the CWS examination during year 16 (n = 1163) (25).
Our study sample included 1127 of these 1163 women selected on the basis of participation at both the year-2 (ages 20 to 32) and year-16 (ages 34 to 46) examinations, from which the determination of PCOS status was ascertained, as detailed below. The year-2 examination was considered baseline in the current study.
Establishing PCOS diagnoses
The schedule of events is depicted in Fig. 1. U.S. National Institutes of Health (NIH) criteria, requiring hyperandrogenism and oligomenorrhea (26), were used to ascertain PCOS diagnoses at baseline (year-2 examination, ages 20 to 32). Hyperandrogenism comprised biochemical and clinical components. Subjects met criteria for biochemical hyperandrogenism on the basis of baseline serum androgens exceeding the 75th percentile, corresponding to total testosterone >53 ng/dL and/or free testosterone >0.38 ng/dL, with the goal of ascertaining a realistic prevalence of PCOS (27). Androgens were assayed by the Obstetrics/Gynecology Research and Diagnostic Laboratory at the University of Alabama, Birmingham, from banked specimens. Total testosterone was measured via direct chemiluminescent-competitive immunoassay on the Beckman Access Automated System (Beckman Coulter, Fullerton, CA). Free testosterone was calculated from total testosterone and sex hormone-binding globulin concentrations, using the law of mass action (28). Sex hormone-binding globulin was determined by equilibrium analysis. Clinical hyperandrogenism considered hirsutism, as indicated by self-report. At the year-16 CWS examination, subjects completed questionnaires recalling symptoms of body hair growth they experienced between the ages of 20 and 30. Women reporting unwanted body hair growth in androgen-sensitive regions, excluding the leg and underarm, were considered to meet evidence for clinical hyperandrogenism (25).
Figure 1.
Schedule of study events. The schedule of study events by examination year (Y; e.g., Y0, Y2, Y5, etc.) is depicted. PCOS diagnoses were made at the Y2 examination, considered as baseline, at which time, women were 20 to 32 years old. Fasting glucose and insulin were banked at Y7 and the CWS questionnaires distributed at Y16. The Center for Epidemiologic Studies-Depression (CES-D) scale was repeated in 5-year intervals from Y5 to Y30. Time-varying covariates, considered in the mixed-effects models [body mass index (BMI), exercise expenditure, education level], were also repeatedly assessed at each examination. *Oligomenorrhea and hirsutism symptoms experienced during women’s 20s (i.e., time of Y2 examination) were later recalled at the Y16 CWS questionnaire.
Menstrual history was also queried at the year-16 CWS examination. Women indicated whether their cycles were regular vs irregular, as well as their typical cycle intervals between the ages of 20 and 30 years. In accordance with a previously published classification schema, women indicating regular or irregular cycles ≥32 to 45 days apart were considered to fulfill the oligomenorrhea criterion on the basis of the available categorized menstrual cycle history data (25, 29).
Women who did not meet the above criteria for both hyperandrogenism and oligomenorrhea were considered non-PCOS control subjects.
Data collection
Sociodemographic variables (age, race, education, health behaviors) were collected by self report and interviewer-administered questionnaires. Education was categorized and coded as follows: 0: <high school, 1: high school graduate, 2: some college, 3: college graduate, 4: graduate school attendance. A detailed schedule of questionnaire data and categorical variable groupings is available online (30). Measurements of weight, height, and waist circumference were systematically obtained (31). Body mass index (BMI) was calculated as kilogram per square meter. Physical activity was reported as total exercise units based on the CARDIA Physical Activity History Questionnaire, which considers frequency and intensity of a variety of physical activities (32). Other health-related behaviors, including current alcohol consumption and tobacco smoking, were coded as binary variables (0: none; 1: any).
Outcome
The Center for Epidemiologic Studies-Depression (CES-D) scale assessed depressive symptoms (33). This validated questionnaire was self-administered at years 5, 10, 15, 20, 25, and 30. The CES-D is a 20-item survey querying the frequency of depression symptoms experienced during the prior week, such as restless sleep, loss of appetite, and feeling lonely. Each item is scored from 0 (rarely or none of the time) to 3 (most or almost all of the time), yielding a total score range of 0 to 60, with higher scores indicating greater depressive symptom burden. The CES-D has been validated in a variety of populations, is sensitive to changes over time, and can be administered to diverse arrays of subjects (34).
Statistical analysis
Data were tested for normality. Descriptive statistics were provided. Baseline characteristics were compared using two-sided t tests, χ2, or Fisher’s exact testing as appropriate. Baseline variables were measured at year 2, except for metabolic serum testing (glucose, insulin, and C-reactive protein), which were assayed from specimens banked at year 7.
To determine whether depression-symptom scores vary across the lifespan in women with PCOS compared with controls, mixed-effects models were used with patient identification as a random factor; CES-D score was a repeated-measure outcome. To determine the appropriate covariance structure, we used graphical methods and correlation matrices; an exchangeable correlation structure was determined to be most appropriate. A univariate model was first explored. Next, covariates, selected on the basis of prior knowledge and literature, as well as results of the preceding univariate analyses, were incorporated into the model. Time-varying covariates considered as fixed effects included the following: BMI, exercise expenditure, education level, and year. Other fixed-effects covariates unchanging over time included the following: PCOS status, baseline age, race, and study center. The restricted maximum-likelihood estimation option was used.
To explore whether the trajectory of depression symptoms varied on the basis of a PCOS diagnosis, we used a mixed-effects model with an interaction term between year and PCOS status. Linear modeling of examination year was determined to be appropriate on the basis of examination of individual plots of the trajectory of CES-D scores, lowess smoother plots of CES-D scores over time by PCOS status, and introduction of categorical time term into the model.
In light of the significant association between race and CES-D scores identified in the mixed-effects models, we sought to characterize further the possible impact of race on depression scores by PCOS status. Mixed models were repeated after stratification by race. A model that included an interaction term between PCOS and race was assessed to determine whether the impact of PCOS on depression scores varied on the basis of race.
Model diagnostics incorporated examinations of residuals; no violations of model assumptions were identified. In a sensitivity analysis, we considered a log transformation of CES-D scores as the outcome to normalize this outcome variable further. This iteration yielded the same conclusions as prior analyses; we ultimately elected to use raw CES-D scores to avoid cumbersome interpretation of predictor coefficients.
