Abstract
Background
In multiple sclerosis (MS) regional gray matter (GM) atrophy has been associated to disability progression.
Objective
The aim of this study was to compare regional GM volume changes in relapsing-remitting MS (RRMS) patients with progressive and stable disability using voxel-based morphometry (VBM).
Methods
Baseline and year one follow-up 3D T1-weighted MRI data of RRMS patients were acquired on two 1.5 Tesla scanners. Patients were pair-wise matched with respect to age, gender, disease duration, medication, scanner, and baseline EDSS into 13 pairs with either progressive EDSS (≥ 1 point change y-1) or stable EDSS as well as into 29 pairs with either progressive MSFC (≥ 0.25% decrease y-1 in any component) or stable MSFC. Longitudinal regional differences in GM volumes were analyzed in progressive and stable EDSS and MSFC groups, respectively, using VBM.
Results
Significant GM volume reductions occurred in the right precuneus in the progressive EDSS group. Differential between-group effects occurred in the right precuneus and in the postcentral gyrus. Further longitudinal GM volume reductions occurred in the right orbicular gyrus in the progressive MSFC group, but no between-group differences were observed. (non-stationary cluster-wise inference, all Pcorrected<0.05)
Conclusion
The results suggest a direct association of disability progression and regional GM atrophy in RRMS.
Keywords: Multiple sclerosis, MRI, voxel-based morphometry, gray matter atrophy, relapsing-remitting, disability progression, Expanded Disability Status Scale, Multiple Sclerosis Functional Composite
Introduction
Historically classified as a white matter (WM) disease, multiple sclerosis (MS) has been associated with both gray matter (GM) lesions and GM atrophy in magnetic resonance imaging (MRI) [1–3]. GM atrophy develops faster than WM atrophy and predominates in early disease phases [4].
Previous studies have shown that global as well as regional GM volume loss in fronto-temporo-parietal regions correlate with disability in relapsing-remitting MS (RRMS) patients [2, 3, 5]. Previous longitudinal voxel-based morphometry (VBM) studies indicate progressive regional GM atrophy in RRMS [6, 7]. However, there are few studies combining GM atrophy and disability progression (Table 1) [8–12]. To the best of our knowledge, only two longitudinal studies directly addressed the relationship of regional GM atrophy and disability progression in MS patients [10, 13].
Table 1. Longitudinal neuroimaging studies relating GM atrophy to disability.
Expanded disability status scale (EDSS) and multiple sclerosis functional composite (MSFC) are amongst the most widely used and clinically relevant disability outcome measures [14]. EDSS includes a wide range of neurological functions and enables comparison of features on a scale of 0-10 for individual and groups of patients over time. MSFC is a standardized, quantitative, assessment instrument of three domains, i.e. ambulation, upper-extremity function, and cognition, enables direct comparison within a study, and correlates with EDSS [14].
In the present study, we investigated regional GM volume changes in RRMS patients with stable disability compared to patients with progressive disability using VBM, a non-biased measure of regional differences of GM volumes in the whole brain [15, 16].
We compared regional GM volume changes over one year in groups of i) RRMS patients with progressive EDSS versus stable EDSS, and ii) patients with progressive MSFC versus stable MSFC.
Based on previous data [10, 13], we hypothesized that the development of GM volume reductions is related to progressive disability in RRMS. We expected to find a closer relationship between regional GM volume reductions and disability in RRMS patients who progressed over the interscan interval compared to patients with stable disability. In particular, we expected changes in specific fronto-temporo-parietal cortical areas [3, 5, 10, 17] reflecting clinical dysfunction in specific functional domains captured by EDSS and MSFC, respectively.
Materials and methods
Patients
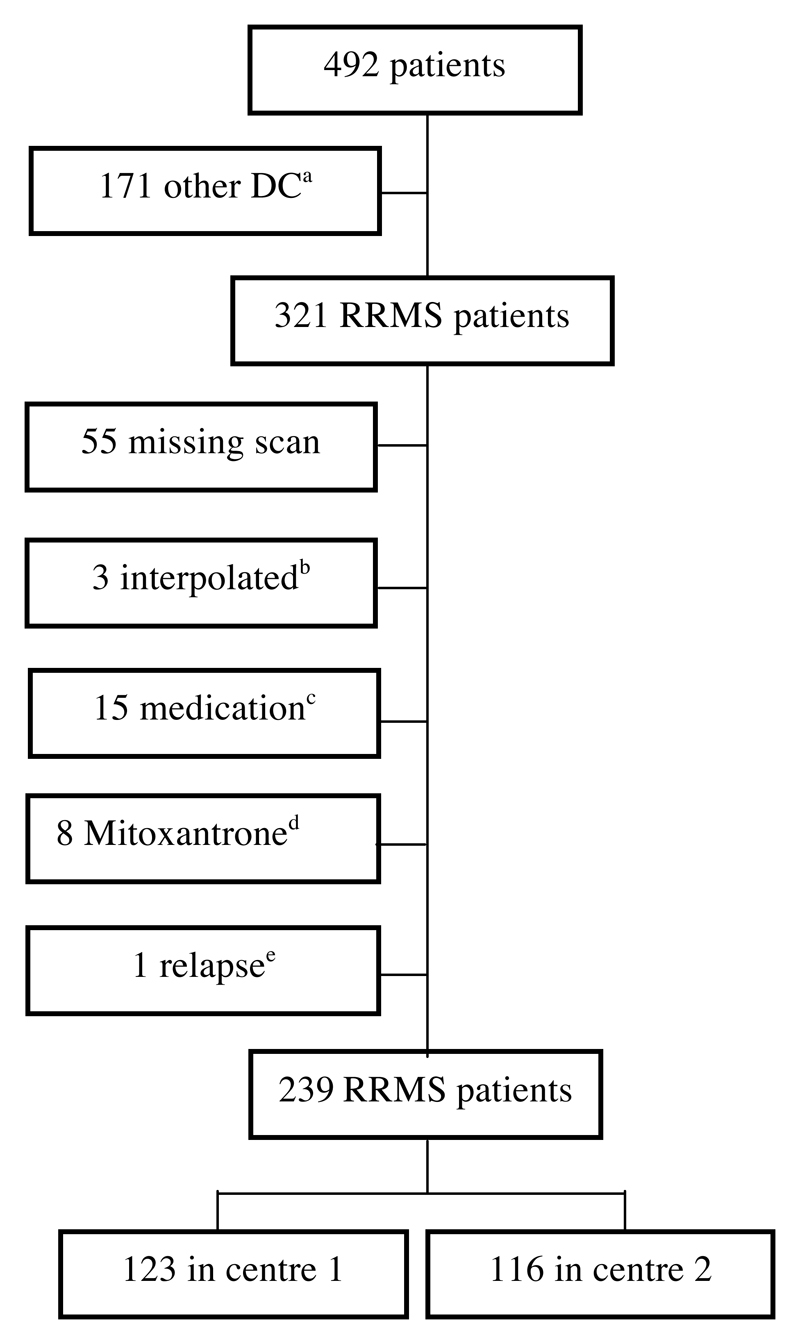
We selected matched pairs of subjects from a total of 239 Caucasian patients (74 men, 165 women) with a diagnosis of clinically definite RRMS [18]. The subjects are from the case-control study for genotype-phenotype associations in MS (“GeneMSA”; GSK UK), recruited in two clinical centres participating in the GeneMSA consortium followed for three years. Strict exclusion criteria were set (Figure 1). There is overlap between subjects in this study and those used in previous MR structural imaging studies [6, 19]. The study was approved by the local ethical standards committee and written informed consent was obtained from each subject.
