Abstract
Glucose is an important preferential substrate for energy metabolism during acute coronary syndrome (ACS) attack, although insulin resistance (IR) increases during ACS. Increasing evidence indicates that natriuretic peptides (NP) regulate glucose homeostasis. We investigated possible compensatory actions of NP in collaboration with other neurohumoral factors that facilitate glucose utilization during ACS. The study population consisted of 1072 consecutive cases with ischemic heart disease who underwent cardiac catheterization (ACS, n = 216; non-ACS, n = 856). Among ACS subjects, biochemical data after acute-phase treatment were available in 91 cases, defined as ACS-remission phase (ACS-rem). Path models based on covariance structure analyses were proposed to clarify the direct contribution of B-type NP (BNP) and noradrenaline to glucose and HOMA-IR levels while eliminating confounding biases. In non-ACS and ACS-rem subjects, although noradrenaline slightly increased glucose and/or HOMA-IR levels (P < 0.03), BNP did not significantly affect them. In contrast, in ACS subjects, high noradrenaline was a significant cause of increases in glucose and HOMA-IR levels (P < 0.001), whereas high BNP was a significant cause of decreases in both parameters (P < 0.005). These findings indicate that BNP and noradrenaline coordinately activate glucose metabolism during ACS, with noradrenaline increasing glucose levels, as an energy substrate, while BNP improves IR and promotes glucose utilization.
Subject terms: Unstable angina, Myocardial infarction
Introduction
Although fatty acid oxidation is the predominant metabolic pathway in the normal adult heart, glycolysis and subsequent glucose metabolism become critical for ATP generation and cardiomyocyte survival during ischemic attack of acute coronary syndrome (ACS)1–4. Insulin plays a pivotal role in accelerating the uptake and utilization of glucose in the skeletal muscle as well as in the myocardium. However, we and others have recently reported that insulin resistance (IR) increases in the acute phase of ACS attack5,6. The activation of various counter-regulatory neurohumoral factors, such as catecholamines, cortisol and cytokines, during ACS attack antagonizes the action of insulin, which leads to high hepatic glucose output and IR and eventually causes the development of stress-induced hyperglycemia5,7.
Natriuretic peptides (NPs) are produced in the heart and classically act on the renal and cardiovascular systems8. NPs regulate blood pressure and fluid homeostasis through vasodilatory and diuretic actions and improve cardiac remodeling, mainly through the inhibition of renin-angiotensin-aldosterone and the sympathetic nervous systems9. In addition to its classical action of hemodynamic regulation, there has been increasing evidence to indicate that NPs also regulate energy balance and glucose homeostasis10–14 as well as thermogenesis in adipose tissues, as recently reported by us and others15,16. Furthermore, one study investigating the direct effects of NPs on systemic glucose regulations in humans showed that B-type NP (BNP) administration reduces the plasma glucose concentration17. In addition, another in vitro study showed that NP increases the glucose uptake during hypoxia in cardiomyocytes18. Furthermore, Coué et al. very recently reported that NPs promote the uptake of glucose and enhance glucose metabolism in human adipocytes19.
Given these previous findings, we hypothesized that the compensatory activations of neurohumoral mechanisms, such as NPs and noradrenaline, facilitate glucose utilization, which overwhelms the IR condition during ACS. One of the difficulties faced in researching the correlations between NPs or noradrenaline and glucose is the intractableness of the statistical approach, as these factors are associated with each other (i.e. correlation between NP and noradrenaline) as well as numerous other factors (i.e. correlations with levels of other neurohumoral factors, degree of obesity or hemodynamics), namely confounding variables. Although there are several ways of eliminating these confounding biases, it is still difficult to control for them if multiple potential confounders are present or if the study population is of insufficient size20. A covariance structure analysis is therefore useful for eliminating confounding biases and clarifying possible cause-and-effect relationships5,20–25.
Using a covariance structure analysis together with conventional single and multiple regression analyses, this study was designed to evaluate the possible regulation of plasma glucose levels as well as the degree of IR by noradrenaline and BNP in patients with ACS compared to those with non-ACS.
Results
The characteristics of the study patients
The clinical characteristics of the 216 cases with ACS and the 856 cases with non-ACS are shown in Table 1. The levels of plasma glucose, insulin and homeostasis model assessment of IR (HOMA-IR) were higher and the potassium lower in the ACS subjects than in the non-ACS subjects, consistent with our previous studies5,26. The plasma BNP was only slightly higher, while the plasma noradrenaline was markedly higher in the ACS subjects than in the non-ACS subjects. The LVEF was lower, while the blood pressure was higher in the ACS subjects than in the non-ACS subjects. Among ACS subjects, biochemical data after acute-phase treatment were available in 91 cases, defined as the ACS-remission phase (ACS-rem) (see Methods section). The levels of glucose, insulin, HOMA-IR, BNP and noradrenaline were all decreased during the remission phase of ACS compared to during ischemic attack (Supplementary Table S1).
