Abstract
Purpose
HER2-targeted therapies have substantially improved the outcome of patients with breast cancer, however, they can be associated with cardiac toxicity. Guidelines recommend holding HER2-targeted therapies until resolution of cardiac dysfunction. SAFE-HEaRt is the first trial that prospectively tests whether these therapies can be safely administered without interruptions in patients with cardiac dysfunction.
Methods
Patients with stage I–IV HER2-positive breast cancer candidates for trastuzumab, pertuzumab or ado-trastuzumab emtansine (TDM-1), with left ventricular ejection fraction (LVEF) 40–49% and no symptoms of heart failure (HF) were enrolled. All patients underwent cardiology visits, serial echocardiograms and received beta blockers and ACE inhibitors unless contraindicated. The primary endpoint was completion of the planned HER2-targeted therapies without developing either a cardiac event (CE) defined as HF, myocardial infarction, arrhythmia or cardiac death or significant asymptomatic worsening of LVEF. The study was considered successful if planned oncology therapy completion rate was at least 30%.
Results
Of 31 enrolled patients, 30 were evaluable. Fifteen patients were treated with trastuzumab, 14 with trastuzumab and pertuzumab, and 2 with TDM-1. Mean LVEF was 45% at baseline and 46% at the end of treatment. Twenty-seven patients (90%) completed the planned HER2-targeted therapies. Two patients experienced a CE and 1 had an asymptomatic worsening of LVEF to ≤ 35%.
Conclusion
This study provides safety data of HER2-targeted therapies in patients with breast cancer and reduced LVEF while receiving cardioprotective medications and close cardiac monitoring. Our results demonstrate the importance of collaboration between cardiology and oncology providers to allow for delivery of optimal oncologic care to this unique population.
Electronic supplementary material
The online version of this article (10.1007/s10549-019-05191-2) contains supplementary material, which is available to authorized users.
Keywords: HER2-targeted therapy, Cardiac dysfunction, Cardiac safety, Carvedilol, Breast cancer
Introduction
The initial trials of trastuzumab for metastatic breast cancer revealed increased risk of cardiac toxicity with rates of left ventricular ejection fraction (LVEF) decline ranging from 3 to 27% [1]. Since then, subsequent trials leading to the approval of human epidermal growth factor receptor 2 (HER2)-targeted therapies have employed stringent cardiovascular eligibility criteria, cardiac monitoring schema, and early stopping rules largely based on LV function assessed by LVEF [2–8]. In the adjuvant trastuzumab clinical trials, up to 18% of patients had asymptomatic declines in LVEF and in one study 19% discontinued trastuzumab, although the observed rates of clinical heart failure (HF) were low (0–4.1%) [9–12]. Current Food and Drug Administration (FDA) recommendations for trastuzumab, pertuzumab and ado-trastuzumab emtansine (T-DM1) limit their use to patients whose LVEF prior to treatment exceeds 50% or 55% and advise dose delay or discontinuation in the setting of LVEF decline during treatment [13–16]. The potential impact of withholding or delaying HER2-targeted therapies on oncologic outcomes is of concern, given that all are associated with substantial oncological benefit [1–8].
Retrospective analyses suggest that continuation of trastuzumab in the setting of asymptomatic LV dysfunction may be safe with appropriate cardiac management [17, 18]. To date, there is no available prospective data regarding the safety of trastuzumab, pertuzumab and T-DM1 in the setting of cardiac dysfunction. The SAFE-HEaRt study (ClinicalTrials.gov, Identifier: NCT01904903) is the first prospective study to test the hypothesis that trastuzumab, pertuzumab and T-DM1 can safely be used in patients with compromised LV systolic function, along with cardiac monitoring and treatment with cardioprotective agents [19]. This group of patients at present have limited oncologic treatment options, and therefore, are at risk for adverse cancer outcomes.