Figure 1. Exclusion criteria.
adifferent or unknown disease course; bDICOM data interpolated; cchange in treatment or introduction of interferon or glatiramer acetate ≤ 6 months prior to scan; dMitoxantrone treatment; eclinical relapse with or without corticosteroid treatment within 1 month previous to scan.
Based on previous studies [10, 11, 14] disability progression was defined as an increase of ≥ 1.0 point in EDSS or of ≥ 0.25% for any of the three components in MSFC over one year. Patients who were defined as clinically stable showed no change in EDSS or no change ≥ 0.25% for any of the three components in MSFC over one year [10, 11]. The MSFC is a three-part, quantitative assessment instrument that measures arm, leg, and cognitive function with the 9-Hole Peg Test, the Timed 25-Foot Walk, and the Paced Auditory Serial Addition Test (3-second version, PASAT3), respectively. Patients with fluctuations (i.e. ≥ +/- 1 point) in EDSS in the year before/after scan were excluded. In total, thirty-five patients showed EDSS progression, 75 stable EDSS.
To avoid confounding effects of sex, medication, and age patients with progressive and stable disability were pair-wise matched accordingly. Then each subject with progressive disability was assigned a patient with stable disability that did not differ by more than ± 10 years in age at baseline. Then they were assigned to a subject who differed by less than ± 5 years in disease duration and less than ± 0.5 in EDSS at baseline to rule out potential bias by differences in EDSS or disease duration and subsequently GM atrophy at baseline. Thus, we obtained 19 pairs of RRMS patients with either stable or progressive EDSS.
Only MSFC data of centre one were available (36 patients with progressive MSFC, 87 with stable MSFC). Patients were matched according to sex, medication, age (± 10 years), and disease duration (± 5 years) to establish 29 pairs of RRMS patients stable and progressive in MSFC, respectively. In a subgroup analysis the relationship between cognition and regional GM volume at baseline was assessed by means of the PASAT3 [20].
MR image acquisition
All subjects were scanned three times (baseline, year 1 and year 2 follow-up) using one of two 1.5T MR systems (centre one: Siemens Avanto; centre two: Siemens Vision) with similar protocols. For VBM analysis, 3D-heavily T1-weighted gradient echo images were acquired (TR:7-20.8 ms; TE: 2-4ms; TI: 300-400ms), consisting of isotropic 1 x 1 x 1 mm3 voxels. Additionally, dual echo-T2-weighted images (magnetization-prepared rapid gradient echo ‘MP-RAGE’; TR: 2,000-4,000 ms; TE: 14-20/80-108 ms; interleaved axial 3.0-mm-thick slices with an in-plane resolution of 1.0 x 1.0 mm2) and post-contrast T1-weighted spin-echo images (TR: 467-650 ms, TE: 8-17ms; axial 3.0-mm-thick slices with an in-plane resolution of 1.0 x 1.0 mm2) were obtained.
MR imaging data analysis
MR Imaging Data Analysis has already been extensively described before [19]. Briefly, MR images were analyzed using Statistical Parametric Mapping software (SPM5, version 958) running under the MATLAB 7.0 (R14) environment. The images were processed using the VBM toolbox v1.03 (http://dbm.neuro.uni-jena.de/vbm/) as described in [6]. The method was modified to reduce the influence of MS lesions in the process, which could alter the normalization and segmentation procedures. To prevent WM lesions from being misclassified as GM, lesions identified on T2 images were masked from the three-dimensional MP-RAGE images [19] and outlined on the proton density scans (to calculate 3D binary masks and quantify the area of previously identified brain lesions) [21]. The 3D binary masks were then co-registered to the MP-RAGE to remove the MS lesions.
In the segmentation step, images were spatially normalized into the same stereotactic space. In SPM5, prior probability maps that are relevant to tissue segmentation are warped to the individual brains, making the creation of a customized template unnecessary. The normalization was performed by first estimating the optimum 12-variable affine transformation for matching images and then optimizing the normalization using 16 nonlinear iterations using 6 x 8 x 6 basis functions to account for global non-linear shape differences as described before [19]. To preserve the total within-voxel volume, every voxel’s signal intensity in the segmented GM images was multiplied by the Jacobian determinants derived from the spatial normalization. The analysis of these modulated datasets was used to detect regional differences in absolute tissue volume. The potential bias coming from errors in registration, in segmentation, and in warping has been minimized by visually checking as described elsewhere [19].