Finally, we conducted a sensitivity analysis in which CES-D scores were considered a dichotomous outcome, with a cut-off score of ≥16, indicating a positive depression screen. A multivariate mixed-effects logistic regression model was used to calculate odds ratios for depression risk. To address potential bias related to oral contraceptive use at baseline, we further assessed a model that excluded women on these medications.
Statistical analyses were performed in STATA v14.2 (StataCorp, College Station, TX). A P value of <0.05 set the threshold for statistical significance.
Results
Of the 1127 women included in the study cohort, subject retention plus completion of CES-D questionnaires included the following: 95%, 93%, 99%, 91%, 91%, and 85% at years 5, 10, 15, 20, 25, and 30, respectively.
Eighty-three of 1127 (7.4%) met NIH criteria for PCOS. Of these women, 33 women (40%) were black and 50 women (60%) were white. Baseline characteristics of women with and without PCOS are detailed in Table 1. There was no difference in baseline age or BMI between women with and without PCOS. Women with PCOS were more like to be white, have pursued postgraduate studies, and report higher baseline exercise expenditure. Metabolic serum parameters from year 7-banked specimens, including glucose, insulin, and C-reactive protein, did not vary on the basis of PCOS diagnosis. By design, women with PCOS had higher serum androgens.
Table 1.
Baseline Characteristics of Women With and Without PCOS
| No PCOS (n = 1044) | PCOS (n = 83) | P Value | |
|---|---|---|---|
| Age, y | 27.3 (3.6) | 26.8 (3.7) | 0.20 |
| BMI, kg/m2 | 25.6 (6.3) | 26.3 (6.8) | 0.33 |
| Race, % white | 46 | 60 | 0.01 |
| Education, % | 0.05 | ||
| <HS | 6 | 5 | |
| HS grad | 26 | 22 | |
| Some college | 35 | 33 | |
| College grad | 23 | 19 | |
| Grad school | 10 | 22 | |
| Study center, % | 0.76 | ||
| Birmingham | 24 | 20 | |
| Chicago | 23 | 27 | |
| Minneapolis | 21 | 24 | |
| Oakland | 32 | 29 | |
| Physical activity, exercise units | 294 (224) | 349 (290) | 0.04 |
| Alcohol, % | 82 | 81 | 0.89 |
| Smoking, % | 27 | 26 | 0.95 |
| Glucose,a mg/dL | 87.4 (16.5) | 87.4 (7.4) | 0.99 |
| Insulin,a U/mL | 14.2 (22.5) | 14.6 (7.4) | 0.87 |
| HOMA-IRa | 3.14 (5.57) | 3.21 (1.76) | 0.91 |
| Total cholesterol, mg/dL | 174.9 (32.7) | 180.0 (31.2) | 0.18 |
| LDL cholesterol, mg/dL | 104.2 (29.2) | 110.4 (29.4) | 0.07 |
| HDL cholesterol, mg/dL | 55.4 (13.7) | 54.4 (12.2) | 0.50 |
| Triglycerides, mg/dL | 74.4 (47.1) | 73.9 (45.4) | 0.92 |
| CRP,a μg/dL | 3.74 (6.18) | 2.76 (3.13) | 0.17 |
| Total testosterone, ng/dL | 41.0 (46.6) | 73.7 (84.2) | <0.001 |
| Free testosterone, ng/dL | 0.29 (0.52) | 0.52 (0.44) | <0.001 |
Mean (SD) or percent as indicated. P values derived from two-sided t test, χ2, or Fisher’s exact as appropriate.
Abbreviations: CRP, C-reactive protein; grad, graduate; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; HS, high school; LDL, low-density lipoprotein.
Glucose, insulin, HOMA-IR, and CRP measured at year-7 examination.
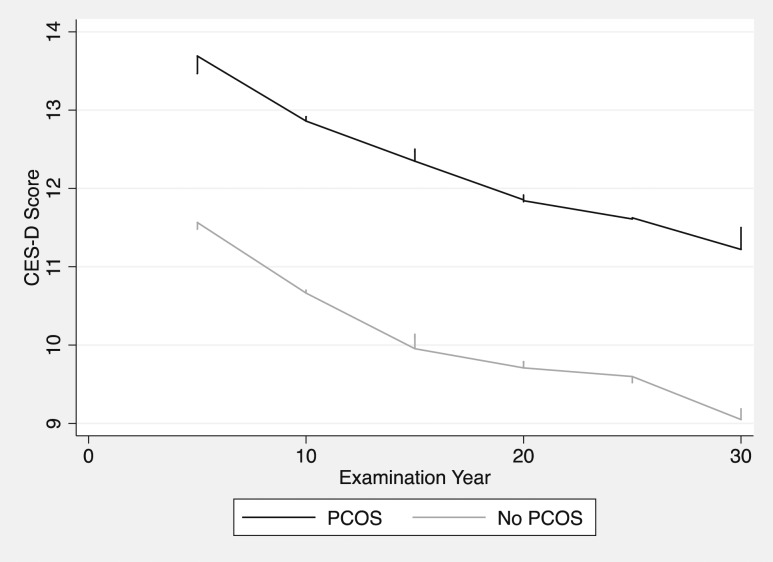
CES-D scores were examined by PCOS status across the lifespan (Fig. 2). Women with PCOS had higher CES-D scores at all time points (i.e., increased depression symptoms).
Figure 2.
Depression scores over time stratified by PCOS diagnosis. Lowess smoother best-fit models of CES-D scores across the lifespan stratified by PCOS status. First CES-D administered at year-5 examination (corresponding to ages 23 to 35); final CES-D administered at year-30 examination (corresponding to ages 48 to 60).
Mixed-effects models quantified the effect of PCOS on lifetime depression symptom scores (Table 2). In an unadjusted model, a PCOS diagnosis was associated with a 2.1-point increase on lifetime CES-D scores (95% CI 1.09, 3.19; P < 0.001). This association remained statistically significant after adjustment for covariates, including age, BMI, race, education, exercise output, and study center [coefficient (coef) 2.51; 95% CI 1.49, 3.54; P < 0.001; Table 2]. In this model, increasing age, education, and exercise output were each independently associated with reductions in CES-D scores. White women had significantly lower depression scores compared with black women after accounting for these potential confounders (coef −1.80; 95% CI −2.41, −1.20; P < 0.001). Recruitment site was not associated with CES-D scores.