Materials and methods
Trial design
This is a pilot study assessing the cardiac safety of trastuzumab, pertuzumab and T-DM1 in patients with cardiac dysfunction. The trial was conducted at three centers in the U.S with accrual from October 2013 to December 2017. Details of the study design have been previously published [19]. All patients signed informed consent. Data were reviewed by a cardiac review panel composed of 3 board-certified cardiologists with expertise in echocardiography every 3 months and by a data safety monitoring board every 6 months. An Investigational New Drug (IND) Application was obtained from the FDA for trastuzumab, pertuzumab and T-DM1 (IND Number 118811). Minimal duration of planned oncology therapy was 3 months. Patients could be on study for a maximum of 12 months and were followed for 6 months after completion of study treatment. The protocol was amended in 2017 to allow contact with patients for long-term follow up. The study was conducted with approval from the MedStar Health Research Institute (MHRI)-Georgetown University Oncology Institutional Review Board (IRB) and the Memorial Sloan Kettering Cancer Center IRB.
Eligibility
Participants with stage I-IV breast cancer who were receiving or planning to receive trastuzumab, trastuzumab with pertuzumab or T-DM1 in the (neo)adjuvant or metastatic setting and had LVEF 40 to 49% prior to study participation, were eligible. Baseline LVEF was confirmed by the MHRI Echocardiography Core Laboratory (Core Lab) [19]. Previous HER2-targeted therapies and anthracyclines were allowed; however, the most recent anthracycline administration had to be completed more than 50 days prior to enrollment. Exclusion criteria included symptomatic HF or HF hospitalization for HF within the last 12 months. During screening, all patients were evaluated by study cardiologists to exclude coronary ischemia and/or other treatable causes of HF. Stress testing and coronary artery imaging were performed at the discretion of the study cardiologists.
Intervention
Patients received HER2-targeted therapy with or without chemotherapy or endocrine therapy as per the treating oncologist’s choice concurrently with the study-mandated cardiac monitoring and cardioprotective medications. Trastuzumab and pertuzumab doses were not reduced. T-DM1 dose was reduced per standard oncologic criteria, but not adjusted for cardiac reasons. Doses of chemotherapy were adjusted as indicated at the discretion of the oncologist.
Cardiac treatment with beta blockers (BB), angiotensin-converting enzyme (ACE) inhibitors (ACEi) or angiotensin II receptor blockers (ARBs) was managed by study cardiologists and initiated in all patients who did not have contraindications prior to the start of on-study HER2-targeted therapy. BB was initiated prior to the first dose of on-study HER2-targeted therapy and subsequently titrated to the maximum tolerated dose. If this was achieved, an ACEi was added and increased as tolerated to the maximum dose. The algorithm detailing the cardiac medication titration has been previously published [19]. All patients were given home blood pressure (BP) cuffs to monitor daily BP to facilitate dose titration. Carvedilol was the preferred BB and was initiated first, followed by ramipril (ACEi) or candesartan (ARB). Patients who were previously on a different BB were changed to carvedilol; however, other ACEi or ARBs were allowed and continued if they were part of patient’s medications prior to the trial.
Cardiac assessments included echocardiograms and cardiology visits at baseline, 6 weeks and 12 weeks and then every 12 weeks while on study. Echocardiograms were repeated at the end of treatment (EOT) and 6 months post EOT. All echocardiograms were acquired following a study-specific protocol and were independently reviewed by the Core Lab, blinded to any clinical information. LVEF was assessed by 3D, 2D biplane Simpson’s method and visual estimate following American Society of Echocardiography guidelines [20]. All available LVEF values were included on the case report form, sent to the investigators and used for clinical decision-making, following a hierarchical approach:
if 3D measurement LVEF was available it was used as LVEF of record for that study;
if 3D measurement was not available, 2D LVEF was used; and
if neither 3D or 2D measurements were available, visual estimated LVEF was used.
