Abstract
FSH glycosylation varies in two functionally important aspects: microheterogeneity, resulting from oligosaccharide structure variation, and macroheterogeneity, arising from partial FSHβ subunit glycosylation. Although advances in mass spectrometry permit extensive characterization of FSH glycan populations, microheterogeneity remains difficult to illustrate, and comparisons between different studies are challenging because no standard format exists for rendering oligosaccharide structures. FSH microheterogeneity is illustrated using a consistent glycan diagram format to illustrate the large array of structures associated with one hormone. This is extended to commercially available recombinant FSH preparations, which exhibit greatly reduced microheterogeneity at three of four glycosylation sites. Macroheterogeneity is demonstrated by electrophoretic mobility shifts due to the absence of FSHβ glycans that can be assessed by Western blotting of immunopurified FSH. Initially, macroheterogeneity was hoped to matter more than microheterogeneity. However, it now appears that both forms of carbohydrate heterogeneity have to be taken into consideration. FSH glycosylation can reduce its apparent affinity for its cognate receptor by delaying initial interaction with the receptor and limiting access to all of the available binding sites. This is followed by impaired cellular signaling responses that may be related to reduced receptor occupancy or biased signaling. To resolve these alternatives, well-characterized FSH glycoform preparations are necessary.
FSH is a pituitary gonadotropin that plays a central role in reproduction. In females, FSH stimulates antrum formation in secondary follicles, growth and maturation in antral follicles, and it prepares the latter for ovulation in response to the LH surge (1). In males, FSH stimulates Sertoli cell proliferation during testicular development and maintains Sertoli cell function in the mature testis (2). Whereas FSH is essential for female fertility, in some species, such as mice, FSH is clearly not essential in the male, as Fshb-null males retain complete fertility (3). In other species, such as humans, FSH may be required for male fertility, as some men deficient in FSH or in FSH receptor (FSHR) function are azoospermatic (4). However, others produce sperm, making the necessity for FSH action in the human testis uncertain (5). In recent years FSH has been suggested to contribute to bone loss in rodent models (6) and perhaps in humans. Recently, it has been proposed to play a role in promoting obesity (7). FSH involvement in these nontraditional targets is controversial (8), yet may be affected by FSH glycosylation.
FSH biological activity in the gonads is dependent on glycosylation, not only as a critical factor in determining survival in the circulation (9), but also in stimulating cellular signaling via FSHR activation, particularly when signaling through the Gαs/cAMP/PKA pathway (10, 11). Very few data exist for the impact of FSH glycosylation on other, more recently identified FSHR-activated pathways (12, 13). FSH glycosylation appears to change in response to changing physiological states, including sex (14), puberty (15, 16), reproductive cycles (17, 18), as well as with age (14, 19, 20). Accordingly, the biologic activity of circulating FSH can vary under changing physiological conditions (21–26). The mechanisms underlying carbohydrate modulation of FSH activity are poorly understood. A major challenge is the heterogeneous population of oligosaccharides found in FSH preparations. The lack of a consensus format for representing the patterns of branching and branch termination, which are the two major sources of oligosaccharide structural variation, makes it difficult to compare one study with another. Glycan structural diagrams combining monosaccharide symbols proposed by the Consortium for Functional Glycomics (27) with solid and dashed lines at defined angles representing linkage as employed by the Oxford Glycobiology Institute (28, 29) will be used throughout. This hybrid format is an accepted alternative to the Consortium for Functional Glycomics system (27).
Classic FSH isoforms were thought to reflect only oligosaccharide negatively charged microheterogeneity reflecting sialic acid content (30–32). However, sulfate and phosphate provide alternative negatively charged groups, although sialic acid predominates. Moreover, several oligosaccharide structures can possess the same negative charge. Macroheterogeneity, resulting from the absence of one FSHβ N-glycan, provides another mechanism for significantly altering FSH glycosylation (33, 34). FSH variants resulting from macroheterogeneity are designated glycoforms (35, 36). The challenge provided by glycoforms is that there is no direct correspondence between them and classic charge-based FSH isoforms (32).
FSH Peptide and Carbohydrate Moieties
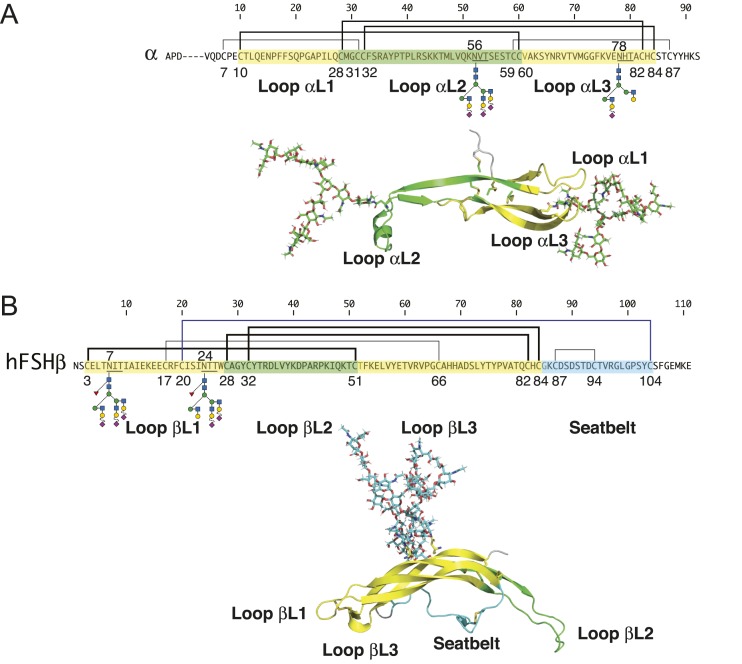
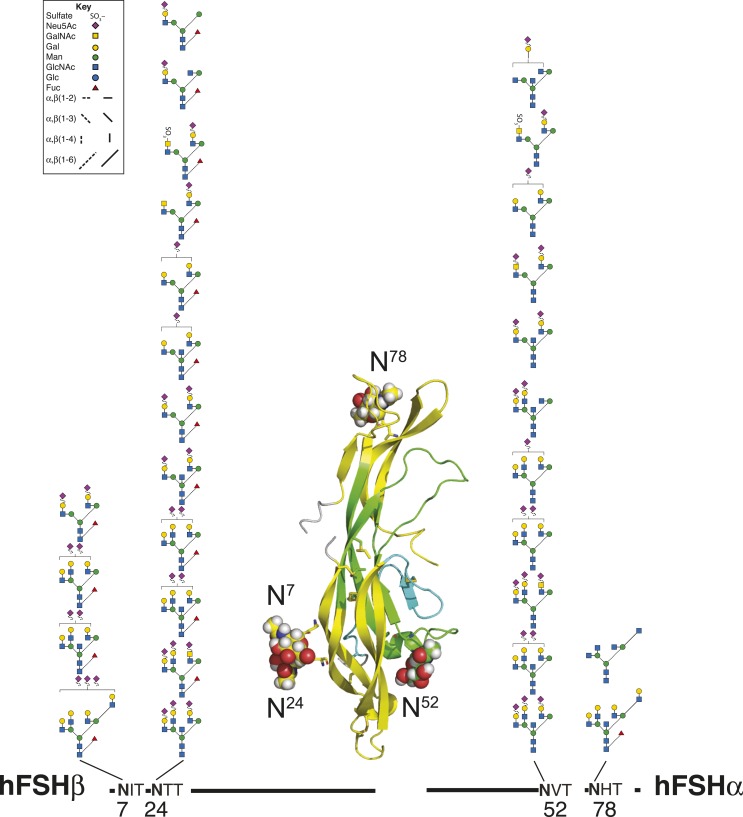
FSH is a heterodimeric glycoprotein comprised of a common α-subunit noncovalently associated with a hormone-specific FSHβ subunit (37). Both subunits are N-glycosylated and, similar to other cystine knot proteins (38), their tertiary structure is dominated by three loops defined by the three Cys knot motif disulfide bonds (Fig. 1). Two loops, designated L1 and L3, are located on one side of the Cys knot, whereas the middle loop, L2, is found on the opposite side. FSHβ also possesses a fourth loop, the so-called seatbelt loop, which is involved in heterodimer formation. The FSH heterodimer comprises antiparallel subunits stabilized by the FSHβ seatbelt loop embracing the FSHα L2 loop. Both α-subunit N-glycans are found on different loops, αL2 and αL3, in the C-terminal half of the primary structure (Fig. 1A), but at opposite ends of the tertiary structure long axis. As these glycans extend the long axis of the elliptically shaped FSH heterodimer (38), their orientation along this axis may account for the nonsignificant increases in metabolic clearance when either one or both are deleted from FSH derivatives (9). Both FSHβ N-glycans are located in the βL1 loop in the N-terminal quarter of the primary structure and side by side in the tertiary structure (Fig. 1B), where they more than double the narrow axis of FSH. Each FSHβ oligosaccharide contributes significantly to the circulatory survival of FSH, and the combined effects are synergistic (9). Although the α-subunit polypeptide moieties derived from the common CGA gene are identical for FSH and the other glycoprotein hormones, LH, TSH, and chorionic gonadotropin (CG), the oligosaccharide populations decorating both N-glycosylation sites distinguish FSHα from α-subunits derived from these other glycoprotein hormones as well as the free α-subunit (39, 40).
Figure 1.
FSH subunit primary structures. (A) FSHα primary and tertiary structures. (B) FSHβ primary and tertiary structures. Amino acid sequences of FSHα and FSHβ subunits indicated by single-letter amino acid code. Cystine knot loops in primary and tertiary structures are indicated by yellow highlighting for loops L1 and L3 and green for loop L2. The seatbelt loop is colored cyan. Lines above the subunit sequences connecting Cys residues (C) indicate disulfide bonds. Cys residue numbers are shown below. Cys knot disulfides are indicated by thick lines. N-glycosylation site sequons of the type NXT (N, Asn; X, any residue except Pro; T, Thr) are underlined, and oligosaccharide structures are shown below each subunit. Key to oligosaccharide structures can be found in Fig. 3. FSH subunit three-dimensional structures were extracted from pdb file 4ay9 using PyMol. Known FSH glycans were added to the FSH polypeptide backbone using GLYCAM.
FSH Glycan Functions Revealed by Deglycosylation or Mutation
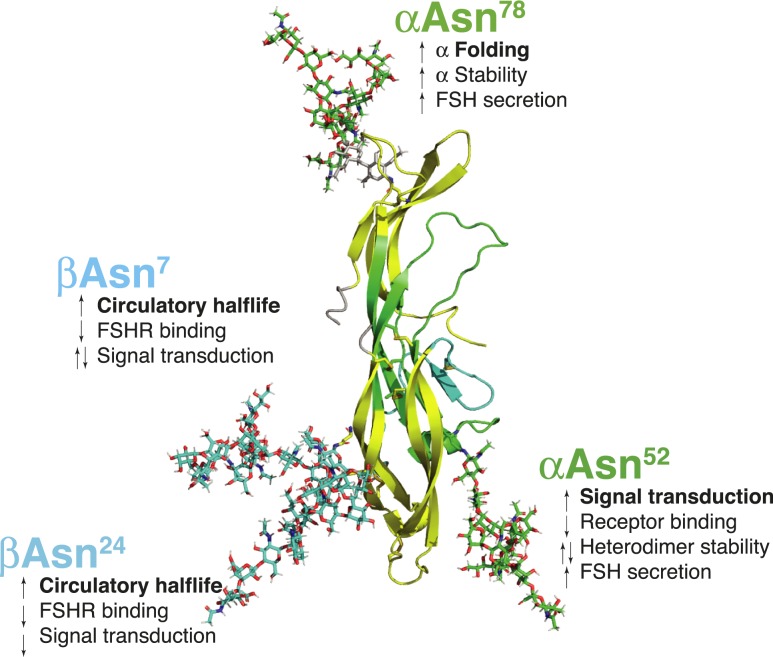
Functions of individual FSH glycans at each of four N-glycosylation sites are summarized in Fig. 2. FSHα subunit oligosaccharides are essential for α-subunit folding (41), α-subunit stability (42), heterodimer stability (43), full FSHR activation (10, 44, 45), and at least one may modulate FSHR binding (10, 44). FSHβ subunit N-glycans both contribute significantly to metabolic clearance (9). Conflicting reports exist for the effects of these glycans on FSHR binding and signaling (9–11, 44).
Figure 2.
Functions of specific Asn-linked oligosaccharides in FSH. Polypeptide moiety of FSH extracted from pdb file 1ay9 and the heterodimer rendered as cartoon using PyMol. The subunits are colored as in Fig. 1 (yellow indicates cystine knot loops L1 and L3, cyan indicates the seatbelt loop, green indicates remaining FSHα and FSHβ). Oligosaccharides are rendered as sticks colored green for αAsn52 and αAsn78, cyan for βAsn7 and βAsn24.
FSH Glycosylation Microheterogeneity
FSH microheterogeneity results in size and charge variants of the hormone called FSH isoforms (46–49). Early FSH oligosaccharide structure reports described 11 to 30, largely complex-type glycans, possessing two, three, or four branches, some of which lacked terminal sialic acid residues, and most of which existed as both fucosylated and nonfucosylated forms (50–52). Some low-abundance glycans were hybrid types, which are more commonly associated with LH and TSH (51, 52).