Finally, in order to increase the signal-to-noise ratio and to account for variation in normal gyral anatomy all images were smoothed using a 5-mm-full-width-at half-maximum isotropic Gaussian kernel [6, 19].
Statistical analysis of demographic data
The median and interquartile range, or the mean and standard deviation were used to describe clinical and MRI characteristics. To compare demographic and clinical variables we used chi-squared test, paired t-test, Mann-Whitney-U and Wilcoxon test, respectively. We performed all statistics using SPSS 15.0 (SPSS Inc.) considering a significance level of p<0.05.
Statistical analysis of MRI data
To investigate longitudinal regional differences in GM volume between the groups, normalized modulated GM volumes were analyzed at voxel level by fitting an analysis of covariance (ANCOVA) model. We have chosen a full-factorial design with the centred covariates scanner and global GM volume (GMV) at baseline in order to assess additional nuisance variation due to head size differences. GMV at baseline was calculated by SPM5.
Baseline brain atrophy has been correlated to EDSS in follow-up several years later [11]. Additionally, all analysis were repeated including T2- and T1 lesion volumes as additional covariates. Before entering the linear regression models, T2 and T1 lesion volume was transformed to reduce skew of lesion volume and reduce the impact of outlier lesion volumes using the logarithm (logT2LV, logT1LV).
In a previous study, we found that the effect of scanner was negligible compared to disease-related effects in RRMS patients of the same dataset thus allowing to pool data from two different 1.5 T scanners. However, to address this point, here we have performed additional analyses of 13 patients per group each pair-wise matched for scanner.
We performed F-tests to investigate if there were any longitudinal GM changes in the progressive and stable group followed by subsequent T-tests contrasting the progressive group against the stable group.
Statistical maps were assessed for significance with cluster size interference adjusted for non-stationarity. We used a cluster-defining threshold of P=0.001 uncorrected, and clusters were considered significant at the familywise error (FWE) corrected PFWE<0.05 cluster level, corrected for a whole-brain search. Localization of significant clusters has already been described elsewhere [19]. Spearman rank correlation was used for correlations between regional GM atrophy and PASAT3 at baseline. The regression was run with centred covariates age, gender, and disease duration.
Results
Clinical and MRI (non-VBM) characteristics
The clinical and MRI characteristics of the different patient groups are reported in Table 2 and 3. The groups did not significantly differ regarding their clinical and MRI characteristics, except for disability measures in MSFC and EDSS, for relapses in the EDSS groups, and for T2LV in the MSFC groups.
Table 2. Demographics and clinical characteristics of the different EDSS patient groups.
| Progressive group N=19 N=13 |
Stable group N = 19 N = 13 |
Statistics Progressive vs. stable: P-value |
|
|---|---|---|---|
| Age at BL in years: mean (SD) | 41.2 (8.9) 42.1 (8.6) |
41.4 (8.9) 41.7 (9.3) |
0.863 0.88 |
| Scanner (centre 1/centre 2) | 8/11 (1:1.4) 6/7 (1:1.16) |
11/8 (1:0.7) 6/7 (1:1.16) |
0.418 1 |
| Male/Female (ratio) | 7/12 (1:1.7) 4/9 (1:2.25) |
7/12 (1:1.7) 4/9 (1:2.25) |
1 1 |
| Disease duration (since first symptoms) at BL in months, median (IQR) | 96 (72-216) 132 (84-222) |
84 (60-168) 132 (72-234) |
0.665 0.724 |
| Scan-interval, months, mean (SD) | 12.4 (1.5) 12.1 (1.2) |
12.4 (0.8) 12.4 (0.9) |
0.708 0.545 |
| Drug treatmenta (T/NT) | 10/9 (1:0.9) 8/5 (1:1.6) |
10/9 (1:0.9) 8/5 (1:1.6) |
1 1 |
| Interferon-β-1a/ interferon-β-1b/ glatiramer acetate | 5/3/2 4/2/2 |
9/1/0 7/1/0 |
|
| Relapseb during follow-up (corticosteroid treatment/no treatment) | 12 (9/3) 7 (5/2) |
3 (2/1) 2 (1/1) |
0.012 0.043 |
| EDSS at BL, median (IQR) | 2.5 (2.0-3.0) 2.0 (1.75-3.0) |
2.0 (2.0-3.0) 2.0 (2.0-3.25) |
0.908 0.88 |
| EDSS at fu, median (IQR) | 4.0 (3.0-4.5) 3.5 (2.75-4.25) |
2.0 (2.0-3.0) 2.0 (2.0-3.25) |
<0.001 0.004 |
| Statistics: EDSS change, BL versus y2: P-value | <0.001 <0.001 |
1 1 |
|
| GMV in cm3, mean (SD) at BL | 668.4 (85.2) 653.9 (74.2) |
646.4 (67.4) 630.6 (50.3) |
0.418 0.418 |
| GMV in cm3, mean (SD) at fu | 669.2 (89.4) 658.1 (81.5) |
645.3 (68.0) 632.1 (46.8) |
0.48 0.47 |
| Statistics: GMVC, BL versus y2: P-value | 0.82 0.399 |
0.553 0.132 |
|
| T1LV in ml, median (IQR) at BL | 1.3 (0.4-2.8) 0.9 (0.3-2.8) |
0.5 (0.2-3.7) 0.9 (0.2-5.7) |
0.47 0.687 |
| T1LV in ml, median (IQR) at fu | 1.3 (0.4-3.0) 1.1 (0.3-2.7) |
0.5 (0.2-4.1) 0.8 (0.2-5.8) |
0.488 0.614 |
| Statistics: T1LV change, BL versus y2: P-value | 0.125 0.44 |
0.92 0.968 |
|
| T2LV in ml, median (IQR) at BL | 3.4 (1.8-6.7) 2.7 (2.1-6.3) |
2.5 (1.0-10.1) 5.1 (1.0-18.0) |
0.665 0.762 |
| T2LV load in ml, median (IQR) at fu | 3.1 (1.7-7.9) 2.6 (2.0-6.7) |
2.6 (0.8-11.0) 5.1 (0.9-19.7) |
0.644 0.801 |
| Statistics: T2LV change, BL versus y2: P-value | 0.059 0.296 |
0.062 0.1 |
|
| New-T2-lesions at fu (count, median (IQR)) | 0 (0-4) 0 (0-3.5) |
1 (0-3) 1 (0-3) |
0.644 0.479 |
| New Gd-enhancing lesions at fu (count, median (IQR)) | 0 (0-1) 0 (0-0.5) |
0 (0-1) 0 (0-1) |
0.931 0.801 |
T, treated; NT, not treated; SD, standard deviation; IQR, interquartile range; EDSS, expanded disability status scale; BL, baseline; fu, follow-up scan (1 year). Results of a subgroup with optimally pair-wise matched subjects of n=13 (progressive) vs. n=13 (stable) is presented in italic letters.