Protocol defined recommendations regarding interruption of HER2-targeted therapies based on LVEF were followed [19]. If there was an asymptomatic absolute decline in LVEF of > 10% points from baseline or to ≤ 35%, HER2-targeted therapy was temporarily withheld. This prompted a cardiology assessment of signs and symptoms of HF and a confirmatory study echocardiogram in 2–4 weeks. If repeated study echocardiogram confirmed LVEF decline, the patient came off the study and the event was considered an asymptomatic worsening of LVEF. However, if the repeated echocardiogram showed improvement in LVEF and the holding criteria were no longer met, HER2-targeted therapy was resumed. If a patient developed symptomatic HF at any time confirmed by the study cardiologist, the patient went off study and the event was considered a CE. Interruptions in HER2-targeted therapies according to the study algorithm did not affect chemotherapy and endocrine therapies and decisions regarding continuation of those were at the discretion of the treating oncologist.
Study endpoints
The primary endpoint was the proportion of patients who completed planned HER2-targeted therapy without developing asymptomatic worsening of cardiac function or CE, defined as symptomatic HF; cardiac arrhythmia requiring pharmacological or electrical treatment; myocardial infarction; sudden cardiac death or death due to myocardial infarction, arrhythmia or HF. Asymptomatic worsening of cardiac function was defined as asymptomatic decline in LVEF > 10% points from baseline and/or LVEF ≤ 35% corroborated by a confirmatory echocardiogram 2–4 weeks and was not considered a CE. Planned HER2-targeted therapy was defined according to the treatment intent. In the (neo)adjuvant setting, planned therapy was considered completion of 1 year of trastuzumab with or without pertuzumab. If a patient had already completed part of the 1 year (neo)adjuvant course prior to enrollment in the trial, the planned oncology therapy course was defined as the time at which 1 year of HER2-targeted therapy was completed (with only a portion of this occurring on study). In the metastatic setting, planned HER2-targeted therapy course was defined as 1 year of treatment or the time until which cessation of the treatment regimen occurred due to non-cardiac toxicity or progressive disease requiring change in oncologic therapy.
Statistical methods
The categorical data analysis methods were used for primary analysis of planned HER2-targeted therapy completion without a CE or asymptomatic worsening of cardiac function. An exact confidence interval of the treatment completion rate based on binomial calculation was obtained. The longitudinal generalized linear model was used to assess the LVEF measures and take CE or asymptomatic LVEF decline into consideration. Wilcoxon rank-sum test was used to compare absolute changes in LVEF from baseline to each time point between those with and without cardiac dysfunction. No imputation was done for patients with missing data. Statistical analysis was performed using SAS 9.4 and graphics were generated by RStudio version 0.99.902 and Excel.
Sample size calculation
We estimated that at present only 10% of patients with HER2-positive breast cancer and reduced LVEF receive HER2-targeted therapy. We proposed that if at least 30% of study participants could complete their planned HER2-targeted therapy on study, this would represent a clinically meaningful increase in the proportion of patients receiving a therapy with a substantial oncologic benefit. Therefore, we defined a completion rate of planned oncology therapy of 30% as clinically relevant, and a completion rate of 10% as similar to current practice. A sample size of 30 patients was planned based on the primary endpoint. A two-stage design with an interim analysis for safety after 15 patients were enrolled was planned with 80% power at a significance level of 5% with two-sided P-value. Safety rules also included planned early trial termination in the setting of any cardiac death or three patients experiencing a CE.
Results
Patient characteristics
In total, 36 patients with HER2-positive breast cancer and cardiac dysfunction were screened, 31 patients were enrolled and 30 patients underwent at least one echocardiogram while on study (Fig. 1). One patient withdrew consent after receiving one dose of HER2-targeted therapy on study due to personal reasons. Because she did not complete at least one follow-up echocardiogram while on study, this patient was not included in the analysis. The patients’ characteristics are described in Table 1. Mean age at enrollment was 53.6 (± 12.5) years. Most patients (N = 28) experienced LVEF decline during HER2-targeted therapy that preceded study enrollment. In 10% (N = 3) of patients LV dysfunction was identified prior to the initiation of HER2-targeted therapy.