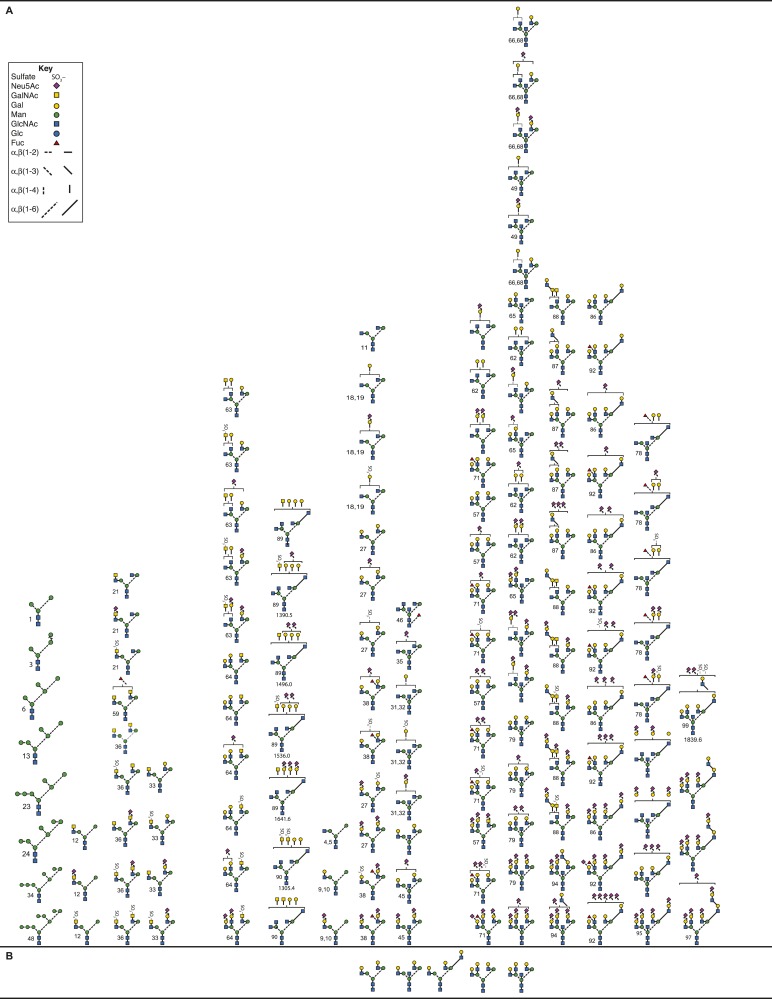
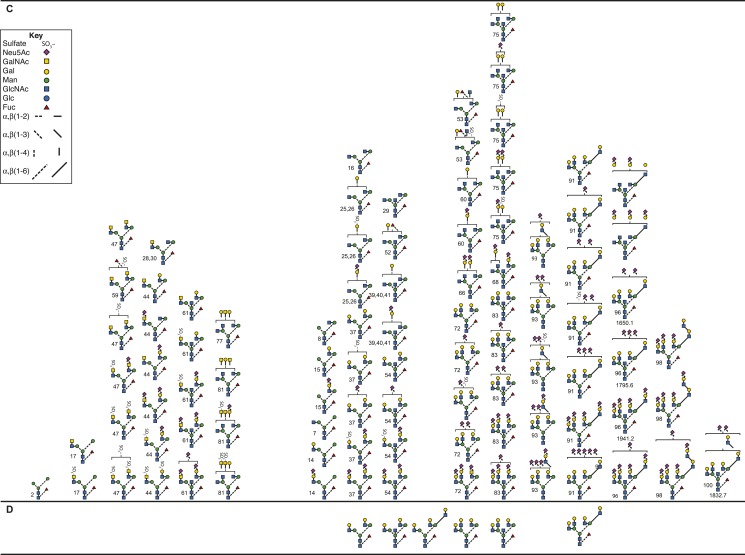
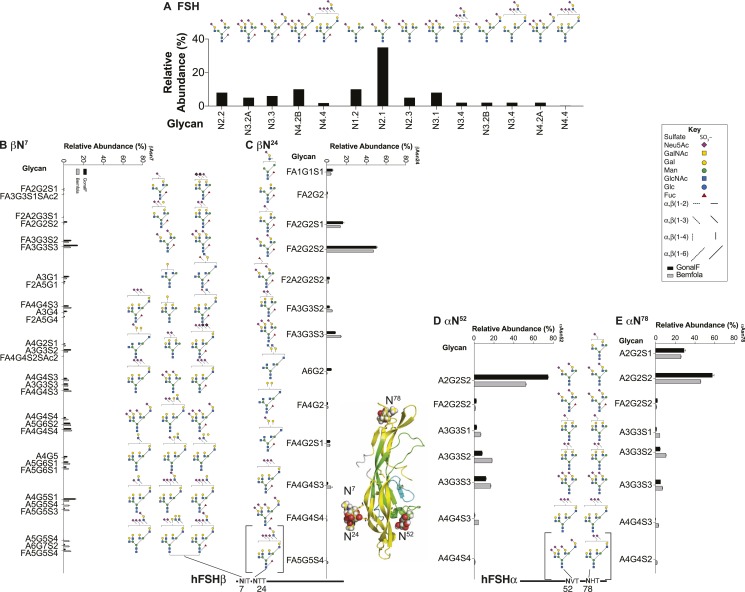
Pituitary FSH, pituitary FSH glycoform, and urinary FSH glycans released from reduced, carboxymethylated samples by PNGaseF digestion, analyzed by nano-electrospray ionization mass spectrometry, revealed an even greater variety of human FSH (hFSH) glycan structures (53, 54). However, the predominance of complex-type glycans, representing 85% to 97% of each population, was confirmed [Table 1 (53, 55–59)]. At least 230 oligosaccharide structures were proposed, including sialylated and sulfated variants of >80 neutral core glycans (Fig. 3). The actual neutral core structures of biantennary or triantennary glycans were detected, albeit in low abundance, but no asialo-tetra-antennary glycans were encountered. Because several oligosaccharide structures can possess the same combination of monosaccharides, there were fewer glycan ions in the spectra than proposed structures. Fragmentation analysis used to confirm ∼70% of the oligosaccharide structures revealed that many of the alternative core glycan structures existed in one FSH glycan preparation or another. Charged glycan variants averaged more than three per core structure, based largely on the number of negatively charged, terminal sialic acid and sulfate groups. Additional heterogeneity arises from N-acetyl-neuraminic acid (Neu5Ac) residue attachment by two different linkages, either α2–3 or α2–6. The latter predominates in pituitary hFSH (52). Sialic acid linkage has been implicated in FSH clearance in rodents, as α2–3-linked Neu5Ac prevents the liver asialoglycoprotein receptor from binding Gal residues in glycans, whereas α2–6-linked Neu5Ac residue does not (60, 61).
Table 1.
Macroheterogeneity and Microheterogeneity in Pituitary, Urinary, and Recombinant hFSH Preparations
| FSH Preparation | Pituitary hFSH (53) | Urinary hFSH (53) | Urinary hFSH (59) | Recombinant GH3-hFSH (55) | Recombinant CHO hFSH (58) | GonalF (56) | GonalF (57) | (Bemfola) (56) | Bemfola (57) | Puregon-HP (59) |
|---|---|---|---|---|---|---|---|---|---|---|
| Glycoform Abundance, % | ||||||||||
| FSH24 | 77 | 86 | +a | 45 | —b | 78.1 | — | — | — | — |
| FSH21 | 23 | 14 | + | 55 | — | 15.8 | — | — | — | — |
| FSH18 | — | — | — | — | — | 6.1 | — | — | — | — |
| FSH15 | — | — | — | — | — | — | — | — | — | — |
| No. oligosaccharide structures | 80 | 67 | 20 | 33 | 10 | 23 | 38 | 27 | 39 | 17 |
| Oligosaccharide Type Abundance, % | ||||||||||
| Oligomannose | 0.3 | 0 | — | 0 | — | 0 | — | — | — | — |
| Complex | 94.2 | 95.9 | 82.6 | 85.3 | 91 | 96.7 | 93.9 | 93.9 | 90 | 94.6 |
| Biantennary | 38.2 | 37.2 | 56.4 | 55.5 | 53 | 63.7 | 60.4 | 53.9 | 47.8 | 72.1 |
| Triantennary(3)c | 41.0 | 44.0 | 26.1 | — | 18 | 21.0 | 22.7 | 23.4 | 26.9 | 12.3 |
| Triantennary(6)d | — | — | — | 29.7 | 8 | — | — | — | — | |
| Tetra-antennary | 15.0 | 14.8 | — | 0 | 12 | 12.0 | 10.8 | 16.6 | 15.3 | — |
| Neutral | 0.3 | 2.2 | — | 12.3 | — | 1.5 | 5.1 | 2.0 | 10.2 | |
| Sialylated | 99.1 | 97.5 | 100 | 87.7 | 91 | 96.7 | 94.4 | 96.7 | 97.8 | 89.8 |
| O-Ac-Neu5Ac or Neu5Gc | — | — | — | — | — | — | 0.1 | — | — | 1.5 |
| Sulfated | 6.5 | 4.2 | — | — | — | — | — | — | — | 3.7 |
| Sialylated/sulfated | 5.9 | 3.9 | — | — | — | — | — | — | — | — |
| Core fucose | 43.0 | 23.9 | 17.0 | 50.6 | 29 | 32.9 | 36.4 | 30.5 | 36.9 | 13.7 |
| Antenna fucose | 0.3 | — | 3.6 | 19.9 | — | — | 2.4 | — | 3.0 | 5.1 |
| Bisect GlcNAc | 32.6 | 23.9 | 13.1 | 47.0 | — | — | — | — | — | — |
| GalNAc | 2.8 | 1.7 | — | 10.5 | — | — | — | — | — | — |
| Lactosamine | 2.1 | 1.3 | — | 3.9 | 5 | 3.0 | 10.0 | 10.1 | 12.6 | — |
Qualitative detection only.
Either not detected or not reported.
Third antenna on Man3 branch.
Third antenna on Man6 branch.
Figure 3.
Pituitary FSH glycans. (A) Enzymatically released human FSH oligosaccharides characterized by nano-electrospray mass spectrometry (53, 54). Only the nonfucosylated forms are shown. Of these, ∼90 have fucosylated counterparts (see Fig. 5C). (B) Chemically released, nonfucosylated human FSH oligosaccharide core structures (50). (C) Enzymatically released human FSH oligosaccharides characterized by nano-electrospray mass spectrometry (53, 54). A total of 91 structures were identified, most of which had nonfucosylated counterparts (Fig. 5A). (D) Chemically released, fucosylated human FSH oligosaccharides (50).
Of the 230 FSH glycans proposed, 139 lacked core-linked fucose (Fig. 3A), whereas 91 were core-fucosylated (Fig. 3C). Virtually all of the core-fucosylated oligosaccharides had nonfucosylated counterparts. There were 100 derivatives of the 11 desialylated FSH oligosaccharide structures initially reported (50). Some glycans exhibited partially synthesized antennae consisting of either unterminated Gal residues, or just the branch initiating GlcNAc residue. Oligosaccharides not reported before included oligomannose-type, either not encountered (50) or present in uncharacterized neutral oligosaccharide fractions (51, 52). These were well characterized owing to their high abundance in the partially glycosylated hFSH variant isolated from purified human LH (hLH) preparations (54) and represent intermediates between the original Glc3Man9GlcNAc2 oligosaccharide transferred en bloc to FSH subunits in the endoplasmic reticulum (ER) and complex glycans assembled in the Golgi cisternae. Hybrid-type glycans fell into two groups, one possessing GalNAc in the complex branch, whereas the others possessed Gal. Complex-type biantennary, triantennary, and tetra-antennary glycans possessing one or more GalNAc residues instead of Gal in at least one antenna were novel. Biantennary and triantennary GalNAc glycans were found in both nonfucosylated and core-fucosylated structures. Tetraantennary GalNAc-including glycans all lacked fucose. The biantennary group included those with GalNAc on both antennae, or GalNAc on one, usually Man3, with Gal on the Man6. The latter group were found in the population possessing core-linked fucose (Fig. 3C), but not in the group lacking core fucose (Fig. 3A). Triantennary glycans in both groups possessed a single GalNAc residue either Man3 or Man6 linked. Tetraantennary glycans were only found in the population lacking core fucose and possessed either one or two GalNAc residues. Lactosamine repeats are often attached to the GlcNAc β1–6 linked to the Man6 branch (62). FSH lactosamine repeats, found on some of the triantennary and tetra-antennary glycans, were located on either Man3 or Man6 branches. Triantennary glycans with the third antenna on the Man6 branch were rare. In contrast, these were the only triantennary glycans observed in recombinant hFSH produced by the rat GH3 cell line [Table 1 and Bousfield et al. (54) and Butnev et al. (55)]. Curiously, sulfate was observed in a few oligosaccharides possessing Gal instead of GalNAc. Additionally, several oligosaccharides possessed one more Neu5Ac residue than did terminal Gal or GalNAc residues in complex branches. Perhaps additional sialic acid residues were linked to GlcNAc residues, as in bovine FSH (51).
Although mass spectrometry greatly expanded the extent of microheterogeneity associated with FSH oligosaccharides, positional information was completely absent. Core fucosylation is typical of >95% of oligosaccharides released from FSHβ, yet present in <5% of those from FSHα. More precise positional information has been obtained by analysis of glycopeptides containing each of the N-glycosylation sites and their attached oligosaccharides. Proteinase K digestion released three– to four–amino acid residue glycopeptides from reduced, carboxymethylated FSH samples (63). Gel filtration eliminated peptides that interfered with mass spectrometry analysis (63). All but Asn-His-Thr were detected by electrospray mass spectrometry. Only a glycosylated tetrapeptide, Glu-Asn-His-Thr, was detected in a subsequent study (32). Overall, 29 glycans were localized: 4 on βAsn7, 12 on βAsn24, 11 on αAsn52, and 2 on αAsn78 (Fig. 4). Twenty-four unique structures were observed, as compared with 230 in the oligosaccharide mass spectra. The uneven yield of site-specific glycans made general conclusions rather tentative, however, with one exception, that is, the restriction of core fucose to FSHβ glycans was supported. The association of greater branching at βAsn7 was suggested by the presence of tetra-antennary glycans, which were absent from the largely biantennary and triantennary βAsn24 and αAsn52 oligosaccharide populations. Proteinase K digestion proved inadequate for LH and CG β-subunits, which possess a high Pro content, thereby making them resistant to proteolysis (63, 64). Better coverage may be obtained from digesting reduced, carboxamidomethylated FSH with chymotrypsin (56) or mixtures of specific and nonspecific proteases (65).
Figure 4.
Site-specific analysis of pituitary FSH N-glycosylation by glycopeptide mass spectrometry. Reduced carboxymethylated hFSH was exhaustively digested with 10% (w/w) proteinase K, and glycopeptides were isolated by gel filtration and analyzed by electrospray mass spectrometry. Composite results are from an analysis of purified hFSH and purified hFSH isoforms separated by chromatofocusing (32, 63).
Recombinant hFSH preparations are currently more readily available than pituitary hFSH and exhibit greater biological activities (14,403 IU/mg) (57) than the latter (National Hormone and Pituitary Program, 8560 IU/mg). This appears partly due to greater FSH content in recombinant FSH preparations as compared with pituitary FSH preparations (55), but it may also reflect reduced glycan branching in the former (Table 1).
PNGaseF-released oligosaccharides from Chinese hamster ovary (CHO) cell–expressed, recombinant hFSH provided by Organon, were characterized by nuclear magnetic resonance (NMR) (58). Biantennary, triantennary (both Man3 and Man6 types), and tetra-antennary oligosaccharide structures possessing α2–3-linked Neu5Ac comprised 91% of the carbohydrate recovered (Fig. 5A). The α2–6-linked Neu5Ac, which comprised the majority in pituitary (52) and urinary hFSH oligosaccharides (59), was not detected. A disialylated, biantennary glycan was the most abundant nonfucosylated structure. The absence of bisecting GlcNAc residues indicated no GlcNAc transferase III activity (66). The relative abundance of nonfucosylated oligosaccharides was 62%, more than twice that of the 29% core-fucosylated structures. Subsequent FSHβ glycopeptide mass spectrometry revealed that βAsn7 possessed both types of glycans [Fig. 5B and Grass et al. (56) and Mastrangeli et al. (57)]. One or more lactosamine repeats were associated with triantennary or tetra-antennary oligosaccharides comprising 4% of the glycans; however, structures were not proposed, and the diagrams in Fig. 5A merely illustrate the glycan types incorporating lactosamine repeats.