No changes in medication during fu.
All patients had been relapse-free and interacted with steroids for at least 1 month at the time of BL and fu scan.
Table 3. Demographics and clinical characteristics of the different MSFC patient groups.
| Progressive group N=29 |
Stable group N=29 |
Statistics Progressive vs. stable: P-value |
|
|---|---|---|---|
| Age at BL in years: mean (SD) | 45.9 (9.3) | 43.0 (10.2) | 0.173 |
| Scanner | Only centre 1 | Only centre 1 | Na |
| Male/Female (ratio) | 7/22 (1:3.14) | 7/22 (1:3.14) | 1 |
| Disease duration (since first symptoms) at BL in months, median (IQR) | 156 (102-228) | 156 (96-228) | 0.852 |
| Scan-interval, months, mean (SD) | 12.3 (1.2) | 12.5 (0.7) | 0.164 |
| Drug treatmenta (T/NT) | 21/8 (1:0.4) | 21/8 (1:0.4) | 1 |
| Interferon-β-1a/ interferon-β-1b/ glatiramer acetate | 13/5/3 | 11/4/6 | |
| Relapseb during follow-up corticosteroid treatment/no treatment) | 8 (7/1) | 11 (7/4) | 0.405 |
| EDSS at BL, median (IQR) | 2.5 (2.0-4.5) | 2.0 (1.5-3.0) | 0.16 |
| EDSS at fu, median (IQR) | 3.0 (2.5-4.5) | 2.0 (1.5-2.5) | 0.001 |
| Statistics: EDSS change, BL versus y2: P-value | 0.041 | 0.655 | |
| GMV in cm3, mean (SD) at BL | 610.3 (76.1) | 625.9 (71) | 0.363 |
| GMV in cm3, mean (SD) at fu | 607.5 (75.3) | 623.9 (69) | 0.255 |
| Statistics: GMVC, BL versus y2: P-value | 0.677 | 0.638 | |
| T1LV in ml, median (IQR) at BL | 1.2 (0.4-3.6) | 0.6 (0.2-1.7) | 0.111 |
| T1LV in ml, median (IQR) at fu | 1.3 (0.3-3.9) | 0.7 (0.2-1.6) | 0.164 |
| Statistics: T1LV change, BL versus y2: P-value | 0.129 | 0.452 | |
| T2LV in ml, median (IQR) at BL | 6.3 (1.6-11.2) | 2.6 (1.0-4.9) | 0.046 |
| T2LV load in ml, median (IQR) at fu | 5.8 (1.6-11.6) | 2.6 (1.0-5.1) | 0.049 |
| Statistics: T2LV change, BL versus y2: P-value | 0.086 | 0.058 | |
| New-T2-lesions at fu (count, median (IQR)) | 0 (0-1.5) | 0 (0-1.5) | 0.459 |
| New Gd-enhancing lesions at fu (count, median (IQR)) | 0 (0-0) | 0 (0-0) | 0.16 |
T, treated; NT, not treated; SD, standard deviation; IQR, interquartile range; EDSS, expanded disability status scale; MSFC, multiple sclerosis functional composite; BL, baseline; fu, follow-up scan (1 year).
No changes in medication during fu.
All patients had been relapse-free and interacted with steroids for at least 1 month at the time of BL and fu scan.
Longitudinal changes in GM volume
EDSS
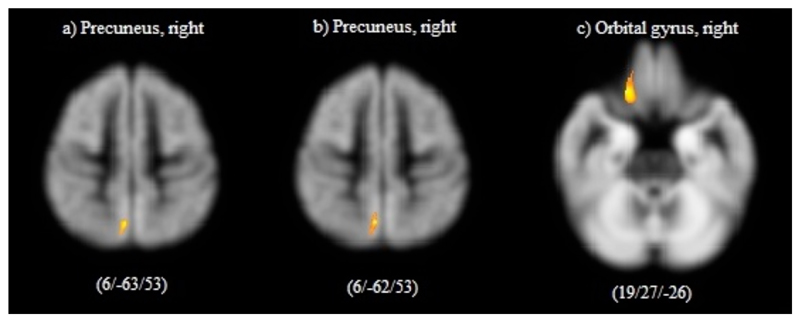
Over one year a significant reduction of cortical GM volume (cluster PFWE<0.05) was found in the right precuneus (Figure 2a, Table 4a) in the progressive group. In contrast, no GM volume changes were found in the group of patients with stable EDSS over time (minimum PFWE=0.283). A statistically significant differential effect of GM volume development between both groups was found in the right precuneus (Figure 3a, Table 4b).
Figure 2. Areas of significant GM volume reductions in patients with progressive disability.
Results refer to significant reduction in gray mater volume between baseline and follow-up scan a) in 19 RRMS patients with progressive EDSS; b) in 13 RRMS patients with progressive EDSS; c) in 29 RRMS patients with progressive MSFC (all P<0.01, corrected for multiple comparison). Images are presented in standard neurological fashion, with the right hemisphere shown on the right of the figure, and vice versa. Coordinates (x, y, and z) refer to the point of maximal change in each cluster in stereotactic space as defined in the MNI atlas.