Fig. 1.
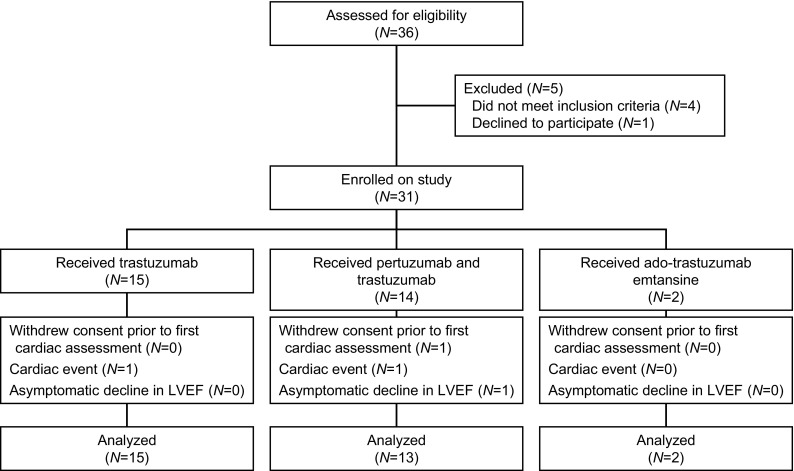
Patient disposition on the SAFE-HEaRt study
Table 1.
Patient demographics
| Variable | N (%) | |
|---|---|---|
| Race/ethnicity | ||
| Non-Latino black | 17 (54.8) | |
| Non-Latino white | 14 (45.2) | |
| Hispanic/Latino | 2 (6.7) | |
| BMI | ||
| Normal (18.5 to < 25) | 5 (16.1) | |
| Overweight (25 to < 30) | 10 (32.3) | |
| Obese (> 30) | 16 (51.6) | |
| Hypertension | 13 (41.9) | |
| Diabetes | 5 (16.1) | |
| Dyslipidemia | 12 (38.7) | |
| Prior radiation therapy | 11 (35.5) | |
| Prior anthracyclines | 17 (54.8) | |
| Prior HER2-targeted therapy | ||
| T | 12 (38.7) | |
| T + P | 17 (54.8) | |
| T-DM1 | 2 (6.4) | |
| Breast cancer stage | ||
| I-III | 18 (58.1) | |
| IV | 13 (41.9) | |
| HER2-targeted therapy received on study | ||
| T | 15 (48.4) | |
| T + P | 14 (45.2) | |
| T-DM1 | 2 (6.4) | |
| Beta blockers on study | Yes | 27 (90) |
| ACEi/ARBs on study | Yes | 21 (70) |
BMI body mass index, T trastuzumab, T + P trastuzumab and pertuzumab, T-DM1 ado-trastuzumab emtansine, ACEi angiotensin converting enzyme (ACE) inhibitors, ARBs angiotensin II receptor blockers
Participants received an average of 11 cycles (range 7–16) of HER2-targeted therapies while on study. Most patients did not receive chemotherapy while on study (63.3%). Regarding cardiac medications, 27 patients (90%) received carvedilol while bradycardia and severe asthma precluded the use of BB in 2 and 1 patients, respectively. Twenty-one patients (70%) received an ACEi or an ARB. In most patients (17/27, 63%), the maximum tolerated carvedilol dose was less than maximal recommended and dose increases were limited by bradycardia. Similarly, for ACEi and ARBs, most patients were not on maximum recommended doses due to relative low BP on home monitoring. The most common AEs reported during study treatment were fatigue and neuropathy, majority being grade 1–2 (Supplementary Table 1).