Table 2.
Mixed-Effects Models Examining the Impact of PCOS Diagnosis on CES-D Scores Across the Lifespan
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Coef (95% CI) | P | Coef (95% CI) | P | |
| PCOS | 2.14 (1.09, 3.19) | <0.001 | 2.51 (1.49, 3.54) | <0.001 |
| Y | −0.08 (−0.10, −0.05) | <0.001 | −0.09 (−0.11, −0.06) | <0.001 |
| BMI, kg/m2 | 0.14 (0.11, 0.18) | <0.001 | 0.08 (0.05, 0.11) | <0.001 |
| Age, y at baseline | −0.21 (−0.28, −0.13) | <0.001 | −0.15 (−0.22, −0.07) | <0.001 |
| Race | <0.001 | <0.001 | ||
| Black | Ref | Ref | ||
| White | −2.89 (−3.43, −2.34) | −1.80 (−2.41, −1.20) | ||
| Education | <0.001 | <0.001 | ||
| Some HS | Ref | Ref | ||
| HS grad | −1.94 (−3.11, −0.77) | 0.001 | −1.79 (−2.96, −0.62) | 0.003 |
| Some college | −2.99 (−4.15, −1.82) | <0.001 | −2.90 (−4.06, −1.74) | <0.001 |
| College grad | −4.75 (−5.94, −3.55) | <0.001 | −3.92 (−5.12, −2.71) | <0.001 |
| Grad school | −5.27 (−6.52, −4.03) | <0.001 | −4.15 (−5.42, −2.88) | <0.001 |
| Study center | 0.18 | 0.41 | ||
| Birmingham | Ref | Ref | ||
| Chicago | 0.59 (−0.22, 1.39) | 0.15 | 0.60 (−0.19, 1.38) | 0.13 |
| Minneapolis | −0.29 (−1.11, 0.53) | 0.49 | 0.52 (−0.31, 1.34) | 0.22 |
| Oakland | −0.05 (−0.80, 0.70) | 0.90 | 0.54 (−0.19, 1.28) | 0.15 |
| Exercise output, 100 units | −0.19 (−0.27, −0.10) | <0.001 | −0.09 (−0.18, −0.00) | 0.04 |
Multivariate mixed-effects model includes all listed covariates. Women were 20 to 32 y of age at baseline, followed through 48 to 60 y of age.
Abbreviations: grad, graduate; HS, high school; Ref, Referent.
The trajectory of CES-D scores with aging was examined in women with and without PCOS (Fig. 2). A mixed-effects model, including the same covariates as the prior multivariate model (Table 2), with the addition of an interaction term between year and PCOS status, was used to determine whether the trajectory of depression symptoms varied on the basis of PCOS status. The trend test for the interaction term was not significant (χ2 = 5.84, P = 0.32); thus, we failed to observe a difference in depression-symptom trajectory across the lifespan in women with vs without PCOS. A graphical depiction of CES-D score trajectories (Fig. 2) visually corroborates this quantitative analysis.
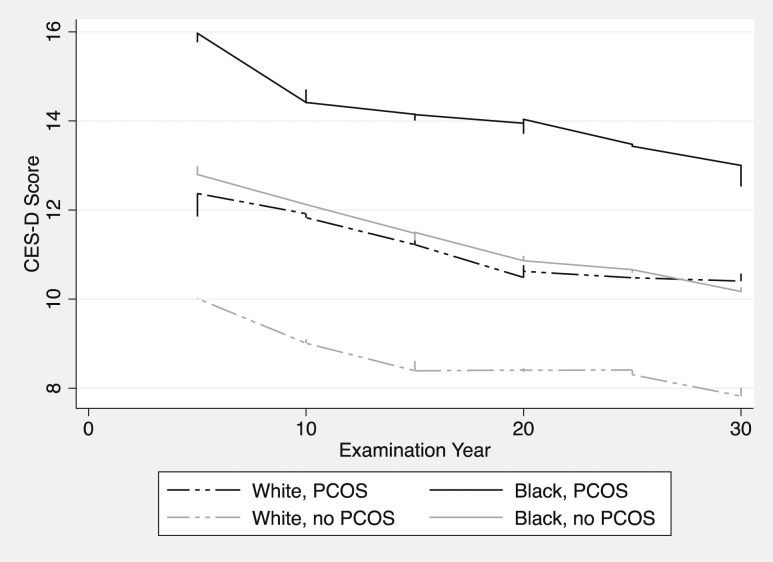
In light of the significant association between race and CES-D scores noted in the multivariate mixed model (Table 2), racial effects were further explored. Visually, we observed an upward shift in depression scores in black women with and without PCOS relative to their white counterparts (Fig. 3). A multivariate mixed-effects model, including an interaction term between PCOS and race, failed to identify a differential impact of PCOS on depression scores in black vs white women (trend test for interaction term: χ2 = 0.17, P = 0.68), suggesting a similar increase in CES-D scores for women with PCOS of either racial background.
Figure 3.
Depression scores over time stratified by PCOS and race. Lowess smoother best-fit models of CES-D scores across the lifespan, stratified by race and PCOS status. First CES-D administered at year-5 examination (corresponding to ages 23 to 35); final CES-D administered at year 30 examination (corresponding to ages 48 to 60).
Baseline characteristics of black and white women were compared (Table 3), and then the mixed-effects models (Table 2) were repeated after stratification by race (Tables 4 and 5). Similar associations were observed in each group. Exceptions were that BMI was positively associated with CES-D scores in white but not black women, whereas baseline age had a negative association with depression scores in black women, but only a trend existed among white women. Exercise was no longer a statistically significant correlate of CES-D scores in this subgroup analysis.
Table 3.