Figure 5.
Analysis of recombinant CHO-expressed hFSH glycosylation by NMR and glycopeptide mass spectrometry. (A) PNGaseF-released oligosaccharides from recombinant FSH provided by Organon. Structures were determined by NMR (58). Glycans with lactosamine repeats were detected, but complete structures were not defined. The bar graph shows relative glycan abundance determined by NMR. (B–E) Reduced, carboxamidomethylated recombinant hFSH samples were digested with 5% (w/w) chymotrypsin and glycopeptides analyzed by ethylene bridged hybrid amide chromatography–electrospray ionization mass spectrometry (57). Oligosaccharide structural diagrams are shown, and the adjacent graphs show relative abundance of each. The bars are means of either duplicate (Bemfola) or triplicate (GonalF) determinations (±SD). (B) GonalF and Bemfola βAsn7 glycosylation. (C) βAsn24 glycosylation. (D) αAsn52 glycosylation. (E) αAsn78 glycosylation. The bracketed oligosaccharides were found in Bemfola but not in GonalF. The remainder were found in both.
Mass spectrometry has been employed to characterize total glycans released from recombinant FSH (67), recombinant FSHα and FSHβ subunits (68), and chymotryptic peptides derived from reduced, carboxamidomethylated preparations (56, 57, 59). Two of the latter studies evaluated glycopeptides in three batches of GonalF and one or two batches of the biosimilar Bemfola (56, 57). The results of the second study are summarized in Fig. 5B–5E. The glycan populations of GonalF and Bemfola were qualitatively similar in that the latter possessed only three glycans not found in GonalF (Fig. 5C–5E). Quantitatively, Bemfola possessed more triantennary and tetra-antennary glycans than did GonalF (Table 1). By far, the most abundant oligosaccharides attached to αAsn52, αAsn78, and βAsn24 were disialylated, biantennary, differing only in core fucose, which the FSHβ glycan possessed and both FSHα glycans lacked (Fig. 5B–5E). Only 9 glycan structures were found on FSHα, whereas βAsn24 possessed 12 glycans and βAsn7 had 29. The latter included significant amounts of tetra-antennary glycans, particularly Bemfola, which exhibited a reduction in biantennary glycans as a result of being better endowed with triantennary and tetra-antennary glycans. Triantennary glycans possessing a lactosamine repeat, which had been identified in an earlier FSH study by fast atom bombardment mass spectrometry fragment ions at m/z 1274 and 913 representing sialic acid1–hexose2–N-acetylhexosamine2 and hexose2–N-acetylhexosamine2 fragments (68), were localized to βAsn7. Tetra-antennary glycans possessing lactosamine repeats were also identified (56, 57). Missing in the mass spectrometry studies were triantennary glycans in which the third antenna was on the Man6 branch, rather than Man3, as indicated by NMR [Fig. 5A and Hard et al. (58)].
Another study of chymotryptic glycopeptides derived from Puregon, a CHO cell–expressed recombinant FSH preparation, reported 22 to 26 FSHβ glycans at each glycosylation site and 15 to 18 glycans at each FSHα glycosylation site (59). However, several proposed structures were unusual; for example, glycans possessing two antenna fucose residues on the same antenna, or sulfated sialic acids. Caution is indicated as the authors appear to have deduced the structures of the N-linked glycans mainly by comparing glucose unit values with those available in GlycoBase (69) and with some very poor-quality fragmentation spectra, which do not contain sufficient information to assign the structures shown. The problem with GlycoBase is that it only contains a limited number of glycans, whose structures were mainly deduced by exoglycosidase digestions and HPLC retention times. The latter, even when expressed as glucose unit values, are not absolutely reproducible. Exoglycosidase digestions do not differentiate isomers, and it is difficult to confirm the presence of a bisecting GlcNAc because of the lack of a suitable enzyme. For urinary hFSH, fewer glycans were reported, 10 to 25 at each site, even though total oligosaccharide analyses in other studies indicate greater heterogeneity in urinary hFSH than in recombinant hFSH (53–55).
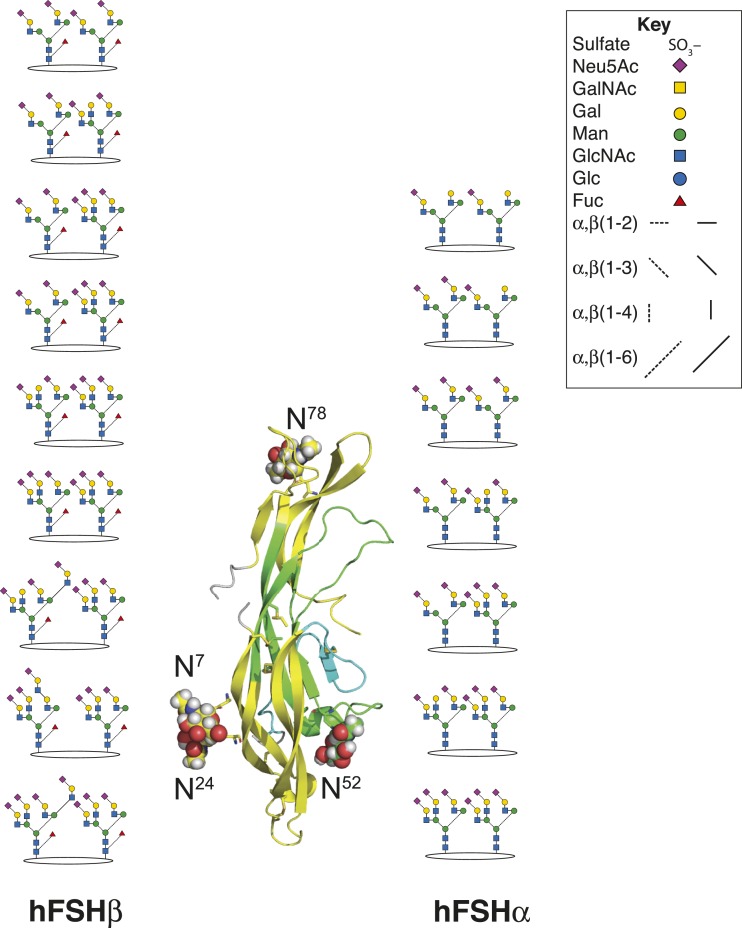
Defining combinations of the three to four N-glycans in FSH preparations is important for developing a complete understanding of FSH glycosylation. Currently, only subunit data are available. Pairs of RCM-FSHα glycans were predicted from its mass spectrum, whereas the RCM-FSHβ spectrum proved unsuitable (68). Reduction and carboxamidomethylation yielded both FSH subunit derivatives that permitted prediction from subunit mass spectra and knowledge of glycan populations (56, 57, 59). A recent study (57) reported seven FSHα masses indicating three variants with two biantennary glycans, two with one biantennary and one triantennary glycan, and two with two triantennary glycans (Fig. 6). The FSHβ subunit ions indicated a pair of biantennary glycans, three with one biantennary and one triantennary glycan, two with both triantennary glycans, and three with one triantennary glycan paired with either a lactosamine repeat–containing triantennary or tetra-antennary glycan. Low pH conditions for liquid chromatography–mass spectrometry (LC-MS) dissociate FSH into subunits, creating a major technical barrier that may be overcome by chemical crosslinking, as has been applied to ovine LH (70).
Figure 6.
FSH subunit glycan pairs. LC-MS of reduced, carboxamidomethyl GonalF provided several pairs of FSH subunit glycan masses (56). In contrast to the original analysis of subunit glycosylation, ions consistent with two triantennary glycans were observed for both subunits.
Macroheterogeneity in the FSHβ Subunit
Macroheterogeneity, the absence of one or more N-glycans, was initially reported for human CG (hCG)β lacking either Asn13 or Asn30 carbohydrates, which exhibited altered electrophoretic mobility (71). The first report of partially glycosylated FSH involved recombinant bovine FSHβ (72). Following SDS-PAGE, FSH subunits appear as a very broad band when stained with Coomassie brilliant blue, thereby obscuring FSHβ mobility shifts (33). Matrix-assisted laser desorption ionization–mass spectrometry (MALDI-MS) and SDS-PAGE revealed a partially glycosylated eFSHβ subunit in purified β-subunit preparations (73). Western blotting with monoclonal antibody RFSH20 (74) revealed two human FSHβ subunit variants, a 24,000 relative molecular mass (Mr) band (24k-FSHβ) and a 21,000 Mr band (21k-FSHβ), while the FSHα subunit migrated as an intermediate sized band (33).
Four variants of human FSHβ have been reported: the 24k-FSHβ, mentioned above, which possesses both Asn7 and Asn24 glycans (33), the 21k-FSHβ, which possesses only Asn7 glycans (34, 55), an 18,000 Mr FSHβ band that possesses only Asn24 glycans (34, 55), and a 15,000 Mr FSHβ band (15k-FSHβ) that lacks both glycan populations (33, 75). We identify the corresponding FSH glycoforms by the FSHβ variant that each possesses, thus fully glycosylated FSH24 or hypoglycosylated FSH21, FSH18, and FSH15, respectively (35). FSH24 and FSH21 appear to be the most abundant and are readily detected in purified, pooled pituitary FSH preparations (33), individual pituitary FSH (53), pooled postmenopausal urinary gonadotropin preparations (53), and individual urine sample FSH (53). FSH18 appears when FSH21 is purified, and hypoglycosylated FSH preparations consisting of both glycoforms are designated as FSH21/18 (34). MALDI-MS analysis of two FSHβ preparations revealed nonglycosylated 15k-FSHβ. Western blotting detected only 21k-FSHβ in one preparation, whereas MALDI-MS revealed only 15k-FSHβ. In a 24k-FSHβ preparation, a small amount of nonglycosylated 15k-FSHβ was detected by MALDI-MS analysis of a 24k-FSHβ preparation (33). The 15k-FSHβ subunit has recently been observed in pituitaries from transgenic mice expressing a double glycosylation site mutant FSHB transgene (75).
In principle, mass spectrometry is a much more sensitive approach for measuring FSH glycoform abundance than Western blots. However, MALDI-MS of pituitary and urinary FSH is confounded by subunit dissociation. FSHα ions partially obscure pituitary FSHβ glycoform ions (59, 76) and are coincident with those of recombinant FSHβ (59). FSH subunits purified by reverse-phase HPLC (68) produce only fully glycosylated 21k-FSHβ (33). Fast atom bombardment mass spectrometry of PNGaseF-treated tryptic FSHβ peptides supported the quantitative glycosylation of both FSHβ Asn residues 7 and 24. Methyl esterification revealed that the 19-35 peptide possessed one more carboxyl group than predicted by its sequence, and the masses of underivatized N-terminal peptides 1-18 and 3-18 were one mass unit larger due to conversion of glycosylated Asn resides to Asp by PNGaseF (68, 77). In the course of chymotryptic glycopeptide analysis, 4.5% to 7.5% nonglycosylated peptides bearing Asn7 and 15.8% bearing Asn24 were encountered, suggesting that as much as 20% to 23% GonalF was hypoglycosylated (61). This was close to the 21% hypoglycosylated FSHβ reported by Western blotting of GonalF (55). A subsequent study of reduced, carboxamidomethylated GonalF chymotryptic digests did not report nonglycosylated glycopeptides (57). A third study reported an unaltered FSHβ chymotryptic peptide following PNGaseF digestion, indicating a nonglycosylated Asn24 in a urinary FSH preparation, but not in Puregon (59). The relative abundance of the unmodified peptide was not reported.
Three groups reported expression of recombinant, glycosylation site mutant versions of two to three hypoglycosylated FSH glycoforms, FSH21, FSH18, and FSH15 (10, 44, 78). Although quantitative data were not reported, the FSHα glycosylation site mutations reduced recombinant hFSH yield more than did the FSHβ mutations (10). Based on FSH immunoreactivity measured in Sephadex G-75 fractions of medium conditioned by Cos7 cells expressing either wild-type (wt) or one of the FSHβ glycosylation site mutants, βN7Q, βN24Q, or βN7,24Q, yields of mutant recombinant FSH were 45%, 69%, and 73% that of the wt, respectively (44). In contrast to expression in transformed cell lines, mouse gonadotropes did not secrete much, if any, FSH15 (75). Accordingly, FSH24, FSH21, and FSH18 are probably the physiologically relevant FSH glycoforms (36).
The cellular mechanism that yields FSH glycoforms seems to be the asparagine (N)-linked glycosylation site skipping by oligosaccharyl transferase (OST). Automated Edman degradation of hypoglycosylated FSHβ preparations (mixtures of 18k- and 21k-FSHβ) revealed phenylthiohydantoin (PhNCS)-Asn at cycles corresponding to Asn7 and Asn24 (33, 55). No PhNCS derivative was observed at Edman cycles corresponding to these residues in 24k-FSHβ, because glycosylated Asn residues are not extracted from the Edman chemistry cartridge of a typical automated gas-phase or pulsed liquid sequencer due to excess hydrophilic character conferred by the oligosaccharide (33). Solid-phase Edman degradation produced a cluster of PhNCS-Asn/carbohydrate peaks when N-glycosylation sites were encountered owing to a more robust extraction with trifluoroacetic acid (39). The presence of PhNCS-Asn at cycles corresponding to N-glycosylation sites indicated that OST failed to transfer the preformed oligosaccharide precursor, Glc3Man9GlcNAc2, from dolichol pyrophosphate to FSHβ at either one of these Asn residues (79, 80). Transient glycosylation followed by enzymatic deglycosylation, as reported for concanavalin A (81), produces a PhNCS-Asp derivative at Edman cycles corresponding to deglycosylated Asn residues (77). It is an unlikely mechanism because folded FSHβ subunits are insensitive to PNGaseF digestion (82), and PhNCS-Asp, although readily differentiated from PhNCS-Asn (39), is not above background when sequencing partially glycosylated FSHβ variants (55).