LVEF results and cardiac events
Mean LVEF measurements at baseline, 6 weeks, 12 weeks and every 12 weeks thereafter, EOT and 6 months post-EOT are shown in Fig. 2. Overall, there were no differences in mean LVEF at baseline (44.8% ± 2.6) compared to EOT (45.7% ± 6.3) and 6-month post EOT (47.6% ± 4.5). Nine patients (33.3%) with metastatic disease continued HER2-targeted therapies beyond study participation. There was no difference in the LVEF 6 months post EOT between patients who remained on HER2-targeted therapies after study completion and patients who did not (P = 0.22) (Supplementary Table 2).
Fig. 2.

a Left ventricular ejection fraction (LVEF) variations during treatment. b LVEF at baseline, end of treatment (EOT) and 6 months later (Post-EOT). Abbreviations: W0 week 0, W6 week 6, W12 week 12, W24 week 24, W36 week 36; W48 week 48, SD Standard deviation
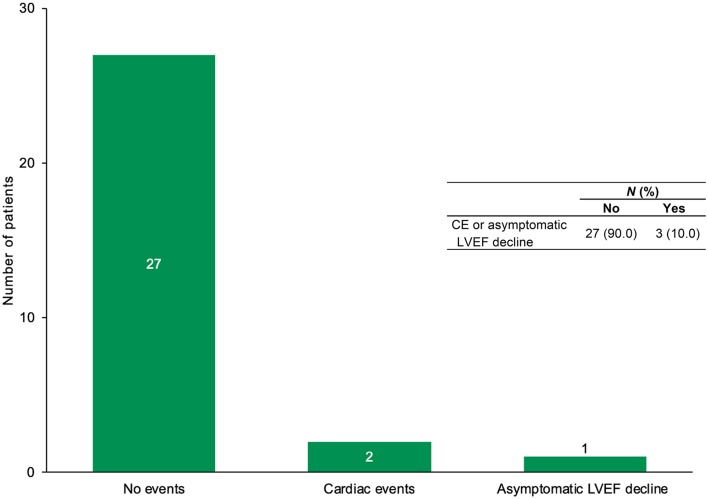
Twenty-seven patients (90%) completed their planned HER2-targeted therapies without developing a CE or protocol-defined asymptomatic decline in LVEF (two-sided 95% CI 73.4–97.9%). Of the three patients who did not complete their planned oncologic therapy, 2 developed symptomatic HF, meeting criteria for a CE, and 1 had a protocol defined asymptomatic LVEF decline to 32% (Fig. 3). All three were taken off study. One of these three patients is alive and continues follow-up and two died due to disease progression at 5 and 16 months after the last study treatment (Supplementary Table 3). Patients who developed a CE or asymptomatic LVEF decline were on study on average 229 days. Baseline LVEF was not different between patients who developed a CE or asymptomatic LVEF decline and those who did not (P = 0.1); however, median LVEF was significantly lower at weeks 6, 24 and EOT among those who developed a CE or asymptomatic decline in LVEF compared to those who did not (P < 0.05) (Table 2). There were no cardiac deaths on study. We performed univariate analyses to examine factors associated with development of a CE or asymptomatic decline in LVEF. Age, co-morbidities, prior anthracyclines or radiation, cancer stage, type of HER2-targeted therapy and cardiac medications on study were not associated with CE.
Fig. 3.
Cardiac event (CE) or asymptomatic decline of left ventricular ejection fraction (LVEF) in the study population
Table 2.