Baseline Characteristics of Black vs White Women
| Black (n = 596) | White (n = 531) | P Value | |
|---|---|---|---|
| Age, y | 26.8 (3.9) | 27.8 (3.3) | <0.001 |
| BMI, kg/m2 | 27.2 (7.0) | 23.9 (4.8) | <0.001 |
| Education, % | <0.001 | ||
| <HS | 8 | 3 | |
| HS grad | 30 | 21 | |
| Some college | 45 | 24 | |
| College grad | 14 | 33 | |
| Grad school | 3 | 19 | |
| Study center, % | <0.001 | ||
| Birmingham | 28 | 19 | |
| Chicago | 26 | 21 | |
| Minneapolis | 13 | 31 | |
| Oakland | 33 | 30 | |
| Physical activity, exercise units | 242 (206) | 360 (239) | <0.001 |
| Alcohol, % | 75 | 90 | <0.001 |
| Smoking, % | 30 | 24 | 0.95 |
| Glucose,a mg/dL | 88.2 (18.9) | 86.5 (12.1) | 0.09 |
| Insulin,a U/mL | 16.9 (29.7) | 11.3 (10.8) | <0.001 |
| HOMA-IRa | 3.80 (7.34) | 2.46 (1.52) | <0.001 |
| Total cholesterol, mg/dL | 175.4 (32.2) | 175.1 (33.0) | 0.90 |
| LDL cholesterol, mg/dL | 105.8 (29.5) | 103.3 (29.0) | 0.16 |
| HDL cholesterol, mg/dL | 54.7 (13.8) | 56.0 (13.3) | 0.13 |
| Triglycerides, mg/dL | 72.5 (48.4) | 76.4 (43.4) | 0.17 |
| CRP,a μg/dL | 4.45 (6.56) | 2.83 (5.24) | <0.001 |
| Total testosterone, ng/dL | 43.4 (48.4) | 43.6 (54.0) | 0.95 |
| Free testosterone, ng/dL | 0.30 (0.40) | 0.32 (0.62) | 0.56 |
| Hirsutism, % | 18 | 21 | 0.21 |
| Oligomenorrhea, % | 9 | 17 | <0.001 |
Mean (SD) or percent as indicated. P values derived from two-sided t test, χ2, or Fisher’s exact as appropriate.
Abbreviations: CRP, C-reactive protein; grad, graduate; HOMA-IR, homeostasis model assessment of insulin resistance; HS, high school.
Glucose, insulin, HOMA-IR, and CRP measured at year-7 examination.
Table 4.
Mixed-Effects Models Examining the Impact of PCOS Diagnosis on CES-D Scores Across the Lifespan—White Stratum
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Coef (95% CI) | P | Coef (95% CI) | P | |
| PCOS | 2.59 (1.32, 3.87) | <0.001 | 2.62 (1.37, 3.87) | <0.001 |
| Y | −0.05 (−0.08, −0.01) | 0.01 | −0.07 (−0.10, −0.03) | <0.001 |
| BMI | 0.19 (0.13, 0.24) | <0.001 | 0.15 (0.09, 0.20) | <0.001 |
| Age, years at baseline | −0.14 (−0.25, −0.02) | 0.02 | −0.11 (−0.22, 0.01) | 0.07 |
| Education | <0.001 | <0.001 | ||
| Some HS | Ref | Ref | ||
| HS grad | −1.25 (−3.40, 0.90) | 0.26 | −1.75 (−3.88, 0.39) | 0.11 |
| Some college | −3.20 (−5.35, −1.05) | 0.004 | −3.65 (−5.79, −1.52) | 0.001 |
| College grad | −4.59 (−6.73, −2.45) | <0.001 | −4.61 (−6.74, −2.48) | <0.001 |
| Grad school | −4.50 (−6.66, −2.34) | <0.001 | −4.66 (−6.81, −2.51) | <0.001 |
| Study center | 0.88 | 0.43 | ||
| Birmingham | Ref | Ref | ||
| Chicago | 0.35 (−0.85, 1.55) | 0.57 | 0.81 (−0.37, 1.98) | 0.18 |
| Minneapolis | 0.42 (−0.68, 1.54) | 0.45 | 0.56 (−0.54, 1.65) | 0.32 |
| Oakland | 0.17 (−0.94, 1.28) | 0.77 | 0.88 (−0.22, 1.99) | 0.12 |
| Exercise output, 100 units | −0.16 (−0.27, −0.05) | <0.01 | −0.09 (−0.20, 0.02) | 0.11 |
Multivariate mixed-effects model includes all listed covariates. Women were 20 to 32 y of age at baseline, followed through 48 to 60 y of age.
Abbreviations: grad, graduate; HS, high school; Ref, Referent.
Table 5.
Mixed-Effects Models Examining the Impact of PCOS Diagnosis on CES-D Scores Across the Lifespan—Black Stratum
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Coef (95% CI) | P | Coef (95% CI) | P | |
| PCOS | 2.54 (0.82, 4.26) | 0.004 | 2.25 (0.54, 3.95) | 0.01 |
| Y | −0.10 (−0.14, −0.07) | <0.001 | −0.10 (−0.14, −0.07) | <0.001 |
| BMI | 0.05 (0.00, 0.09) | 0.04 | 0.04 (−0.01, 0.09) | 0.10 |
| Age at baseline | −0.16 (−0.26, −0.06) | <0.01 | −0.17 (−0.27, −0.07) | 0.001 |
| Education | <0.001 | <0.001 | ||
| Some HS | Ref | Ref | ||
| HS grad | −2.13 (−3.57, −0.70) | 0.004 | −2.05 (−3.49, −0.60) | 0.005 |
| Some college | −2.77 (−4.18, −1.36) | <0.001 | −2.70 (−4.13, −1.28) | <0.001 |
| College grad | −3.76 (−5.28, −2.24) | <0.001 | −3.57 (−5.11, −2.04) | <0.001 |
| Grad school | −4.37 (−6.12, −2.62) | <0.001 | −4.18 (−5.95, −2.41) | <0.001 |
| Study center | 0.18 | 0.68 | ||
| Birmingham | Ref | Ref | ||
| Chicago | 1.01 (−0.06, 2.09) | 0.06 | 0.59 (−0.47, 1.66) | 0.27 |
| Minneapolis | 0.92 (−0.40, 2.23) | 0.17 | 0.64 (−0.67, 1.95) | 0.34 |
| Oakland | 0.17 (−0.84, 1.18) | 0.74 | 0.32 (−0.68, 1.33) | 0.53 |
| Exercise output, 100 units | −0.07 (−0.20, 0.07) | 0.34 | −0.06 (−0.20, 0.07) | 0.35 |
Multivariate mixed-effects model includes all listed covariates. Women were 20 to 32 y of age at baseline, followed through 48 to 60 y of age.