Glycosylation and FSH Biosynthesis
OST is found in all domains of life. Both bacterial PglB and archaeal AglB consist of single catalytic subunit OSTs (83, 84). The crystal structure of PglB revealed a requirement for unfolded protein structure, as the glycopeptide precursor makes a sharp turn while being scanned in the OST active site (83). This feature has been confirmed in the structure of multisubunit eukaryotic OST (85). Mammals possess two major OST complexes consisting of eight to nine subunits encoded by a dozen genes (85). Six noncatalytic subunits are common to both OST complexes: essential common subunits, ribophorin I/II, DAD1, DDOST, and nonessential common subunits OST4 and TMEM258. The OST complexes are largely differentiated by their catalytic subunit, staurosporine and temperature sensitivity 3 (STT3). The Sec61 translocon-associated, glycosylation site skip-prone isoform is designated STT3A, whereas the other, more versatile variant, is STT3B (86). The former performs N-glycosylation only cotranslationally, whereas the latter can perform N-glycosylation both cotranslationally and posttranslationally (87). Moreover, STT3B often glycosylates Asn residues skipped by STT3A (87). Two subunits, KRTCAP2 and OSTC, are associated only with STT3A. OSTC couples the OST complex to the Sec61 translocon (85). Two oxidoreductase subunits, TUSC3 or MAGT1, are associated with the STT3B-containing OST complex in a mutually exclusive manner (88, 89).
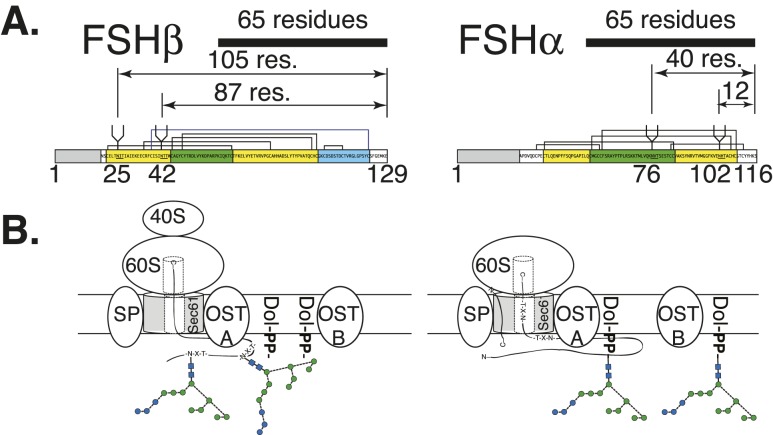
Studies with model glycoproteins have identified several structural characteristics associated with STT3A skipping potential N-glycosylation sites. However, glycosylation sites skipped in FSHβ subunit variants are not consistent with partial N-glycosylation of model glycoproteins (86). For example, the distance between the P site in the ribosome and the active site in STT3A (the Sec61 translocon-associated OST) is estimated to be 65 residues (90). Glycosylation sites in the last 75 C-terminal residues are frequently skipped by STT3A, with a steep drop-off in glycosylation efficiency beginning 50 residues from the C terminus (79). FSHα glycosylation sites at Asn52 and Asn78 are only 40 and 12 residues from the C terminus, respectively (Fig. 7), yet they are always N-glycosylated in hormone preparations derived from natural sources. The partially glycosylated Asn7 and Asn24 residues in FSHβ are 105 and 87 residues from the FSHβ C terminus, respectively. FSHβ Asn7 is fairly close to the human FSHβ signal peptide cleavage sites [Cys−1/Asn1 and the preferred Ser2/Cys3 site (33)], which is another region where glycosylation sites are skipped by STT3A (86). Nevertheless, Asn7 is more frequently glycosylated than Asn24, as indicated by the greater abundance of FSH21 (34). Both FSHβ glycosylation sites are located four residues from Cys residues involved in disulfide bond formation (Fig. 7), which is a third protein structural feature that promotes STT3A glycosylation site skipping (88). In contrast, FSHα glycosylation sites are not as close to disulfide bond–associated Cys residues. As both LH and FSH are often synthesized in the same cell (91), a glaring gap in our knowledge is how LH subunits and FSHα are quantitatively N-glycosylated whereas FSHβ is partially glycosylated at the same time in the same cellular compartment.
Figure 7.
Cotranslational and posttranslational glycosylation of FSH subunits. (A) Distance of subunit C terminus from N-glycosylation sites. The 65-residue bar indicates the distance from the ribosomal P site to the Asn residue of the FSH subunit sequon (90). Prepeptide numbering is used for each subunit diagram. (B) Potential cotranslational glycosylation of FSHβ and posttranslational glycosylation of FSHα. OSTA indicates isoform possessing STT3A associated with Sec61 translocon. OSTB indicates isoform possessing STT3B not associated with Sec61 translocon that can perform posttranslational N-glycosylation. For the oligosaccharide diagrams, the blue circles represent Glc residues, green circles represent mannose, and blue squares represent GlcNAc. Connecting lines show linkages as in Fig. 6 key.
Studies involving OST employing either small interfering RNA suppression or CRISPR/Cas-directed knockout procedures to inhibit selected OST subunits show altered OST complex activities toward model proteins (88, 92, 93). This could be a mechanism by which steroid hormones, such as estrogen, progesterone, and testosterone, modulate OST activity. Although there is a report of progesterone regulating OST activity in hybridoma cells (94), it is not known whether expression of any OST subunit genes is regulated directly by steroid hormones. Application of RNA sequencing to fluorescence-labeled transgenic gonadotropes (95), or single-cell RNA sequencing to immortalized gonadotropes (96), or unmodified pituitary cells may help resolve this question. The effects of gonadal steroids on glycoform abundance may be mediated by GnRH, perhaps via the PKC pathway, as yeast PKC was reported to increase Stt3 activity (97). In mammalian cells, this might involve posttranslational glycosylation of skipped sites, as only STT3B possesses potential phosphorylation sites (98).
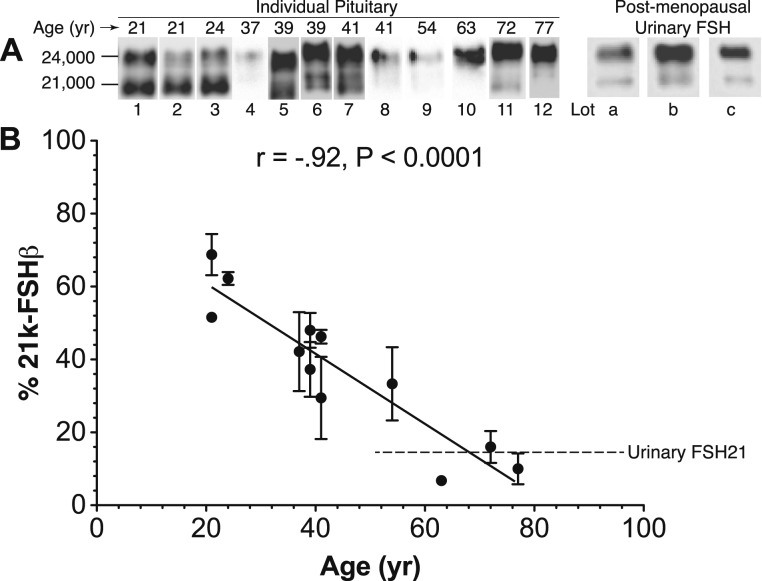
Other than the existence of partially glycosylated FSH variants in pituitary extracts, urinary gonadotropin preparations, and individual urine samples, evidence for physiological regulation of OST activity producing these variants is difficult to obtain. An apparent age-related reduction in the relative abundance of FSH21 in human pituitaries was revealed by Western blotting (Fig. 8) of anti-FSHβ monoclonal antibody 46.3H6.B7-immunopurified FSH samples (99). Extension to serum or urinary FSH has been hampered by the need to purify FSH and the low sensitivity of Western blotting procedures. Differential secretion of serum FSH glycoforms identified by electrophoretic separation and radioimmunoassay has been reported for women 21 to 40 years of age (100). However, these resulted from charge-based isoform measurements, and there does not seem to be a direct relationship between glycoforms and isoforms (32). Indeed, subsequent reports involving partially glycosylated FSH, LH, and TSH added size-based separation by gel filtration (24). Ovariectomy of female rhesus monkeys was attended by increased abundance of high–molecular weight forms of recombinant hFSH immunoreactivity, as assessed by Sephadex G-100 gel filtration (46). Western blots of ovariectomized rhesus pituitary FSH samples revealed the presence of both 24k-FSHβ and 21k-FSHβ (76). However, although Superdex 75 gel filtration chromatograms of the immunopurified rhesus FSH samples were very broad, no high–molecular weight peak was obvious, and the FSHα subunit obscured FSHβ glycoforms in MALDI-MS spectra. Estrogen replacement therapy was shown to eliminate the high–molecular weight pituitary recombinant hFSH found in ovariectomized monkey pituitaries, suggesting that negative feedback in the hypothalamic–pituitary axis can modulate OST activity toward FSHβ (101). More subtle shifts in rat pituitary FSH size following gonadectomy were altered by estrogen replacement in females and testosterone treatment in males (47). Again, because steroid hormones can act at both the pituitary and hypothalamic levels it is not known whether changes in FSH glycosylation are a direct effect on the gonadotrope or an indirect effect via hypothalamic stimulation. For example, GnRH stimulated secretion of less acidic FSH from human pituitaries (102–104).
Figure 8.
Age-related decline in pituitary FSH21 abundance. (A) Representative Western blots of pituitary hFSH isolated from individual human pituitary glands by immunoaffinity followed by gel filtration chromatography. The immunoaffinity column employed anti-FSHβ monoclonal antibody 46.3H6.B7 (99), and the Western blot primary antibody was anti-FSHβ monoclonal RFSH20 (74). The age at autopsy is indicated. Each FSH sample was evaluated in three to four independent Western blots. Postmenopausal hFSH derived from three lots of Pergonal is shown. (B) Linear regression of FSH21 abundance based on relative density of 21k-FSHβ bands. The dotted line indicates the average relative abundance of FSH21 in the Pergonal lots. [Reproduced from Bousfield GR, Butnev VY, Rueda-Santos MA, Brown A, Smalter Hall A, Harvey DJ. Macro and micro heterogeneity in pituitary and urinary follicle-stimulating hormone glycosylation. J Glycomics Lipidomics. 2014;4:125. Copyright: © 2014 Bousfield GR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.]
N-linked oligosaccharides play important roles in glycoprotein biosynthesis, as they recruit lectin domain chaperones, calnexin or calreticulin, to assist in folding (105). Although there are no reports of specific chaperones interacting with FSH subunits, chaperone-mediated folding is likely important for both FSH subunits. For example, the common α-subunit can fold on its own (106–109). Bovine LHα subunit folding requires 75 minutes to achieve near native conformation, as indicated by circular dichroism in vitro (106). Meanwhile, human α-subunit disulfide bond formation is complete in less than 2 minutes in vivo (110). Accordingly, chaperones accelerate even normally spontaneous reactions. Studies with hCGα implicate αAsn78 oligosaccharides with α-subunit folding, as mutation to eliminate this site resulted in <20% α-subunit secreted, with the remainder degraded (41). NMR studies of selectively αAsn52-deglycosylated hCGα revealed that both reducing terminal αAsn78 GlcNAc residues formed hydrogen bonds with αL1 and αL3 loop amino acid residue side chains (111). Reduced mobilities of the Man3 and Man6 residues suggested that they also interacted with the α-subunit loops (42). All of these monosaccharide residues are found in N-glycans regardless of glycan type, antenna number, or structure. The NMR three-dimensional structure of Asn52-deglycosylated hCGα demonstrated that Leu12 and Leu26 in loop αL1 and Tyr65, Val76, His79, and Thr80 in αL3 specifically interact with αAsn78 oligosaccharide (112). The corresponding cystine knot loops βL1 and βL3 in FSHβ are stabilized by a covalent disulfide bond between Cys residues 17 and 66 (38). Heterodimer stability has been associated with αAsn52 glycan because <30% of the mutant hCG dimer lacking this glycan was secreted (41). Moreover, deglycosylation of the homologous αAsn56 in equine FSH reduced the time to reach 50% subunit dissociation at 37°C from 72 hours to <2 hours (43). Three laboratories mutated all four FSH N-glycosylation sites and reported a variety of effects on FSH synthesis and secretion (10, 44, 45). CHO cells expressing single FSHα glycosylation site mutants exhibited reduced secretion rates for FSH heterodimer (10). Mutating βAsn7, βAsn24, or both glycosylation sites to Gln and transient transfection of Cos7 cells resulted in 31% to 55% reduction of hFSH immunoactivity in 96-hour conditioned medium samples, based on Sephadex G-75 peak areas (44). As long as a single glycosylation site was maintained, FSH triple mutants were secreted from CHO cells (10). In contrast, gonadotropes of transgenic mice expressing N7Q/N24Q FSHβ double mutants failed to secrete sufficient FSH15 to restore fertility to transgenic females lacking a functional mouse Fshb gene (75). Real-time quantitative PCR indicated that both wt-FSHB and N7,24Q-FSHB transcripts were expressed at the same levels. Confocal microscopy confirmed expression of FSHβ immunoreactivity in gonadotropes; however, pituitary extract Western blots revealed significantly reduced amounts of 15k-FSHβ protein under reducing conditions and little FSH15 heterodimer under nonreducing conditions. Mouse serum contained very little FSH immunoactivity, and no FSH was detected in pituitary organ culture medium. Thus, for FSHβ subunit folding, oligosaccharide-recruited chaperones appear to be necessary. Eliminating single FSH glycosylation sites appeared to slow biosynthesis of the heterodimer, no matter which subunit was mutated. Asn to Gln mutations at all four glycosylation sites produced no detectable secreted hormone from CHO cells, and that retained intracellularly was barely detectable (10). Mutation of all four Asn resides to Asp produced nonglycosylated FSH, although secretion was only ∼10% that of wt-FSH (45).
Glycosylation and FSH Secretion
An appreciation of the complexities of gonadotropin synthesis, storage, and release can be obtained in reviews focused on sheep and rat gonadotropes (91, 113). It is generally accepted that FSH and LH are differentially secreted, with FSH secretion primarily via the constitutive secretory pathway, whereas LH secretion primarily involves the regulated secretory pathway (113–116). In sheep pituitaries, FSH secretion was estimated to involve 60% to 80% of the pituitary content per 24 hours, but only 1% to 8% of LH (117). Synthesis and release of most pituitary FSH in contrast to storage of the bulk of LH is consistent with relative yields of both gonadotropins in biochemical studies. For sheep, pigs, and cattle, purified FSH yields are low, ranging from 2 to 3 mg/kg pituitary tissue, whereas 100 to 300 mg/kg recoveries of purified LH are readily obtained (118, 119). This pattern is not universal, as horse pituitaries yield 60 mg/kg purified equine FSH and 110 mg/kg purified equine LH (120).