Table 4. Gray matter volume changes and differential effects.
| Area | MNI coordinates of cluster maximum (x y z) | T | Cluster size kE(voxels) | Cluster Pcorrected | Voxel PFWE-corrected | |
|---|---|---|---|---|---|---|
| a) Right parietal lobe | Precuneus, BA7 | 5, -61, 54 | 5.77 | 1771 | <0.001 | 0.025 |
| b) Right parietal lobe | Precuneus, BA7 | 6, -63, 53 | 4.37 | 792 | <0.001 | 0.917 |
| c) Right parietal lobe | Precuneus, BA7 | 6, -62, 53 | 4.78 | 1284 | <0.001 | 0.743 |
| d) Right parietal lobe | Postcentral Gyrus, BA5 | 27, -52, 56 | 5.12 | 877 | <0.001 | 0.404 |
| Precuneus, BA7 | 6, -63, 51 | 4.93 | 1536 | <0.001 | 0.592 | |
| e) Right Frontal Lobe | Orbital Gyrus BA47 | 19, 27, -26 | 4.68 | 956 | 0.001 | 0.438 |
Results refer to a) longitudinal GM volume changes in 19 RRMS patients with progressive EDSS; b) comparison of optimally pair-wise matched groups with progressive (n=19) and stable EDSS (n=19); c) longitudinal GM volume changes in 13 RRMS patients with progressive EDSS; d) comparison of optimally pair-wise and centre matched groups with progressive (n=13) and stable EDSS (n=13); e) longitudinal GM volume changes in 29 RRMS patients with progressive MSFC over one year (all Cluster pcorrected < 0.05). Coordinates (x, y, and z) refer to the point of maximal change in each cluster in stereotactic space as defined in the MNI atlas.
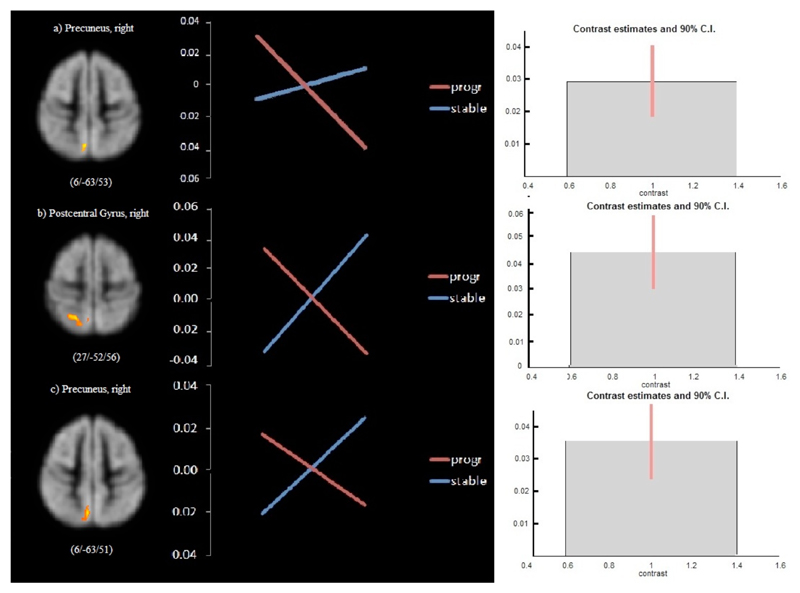
Figure 3. Comparison of longitudinal regional GM volume changes in RRMS patients with progressive disability and stable disability.
For each individual region from left to right: (1) Superimposed image of the significant GM volume differences (P<0.01 corrected) onto an MNI-template. (2) Mean signal intensities (‘eigenvalues’) for the interaction between the effects of disability (progressive vs. stable) and time (baseline vs. follow-up scan) in a variety of cortical regions. (3) Contrast estimates and 90% confidence intervals for the differential effects. Results refer to a) pair-wise matched RRMS patients with progressive (n=19) and stable (n=19) EDSS; b) and c) optimally pair-wise matched RRMS patients with progressive (n=13) and stable (n=13) EDSS. Images are presented in standard radiological fashion, with the right hemisphere shown on the left of the figure, and vice versa. Coordinates (x, y, and z) refer to the point of maximal change in each cluster in stereotactic space as defined in the MNI atlas.
The results of the centre-matched patients basically confirmed those of the first analysis showing regional GM volume reduction in the right precuneus in patients with progressive EDSS, but no change in the stable group (Figure 2b, Table 4c). Statistically significant differential effects between RRMS patients with progressive and those with stable EDSS were also found in the right precuneus, and additionally in the right postcentral gyrus (Figure 3b and 3c, Table 4d).
MSFC
In the group with progressive MSFC cortical GM volume decreased significantly (cluster PFWE<0.05) in the right orbital gyrus (Figure 2c, Table 4e). No GM volume changes in the stable group (minimum PFWE=0.848) and no between-group differences (minimum PFWE=0.663) were found.
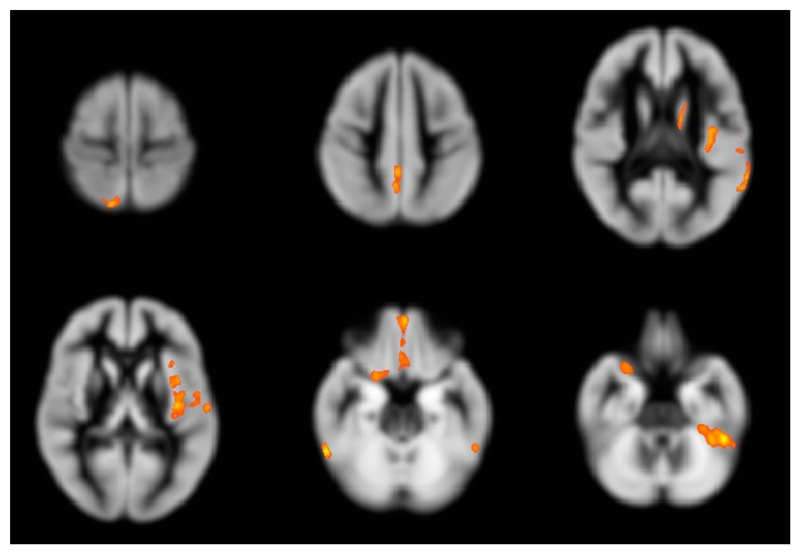
In the total of 123 patients, we also found moderate correlations between the PASAT3 test and GM volumes at baseline in a number of cortical and deep GM areas. (Figure 4, Table 5).