Abbreviations: grad, graduate; HS, high school; Ref, Referent.
In a model excluding women on hormonal contraceptives at baseline (35%), our primary results were unchanged.
We did not identify an association between androgens and CES-D scores nor between isolated hyperandrogenism and CES-D scores.
Finally, a sensitivity analysis incorporating a mixed-effects logistic regression model was performed to verify whether the observed differences in depression scores between women with and without PCOS further translated into differences in rates of positive depression screens, using a cut-off score of ≥16 (Table 6). In this analysis, we confirmed that women with PCOS were at ×2.1 increased odds of positive depression screens compared with women without PCOS (OR 2.11; 95% CI 1.24, 3.58; P < 0.01).
Table 6.
Mixed-Effects Logistic Regression Models Examining the Impact of PCOS Diagnosis on Positive Depression Screens (CES-D ≥ 16) Across the Lifespan
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| PCOS | 1.79 (1.02, 3.14) | 0.04 | 2.11 (1.24, 3.58) | <0.01 |
| Y | 0.98 (0.97, 0.99) | <0.001 | 0.97 (0.96, 0.98) | <0.001 |
| BMI, kg/m2 | 1.04 (1.02, 1.06) | <0.001 | 1.02 (1.01, 1.04) | <0.01 |
| Age, y at baseline | 0.93 (0.89, 0.97) | <0.001 | 0.95 (0.91, 0.99) | 0.01 |
| Race | <0.001 | 0.001 | ||
| Black | Ref | Ref | ||
| White | 0.38 (0.28, 0.51) | 0.58 (0.42, 0.79) | ||
| Education | <0.001 | <0.001 | ||
| Some HS | Ref | Ref | ||
| HS grad | 0.52 (0.31, 0.88) | 0.01 | 0.54 (0.32, 0.91) | 0.02 |
| Some college | 0.39 (0.23, 0.66) | <0.001 | 0.40 (0.24, 0.68) | 0.001 |
| College grad | 0.22 (0.13, 0.39) | <0.001 | 0.28 (0.16, 0.49) | <0.001 |
| Grad school | 0.17 (0.09, 0.30) | <0.001 | 0.23 (0.13, 0.41) | <0.001 |
| Study center | 0.25 | 0.95 | ||
| Birmingham | Ref | Ref | ||
| Chicago | 1.06 (0.69, 1.64) | 0.79 | 1.08 (0.72, 1.62) | 0.73 |
| Minneapolis | 0.74 (0.47, 1.16) | 0.18 | 0.98 (0.64, 1.52) | 0.93 |
| Oakland | 0.77 (0.51, 1.16) | 0.21 | 0.96 (0.65, 1.41) | 0.84 |
| Exercise output, 100 units | 0.92 (0.89, 0.97) | <0.001 | 0.95 (0.91, 0.99) | 0.03 |
Multivariate mixed-effects model includes all listed covariates. Women were 20 to 32 y of age at baseline, followed through 48 to 60 y of age.
Abbreviations: grad, graduate; HS, high school; Ref, Referent.
Discussion
PCOS, once considered primarily a gynecologic disorder, is becoming increasingly recognized as a multisystem syndrome with myriad clinical implications affecting the whole individual (22). Psychological comorbidities, including depression, are a major priority to address in both research and clinical realms to improve the quality of life women with PCOS (22).
Whereas important leaps forward have been made in characterizing the increased prevalence of depression in clinical PCOS cohorts, crucial gaps in the literature remain. One such void we sought to address in the current study is an evaluation of the depression burden of women with PCOS “in the wild.” The vast majority of studies that explore the psychological burdens of PCOS have been undertaken in populations of women presenting for clinical care. It has been suggested that physical stigmata of PCOS, such as hirsutism and overweight/obesity, or other associated clinical sequelae, such as infertility, contribute to a depressed mood in women with PCOS, and likewise, depressive symptoms may amplify how PCOS symptoms are experienced, making it more likely the patient will present for care (35, 36). Either way, investigations that focus only on women presenting to care for PCOS may provide an inflated estimate of depression compared with the PCOS population at large. Previous studies in community-based samples are limited by small sample sizes (37), self-report of PCOS diagnosis (13, 38), and cross-sectional design (13, 37, 38). We thus used the CARDIA cohort as a model for community-dwelling women with PCOS features, including oligomenorrhea and hyperandrogenism, to ascertain an improved estimate of depressive symptoms in women with PCOS over time.
Indeed, we observed a consistent elevation in CES-D scores across the lifespan in this unselected sample of women identified to have PCOS, independent of important potential confounders, including BMI. The magnitude of this difference was between two and three points on the CES-D scale. When we considered CES-D scores dichotomously using an established clinical cut-off score, we reiterated our findings of increasing depression risk among women with PCOS. Thus, our findings augment and extend the established literature regarding depression risk in clinical PCOS populations (8–12), strengthening the notion that depressed mood is a long-term psychological comorbidity of PCOS pathophysiology.
The prospective CARDIA cohort also served as a model for assessing the longitudinal course of depression symptoms in women with PCOS. To date, prior prospective longitudinal literature on psychological well-being in women with PCOS is extremely limited. One American study followed 60 women with PCOS for an average of 1.8 years and reported a stable depression prevalence of 33% and 40% at baseline and follow-up (14); average baseline age was 32 years. The data from our PCOS clinical cohort yielded strikingly similar results; prevalence of depression was 36% and 32% at baseline and follow-up, respectively, among 163 women followed prospectively for an average of 5.5 years at our center (15). Average baseline age was 29.0 years in our cohort (15). The only other published longitudinal data are derived from the aforementioned Northern Finland Birth Cohort 1966 in which women with self-reported oligomenorrhea and hirsutism had similar rates of depression at ages 31 and 46 years (16). Our present investigation challenges the prior literature, with instead, the observation of a continuous decline in depression scores over the course of 25 years. This difference might stem from the prolonged duration of follow-up and extended age range into the postmenopausal years (age 48 to 60 at time of final year-30 examination) in the current study. Our findings in the PCOS subjects mirrored the trajectory of decline in symptoms seen in non-PCOS, although PCOS subjects maintained increased depression scores compared with their peers across all study time points. Overall, our evidence for both populations echoes epidemiologic evidence that suggests depression risk progressively ameliorates with aging (17, 18), particularly after age 45 (18).