In the rat pituitary, dual LH and FSH immunostaining revealed three populations of gonadotropes with regard to gonadotropin content, 21% to 35% for LH-only, 10% to 22% for FSH-only monohormonal cells, and 45% to 69% for bihormonal cells (114). The relative proportions are dynamic in cycling females, with the proportion of bihormonal cells reaching a maximum just before the preovulatory gonadotropin surge (114). Sheep gonadotropes fall into two groups, LH-only and bihormonal, with only the occasional FSH-only cell detected at the end of the preovulatory surge (121). At the light microscope level, FSH and LH appear differentially packaged in bihormonal gonadotropes, as they respond differentially to GnRH stimulation by aggregating in different regions of the cell (122). Immunogold labeling of rat gonadotropes detected both LH and FSH in secretory vesicles during all stages of the estrus cycle (114). Monohormonal LH vesicles were also observed and reached a maximum prior to the LH surge (114), whereas FSH-only vesicle abundance increased prior to rises in serum FSH (114). In contrast, bihormonal sheep gonadotrope secretory granules were either LH-only or both LH and FSH (123). The latter are consistent with GnRH stimulation of simultaneous LH and FSH pulses observed in sheep portal blood (124). The nearly complete absence of FSH-only granules is inconsistent with GnRH-independent FSH pulses lacking a corresponding LH pulse in the same study, but it may reflect the use of intact animals in one study (123) vs ovariectomized animals in the other (124). Constitutive FSH release may render FSH-only granules very rare. The immediacy of FSH synthesis and secretion may permit changes in glycosylation to influence current physiological change, whereas storage of LH may result in glycosylation patterns that differ from conditions prevailing at the time of release.
Discovery of sulfated oligosaccharides on LH led to studies regarding carbohydrate-mediated intracellular targeting; however, these studies showed no carbohydrate-dependent sorting mechanisms (125). LH secretion by the regulated secretory pathway appears dependent on a C-terminal heptapeptide, Leu-Ser-Gly-Leu-Leu-Phe-Leu (126). This sequence was not detected during sequence determination by Edman degradation, as the C-terminal sequence was poorly defined beyond the last Cys residue (127–129). First predicted by sequencing the LHB gene (130), evaluation of LH peptides by mass spectrometry identified this peptide in LHβ derived from recombinant LH (68). LH and FSH appear to diverge early in the secretory pathway, rather than later in the trans-Golgi network, where much protein sorting is accomplished. Expressed in the absence of α-subunit, FSHβ appeared in peripheral ER, where its distribution resembled that of calnexin (131). LHβ was sequestered in a perinuclear ER compartment where it was significantly colocalized with the HSP70 family chaperone BiP (immunological heavy chain–binding protein, or HSPA5). Coexpression of the α-subunit with LHβ resulted in displacement of the latter to the peripheral ER, most likely as a heterodimer. As LH and FSH glycan populations are quite different in their branching patterns, their paths may remain separate. Their exposure in the medial Golgi to oligosaccharide branch-initiating GlcNAc transferases I to VI (132) appears to differ, resulting in more extensive branching of FSH glycans than LH (133). Adding the seven-residue, C-terminal LHβ peptide to the FSHβ C terminus directed the mutant FSH to the regulated secretory pathway (134). How the mutant FSH N-glycan population compares with that of wt-FSH is unknown.
Role of Glycosylation on FSH Clearance
Effects of individual FSH glycosylation sites on FSH clearance have been tested with recombinant hFSH expressed in CHO cells (9). Although loss of either one or both α-subunit oligosaccharides appeared to accelerate clearance, the results were not significant. Furthermore, there was no significant loss of in vivo biological activity in the Steelman–Pohley assay for either mutant FSH derivative as compared with wt-FSH. Each FSHβ N-glycan significantly increased clearance and reduced in vivo biologic activity, whereas mutation of both sites dramatically increased clearance and appeared to eliminate FSH activity in the Steelman–Pohley assay.
Recombinant FSH expression by CHO cell lines deficient for GlcNAc transferase I (Man5GlcNAc2 oligosaccharides) or sialic acid (complex glycans terminated with Gal) resulted in rapid clearance from serum, effectively eliminating in vivo biological activity as measured by ovarian aromatase expression (78). In vitro steroidogenic activity was unaffected by either change in oligosaccharide structure. Selective inhibition of ST3 β-galactoside α2–3-sialyltransferase (ST3GAL) expression by estrogen has been demonstrated in female rat pituitary glands (135). In contrast, ST6 β-galactoside α2–6-sialyltransferase (ST6GAL) expression remained unaltered. The reduction in ST3GAL may shift the ratio of α2–6 to α2–3 linkages in favor of α2–6, thereby increasing clearance rates, as α2–3-linked sialic acid appears more effective than α2–6-linked sialic acid at preventing FSH clearance in the liver (61). A caveat in humans is the reduced impact of the liver Ashwell asialoglycoprotein receptor on glycoprotein clearance as compared with rodents (136).
FSHβ Carbohydrate Effects on FSHR Binding
Chemical deglycosylation of FSH showed little or no impact on receptor binding (137, 138). FSH scarcity precluded studying the effects of deglycosylation on each subunit. Site-directed mutagenesis provided FSH glycoform models, but it also provided a cautionary tale, as mutations near the βAsn24 site appeared to alter FSH conformation (44). Individual mutation of either the βAsn7 or βAsn24 glycosylation sites increased receptor binding affinity in one study (44) and left it unchanged in another (10) Three groups reported mutating both FSHβ glycosylation sites increased FSHR binding (10, 44, 45). A report involving Cos7-expressed FSH glycosylation mutants and human FSHR expressed in Y-1 cells found no increase in FSHR binding by elimination of αAsn78, βAsn7, or βAsn24 oligosaccharides, but a 2.4-fold increase following elimination of glycosylation at αAsn52 (11). In FSH glycoforms, partial hFSHβ glycosylation increased FSHR binding 3- to 20-fold (34, 55). The biggest difference in FSHR binding was associated with major changes in oligosaccharide structure, as the FSH21/18 preparation was decorated with oligomannose glycans (54). Although it is not known whether fucosylation is restricted to the FSHβ subunit in this preparation, as it appears to be in complex glycan-decorated FSH preparations, it is tempting to speculate a site-specific location for these glycans on αAsn52, implying altered binding associated with a specific glycan structure. Alternatively, recombinant FSH expressed by a CHO cell line lacking GlcNAc transferase I, thereby decorated with oligomannose type glycans at all four sites, retained equivalent FSHR binding activity as wt-recombinant FSH (78).
During kinetic receptor-binding studies involving FSH glycoforms, FSH21 engaged the FSHR immediately, occupied more binding sites, and reached maximum binding an hour before FSH24 (34). The latter exhibited a >30-minute lag before significant FSHR binding was observed, and then binding proceeded at the same rate as FSH21, but required a total of 4 hours before reaching a submaximal level as compared with that which FSH21 attained in 3 hours. Saturation binding studies revealed that FSH21 occupied twofold to threefold more receptors than did FSH24. The mechanisms involved in carbohydrate inhibition of FSHR binding are unknown. It is unlikely that FSH oligosaccharides influence binding to the high-affinity site in the FSHR extracellular domain (FSHRECD), as both FSH/FSHRECD structures show the FSH polypeptide moiety between the receptor and the carbohydrate (139, 140). Neighboring FSHRs may be involved instead.
Cocrystallization of FSH with the entire FSHRECD produced FSH/FSHRECD trimers (140). The endoglycosidase F-digested FSH and FSHRECD preparations employed in the crystallization studies possessed only single GlcNAc residues at each glycosylation site. The organization of the FSHR trimers in the model suggested that a biantennary αAsn52 oligosaccharide would prevent more than one FSH ligand binding to an FSHR trimer at a time. Support for this concept was provided by saturation binding studies with wt-recombinant hFSH and αAsn52 glycosylation site mutant hFSH, as the latter occupied threefold as many sites as did wt-hFSH (141). It was noted that FSHR allosteric modulators increased FSH binding threefold (142). The nature of the allosteric modulator did not matter, as a negative allosteric modulator (143), positive allosteric modulator (144), and nonfunctional allosteric modulator [an LH allosteric modulator that also bound FSHR, but did not affect FSHR activity (145)] all increased 125I-hFSH binding threefold. In a subsequent report, an allosteric FSH modulator increased FSHR binding to β-arrestin threefold (141). These data suggested a common allosteric effect, dissociating FSHR trimers, thereby making more monomeric FSHR available for glycosylated FSH to bind. Although it is well-established that FSHR exits as at least dimers (146), studies of negative cooperativity involving glycoprotein hormone receptors suggest that at least a portion of FSHRs are monomeric (147). Application of super-resolution light microscopic procedures, such as photo-activated dyes and localization microscopy used to quantify LH receptor oligomerization in the cell membrane (148), may be useful to clarify FSHR oligomer patterns.
Glycosylation and FSH Signaling
Studies of signaling by glycosylation-impaired FSH have largely been restricted to the Gs–cAMP–PKA–CREB steroidogenesis pathway. The primary role of FSH αAsn52 in FSH-mediated steroidogenesis, initially recognized in hCG (149), has been consistently demonstrated for FSH in rat gonadal cells, whereas αAsn78 glycosylation appeared to have no impact on FSH biological activity when each glycan was eliminated by itself and tested against rat FSH target cells (10, 44, 45). In Y-1 cells that expressed human FSHR, only elimination of αAsn52 oligosaccharide in Cos7-expressed recombinant hFSH reduced steroidogenesis when FSH concentrations yielding equivalent FSHR binding were employed (11). For FSHβ glycosylation sites, elimination of either one or both Asn7 and Asn24 glycans increased steroidogenic potency in rat granulosa cells (44). Experiments involving βAsn7 were fairly straightforward, as Thr9 mutation to Ser preserved steroidogenic activity along with N-glycosylation. Mutation at Thr26 reduced signaling regardless of whether the mutation preserved glycosylation of Asn24 (44). These results suggest naturally occurring FSH21 is preferable to βAsn24 glycosylation site mutants. In Y-1 cells, there was no effect of eliminating either βAsn7 or βAsn24 glycosylation on steroidogenesis for single glycosylation site mutants, although double mutants were not tested (11). A potentially significant difference in the responsiveness of gonadal granulosa cells and Sertoli cells, as compared with Y-1 cells, is the greater number of receptors expressed by Y-1 cells as compared with physiological target cells. In a human ovarian granulosa cell line, recombinant FSH21/18 was more effective than FSH24 in stimulating cAMP accumulation, PKA activation as indicated by PKA substrate phosphorylation, and CREB phosphorylation (150). FSH21/18 also stimulated more CRE-mediated transcriptional activity, androgen conversion to estradiol by aromatase, and progesterone biosynthesis. In Fshb-null mice, FSH21/18 exhibited significant in vivo biological activity, often exceeding that of FSH24 (151).
FSHR has been demonstrated to activate additional cellular signal pathways via other trimeric G proteins, such as Gi and Gq, by β-arrestin, as modulated by GRK5/6 or GRK2/3, and by pleckstrin homology domain, phosphotyrosine-binding domain, and leucine zipper motif isoform 1 (APPL1) (152, 153). Whereas pituitary and recombinant hFSH preparations provoked the usual sigmoidal dose-response curves for cAMP accumulation in a CHO cell line stably transfected with human FSHR, chemically deglycosylated and baculovirus-expressed (oligomannose glycans only) FSH preparations provoked biphasic responses (13). The increases in cAMP occurred at the same concentrations as those for both pituitary and recombinant FSH, whereas the decreases occurred at 1000-fold higher FSH concentrations. Stimulation of Gs with cholera toxin increased cAMP accumulation over that stimulated by a fixed concentration of pituitary FSH, but it did not prevent its decrease in the face of high concentrations of baculovirus-derived FSH. Inhibition of Gi with pertussis toxin did not affect the pituitary FSH-stimulated cAMP accumulation, but it prevented the cAMP decrease at elevated baculovirus FSH concentrations, indicating Gi involvement, but only at higher FSH concentrations. In a separate study, baculovirus recombinant hFSH provoked a biphasic progesterone response in rat granulosa cells, but an αAsn56-deglycosylated eFSH derivative provoked a sigmoidal response (154). Impaired Gs signaling was indicated by the 10-fold greater FSH derivative concentration needed to stimulate progesterone synthesis and submaximal steroid accumulation. The absence of biphasic response suggested impaired Gi activation as well as impaired Gs activation by the partially glycosylated FSH derivative.
Biased signaling via the FSHR was reported for an equine LH (eLH) derivative that modified both O- and N-glycosylation. Removal of the eLHβ C-terminal 25 residues and eLHα Asn56 oligosaccharide (eLHβt) resulted in maintenance of FSHR binding, but complete loss of steroidogenic activity (155). Both progesterone and estrogen synthesis by DES-primed rat granulosa cells were completely eliminated by these two modifications to eLH (155). However, significant FSHR internalization was noted, despite that the FSHR activation of the PKA pathway appeared completely compromised (12). Recruitment of β-arrestin by eLHβt/FSHR binding in a cAMP-independent manner in turn activated ERK (12).
Studies using microarrays to evaluate global granulosa cell gene expression changes in response to FSH employed either recombinant hFSH isoforms (156) or compared recombinant hFSH (GonalF) with menopausal gonadotropins (urinary hFSH, hLH, and hCG) (157). It is likely that isoelectric focusing produced high and low sialic acid content FSH preparations only at βAsn7 because of the lack of heterogeneity at the other three glycosylation sites (Fig. 5). However, the hybrid/oligomannose population derived from concanavalin A lectin chromatography cannot be defined, as these glycans were missing from glycopeptide analyses. Accordingly, their structures and locations are unknown. Accordingly, significant differences in KGN granulosa cell gene expression cannot be related to glycosylation patterns (156). Comparison of the differences in branching between recombinant hFSH, once again, GonalF, and urinary FSH in menopausal gonadotropin stimulation of human granulosa cells derived from in vitro fertilization overlooked the contributions of hLH and hCG, as these cells express both FSH and LH receptors (157).
Conclusions
Significant progress has been made in defining FSH microheterogeneity using mass spectrometry, which offers both the high resolution and sensitivity needed to characterize the many glycans attached to FSH preparations. The addition of quantitative measures of glycan abundance helps measure changes in FSH glycosylation that could only be guessed based on differences in charge. Challenges still remain such as the structural ambiguity regarding position and linkage in the many partially sialylated glycans that decorate FSH. Subunit dissociation and preferential ionization of the FSHα subunit provide major challenges for using mass spectrometry to measure FSH glycoform abundance. Although conflicting data exist, FSH oligosaccharides appear to hinder high-affinity binding to FSHR; however, the nature of this interference remains to be determined. Structure determination for the entire FSHR with and without FSH ligand may reveal as yet unknown interactions between carbohydrates and the transmembrane domain of the receptor. FSHR oligomerization may be responsible for glycan inhibition of binding, which will require structures of FSHR complexes rather than individual receptors. Additional FSHR-activated signaling pathways remain to be investigated to evaluate the effects of FSH glycosylation on them. These studies will require production of well-characterized FSH glycoform preparations, which remains a major challenge.