Figure 4. Areas of regional gray matter volume at baseline which correlated significantly with PASAT at baseline.
Significant correlations were found in the left orbital gyrus, the left superior temporal gyrus, the left and right fusiform gyrus, the left insula, the left caudate body, the right inferior frontal gyrus, the right superior parietal lobule, and the right precuneus. Images are presented in standard neurological fashion, with the right hemisphere shown on the right of the figure, and vice versa.
Table 5. Univariate correlation coefficients between PASAT and baseline measures of regional gray matter volume.
| GM area | MNI coordinates (x y z) | Cluster Size kE (voxels) | Ra |
|---|---|---|---|
| Left frontal lobe, orbital gyrus, BA11 | -1, 53, -23 | 1479 | 0.466** |
| Left superior temporal gyrus, BA22 | -61, -54, 14 | 1510 | 0.537** |
| Left temporal lobe, fusiform gyrus, BA36 | -47, -41, -28 | 1551 | 0.487** |
| Left Insula, BA13 | -35, -9, 14 | 5597 | 0.513** |
| Left caudate body | -15, -4, 19 | 643 | 0.460** |
| Right inferior frontal gyrus, BA47 | 21, 10, -21 | 812 | 0.471** |
| Right temporal lobe, fusiform gyrus, BA37 | 59, -50, -23 | 767 | 0.494** |
| Right superior parietal lobule, BA7 | 13, -66, 61 | 911 | 0.473** |
| Right parietal lobe, Precuneus, BA7 | 5, -52, 44 | 1559 | 0.484** |
PASAT, Paced Auditory Serial Addition Test; BA, Brodmann area. Results of 123 patients of centre 1 (mean age: 43 years; median disease duration: 10 years; median baseline EDSS: 2.0).
aDerived from the mean signal intensity values of each significant cluster. Significance levels: **<0.01
Discussion
In general, GM atrophy has been reported to relate more to disability and cognitive impairment [2, 3, 22] than WM atrophy [11]. However, to some extent specific lesion location seems to be associated with symptom development in MS [23], which might partially explain the ‘clinico-radiologic paradox’ of modest association of clinical and radiological findings [24]. The rate of brain atrophy during the RRMS disease course seems to correlate better with future clinical disability than disability measures at that time [25]. Moreover, it is yet undetermined if GM atrophy is the result of GM pathology (e.g. CLs) and/or indirectly the result of Wallerian degeneration due to an increasing WM lesion burden or WM lesions in specific tracts.
In the present study, neither in the progressive nor in the stable EDSS and MSFC groups significant global GMV changes were found over one year. In contrast, regional GM volume reductions occured in the right parietal lobe which is consistent with previous work showing significant loss of apparent cGM thickness over one year in parietal and precentral areas in progressive disability compared to stable disability [10]. However, in the latter study, MS patients with progressive disability had 29% higher baseline EDSS, 303% higher T2 lesion volumes, and a combined analysis of RRMS and SPMS patients was performed. Our finding of an effect in the right hemisphere is generally consistent with another study of different MS types, although the patients in that study had larger hyperintense lesion volumes (by 64%) and a shorter mean disease duration of 1.8 years [13]. Furthermore, a less specific and sophisticated method was used in this study.
Strikingly, consistent with own previous results in similar samples of the same dataset [6, 19], no significant GM volume changes occurred in the motor cortices during follow up. The patients in the present sample might have reached a steady state in GM atrophy in motor cortex due to their good adherence to an adequate therapy with immunomodulatory drugs, and their relatively advanced disease duration and age. Soft factors such as fatigue are also included in the EDSS. Thus, progression in disability measured by EDSS may partly be attributed to fatigue. Previous studies reported cortical atrophy of the parietal lobe related to fatigue [26, 27]. The authors suggested that dysfunctions in higher-order aspects of motor control and attentional system play a major role in determining fatigue in MS. A further study found a correlation between fatigue and GM atrophy in the primary sensorimotor area [28]. Interestingly, we found a relationship between EDSS progression and regional GM atrophy in the right postcentral gyrus and right precuneus.
Although a previous study reported GM atrophy to better correlate with MSFC than EDSS, in the present study we only found between-group differences in disability described by EDSS [11]. Furthermore, the authors found a relatively low concordance between MSFC and EDSS progression (Table 6) [11].
Table 6. Relationship between MSFC and EDSS progression in centre 1.
| Progressive EDSS | Stable EDSS | Total | |
|---|---|---|---|
| Progressive MSFC | 8 | 28 | 36 (29%) |
| Stable MSFC | 17 | 70 | 87 (71%) |
| Total | 25 (20%) | 98 (80%) |
In the present study, PASAT3 at baseline correlated with a pattern of widespread regional GM volumes in fronto-temporo-parietal cortices and deep GM. PASAT is testing executive function, working memory, information processing, and attention [29]. Previous MS studies reported left frontal and temporal atrophy to be related to auditory and verbal memory, right frontal atrophy to impairment in episodic and working memory, left and right temporal atrophy to visual/spatial memory, frontal temporal atrophy to learning consistency, precuneus and superior parietal lobule to performance of simple calculation and arithmetic tasks [29, 30]. Such highly interconnected areas might prone to be affected by axonal degeneration in the WM [5].
Methodological issues and limitations
Generally, inter-individual differences in WM lesion patterns can influence GM volume reductions. However, it is rather unlikely that the observed effects are related to different distribution of new lesions in the two groups, because the number of new lesions is very small and not significantly different between groups, and the drugs used in this study are known to equally well prevent the occurrence of new inflammatory lesions [19]. Potential differences in lesions or atrophy of the spinal cord or brain stem, respectively, may have contributed to differences in disability as well as in cortical GM volume between the two groups as MR data of spinal cords were not available for our study. A previous study showed an association of cervical cord atrophy and physical disability [31], but cortical GM atrophy and cervical cord atrophy was also reported to be independently associated with long-term disability in MS [32].