According to the 2018 Androgen Excess–PCOS Society evidence-based guideline, there has been “no study comparing two or more ethnicities using the same PCOS selection criteria and screening tools for depression.” The CARDIA cohort enabled a preliminary exploration of this issue in white and black Americans. Like other previous CARDIA follow-up studies (25, 29), we identified a disproportionate percentage of white women in the group classified as having PCOS, although our cohort selection strategy yielded a relatively more balanced PCOS cohort (60% white vs 70% white in prior reports). International reports on the prevalence of PCOS in varied populations have revealed impressively similar figures across groups, generally ranging between 6% and 9%, based on NIH criteria (1, 27, 39–41); some authors surmise that PCOS genotypes preceded the emergence of racial diversity accordingly (42). Thus, we cannot explain this racial difference in prevalence outside of perhaps random chance, small numbers, or a potential systematic difference in responding to survey questions regarding menstrual and/or unwanted hair histories.
However, racial disparities and the discrepancies in racial compositions of the PCOS and non-PCOS groups may have contributed to the surprising failure to identify a difference in BMI or other metabolic traits between PCOS and non-PCOS groups. Black women, on average, had significantly higher BMIs than white women (Table 3), with concordant deleterious effects on metabolic features. Given that the PCOS group was skewed toward white women and non-PCOS slightly toward black women, this may have partially masked PCOS-related metabolic alterations. Alternatively, the relative superior metabolic health prevalent at the time of baseline data collection (1987 to 1988) compared to present day may account for limited skew in metabolic parameters.
Regardless, our results suggest that PCOS has a similar impact on depression scores in both white and black women, increasing scores by a magnitude between two and three points above their non-PCOS peers. However, black women with and without PCOS experienced an approximate two-point upward shift in depression scores relative to their white counterparts across the lifespan (Fig. 3). These differences persisted after controlling for potential socioeconomic confounders, including education, exercise behavior, and BMI. The increased depression scores in black vs white Americans have been previously characterized in the CARDIA cohort (43–45) and the population at large (46–49); however, whether race modulates the possible impact of PCOS on depression risk scores has not been reported.
Additional targeted research should further clarify how the psychological sequelae of PCOS manifest in a variety of racial and ethnic groups. Providers should meanwhile acknowledge that whereas PCOS appears to play a similar role in the increase of CES-D scores in black and white women, black women may constitute a higher risk group for depression.
Strengths, limitations, and future directions
Considerable strengths of our study include its prospective design with long-term follow-up and notable retention across the lifespan. A large number of study participants were recruited at four representative, diverse locations across the United States, with excellent representation and inclusion of black women. Laboratory tests were systematically assayed at a single center; rigorous collection of clinical covariates was undertaken.
Limitations include factors inherent in reliance on self report for features of PCOS. Subjects may have been vulnerable to recall bias when reporting body hair and menstrual patterns between ages 20 and 30. Clinical data of hirsutism scores at baseline were unavailable. The use of serum androgens provided an additional corroborating diagnostic feature. Whereas the precise cut-off for assay percentile is a matter of some controversy (50), ours was set with priority placed on the identification of a cohort of women with PCOS features and a realistic prevalence for our results to be applicable to a U.S. population (27). In addition, oral contraceptive use at baseline might impact serum androgens. Yet, a sensitivity analysis excluding women on oral contraceptives yielded the same results. Furthermore, such sources of misclassification bias would bias toward the null. Another limitation is that the menstrual categories queried in the CWS questionnaire were somewhat cumbersome to apply to a PCOS case in that the longest cycle interval categories were 32 to 45 days or >45 days. Although we considered women in both categories to be oligomenorrheic, the former category bridges physiologic and oligo-ovulatory ranges. An additional limitation is the lack of contemporaneous transvaginal ultrasound data at the baseline examination to determine PCOS cases by Rotterdam criteria, a common clinical diagnostic approach today. As such, our findings cannot be generalized to a PCOS population identified by Rotterdam criteria. A questionnaire (CES-D) was used to evaluate depression symptoms rather than the gold standard structured clinical interview diagnostic. However, the CES-D is a reliable and validated instrument, extensively used to assess depression, with demonstrated sensitivity to changes in depression symptoms over time (33, 51–56). Finally, our data do not elucidate the potential role of menopausal symptoms on CES-D scores, which should be queried in subsequent research. Future prospective studies should involve rigorous clinician-administered diagnoses of both PCOS and depression, with transvaginal ultrasonography and structured clinical interviews, respectively.
In conclusion, in a large prospective cohort of population-based black and white women, not presenting to clinical care for treatment of bothersome PCOS symptoms, we identified an elevated depression symptom burden across the lifespan in women with PCOS compared with controls. Depression scores decreased with aging in both PCOS and non-PCOS groups. Black women had higher CES-D scores compared with white women, with and without PCOS. Our findings suggest an intrinsic process in PCOS pathophysiology that contributes to depressed mood.
Acknowledgments
This manuscript has been reviewed by CARDIA for scientific content.