Acknowledgments
Financial Support: This work was supported by National Institutes of Health/National Institute on Aging Grant AG-029531 (to G.R.B.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- 15k-FSHβ
15,000 relative molecular mass FSHβ band
- 21k-FSHβ
21,000 relative molecular mass FSHβ band
- 24k-FSHβ
24,000 relative molecular mass FSHβ band
- APPL1
adapter protein, phosphotyrosine interacting with PH domain and leucine zipper motif isoform 1
- CG
chorionic gonadotropin
- CHO
Chinese hamster ovary
- eLH
equine LH
- ER
endoplasmic reticulum
- FSHR
FSH receptor
- FSHRECD
FSH receptor extracellular domain
- hCG
human chorionic gonadotropin
- hFSH
human FSH
- hLH
human LH
- LC-MS
liquid chromatography–mass spectrometry
- MALDI-MS
matrix-assisted laser desorption ionization–mass spectrometry
- Mr
relative molecular mass
- Neu5Ac
N-acetyl-neuraminic acid
- Neu5Gc
N-glycolyl-neuraminic acid
- NMR
nuclear magnetic resonance
- O-Ac-Neu5Ac
O-acetyl-N-acetyl-neuraminic acid
- OST
oligosaccharyl transferase
- PhNCS
phenylthiohydantoin
- STT3
staurosporine and temperature sensitivity 3
- wt
wild-type
References and Notes
- 1. Hunzicker-Dunn M, Mayo KE. Gonadotropin signaling in the ovary. In: Plant TM, Zeleznik AJ, eds. Knobil and Neill’s Physiology of Reproduction. Vol 1. 4th ed. Amsterdam, Netherlands: Elsevier; 2015:895–946. [Google Scholar]
- 2. Lee LB, Walker WH. Hormone signaling in the testis. In: Plant TM, Zeleznik AJ, eds. Knobil and Neill’s Physiology of Reproduction. Vol 1. 4th ed. Amsterdam, Netherlands: Elsevier; 2015:637–690. [Google Scholar]
- 3. Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15(2):201–204. [DOI] [PubMed] [Google Scholar]
- 4. Zheng J, Mao J, Cui M, Liu Z, Wang X, Xiong S, Nie M, Wu X. Novel FSHβ mutation in a male patient with isolated FSH deficiency and infertility. Eur J Med Genet. 2017;60(6):335–339. [DOI] [PubMed] [Google Scholar]
- 5. Huhtaniemi I. A short evolutionary history of FSH-stimulated spermatogenesis. Hormones (Athens). 2015;14(4):468–478. [DOI] [PubMed] [Google Scholar]
- 6. Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, Zaidi S, Zhu LL, Yaroslavskiy BB, Zhou H, Zallone A, Sairam MR, Kumar TR, Bo W, Braun J, Cardoso-Landa L, Schaffler MB, Moonga BS, Blair HC, Zaidi M. FSH directly regulates bone mass. Cell. 2006;125(2):247–260. [DOI] [PubMed] [Google Scholar]
- 7. Zaidi M, New MI, Blair HC, Zallone A, Baliram R, Davies TF, Cardozo C, Iqbal J, Sun L, Rosen CJ, Yuen T. Actions of pituitary hormones beyond traditional targets. J Endocrinol. 2018;237(3):R83–R98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stelmaszewska J, Chrusciel M, Doroszko M, Akerfelt M, Ponikwicka-Tyszko D, Nees M, Frentsch M, Li X, Kero J, Huhtaniemi I, Wolczynski S, Rahman NA. Revisiting the expression and function of follicle-stimulation hormone receptor in human umbilical vein endothelial cells. Sci Rep. 2016;6:37095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bishop LA, Nguyen TV, Schofield PR. Both of the β-subunit carbohydrate residues of follicle-stimulating hormone determine the metabolic clearance rate and in vivo potency. Endocrinology. 1995;136(6):2635–2640. [DOI] [PubMed] [Google Scholar]
- 10. Bishop LA, Robertson DM, Cahir N, Schofield PR. Specific roles for the asparagine-linked carbohydrate residues of recombinant human follicle stimulating hormone in receptor binding and signal transduction. Mol Endocrinol. 1994;8(6):722–731. [DOI] [PubMed] [Google Scholar]
- 11. Valove FM, Finch C, Anasti JN, Froehlich J, Flack MR. Receptor binding and signal transduction are dissociable functions requiring different sites on follicle-stimulating hormone. Endocrinology. 1994;135(6):2657–2661. [DOI] [PubMed] [Google Scholar]
- 12. Wehbi V, Tranchant T, Durand G, Musnier A, Decourtye J, Piketty V, Butnev VY, Bousfield GR, Crépieux P, Maurel MC, Reiter E. Partially deglycosylated equine LH preferentially activates β-arrestin-dependent signaling at the follicle-stimulating hormone receptor. Mol Endocrinol. 2010;24(3):561–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arey BJ, Stevis PE, Deecher DC, Shen ES, Frail DE, Negro-Vilar A, Lopez FJ. Induction of promiscuous G protein coupling of the follicle-stimulating hormone (FSH) receptor: a novel mechanism for transducing pleiotropic actions of FSH isoforms. Mol Endocrinol. 1997;11(5):517–526. [DOI] [PubMed] [Google Scholar]
- 14. Wide L. Male and female forms of human follicle-stimulating hormone in serum. J Clin Endocrinol Metab. 1982;55(4):682–688. [DOI] [PubMed] [Google Scholar]
- 15. Phillips DJ, Albertsson-Wikland K, Eriksson K, Wide L. Changes in the isoforms of luteinizing hormone and follicle-stimulating hormone during puberty in normal children. J Clin Endocrinol Metab. 1997;82(9):3103–3106. [DOI] [PubMed] [Google Scholar]
- 16. Ulloa-Aguirre A, Mejia JJ, Dominguez R, Guevara-Aguirre J, Diaz-Sánchez V, Larrea F. Microheterogeneity of anterior pituitary FSH in the male rat: isoelectric focusing pattern throughout sexual maturation. J Endocrinol. 1986;110(3):539–549. [DOI] [PubMed] [Google Scholar]
- 17. Wide L, Bakos O. More basic forms of both human follicle-stimulating hormone and luteinizing hormone in serum at midcycle compared with the follicular or luteal phase. J Clin Endocrinol Metab. 1993;76(4):885–889. [DOI] [PubMed] [Google Scholar]
- 18. Zambrano E, Olivares A, Mendez JP, Guerrero L, Díaz-Cueto L, Veldhuis JD, Ulloa-Aguirre A. Dynamics of basal and gonadotropin-releasing hormone-releasable serum follicle-stimulating hormone charge isoform distribution throughout the human menstrual cycle. J Clin Endocrinol Metab. 1995;80(5):1647–1656. [DOI] [PubMed] [Google Scholar]
- 19. Wide L. Follicle-stimulating hormones in anterior pituitary glands from children and adults differ in relation to sex and age. J Endocrinol. 1989;123(3):519–529. [DOI] [PubMed] [Google Scholar]
- 20. Creus S, Pellizzari E, Cigorraga SB, Campo S. FSH isoforms: bio and immuno-activities in post-menopausal and normal menstruating women. Clin Endocrinol (Oxf). 1996;44(2):181–189. [DOI] [PubMed] [Google Scholar]
- 21. Vitt UA, Kloosterboer HJ, Rose UM, Mulders JW, Kiesel PS, Bete S, Nayudu PL. Isoforms of human recombinant follicle-stimulating hormone: comparison of effects on murine follicle development in vitro. Biol Reprod. 1998;59(4):854–861. [DOI] [PubMed] [Google Scholar]
- 22. Yding Andersen C, Leonardsen L, Ulloa-Aguirre A, Barrios-De-Tomasi J, Moore L, Byskov AG. FSH-induced resumption of meiosis in mouse oocytes: effect of different isoforms. Mol Hum Reprod. 1999;5(8):726–731. [DOI] [PubMed] [Google Scholar]
- 23. Wide L, Hobson BM. Qualitative difference in follicle-stimulating hormone activity in the pituitaries of young women compared to that of men and elderly women. J Clin Endocrinol Metab. 1983;56(2):371–375. [DOI] [PubMed] [Google Scholar]
- 24. Wide L, Eriksson K. Low-glycosylated forms of both FSH and LH play major roles in the natural ovarian stimulation. Ups J Med Sci. 2018;123(2):100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Timossi C, Damián-Matsumura P, Dominguez-González A, Ulloa-Aguirre A. A less acidic human follicle-stimulating hormone preparation induces tissue-type plasminogen activator enzyme activity earlier than a predominantly acidic analogue in phenobarbital-blocked pro-oestrous rats. Mol Hum Reprod. 1998;4(11):1032–1038. [DOI] [PubMed] [Google Scholar]
- 26. Timossi CM, Barrios de Tomasi J, Zambrano E, González R, Ulloa-Aguirre A. A naturally occurring basically charged human follicle-stimulating hormone (FSH) variant inhibits FSH-induced androgen aromatization and tissue-type plasminogen activator enzyme activity in vitro. Neuroendocrinology. 1998;67(2):153–163. [DOI] [PubMed] [Google Scholar]
- 27. Varki A, Cummings RD, Aebi M, Packer NH, Seeberger PH, Esko JD, Stanley P, Hart G, Darvill A, Kinoshita T, Prestegard JJ, Schnaar RL, Freeze HH, Marth JD, Bertozzi CR, Etzler ME, Frank M, Vliegenthart JF, Lütteke T, Perez S, Bolton E, Rudd P, Paulson J, Kanehisa M, Toukach P, Aoki-Kinoshita KF, Dell A, Narimatsu H, York W, Taniguchi N, Kornfeld S. Symbol nomenclature for graphical representation of glycans. Glycobiology. 2015;25(12):1323–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harvey DJ, Merry AH, Royle L, Campbell MP, Dwek RA, Rudd PM. Proposal for a standard system for drawing structural diagrams of N- and O-linked carbohydrates and related compounds. Proteomics. 2009;9(15):3796–3801. [DOI] [PubMed] [Google Scholar]
- 29. Harvey DJ, Merry AH, Royle L, Campbell MP, Rudd PM. Symbol nomenclature for representing glycan structures: extension to cover different carbohydrate types. Proteomics. 2011;11(22):4291–4295. [DOI] [PubMed] [Google Scholar]
- 30. Stanton PG, Robertson DM, Burgon PG, Schmauk-White B, Hearn MT. Isolation and physicochemical characterization of human follicle-stimulating hormone isoforms. Endocrinology. 1992;130(5):2820–2832. [DOI] [PubMed] [Google Scholar]
- 31. Stanton PG, Shen Z, Kecorius EA, Burgon PG, Robertson DM, Hearn MT. Application of a sensitive HPLC-based fluorometric assay to determine the sialic acid content of human gonadotropin isoforms. J Biochem Biophys Methods. 1995;30(1):37–48. [DOI] [PubMed] [Google Scholar]
- 32. Bousfield GR, Butnev VY, Bidart JM, Dalpathado D, Irungu J, Desaire H. Chromatofocusing fails to separate hFSH isoforms on the basis of glycan structure. Biochemistry. 2008;47(6):1708–1720. [DOI] [PubMed] [Google Scholar]
- 33. Walton WJ, Nguyen VT, Butnev VY, Singh V, Moore WT, Bousfield GR. Characterization of human FSH isoforms reveals a nonglycosylated β-subunit in addition to the conventional glycosylated β-subunit. J Clin Endocrinol Metab. 2001;86(8):3675–3685. [DOI] [PubMed] [Google Scholar]
- 34. Bousfield GR, Butnev VY, Butnev VY, Hiromasa Y, Harvey DJ, May JV. Hypo-glycosylated human follicle-stimulating hormone (hFSH21/18) is much more active in vitro than fully-glycosylated hFSH (hFSH24). Mol Cell Endocrinol. 2014;382(2):989–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Davis JS, Kumar TR, May JV, Bousfield GR. Naturally occurring follicle-stimulating hormone glycosylation variants. J Glycomics Lipidomics. 2014;4(1):e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bousfield GR, May JV, Davis JS, Dias JA, Kumar TR. In vivo and in vitro impact of carbohydrate variation on follicle-stimulating hormone function. Front Endocrinol (Lausanne). 2018;9:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ulloa-Aguirre A, Dias JA, Bousfield GR. Gonadotropins. In: Simoni M, Huhtaniemi I, eds. Endocrinology of the Testis and Male Reproduction. Cham, Switzerland: Springer; 2017:1–52. [Google Scholar]
- 38. Fox KM, Dias JA, Van Roey P. Three-dimensional structure of human follicle-stimulating hormone. Mol Endocrinol. 2001;15(3):378–389. [DOI] [PubMed] [Google Scholar]
- 39. Gotschall RR, Bousfield GR. Oligosaccharide mapping reveals hormone-specific glycosylation patterns on equine gonadotropin α-subunit Asn56. Endocrinology. 1996;137(6):2543–2557. [DOI] [PubMed] [Google Scholar]
- 40. Maghuin-Rogister G, Closset J, Hennen G. Differences in the carbohydrate portion of the α subunit of porcine lutropin (LH), follitropin (FSH) and thyrotropin (TSH). FEBS Lett. 1975;60(2):263–266. [DOI] [PubMed] [Google Scholar]
- 41. Matzuk MM, Boime I. The role of the asparagine-linked oligosaccharides of the α subunit in the secretion and assembly of human chorionic gonadotrophin. J Cell Biol. 1988;106(4):1049–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van Zuylen CWEM, Kamerling JP, Vliegenthart JFG. Glycosylation beyond the Asn78-linked GlcNAc residue has a significant enhancing effect on the stability of the α subunit of human chorionic gonadotropin. Biochem Biophys Res Commun. 1997;232(1):117–120. [DOI] [PubMed] [Google Scholar]
- 43. Bousfield GR, Butnev VY, Butnev VY, Nguyen VT, Gray CM, Dias JA, MacColl R, Eisele L, Harvey DJ. Differential effects of α subunit asparagine56 oligosaccharide structure on equine lutropin and follitropin hybrid conformation and receptor-binding activity. Biochemistry. 2004;43(33):10817–10833. [DOI] [PubMed] [Google Scholar]
- 44. Flack MR, Froehlich J, Bennet AP, Anasti J, Nisula BC. Site-directed mutagenesis defines the individual roles of the glycosylation sites on follicle-stimulating hormone. J Biol Chem. 1994;269(19):14015–14020. [PubMed] [Google Scholar]
- 45. Keene JL, Nishimori K, Galway AB, Matzuk MM, Hsueh A, Boime I. Recombinant deglycosylated human FSH is an antagonist of human FSH action in cultured rat granulosa-cells. Endocrine. 1994;2(3):175–180. [Google Scholar]
- 46. Peckham WD, Yamaji T, Dierschke DJ, Knobil E. Gonadal function and the biological and physicochemical properties of follicle stimulating hormone. Endocrinology. 1973;92(6):1660–1666. [DOI] [PubMed] [Google Scholar]