Progression of T2 and T1 lesion volume has been related to regional GM atrophy [6] but seems to be not relevant in the present study, where no significant WM lesion volume changes over time were found. Furthermore, GM volume changes may be mimict by GM volume fluctuations: Gliosis or pharmaceutical effects may increase brain volume; underlying co-existing pathological processes independent of MS, or effects of certain pharmaceuticals may reduce regional brain volume. There is not yet sufficient evidence, however, on the association of medication and regional GM volume change. Anti-inflammatory drugs are also suggested to slow brain atrophy during the RRMS disease stage [33]. To reduce such confounding effects, patients of the two groups were matched with respect to medication, but not with respect to the active ingredient. To rule out potential “pseudoatrophy” (accelerated brain atrophy and decrease in the number of gadolinium-enhancing lesions due to anti-inflammatory effects of medication)[34], here we set strict exclusion criteria concerning use and change of immunomodulatory drugs as well as of corticosteroids.
In a previous longitudinal VBM study, we provided evidence that pooling of MRI data of our two scanners provides reliable results, because pooling of MRI data of different scanners might introduce a systematic error. This issue is extensively discussed elsewhere [19] In spite of pairwise matching with respect to age, the effect of aging cannot fully be ruled out [19] because aging-related GM volume changes interfere with disease specific effects and they do not occur in a linear way.
EDSS is a method summing up several compounds to quantify disability with emphasis on the evaluation ambulation, only marginally assessing cognition. A global approach is not the most sensitive tool to look for regional GM volume changes since patients with progressive EDSS may show progression in different functional systems thus affecting different regions of the cortex. In the present study, several shortcomings of EDSS [14] have been considered. Only patients with a baseline EDSS <5.0 were included, because the clinical importance of a 1.0 point change varies according to the starting score [14]. Sustained improvement in EDSS occurring very frequently in RRMS might contribute to imprecision in disability progression measurement by EDSS. Thus, to control for EDSS fluctuations, we assessed confirmed disability progression one year after follow-up [14]. Furthermore, intra- and inter-examiner reliability was improved by using a standardised version of the EDSS.
The lacking association of MSFC progression and GM atrophy in our data might be due to the relatively small cohort. Furthermore, MSFC still lacks an accepted definition of clinically meaningful change [35]. To enable comparison across studies, in the present study significant worsening in MSFC was defined as a 25% change in one component sustained over one year. Noteworthy, the influence of floor and ceiling effects on MSFC progression was not ruled out [14].
Conclusion
The results suggest that cortical regional GM volume reductions in the right precuneus and in the right postcentral gyrus are associated with disability progression as measured by EDSS. Future longer-term studies should clarify the association of regional GM atrophy to disability progression in more specific clinical functions, such as motor and cognitive impairment, depression, or fatigue.
Acknowledgements
The authors thank the Head of GlaxoSmithKline Clinical Imaging Centre, Professor Paul Matthews, and the Swiss MS Society.
Footnotes
Conflict of Interest Statement
All authors declare no conflicts of interest.
References
- 1.Miller DH, Barkhof F, Frank JA, et al. Measurement of atrophy in multiple sclerosis: pathological basis, methodological aspects and clinical relevance. Brain. 2002;125:1676–1695. doi: 10.1093/brain/awf177. [DOI] [PubMed] [Google Scholar]
- 2.De Stefano N, Matthews PM, Filippi M, et al. Evidence of early cortical atrophy in MS: relevance to white matter changes and disability. Neurology. 2003;60:1157–1162. doi: 10.1212/01.wnl.0000055926.69643.03. [DOI] [PubMed] [Google Scholar]
- 3.Sailer M, Fischl B, Salat D, et al. Focal thinning of the cerebral cortex in multiple sclerosis. Brain. 2003;126:1734–1744. doi: 10.1093/brain/awg175. [DOI] [PubMed] [Google Scholar]
- 4.Chard DT, Griffin CM, Rashid W, et al. Progressive grey matter atrophy in clinically early relapsing-remitting multiple sclerosis. Mult Scler J. 2004;10:387–391. doi: 10.1191/1352458504ms1050oa. [DOI] [PubMed] [Google Scholar]
- 5.Charil A, Dagher A, Lerch JP, et al. Focal cortical atrophy in multiple sclerosis: relation to lesion load and disability. Neuroimage. 2007;34:509–517. doi: 10.1016/j.neuroimage.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Bendfeldt K, Kuster P, Traud S, et al. Association of regional gray matter volume loss and progression of white matter lesions in multiple sclerosis - A longitudinal voxel-based morphometry study. Neuroimage. 2009;45:60–67. doi: 10.1016/j.neuroimage.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Pagani E, Rocca MA, Gallo A, et al. Regional brain atrophy evolves differently in patients with multiple sclerosis according to clinical phenotype. AJNR Am J Neuroradiol. 2005;26:341–346. [PMC free article] [PubMed] [Google Scholar]
- 8.Audoin B, Davies GR, Finisku L, et al. Localization of grey matter atrophy in early RRMS : A longitudinal study. J Neurol. 2006;253:1495–1501. doi: 10.1007/s00415-006-0264-2. [DOI] [PubMed] [Google Scholar]
- 9.Calabrese M, Rocca MA, Atzori M, et al. A 3-year magnetic resonance imaging study of cortical lesions in relapse-onset multiple sclerosis. Ann Neurol. 67:376–383. doi: 10.1002/ana.21906. [DOI] [PubMed] [Google Scholar]