Financial Support: The CARDIA study is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI), NIH, in collaboration with the University of Alabama at Birmingham (HHSN268201800005I and HHSN268201800007I), Northwestern University (HHSN268201800003I), University of Minnesota (HHSN268201800006I), and Kaiser Foundation Research Institute (HHSN268201800004I).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- BMI
body mass index
- CARDIA
Coronary Artery Risk Development in Young Adults
- CES-D
Center for Epidemiologic Studies-Depression
- coef
coefficient
- CWS
CARDIA Women’s Study
- NIH
U.S. National Institutes of Health
- PCOS
polycystic ovary syndrome
References and Notes
- 1. March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25(2):544–551. [DOI] [PubMed] [Google Scholar]
- 2. Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31(12):2841–2855. [DOI] [PubMed] [Google Scholar]
- 3. Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril. 2016;106(1):6–15. [DOI] [PubMed] [Google Scholar]
- 4. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25. [DOI] [PubMed] [Google Scholar]
- 5. Cassar S, Misso ML, Hopkins WG, Shaw CS, Teede HJ, Stepto NK. Insulin resistance in polycystic ovary syndrome: a systematic review and meta-analysis of euglycaemic-hyperinsulinaemic clamp studies. Hum Reprod. 2016;31(11):2619–2631. [DOI] [PubMed] [Google Scholar]
- 6. Rubin KH, Glintborg D, Nybo M, Abrahamsen B, Andersen M. Development and risk factors of type 2 diabetes in a nationwide population of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102(10):3848–3857. [DOI] [PubMed] [Google Scholar]
- 7. Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2010;16(4):347–363. [DOI] [PubMed] [Google Scholar]
- 8. Barry JA, Kuczmierczyk AR, Hardiman PJ. Anxiety and depression in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2011;26(9):2442–2451. [DOI] [PubMed] [Google Scholar]
- 9. Veltman-Verhulst SM, Boivin J, Eijkemans MJ, Fauser BJ. Emotional distress is a common risk in women with polycystic ovary syndrome: a systematic review and meta-analysis of 28 studies. Hum Reprod Update. 2012;18(6):638–651. [DOI] [PubMed] [Google Scholar]
- 10. Dokras A, Clifton S, Futterweit W, Wild R. Increased prevalence of anxiety symptoms in women with polycystic ovary syndrome: systematic review and meta-analysis. Fertil Steril. 2012;97(1):225–230.e2. [DOI] [PubMed] [Google Scholar]
- 11. Hollinrake E, Abreu A, Maifeld M, Van Voorhis BJ, Dokras A. Increased risk of depressive disorders in women with polycystic ovary syndrome. Fertil Steril. 2007;87(6):1369–1376. [DOI] [PubMed] [Google Scholar]
- 12. Cooney LG, Lee I, Sammel MD, Dokras A. High prevalence of moderate and severe depressive and anxiety symptoms in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2017;32(5):1075–1091. [DOI] [PubMed] [Google Scholar]
- 13. Damone AL, Joham AE, Loxton D, Earnest A, Teede HJ, Moran LJ. Depression, anxiety and perceived stress in women with and without PCOS: a community-based study [published online ahead of print 22 August 2018]. Psychol Med. doi: 10.1017/S0033291718002076. [DOI] [PubMed] [Google Scholar]
- 14. Kerchner A, Lester W, Stuart SP, Dokras A. Risk of depression and other mental health disorders in women with polycystic ovary syndrome: a longitudinal study. Fertil Steril. 2009;91(1):207–212. [DOI] [PubMed] [Google Scholar]
- 15. Greenwood EA, Pasch LP, Shinkai K, Cedars MI, Huddleston HG. Clinical course of depression symptoms and predictors of enduring depression risk in women with polycystic ovary syndrome: results of a longitudinal study. Fertil Steril. 2019;111(1):147–156. [DOI] [PubMed] [Google Scholar]
- 16. Karjula S, Morin-Papunen L, Auvinen J, Ruokonen A, Puukka K, Franks S, Järvelin MR, Tapanainen JS, Jokelainen J, Miettunen J, Piltonen TT. Psychological distress is more prevalent in fertile age and premenopausal women with PCOS symptoms: 15-year follow-up. J Clin Endocrinol Metab. 2017;102(6):1861–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eaton WW, Anthony JC, Gallo J, Cai G, Tien A, Romanoski A, Lyketsos C, Chen LS. Natural history of diagnostic interview schedule/DSM-IV major depression. The Baltimore Epidemiologic Catchment Area follow-up. Arch Gen Psychiatry. 1997;54(11):993–999. [DOI] [PubMed] [Google Scholar]
- 18. Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS; National Comorbidity Survey Replication. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289(23):3095–3105. [DOI] [PubMed] [Google Scholar]
- 19. Brown ZA, Louwers YV, Fong SL, Valkenburg O, Birnie E, de Jong FH, Fauser BC, Laven JS. The phenotype of polycystic ovary syndrome ameliorates with aging. Fertil Steril. 2011;96(5):1259–1265. [DOI] [PubMed] [Google Scholar]
- 20. Hsu MI. Changes in the PCOS phenotype with age. Steroids. 2013;78(8):761–766. [DOI] [PubMed] [Google Scholar]
- 21. Johnstone EB, Davis G, Zane LT, Cedars MI, Huddleston HG. Age-related differences in the reproductive and metabolic implications of polycystic ovarian syndrome: findings in an obese, United States population. Gynecol Endocrinol. 2012;28(10):819–822. [DOI] [PubMed] [Google Scholar]
- 22. Dokras A, Stener-Victorin E, Yildiz BO, Li R, Ottey S, Shah D, Epperson N, Teede H. Androgen Excess-Polycystic Ovary Syndrome Society: position statement on depression, anxiety, quality of life, and eating disorders in polycystic ovary syndrome. Fertil Steril. 2018;109(5):888–899. [DOI] [PubMed] [Google Scholar]
- 23. Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, Piltonen T, Norman RJ; International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2018;33(9):1602–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR Jr, Liu K, Savage PJ. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105–1116. [DOI] [PubMed] [Google Scholar]
- 25. Wang ET, Calderon-Margalit R, Cedars MI, Daviglus ML, Merkin SS, Schreiner PJ, Sternfeld B, Wellons M, Schwartz SM, Lewis CE, Williams OD, Siscovick DS, Bibbins-Domingo K. Polycystic ovary syndrome and risk for long-term diabetes and dyslipidemia. Obstet Gynecol. 2011;117(1):6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome; towards a rational approach. In: Dunaif A, Givens JR, Haseltine F, eds. Polycystic Ovary Syndrome. Boston, MA: Blackwell Scientific; 1992:377–384. [Google Scholar]
- 27. Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89(6):2745–2749. [DOI] [PubMed] [Google Scholar]
- 28. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666–3672. [DOI] [PubMed] [Google Scholar]
- 29. Wang ET, Ku IA, Shah SJ, Daviglus ML, Schreiner PJ, Konety SH, Williams OD, Siscovick D, Bibbins-Domingo K. Polycystic ovary syndrome is associated with higher left ventricular mass index: the CARDIA women’s study. J Clin Endocrinol Metab. 2012;97(12):4656–4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. National Heart Lung, and Blood Institute (NHLBI) of the National Institutes of Health (NIH). CARDIA: Coronary Artery Risk Development in Young Adults. Available at: https://www.cardia.dopm.uab.edu/. Accessed 30 October 2018.