Left ventricular ejection fraction (LVEF) variations according to the development of a cardiac event (CE) or protocol defined decrease in LVEF (Wilcoxon rank-sum test)
| Time point | Median LVEF (assessed by core lab), % [IQR] | P value | ||
|---|---|---|---|---|
| Overall study population (N = 30) | No events (N = 27) | CE or asymptomatic decline in LVEF (N = 3) | ||
| Baseline | 45.0 [43, 47] | 46.0 [43, 47] | 43.0 [40, 44] | 0.10 |
| 6 weeks | 45.0 [43, 47] | 45.0 [43, 48] | 41.0 [40, 43] | 0.04 |
| 12 weeks | 47.5 [44, 53] | 49.0 [44, 53] | 41.0 [35, 45] | 0.07 |
| 24 weeks | 45.0 [41, 48] | 46.0 [41, 49] | 31.5 [25, 38] | 0.04 |
| End of treatment (EOT) | 48.0 [42, 50] | 48.0 [43, 50] | 31.0 [29, 37] | 0.01 |
| 6 months post-EOT | 47.0 [45, 50] | 47.0 [45, 50] | 43.5 [41, 46] | 0.16 |
IQR interquartile range
Discussion
The SAFE-HEaRt trial is the first prospective study to demonstrate safety of HER2-targeted therapies in patients with reduced cardiac function defined as LVEF between 40 and 49% with concomitant treatment with carvedilol and renin-angiotensin inhibitors. The trial met its primary endpoint with 27 patients (90%) completing planned oncologic therapy without developing a CE or asymptomatic decline in LVEF, with a two-sided 95% CI 73.4–97.9%. These results support the concept that through cardiology and oncology collaboration, this patient population can receive optimal cancer therapy while minimizing the risk of poor cardiac outcomes.
The survival impact of withholding or delaying HER2-targeted therapies on oncologic outcomes is not well defined. Current FDA approved package inserts for trastuzumab, pertuzumab and T-DM1 recommend holding these drugs in the presence of cardiomyopathy pre-treatment or LVEF decreases during treatment as follows: absolute decrease in LVEF ≥ 16% from pre-treatment values or LVEF ≤ 50% and ≥ 10% absolute decrease from baseline (trastuzumab); LVEF < 40% or LVEF of 40–45% with 10% or greater absolute decrease below pretreatment values (pertuzumab and T-DM1) [14–16]. Interestingly, there is no prospective data supporting the different cut-offs. In the SAFE-HEaRt study, after careful discussion with cardiologists and oncologists, the cut-off to hold therapy was further lowered. According to the current package inserts, most patients enrolled in this study would have required interruption in HER2-targeted therapies until LVEF recovery or discontinuation of therapy.
The SAFE-HEaRt study aimed at enrolling “real world patients” with racial and ethnic diversity (over 65% of patients self-identified as non-Latino Black or Latino) and a high incidence of co-morbidities including advanced age, hypertension and previous receipt of anthracyclines [9–11]. Indeed, the participants in the SAFE-HEaRt study fall into the category of “high risk” based on the American Society of Clinical Oncology Clinical Practice Guideline for Prevention and Monitoring of Cardiac Dysfunction [21] and our study provides a model for successful co-management of patients by the oncologists and cardiologists. Recent studies reported benefit of ACEi and/or BB as primary prevention strategy in patients with normal LVEF during treatment with epirubicin [22] or trastuzumab [23], however, these trials excluded patients with lower LVEF who may potentially have the greatest benefit from this type of cardiac intervention. Another strength of our study is the central LVEF assessment by the Core Lab. The importance of core laboratory cardiac imaging is well known in cardiology research given differences among imaging and has only recently been investigated in oncology clinical trials [24, 25].
The limitations of this trial are the small sample size, lack of randomization and the heterogeneity of the study population. The fact that the study was conducted at three large centers with both cardiology and oncology expertise may limit its generalizability in centers without readily available expertise in cardio-oncology. Future steps include long-term follow-up and larger implementation studies in the community setting.
In summary, the SAFE-HEaRt is the first study to provide prospective data on the safety of the use of HER2-targeted therapies in patients with breast cancer and compromised cardiac function. The results provide the basis for clinical practice changes supporting the use of HER2-targeted therapies in selected asymptomatic patients with LVEF between 40–49% in close collaboration with cardiology. This conclusion is particularly important given the major advance that HER2-targeted therapies constitute and the potential negative impact on survival of delaying or discontinuing these therapies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to acknowledge Blaise Springfield for graphic design and editorial support; Kathryn Bailey for regulatory support; the members of the cardiac review panel, Drs. Umberto Campia, Rachel Marcus and Neil J. Weissman; as well as the ad hoc member of the Data Safety Management Committee, Dr. Michael Ewer.