- 47. Bogdanove EM, Campbell GT, Peckham WD. FSH pleomorphism in the rat—regulation by gonadal steroids. Endocr Res Commun. 1974;1(1):87–99. [DOI] [PubMed] [Google Scholar]
- 48. Ulloa-Aguirre A, Chappel SC. Multiple species of follicle-stimulating hormone exist within the anterior pituitary gland of male golden hamsters. J Endocrinol. 1982;95(2):257–266. [DOI] [PubMed] [Google Scholar]
- 49. Wide L. Electrophoretic and gel chromatographic analyses of follicle-stimulating hormone in human serum. Ups J Med Sci. 1981;86(3):249–258. [DOI] [PubMed] [Google Scholar]
- 50. Renwick AG, Mizuochi T, Kochibe N, Kobata A. The asparagine-linked sugar chains of human follicle-stimulating hormone. J Biochem. 1987;101(5):1209–1221. [DOI] [PubMed] [Google Scholar]
- 51. Green ED, Baenziger JU. Asparagine-linked oligosaccharides on lutropin, follitropin, and thyrotropin. I. Structural elucidation of the sulfated and sialylated oligosaccharides on bovine, ovine, and human pituitary glycoprotein hormones. J Biol Chem. 1988;263(1):25–35. [PubMed] [Google Scholar]
- 52. Green ED, Baenziger JU. Asparagine-linked oligosaccharides on lutropin, follitropin, and thyrotropin. II. Distributions of sulfated and sialylated oligosaccharides on bovine, ovine, and human pituitary glycoprotein hormones. J Biol Chem. 1988;263(1):36–44. [PubMed] [Google Scholar]
- 53. Bousfield GR, Butnev VY, Rueda-Santos MA, Brown A, Hall AS, Harvey DJ. Macro- and micro-heterogeneity in pituitary and urinary follicle-stimulating hormone glycosylation. J Glycomics Lipidomics. 2014;4:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bousfield GR, Butnev VY, White WK, Hall AS, Harvey DJ. Comparison of follicle-stimulating hormone glycosylation microheterogeneity by quantitative negative mode nano-electrospray mass spectrometry of peptide-N glycanase-released oligosaccharides. J Glycomics Lipidomics. 2015;5(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Butnev VY, Butnev VY, May JV, Shuai B, Tran P, White WK, Brown A, Smalter Hall A, Harvey DJ, Bousfield GR. Production, purification, and characterization of recombinant hFSH glycoforms for functional studies. Mol Cell Endocrinol. 2015;405:42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Grass J, Pabst M, Chang M, Wozny M, Altmann F. Analysis of recombinant human follicle-stimulating hormone (FSH) by mass spectrometric approaches. Anal Bioanal Chem. 2011;400(8):2427–2438. [DOI] [PubMed] [Google Scholar]
- 57. Mastrangeli R, Satwekar A, Cutillo F, Ciampolillo C, Palinsky W, Longobardi S. In-vivo biological activity and glycosylation analysis of a biosimilar recombinant human follicle-stimulating hormone product (Bemfola) compared with its reference medicinal product (GONAL-f). PLoS One. 2017;12(9):e0184139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hård K, Mekking A, Damm JB, Kamerling JP, de Boer W, Wijnands RA, Vliegenthart JFG. Isolation and structure determination of the intact sialylated N-linked carbohydrate chains of recombinant human follitropin expressed in Chinese hamster ovary cells. Eur J Biochem. 1990;193(1):263–271. [DOI] [PubMed] [Google Scholar]
- 59. Wang H, Chen X, Zhang X, Zhang W, Li Y, Yin H, Shao H, Chen G. Comparative assessment of glycosylation of a recombinant human FSH and a highly purified FSH extracted from human urine. J Proteome Res. 2016;15(3):923–932. [DOI] [PubMed] [Google Scholar]
- 60. Park EI, Manzella SM, Baenziger JU. Rapid clearance of sialylated glycoproteins by the asialoglycoprotein receptor. J Biol Chem. 2003;278(7):4597–4602. [DOI] [PubMed] [Google Scholar]
- 61. Steirer LM, Park EI, Townsend RR, Baenziger JU. The asialoglycoprotein receptor regulates levels of plasma glycoproteins terminating with sialic acid α2,6-galactose. J Biol Chem. 2009;284(6):3777–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Stanley P, Cummings RD. Structures common to different glycans. In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GH, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, Schnaar RL, Seeberger PH, eds. Essentials of Glycobiology. 3rd ed.Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2017:161–178. [Google Scholar]
- 63. Dalpathado DS, Irungu J, Go EP, Butnev VY, Norton K, Bousfield GR, Desaire H. Comparative glycomics of the glycoprotein follicle stimulating hormone: glycopeptide analysis of isolates from two mammalian species. Biochemistry. 2006;45(28):8665–8673. [DOI] [PubMed] [Google Scholar]
- 64. Irungu J, Dalpathado DS, Go EP, Jiang H, Ha HV, Bousfield GR, Desaire H. Method for characterizing sulfated glycoproteins in a glycosylation site-specific fashion, using ion pairing and tandem mass spectrometry. Anal Chem. 2006;78(4):1181–1190. [DOI] [PubMed] [Google Scholar]
- 65. Cao L, Diedrich JK, Ma Y, Wang N, Pauthner M, Park SR, Delahunty CM, McLellan JS, Burton DR, Yates JR, Paulson JC. Global site-specific analysis of glycoprotein N-glycan processing. Nat Protoc. 2018;13(6):1196–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Brockhausen I, Narasimhan S, Schachter H. The biosynthesis of highly branched N-glycans: studies on the sequential pathway and functional role of N-acetylglucosaminyltransferases I, II, III, IV, V and VI. Biochimie. 1988;70(11):1521–1533. [DOI] [PubMed] [Google Scholar]
- 67. Gervais A, Hammel YA, Pelloux S, Lepage P, Baer G, Carte N, Sorokine O, Strub J-M, Koerner R, Leize E, Van Dorsselaer A. Glycosylation of human recombinant gonadotrophins: characterization and batch-to-batch consistency. Glycobiology. 2003;13(3):179–189. [DOI] [PubMed] [Google Scholar]
- 68. Amoresano A, Siciliano R, Orrù S, Napoleoni R, Altarocca V, De Luca E, Sirna A, Pucci P. Structural characterisation of human recombinant glycohormones follitropin, lutropin and choriogonadotropin expressed in Chinese hamster ovary cells. Eur J Biochem. 1996;242(3):608–618. [DOI] [PubMed] [Google Scholar]
- 69. Campbell MP, Royle L, Radcliffe CM, Dwek RA, Rudd PM. GlycoBase and autoGU: tools for HPLC-based glycan analysis. Bioinformatics. 2008;24(9):1214–1216. [DOI] [PubMed] [Google Scholar]
- 70. Farmer TB, Caprioli RM. Assessing the multimeric states of proteins: studies using laser desorption mass spectrometry. Biol Mass Spectrom. 1991;20(12):796–800. [DOI] [PubMed] [Google Scholar]
- 71. Matzuk MM, Krieger M, Corless CL, Boime I. Effects of preventing O-glycosylation on the secretion of human chorionic gonadotropin in Chinese hamster ovary cells. Proc Natl Acad Sci USA. 1987;84(18):6354–6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chappel S, Beck A, Nugent N, Hyman L, Zabrecky J, Maurer R. Production of bovine follicle stimulating hormone (FSH) by recombinant DNA technology. In: Proceedings of the 69th Annual Meeting of the Endocrine Society; 10–12 June 1987; Indianapolis, IN. Abstract 130. [Google Scholar]
- 73. Bousfield GR, Butnev VY, Gotschall RR, Baker VL, Moore WT. Structural features of mammalian gonadotropins. Mol Cell Endocrinol. 1996;125(1–2):3–19. [DOI] [PubMed] [Google Scholar]
- 74.RRID:AB_2800424, https://scicrunch.org/resolver/AB_2800424.
- 75. Wang H, Butnev V, Bousfield GR, Kumar TR. A human FSHB transgene encoding the double N-glycosylation mutant (Asn7Δ Asn24Δ) FSHβ subunit fails to rescue Fshb null mice. Mol Cell Endocrinol. 2016;426:113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bousfield GR, Butnev VY, Walton WJ, Nguyen VT, Huneidi J, Singh V, Kolli VS, Harvey DJ, Rance NE. All-or-none N-glycosylation in primate follicle-stimulating hormone β-subunits. Mol Cell Endocrinol. 2007;260-262:40–48. [DOI] [PubMed] [Google Scholar]
- 77. Plummer TH Jr, Tarentino AL. Facile cleavage of complex oligosaccharides from glycopeptides by almond emulsin peptide: N-glycosidase. J Biol Chem. 1981;256(20):10243–10246. [PubMed] [Google Scholar]
- 78. Galway AB, Hsueh AJ, Keene JL, Yamoto M, Fauser BCJM, Boime I. In vitro and in vivo bioactivity of recombinant human follicle-stimulating hormone and partially deglycosylated variants secreted by transfected eukaryotic cell lines. Endocrinology. 1990;127(1):93–100. [DOI] [PubMed] [Google Scholar]
- 79. Shrimal S, Trueman SF, Gilmore R. Extreme C-terminal sites are posttranslocationally glycosylated by the STT3B isoform of the OST. J Cell Biol. 2013;201(1):81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Shrimal S, Cherepanova NA, Gilmore R. Cotranslational and posttranslocational N-glycosylation of proteins in the endoplasmic reticulum. Semin Cell Dev Biol. 2015;41:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sheldon PS, Bowles DJ. The glycoprotein precursor of concanavalin A is converted to an active lectin by deglycosylation. EMBO J. 1992;11(4):1297–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Butnev VY, May JV, Butnev VY, White WK, Bousfield GR. Culture conditions influence FSHα subunit N-glycosylation. In: Proceedings of the 50th Annual Meeting of the Society for the Study of Reproduction; 13–16 July 2017; Washington, DC. Abstract 402. [Google Scholar]
- 83. Lizak C, Gerber S, Numao S, Aebi M, Locher KP. X-ray structure of a bacterial oligosaccharyltransferase. Nature. 2011;474(7351):350–355. [DOI] [PubMed] [Google Scholar]
- 84. Matsumoto S, Shimada A, Nyirenda J, Igura M, Kawano Y, Kohda D. Crystal structures of an archaeal oligosaccharyltransferase provide insights into the catalytic cycle of N-linked protein glycosylation. Proc Natl Acad Sci USA. 2013;110(44):17868–17873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Braunger K, Pfeffer S, Shrimal S, Gilmore R, Berninghausen O, Mandon EC, Becker T, Förster F, Beckmann R. Structural basis for coupling protein transport and N-glycosylation at the mammalian endoplasmic reticulum. Science. 2018;360(6385):215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kelleher DJ, Karaoglu D, Mandon EC, Gilmore R. Oligosaccharyltransferase isoforms that contain different catalytic STT3 subunits have distinct enzymatic properties. Mol Cell. 2003;12(1):101–111. [DOI] [PubMed] [Google Scholar]
- 87. Ruiz-Canada C, Kelleher DJ, Gilmore R. Cotranslational and posttranslational N-glycosylation of polypeptides by distinct mammalian OST isoforms. Cell. 2009;136(2):272–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Cherepanova NA, Shrimal S, Gilmore R. Oxidoreductase activity is necessary for N-glycosylation of cysteine-proximal acceptor sites in glycoproteins. J Cell Biol. 2014;206(4):525–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yan A, Lennarz WJ. Two oligosaccharyl transferase complexes exist in yeast and associate with two different translocons. Glycobiology. 2005;15(12):1407–1415. [DOI] [PubMed] [Google Scholar]
- 90. Whitley P, Nilsson IM, von Heijne G. A nascent secretory protein may traverse the ribosome/endoplasmic reticulum translocase complex as an extended chain. J Biol Chem. 1996;271(11):6241–6244. [DOI] [PubMed] [Google Scholar]
- 91. Childs GV. Gonadotropes and lactotropes. In: Neill J, ed. Knobil and Neill’s Physiology of Reproduction. Vol 1 St. Louis, MO: Academic Press; 2006:1483–1579. [Google Scholar]
- 92. Shrimal S, Gilmore R. Reduced expression of the oligosaccharyltransferase exacerbates protein hypoglycosylation in cells lacking the fully assembled oligosaccharide donor. Glycobiology. 2015;25(7):774–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Cherepanova NA, Gilmore R. Mammalian cells lacking either the cotranslational or posttranslocational oligosaccharyltransferase complex display substrate-dependent defects in asparagine linked glycosylation. Sci Rep. 2016;6(1):20946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Prados MB, La Blunda J, Szekeres-Bartho J, Caramelo J, Miranda S. Progesterone induces a switch in oligosaccharyltransferase isoform expression: consequences on IgG N-glycosylation. Immunol Lett. 2011;137(1-2):28–37. [DOI] [PubMed] [Google Scholar]
- 95. Qiao S, Nordström K, Muijs L, Gasparoni G, Tierling S, Krause E, Walter J, Boehm U. Molecular plasticity of male and female murine gonadotropes revealed by mRNA sequencing. Endocrinology. 2016;157(3):1082–1093. [DOI] [PubMed] [Google Scholar]
- 96. Ruf-Zamojski F, Ge Y, Nair V, Zamojski M, Pincas H, Toufaily C, Tome-Garcia J, Stoeckius M, Stephenson W, Smith GR, Bernard DJ, Tsankova NM, Hartmann BM, Fribourg M, Smibert P, Swerdlow H, Turgeon JL, Sealfon SC. Single-cell stabilization method identifies gonadotrope transcriptional dynamics and pituitary cell type heterogeneity. Nucleic Acids Res. 2018;46(21):11370–11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Park H, Lennarz WJ. Evidence for interaction of yeast protein kinase C with several subunits of oligosaccharyl transferase. Glycobiology. 2000;10(7):737–744. [DOI] [PubMed] [Google Scholar]
- 98. UniProt Consortium T. UniProt: the universal protein knowledgebase. Nucleic Acids Res. 2018;46(5):2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.RRID. AB_2800427, https://scicrunch.org/resolver/AB_2800427.