- 10.Chen JT, Narayanan S, Collins DL, et al. Relating neocortical pathology to disability progression in multiple sclerosis using MRI. Neuroimage. 2004;23:1168–1175. doi: 10.1016/j.neuroimage.2004.07.046. [DOI] [PubMed] [Google Scholar]
- 11.Rudick RA, Lee JC, Nakamura K, et al. Gray matter atrophy correlates with MS disability progression measured with MSFC but not EDSS. J Neurol Sci. 2009;282:106–111. doi: 10.1016/j.jns.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher E, Lee JC, Nakamura K, et al. Gray matter atrophy in multiple sclerosis: a longitudinal study. Ann Neurol. 2008;64:255–265. doi: 10.1002/ana.21436. [DOI] [PubMed] [Google Scholar]
- 13.Jasperse B, Vrenken H, Sanz-Arigita E, et al. Regional brain atrophy development is related to specific aspects of clinical dysfunction in multiple sclerosis. Neuroimage. 2007;38:529–537. doi: 10.1016/j.neuroimage.2007.07.056. [DOI] [PubMed] [Google Scholar]
- 14.Cohen JA, Reingold SC, Polman CH, et al. Disability outcome measures in multiple sclerosis clinical trials: current status and future prospects. Lancet Neurol. 2012;11:467–476. doi: 10.1016/S1474-4422(12)70059-5. [DOI] [PubMed] [Google Scholar]
- 15.Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 16.Good CD, Johnsrude IS, Ashburner J, et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 17.Bakshi R, Benedict RH, Bermel RA, et al. Regional brain atrophy is associated with physical disability in multiple sclerosis: semiquantitative magnetic resonance imaging and relationship to clinical findings. J Neuroimaging. 2001;11:129–136. doi: 10.1111/j.1552-6569.2001.tb00022.x. [DOI] [PubMed] [Google Scholar]
- 18.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the "McDonald Criteria". Ann Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 19.Bendfeldt K, Hofstetter L, Kuster P, et al. Longitudinal gray matter changes in multiple sclerosis-Differential scanner and overall disease-related effects. Hum Brain Mapp. 2012;33:1225–1245. doi: 10.1002/hbm.21279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Penner IK, S B, Calabrese P, Freedman MS, et al. Effects of interferon beta-1b on cognitive performance in patients with a first event suggestive of multiple sclerosis. Mult Scler J. 2012;18:1466–1471. doi: 10.1177/1352458512442438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kappos L, Radue EW, O'Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2006;362:387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 22.Chard DT, Griffin CM, Parker GJ, et al. Brain atrophy in clinically early relapsing-remitting multiple sclerosis. Brain. 2002;125:327–337. doi: 10.1093/brain/awf025. [DOI] [PubMed] [Google Scholar]
- 23.Kincses ZT, Ropele S, Jenkinson M, et al. Lesion probability mapping to explain clinical deficits and cognitive performance in multiple sclerosis. Mult Scler J. 2011;17:681–689. doi: 10.1177/1352458510391342. [DOI] [PubMed] [Google Scholar]
- 24.Barkhof F. The clinico-radiological paradox in multiple sclerosis revisited. Curr Opin Neurol. 2002;15:239–245. doi: 10.1097/00019052-200206000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Fisher E, Rudick RA, Simon JH, et al. Eight-year follow-up study of brain atrophy in patients with MS. Neurology. 2002;59:1412–1420. doi: 10.1212/01.wnl.0000036271.49066.06. [DOI] [PubMed] [Google Scholar]
- 26.Pellicano C, Gallo A, Li X, et al. Relationship of cortical atrophy to fatigue in patients with multiple sclerosis. Arch Neurol. 2010;67:447–453. doi: 10.1001/archneurol.2010.48. [DOI] [PubMed] [Google Scholar]
- 27.Calabrese M, Rinaldi F, Grossi P, et al. Basal ganglia and frontal/parietal cortical atrophy is associated with fatigue in relapsing-remitting multiple sclerosis. Mult Scler J. 2010;16:1220–1228. doi: 10.1177/1352458510376405. [DOI] [PubMed] [Google Scholar]
- 28.Riccitelli G, Rocca MA, Forn C, et al. Voxelwise assessment of the regional distribution of damage in the brains of patients with multiple sclerosis and fatigue. AJNR Am J Neuroradiol. 2011;32:874–879. doi: 10.3174/ajnr.A2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morgen K, Sammer G, Courtney SM, et al. Evidence for a direct association between cortical atrophy and cognitive impairment in relapsing-remitting MS. Neuroimage. 2006;30:891–898. doi: 10.1016/j.neuroimage.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 30.Tekok-Kilic A, Benedict RH, Weinstock-Guttman B, et al. Independent contributions of cortical gray matter atrophy and ventricle enlargment of predicting neuropsychological impairment in multiple sclerosis. Neuroimage. 2007;36:1294–1300. doi: 10.1016/j.neuroimage.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 31.Cohen AB, Neema M, Arora A, et al. The Relationships among MRI-Defined Spinal Cord Involvement, Brain Involvement, and Disability in Multiple Sclerosis. J Neuroimaging. 2012;22:122–128. doi: 10.1111/j.1552-6569.2011.00589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonati U, Fisniku LK, Altmann DR, et al. Cervical cord and brain grey matter atrophy independently associate with long-term MS disability. J Neurol Neurosurg Psychiatry. 2011;82:471–472. doi: 10.1136/jnnp.2010.205021. [DOI] [PubMed] [Google Scholar]
- 33.Filippi M, Rovaris M, Inglese M, et al. Interferon beta-1a for brain tissue loss in patients at presentation with syndromes suggestive of multiple sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:1489–1496. doi: 10.1016/S0140-6736(04)17271-1. [DOI] [PubMed] [Google Scholar]
- 34.Barkhof F, Calabresi PA, Miller DH, et al. Imaging outcomes for neuroprotection and repair in multiple sclerosis trials. Nat Rev Neurol. 2009;5:256–266. doi: 10.1038/nrneurol.2009.41. [DOI] [PubMed] [Google Scholar]
- 35.Polman CH, Rudick RA. The multiple sclerosis functional composite: a clinically meaningful measure of disability. Neurology. 2010;74:S8–15. doi: 10.1212/WNL.0b013e3181dbb571. [DOI] [PubMed] [Google Scholar]