Funding
This work was supported by Genentech, Inc., a member of the Roche group [ML28685]; the National Cancer Institute at the National Institutes of Health [P30CA051008 to Site P.I., L.M. Weiner]; a Conquer Cancer Foundation of ASCO Young Investigator Award, supported by The Breast Cancer Research Foundation [F.L.]; a Georgetown-Howard Universities Center for Clinical & Translational Science Post-doctoral KL2 Award [A.B.]; and Genentech, Inc. provided study drugs: ado-trastuzumab emtansine (TDM-1), pertuzumab, and trastuzumab in-kind. Any opinions, findings, and conclusions expressed in this work are those of the authors and do not necessarily reflect those of the American Society of Clinical Oncology, the Conquer Cancer Foundation, The Breast Cancer Research Foundation, the National Cancer Institute at the National Institutes of Health, or the National Institutes of Health.
Data availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Compliance with ethical standards
Conflict of interest
F.L. reports personal fees from AstraZeneca, Celgene, Jounce; research grants to institution from Bristol-Myers Squibb, Calithera, Chugai, Genentech/Roche, Immunomedics, Inivata, Pfizer, Regeneron, Tesaro. C.D. reports personal fees from Amgen, Pfizer, Puma Biotechnology, Roche/Genentech; research grants from Amgen, Pfizer, Puma Biotechnology, Roche/Genentech. A.B. reports honoraria from Bristol-Myers Squibb. A.F.U. reports personal fees from Glenmark Pharmaceuticals. KLS reports ownership interest in AbbVie, Abbott Laboratories; honoraria from ASiM CME; research grants to self from Pfizer/NCCN and to institution from AstraZeneca, Pfizer, Galena BioPharma, Novartis, Pfizer, Syndax. P.R.P. reports ownership interest in Immunonet BioSciences; personal fees from Genentech/Roche, Heron Therapeutics, Immunonet BioSciences, OncoPlex Diagnostics, Personalized Cancer Therapy, Pfizer, Puma Biotechnology; research grants to institution from Advanced Cancer Therapeutics, Caris Centers of Excellence, Cascadian Therapeutics, Fabre-Kramer, Genentech/Roche, Pfizer, Pieris Pharmaceuticals; and payments for patents, royalties, or other intellectual property for U.S. Patent Nos. 8,486,413; 8,501,417; 9,023,362; and 294 9,745,377. R.N. reports personal fees from Bristol-Myers Squibb; travel and accommodation from Bristol-Myers Squibb, Caris Life Sciences. P.H. reports employment by AstraZeneca; C.I. reports honoraria from AstraZeneca, Genentech, Roche; personal fees from AstraZeneca, Genentech, NanoString Technologies, Novartis, Pfizer, Puma Biotechnology, Syndax; speakers’ bureau payments from AstraZeneca, Genentech, Pfizer; research grants to institution from Genentech, Novartis, Pfizer, Tesaro; and payments for patents, royalties, or other intellectual property from UpToDate, McGraw Hill. S.M.S. reports honoraria from Novartis, personal fees from Cardinal Health, Daiichi-Sankyo, Eli Lilly & Co., Genentech/Roche, Genomic Health, Inivata, Peiris Pharmaceuticals, Tocagen; research grants to institution from Genentech; travel and accommodation from Caris Centers of Excellence, Daiichi-Sankyo, Eli Lilly & Co., Genentech/Roche, NanoString Technologies; payments for participation on OlympiA IDMC from AstraZeneca. All remaining authors have declared no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