- 100. Wide L, Eriksson K. Dynamic changes in glycosylation and glycan composition of serum FSH and LH during natural ovarian stimulation. Ups J Med Sci. 2013;118(3):153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Peckham WD, Knobil E. The effects of ovariectomy, estrogen replacement, and neuraminidase treatment on the properties of the adenohypophysial glycoprotein hormones of the Rhesus monkey. Endocrinology. 1976;98(4):1054–1060. [DOI] [PubMed] [Google Scholar]
- 102. Wide L, Albertsson-Wikland K. Change in electrophoretic mobility of human follicle-stimulating hormone in serum after administration of gonadotropin-releasing hormone. J Clin Endocrinol Metab. 1990;70(1):271–276. [DOI] [PubMed] [Google Scholar]
- 103. Phillips DJ, Wide L. Serum gonadotropin isoforms become more basic after an exogenous challenge of gonadotropin-releasing hormone in children undergoing pubertal development. J Clin Endocrinol Metab. 1994;79(3):814–819. [DOI] [PubMed] [Google Scholar]
- 104. Wide L, Albertsson-Wikland K, Phillips DJ. More basic isoforms of serum gonadotropins during gonadotropin-releasing hormone agonist therapy in pubertal children. J Clin Endocrinol Metab. 1996;81(1):216–221. [DOI] [PubMed] [Google Scholar]
- 105. Lamriben L, Graham JB, Adams BM, Hebert DN. N-glycan-based ER molecular chaperone and protein quality control system: the calnexin binding cycle. Traffic. 2016;17(4):308–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Giudice LC, Pierce JG. Studies on the disulfide bonds of glycoprotein hormones. Complete reduction and reoxidation of the disulfide bonds of the α subunit of bovine luteinizing hormone. J Biol Chem. 1976;251(20):6392–6399. [PubMed] [Google Scholar]
- 107. Giudice LC, Pierce JG. Studies on the reduction and reoxidation of the disulfide bonds of the alpha and beta subunits of human choriogonadotropin. Biochim Biophys Acta. 1978;533(1):140–146. [DOI] [PubMed] [Google Scholar]
- 108. Strickland TW, Thomason AR, Nilson J, Pierce JG. The common α subunit of bovine glycoprotein hormones: limited formation of native structure by the totally nonglycosylated polypeptide chain. J Cell Biochem. 1985;29(3):225–237. [DOI] [PubMed] [Google Scholar]
- 109. Bousfield GR, Ward DN. Evidence for two folding domains in glycoprotein hormone α-subunits. Endocrinology. 1994;135(2):624–635. [DOI] [PubMed] [Google Scholar]
- 110. Ruddon RW, Krzesicki RF, Norton SE, Beebe JS, Peters BP, Perini F. Detection of a glycosylated, incompletely folded form of chorionic gonadotropin β subunit that is a precursor of hormone assembly in trophoblastic cells. J Biol Chem. 1987;262(26):12533–12540. [PubMed] [Google Scholar]
- 111. Erbel PJA, Karimi-Nejad Y, van Kuik JA, Boelens R, Kamerling JP, Vliegenthart JFG. Effects of the N-linked glycans on the 3D structure of the free α-subunit of human chorionic gonadotropin. Biochemistry. 2000;39(20):6012–6021. [DOI] [PubMed] [Google Scholar]
- 112. Erbel PJ, Karimi-Nejad Y, De Beer T, Boelens R, Kamerling JP, Vliegenthart JF. Solution structure of the alpha-subunit of human chorionic gonadotropin. Eur J Biochem. 1999;260(2):490–498. [DOI] [PubMed] [Google Scholar]
- 113. McNeilly AS, Crawford JL, Taragnat C, Nicol L, McNeilly JR. The differential secretion of FSH and LH: regulation through genes, feedback and packaging. Reprod Suppl. 2003;61:463–476. [PubMed] [Google Scholar]
- 114. Childs GV, Unabia G, Tibolt R, Lloyd JM. Cytological factors that support nonparallel secretion of luteinizing hormone and follicle-stimulating hormone during the estrous cycle. Endocrinology. 1987;121(5):1801–1813. [DOI] [PubMed] [Google Scholar]
- 115. Kumar TR. Gonadotropin re-routing and evolution of estrus cycles. In: Proceedings of the 48th Annual Meeting of the Society for the Study of Reproduction; 18–22 June 2015; San Juan, Puerto Rico. Abstract 60. [Google Scholar]
- 116. Farnworth PG. Gonadotrophin secretion revisited. How many ways can FSH leave a gonadotroph? J Endocrinol. 1995;145(3):387–395. [DOI] [PubMed] [Google Scholar]
- 117. McNeilly AS. The control of FSH secretion. Acta Endocrinol Suppl (Copenh). 1988;288:31–40. [PubMed] [Google Scholar]
- 118. Liu WK, Ward DN. The purification and chemistry of pituitary glycoprotein hormones. Pharmacol Ther B. 1975;1(3):545–570. [DOI] [PubMed] [Google Scholar]
- 119. Cheng KW. Purification and properties of bovine pituitary follitropin. Biochem J. 1976;159(3):651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Bousfield GR, Ward DN. Purification of lutropin and follitropin in high yield from horse pituitary glands. J Biol Chem. 1984;259(3):1911–1921. [PubMed] [Google Scholar]
- 121. Taragnat C, Bernier A, Fontaine J. Gonadotrophin storage patterns in the ewe during the oestrous cycle or after long-term treatment with a GnRH agonist. J Endocrinol. 1998;156(1):149–157. [DOI] [PubMed] [Google Scholar]
- 122. Childs GV. Shifts in gonadotropin storage in cultured gonadotropes following GnRH stimulation, in vitro. Peptides. 1985;6(1):103–107. [DOI] [PubMed] [Google Scholar]
- 123. Crawford JL, McNeilly AS. Co-localisation of gonadotrophins and granins in gonadotrophs at different stages of the oestrous cycle in sheep. J Endocrinol. 2002;174(2):179–194. [DOI] [PubMed] [Google Scholar]
- 124. Padmanabhan V, McFadden K, Mauger DT, Karsch FJ, Midgley AR Jr. Neuroendocrine control of follicle-stimulating hormone (FSH) secretion. I. Direct evidence for separate episodic and basal components of FSH secretion. Endocrinology. 1997;138(1):424–432. [DOI] [PubMed] [Google Scholar]
- 125. Blomquist JF, Baenziger JU. Differential sorting of lutropin and the free α-subunit in cultured bovine pituitary cells. J Biol Chem. 1992;267(29):20798–20803. [PubMed] [Google Scholar]
- 126. Jablonka-Shariff A, Boime I. A dileucine determinant in the carboxyl terminal sequence of the LHβ subunit is implicated in the regulated secretion of lutropin from transfected GH3 cells. Mol Cell Endocrinol. 2011;339(1–2):7–13. [DOI] [PubMed] [Google Scholar]
- 127. Shome B, Parlow AF. The primary structure of the hormone-specific, beta subunit of human pituitary luteinizing hormone (hLH). J Clin Endocrinol Metab. 1973;36(3):618–621. [DOI] [PubMed] [Google Scholar]
- 128. Sairam MR, Li CH. Human pituitary lutropin. Isolation, properties, and the complete amino acid sequence of the β-subunit. Biochim Biophys Acta. 1975;412(1):70–81. [PubMed] [Google Scholar]
- 129. Keutmann HT, Jacobs JW, Bishop WH, Ryan RJ. Human luteinizing hormone: amino-terminal sequence analysis of the β subunit using [35S]phenylisothiocyanate. Hoppe Seylers Z Physiol Chem. 1974;355(2):935–938. [DOI] [PubMed] [Google Scholar]
- 130. Talmadge K, Vamvakopoulos NC, Fiddes JC. Evolution of the genes for the β subunits of human chorionic gonadotropin and luteinizing hormone. Nature. 1984;307(5946):37–40. [DOI] [PubMed] [Google Scholar]
- 131. Jablonka-Shariff A, Boime I. A novel carboxyl-terminal heptapeptide initiates the regulated secretion of LH from unique sub-domains of the ER. PLoS One. 2013;8(5):e65002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54(1):631–664. [DOI] [PubMed] [Google Scholar]
- 133. Weisshaar G, Hiyama J, Renwick AG, Nimtz M. NMR investigations of the N-linked oligosaccharides at individual glycosylation sites of human lutropin. Eur J Biochem. 1991;195(1):257–268. [DOI] [PubMed] [Google Scholar]
- 134. Wang H, Larson M, Jablonka-Shariff A, Pearl CA, Miller WL, Conn PM, Boime I, Kumar TR. Redirecting intracellular trafficking and the secretion pattern of FSH dramatically enhances ovarian function in mice. Proc Natl Acad Sci USA. 2014;111(15):5735–5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Damián-Matsumura P, Zaga V, Maldonado A, Sánchez-Hernández C, Timossi C, Ulloa-Aguirre A. Oestrogens regulate pituitary α2,3-sialyltransferase messenger ribonucleic acid levels in the female rat. J Mol Endocrinol. 1999;23(2):153–165. [DOI] [PubMed] [Google Scholar]
- 136. Poulin P. A single-species approach considering additional physiological information for prediction of hepatic clearance of glycoprotein derivate therapeutics. Clin Pharmacokinet. 2011;50(10):665–674. [DOI] [PubMed] [Google Scholar]
- 137. Calvo FO, Keutmann HT, Bergert ER, Ryan RJ. Deglycosylated human follitropin: characterization and effects on adenosine cyclic 3′,5′-phosphate production in porcine granulosa cells. Biochemistry. 1986;25(13):3938–3943. [DOI] [PubMed] [Google Scholar]
- 138. Manjunath P, Sairam MR, Sairam J. Studies on pituitary follitropin. X. Biochemical, receptor binding and immunological properties of deglycosylated ovine hormone. Mol Cell Endocrinol. 1982;28(2):125–138. [DOI] [PubMed] [Google Scholar]
- 139. Fan QR, Hendrickson WA. Structure of human follicle-stimulating hormone in complex with its receptor. Nature. 2005;433(7023):269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Jiang X, Liu H, Chen X, Chen PH, Fischer D, Sriraman V, Yu HN, Arkinstall S, He X. Structure of follicle-stimulating hormone in complex with the entire ectodomain of its receptor. Proc Natl Acad Sci USA. 2012;109(31):12491–12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Jiang X, Fischer D, Chen X, McKenna SD, Liu H, Sriraman V, Yu HN, Goutopoulos A, Arkinstall S, He X. Evidence for follicle-stimulating hormone receptor as a functional trimer. J Biol Chem. 2014;289(20):14273–14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Jiang X, Dias JA, He X. Structural biology of glycoprotein hormones and their receptors: insights to signaling. Mol Cell Endocrinol. 2014;382(1):424–451. [DOI] [PubMed] [Google Scholar]
- 143. Dias JA, Bonnet B, Weaver BA, Watts J, Kluetzman K, Thomas RM, Poli S, Mutel V, Campo B. A negative allosteric modulator demonstrates biased antagonism of the follicle stimulating hormone receptor. Mol Cell Endocrinol. 2011;333(2):143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. van Koppen CJ, Verbost PM, van de Lagemaat R, Karstens WJ, Loozen HJ, van Achterberg TA, van Amstel MG, Brands JH, van Doornmalen EJ, Wat J, Mulder SJ, Raafs BC, Verkaik S, Hanssen RG, Timmers CM. Signaling of an allosteric, nanomolar potent, low molecular weight agonist for the follicle-stimulating hormone receptor. Biochem Pharmacol. 2013;85(8):1162–1170. [DOI] [PubMed] [Google Scholar]
- 145. Janovick JA, Maya-Núñez G, Ulloa-Aguirre A, Huhtaniemi IT, Dias JA, Verbost P, Conn PM. Increased plasma membrane expression of human follicle-stimulating hormone receptor by a small molecule thienopyr(im)idine. Mol Cell Endocrinol. 2009;298(1–2):84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Thomas RM, Nechamen CA, Mazurkiewicz JE, Muda M, Palmer S, Dias JA. Follice-stimulating hormone receptor forms oligomers and shows evidence of carboxyl-terminal proteolytic processing. Endocrinology. 2007;148(5):1987–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Urizar E, Montanelli L, Loy T, Bonomi M, Swillens S, Gales C, Bouvier M, Smits G, Vassart G, Costagliola S. Glycoprotein hormone receptors: link between receptor homodimerization and negative cooperativity. EMBO J. 2005;24(11):1954–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Jonas KC, Fanelli F, Huhtaniemi IT, Hanyaloglu AC. Single molecule analysis of functionally asymmetric G protein-coupled receptor (GPCR) oligomers reveals diverse spatial and structural assemblies. J Biol Chem. 2015;290(7):3875–3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Matzuk MM, Boime I. Site-specific mutagenesis defines the intracellular role of the asparagine-linked oligosaccharides of chorionic gonadotropin β subunit. J Biol Chem. 1988;263(32):17106–17111. [PubMed] [Google Scholar]
- 150. Jiang C, Hou X, Wang C, May JV, Butnev VY, Bousfield GR, Davis JS. Hypo-glycosylated hFSH has greater bioactivity than fully glycosylated recombinant hFSH in human granulosa cells. J Clin Endocrinol Metab. 2015;100(6):E852–E860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Wang H, May J, Butnev V, Shuai B, May JV, Bousfield GR, Kumar TR. Evaluation of in vivo bioactivities of recombinant hypo- (FSH21/18) and fully- (FSH24) glycosylated human FSH glycoforms in Fshb null mice. Mol Cell Endocrinol. 2016;437:224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Ulloa-Aguirre A, Reiter E, Crépieux P. FSH receptor signaling: complexity of Interactions and signal diversity. Endocrinology. 2018;159(8):3020–3035. [DOI] [PubMed] [Google Scholar]
- 153. Gloaguen P, Crépieux P, Heitzler D, Poupon A, Reiter E. Mapping the follicle-stimulating hormone-induced signaling networks. Front Endocrinol (Lausanne). 2011;2:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Nguyen VT, Singh V, Butnev VY, Gray CM, Westfall S, Davis JS, Dias JA, Bousfield GR. Inositol phosphate stimulation by LH requires the entire α Asn56 oligosaccharide. Mol Cell Endocrinol. 2003;199(1–2):73–86. [DOI] [PubMed] [Google Scholar]
- 155. Butnev VY, Singh V, Nguyen VT, Bousfield GR. Truncated equine LHβ and asparagine56-deglycosylated equine LHα combine to produce a potent follicle-stimulating hormone antagonist. J Endocrinol. 2002;172(3):545–555. [DOI] [PubMed] [Google Scholar]
- 156. Loreti N, Fresno C, Barrera D, Andreone L, Albarran SL, Fernandez EA, Larrea F, Campo S. The glycan structure in recombinant human FSH affects endocrine activity and global gene expression in human granulosa cells. Mol Cell Endocrinol. 2013;366(1):68–80. [DOI] [PubMed] [Google Scholar]
- 157. Grøndahl ML, Borup R, Lee YB, Myrhøj V, Meinertz H, Sørensen S. Differences in gene expression of granulosa cells from women undergoing controlled ovarian hyperstimulation with either recombinant follicle-stimulating hormone or highly purified human menopausal gonadotropin. Fertil Steril. 2009;91(5):1820–1830. [DOI] [PubMed] [Google Scholar]