- 31. Folsom AR, Burke GL, Ballew C, Jacobs DR , Jr, Haskell WL, Donahue RP, Liu KA, Hilner JE. Relation of body fatness and its distribution to cardiovascular risk factors in young blacks and whites. The role of insulin. Am J Epidemiol. 1989;130(5):911–924. [DOI] [PubMed] [Google Scholar]
- 32. Sidney S, Jacobs DR , Jr, Haskell WL, Armstrong MA, Dimicco A, Oberman A, Savage PJ, Slattery ML, Sternfeld B, Van Horn L. Comparison of two methods of assessing physical activity in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol. 1991;133(12):1231–1245. [DOI] [PubMed] [Google Scholar]
- 33. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 34. Roth DL, Ackerman ML, Okonkwo OC, Burgio LD. The four-factor model of depressive symptoms in dementia caregivers: a structural equation model of ethnic differences. Psychol Aging. 2008;23(3):567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pasch L, He SY, Huddleston H, Cedars MI, Beshay A, Zane LT, Shinkai K. Clinician vs self-ratings of hirsutism in patients with polycystic ovarian syndrome: associations with quality of life and depression. JAMA Dermatol. 2016;152(7):783–788. [DOI] [PubMed] [Google Scholar]
- 36. Greenwood EA, Pasch LA, Cedars MI, Legro RS, Huddleston HG; Eunice Kennedy Shriver National Institute of Child Health and Human Development Reproductive Medicine Network. Association among depression, symptom experience, and quality of life in polycystic ovary syndrome. Am J Obstet Gynecol. 2018;219(3):279.e1–279.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jedel E, Waern M, Gustafson D, Landén M, Eriksson E, Holm G, Nilsson L, Lind AK, Janson PO, Stener-Victorin E. Anxiety and depression symptoms in women with polycystic ovary syndrome compared with controls matched for body mass index. Hum Reprod. 2010;25(2):450–456. [DOI] [PubMed] [Google Scholar]
- 38. Deeks AA, Gibson-Helm ME, Paul E, Teede HJ. Is having polycystic ovary syndrome a predictor of poor psychological function including anxiety and depression? Hum Reprod. 2011;26(6):1399–1407. [DOI] [PubMed] [Google Scholar]
- 39. Asunción M, Calvo RM, San Millán JL, Sancho J, Avila S, Escobar-Morreale HF. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab. 2000;85(7):2434–2438. [DOI] [PubMed] [Google Scholar]
- 40. Diamanti-Kandarakis E, Kouli CR, Bergiele AT, Filandra FA, Tsianateli TC, Spina GG, Zapanti ED, Bartzis MI. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: hormonal and metabolic profile. J Clin Endocrinol Metab. 1999;84(11):4006–4011. [DOI] [PubMed] [Google Scholar]
- 41. Moran C, Tena G, Moran S, Ruiz P, Reyna R, Duque X. Prevalence of polycystic ovary syndrome and related disorders in mexican women. Gynecol Obstet Invest. 2010;69(4):274–280. [DOI] [PubMed] [Google Scholar]
- 42. Azziz R, Dumesic DA, Goodarzi MO. Polycystic ovary syndrome: an ancient disorder? Fertil Steril. 2011;95(5):1544–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Whooley MA, Kiefe CI, Chesney MA, Markovitz JH, Matthews K, Hulley SB; CARDIA Study. Depressive symptoms, unemployment, and loss of income: The CARDIA Study. Arch Intern Med. 2002;162(22):2614–2620. [DOI] [PubMed] [Google Scholar]
- 44. Henderson C, Diez Roux AV, Jacobs DR , Jr, Kiefe CI, West D, Williams DR. Neighbourhood characteristics, individual level socioeconomic factors, and depressive symptoms in young adults: the CARDIA study. J Epidemiol Community Health. 2005;59(4):322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Davidson K, Jonas BS, Dixon KE, Markovitz JH. Do depression symptoms predict early hypertension incidence in young adults in the CARDIA study? Arch Intern Med. 2000;160(10):1495–1500. [DOI] [PubMed] [Google Scholar]
- 46. George LK, Lynch SM. Race differences in depressive symptoms: a dynamic perspective on stress exposure and vulnerability. J Health Soc Behav. 2003;44(3):353–369. [PubMed] [Google Scholar]
- 47. Skarupski KA, Mendes de Leon CF, Bienias JL, Barnes LL, Everson-Rose SA, Wilson RS, Evans DA. Black-white differences in depressive symptoms among older adults over time. J Gerontol B Psychol Sci Soc Sci. 2005;60(3):136–142. [DOI] [PubMed] [Google Scholar]
- 48. Cochran DL, Brown DR, McGregor KC. Racial differences in the multiple social roles of older women: implications for depressive symptoms. Gerontologist. 1999;39(4):465–472. [DOI] [PubMed] [Google Scholar]
- 49. Spence NJ, Adkins DE, Dupre ME. Racial differences in depression trajectories among older women: socioeconomic, family, and health influences. J Health Soc Behav. 2011;52(4):444–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ntumy M, Maya E, Lizneva D, Adanu R, Azziz R. The pressing need for standardization in epidemiologic studies of PCOS across the globe. Gynecol Endocrinol. 2019;35(1):1–3. [DOI] [PubMed] [Google Scholar]
- 51. Chin WY, Choi EP, Chan KT, Wong CK. The psychometric properties of the Center for Epidemiologic Studies Depression Scale in Chinese Primary Care Patients: factor structure, construct validity, reliability, sensitivity and responsiveness. PLoS One. 2015;10(8):e0135131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Myers JK, Weissman MM. Use of a self-report symptom scale to detect depression in a community sample. Am J Psychiatry. 1980;137(9):1081–1084. [DOI] [PubMed] [Google Scholar]
- 53. Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol. 1977;106(3):203–214. [DOI] [PubMed] [Google Scholar]
- 54. Barry LC, Abou JJ, Simen AA, Gill TM. Under-treatment of depression in older persons. J Affect Disord. 2012;136(3):789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li X, Wang W, Gao Q, Wu L, Luo Y, Tang Z, Guo X. The trajectories and correlation between physical limitation and depression in elderly residents of Beijing, 1992–2009. PLoS One. 2012;7(8):e42999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Giese-Davis J, Collie K, Rancourt KM, Neri E, Kraemer HC, Spiegel D. Decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer: a secondary analysis. J Clin Oncol. 2011;29(4):413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]