F. Lynce and A. Barac have contributed equally.
References
- 1.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 2.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 3.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 4.Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swain SM, Baselga J, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–734. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swain SM, Ewer MS, Viale G, et al. Pertuzumab, trastuzumab, and standard anthracycline- and taxane-based chemotherapy for the neoadjuvant treatment of patients with HER2- positive localized breast cancer (BERENICE): a phase II, open-label, multicenter, multinational cardiac safety study. Ann Oncol. 2018;29(3):646–653. doi: 10.1093/annonc/mdx773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lynce F, Swain SM. Pertuzumab for the treatment of breast cancer. Cancer Invest. 2014;32(8):430–438. doi: 10.3109/07357907.2014.922570. [DOI] [PubMed] [Google Scholar]
- 9.Tan-Chiu E, Yothers G, Romond E, et al. Assessment of cardiac dysfunction in arandomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy is node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol. 2005;23:7811–7819. doi: 10.1200/JCO.2005.02.4091. [DOI] [PubMed] [Google Scholar]
- 10.Romond EH, Jong JH, Rastoja P, et al. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide (AC) followed by paclitaxel to AC followed by paclitaxel plus trastuzumab as adjuvant therapy for patients with node-positive, HER2-positive breast cancer. J Clin Oncol. 2012;30:3792–3799. doi: 10.1200/JCO.2011.40.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perez EA, Suman VJ, Davidson NE, et al. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 Adjuvant breast Cancer Trial. J Clin Oncol. 2008;26:1231–1238. doi: 10.1200/JCO.2007.13.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA) Ann Oncol. 2013;24(9):2278–2284. doi: 10.1093/annonc/mdt182. [DOI] [PubMed] [Google Scholar]
- 13.Kenigsberg B, Wellstein A, Barac A. Left ventricular dysfunction in cancer treatment: is it relevant? JACC Heart Fail. 2018;6(2):87–95. doi: 10.1016/j.jchf.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 14.Herceptin [package insert]. San Francisco, CA: Genentech, Inc., 2015
- 15.Kadcyla [package insert]. San Francisco, CA: Genentech, Inc., 2016
- 16.Perjeta [package insert]. San Francisco, CA: Genentech, Inc., 2016
- 17.Ewer MS, Vooletich MT, Durand JB, et al. Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J ClinOncol. 2005;23:7820–7826. doi: 10.1200/JCO.2005.13.300. [DOI] [PubMed] [Google Scholar]
- 18.Yu AF, Yadav NU, Eaton AA, et al. Continuous trastuzumab therapy in breast cancer patients with asymptomatic left ventricular dysfunction. Oncologist. 2015;20:1105–1110. doi: 10.1634/theoncologist.2015-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lynce F, Barac A, Tan MT et al., SAFE-HEART: SAFE-HEaRt: rationale and design of a pilot study investigating cardiac safety of HER2 targeted therapy in patients with HER2-positive breast cancer and reduced left ventricular function. Oncologist 2017;22(5):518–525 [DOI] [PMC free article] [PubMed]
- 20.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Amenian SH, Lacchetti C, Barac A, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017;35(8):893–911. doi: 10.1200/JCO.2016.70.5400. [DOI] [PubMed] [Google Scholar]
- 22.Gulati G, Heck SL, Ree AH, et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J. 2016;37:1671–1680. doi: 10.1093/eurheartj/ehw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pituskin E, Mackey JR, Koshman S, et al. Multidisciplinary approach to novel therapies in cardio-oncology research (MANTICORE 101-Breast): a randomized trial for the prevention of trastuzumab-associated cardiotoxicity. J Clin Oncol. 2017;35:870–876. doi: 10.1200/JCO.2016.68.7830. [DOI] [PubMed] [Google Scholar]
- 24.Douglas PS, DeCara JM, Devereux RB, et al. Echocardiographic imaging in clinical trials: American Society of Echocardiography Standards for echocardiography core laboratories: endorsed by the American College of Cardiology Foundation. J Am Soc Echocardiogr. 2009;22(7):755–765. doi: 10.1016/j.echo.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 25.Khouri MG, Ky B, Dunn G, et al. Echocardiography core laboratory reproducibility of cardiac safety assessments in cardio-oncology. J Am Soc Echocardiogr. 2018;31(3):361–371. doi: 10.1016/j.echo.2017.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.