Table 1.
Clinical characteristics.
| Overall (n = 1072) | ACS (n = 216) | Non-ACS (n = 856) | P-Value | |
|---|---|---|---|---|
| Age, years old | 66 ± 11 | 61 ± 13 | 67 ± 11 | <0.001 |
| Gender; Male (%) | 933 (87.0) | 192 (88.9) | 741 (86.6) | 0.364 |
| BMI, kg/m2 | 24.8 ± 3.7 | 25.0 ± 4.2 | 24.7 ± 3.5 | 0.726 |
| Current smoker (%) | 241 (22.5) | 74 (34.3) | 167 (19.5) | <0.001 |
| BP, mmHg | ||||
| Systolic | 131 ± 23 | 134 ± 23 | 131 ± 23 | 0.030 |
| Diastolic | 70 ± 13 | 77 ± 13 | 69 ± 12 | <0.001 |
| Mean | 95 ± 15 | 101 ± 15 | 94 ± 14 | <0.001 |
| eGFR, mL/min/1.73 m2 | 70.3 ± 18.4 | 75.1 ± 19.2 | 69.0 ± 17.9 | <0.001 |
| Na, mmol/L | 139.6 ± 2.3 | 139.1 ± 2.6 | 139.8 ± 2.2 | 0.002 |
| K, mmol/L | 4.1 ± 0.4 | 3.9 ± 0.4 | 4.1 ± 0.3 | <0.001 |
| UA, mg/dL | 5.9 ± 1.3 | 5.8 ± 1.4 | 5.9 ± 1.3 | 0.355 |
| Glucose, mg/dL | 119.8 ± 35.6 | 142.4 ± 53.3 | 114.1 ± 26.8 | <0.001 |
| Insulin, μU/mL | 9.2 ± 10.0 | 14.4 ± 17.2 | 7.9 ± 6.6 | <0.001 |
| HbA1c, % | 6.2 ± 0.9 | 6.2 ± 1.0 | 6.2 ± 0.8 | 0.017 |
| HOMA-IR | 3.0 ± 4.7 | 5.9 ± 8.6 | 2.3 ± 2.7 | <0.001 |
| HOMA- β | 63.2 ± 66.5 | 68.0 ± 57.5 | 62.0 ± 68.5 | 0.643 |
| TG, mg/dL | 122.8 ± 80.2 | 114.7 ± 111.4 | 124.8 ± 70.2 | <0.001 |
| LDL-C, mg/dL | 101.3 ± 30.3 | 121.1 ± 34.6 | 96.3 ± 26.9 | <0.001 |
| HDL-C, mg/dL | 50.7 ± 14.3 | 50.4 ± 13.2 | 50.7 ± 14.5 | 0.788 |
| BNP, pg/mL | 95.6 ± 205.6 | 96.6 ± 196.5 | 95.4 ± 208.0 | 0.042 |
| Noradrenaline, pg/mL | 313.5 ± 190.2 | 473.0 ± 265.3 | 273.3 ± 139.9 | <0.001 |
| LVEF, % | 58.1 ± 10.5 | 54.9 ± 9.6 | 58.9 ± 10.6 | <0.001 |
| Myocardial infarction (%) | — | 112 (51.9) | — | — |
| Unstable angina (%) | — | 104 (48.1) | — | — |
| Diabetes mellitus (%) | 405 (37.8) | 65 (30.1) | 340 (39.7) | 0.009 |
| Hypertension (%) | 781 (72.9) | 130 (60.2) | 651 (76.1) | <0.001 |
| Medication | ||||
| ACE inhibitors (%) | 240 (22.4) | 13 (6.0) | 227 (26.5) | <0.001 |
| ARBs (%) | 386 (36.0) | 52 (24.1) | 334 (39.0) | <0.001 |
| Beta blockers (%) | 458 (42.7) | 43 (19.9) | 415 (48.5) | <0.001 |
| Calcium channel blockers (%) | 558 (52.1) | 71 (32.9) | 487 (56.9) | <0.001 |
| Diuretics (%) | 176 (16.4) | 18 (8.3) | 158 (18.5) | <0.001 |
| Oral antidiabetic agents (%) | 274 (25.6) | 37 (17.1) | 237 (27.7) | 0.001 |
ACE: angiotensin converting enzyme, ACS: Acute Coronary Syndrome, ARBs: angiotensin II type I-receptor blockers, BMI: body mass index, BNP: B-type natriuretic peptide, BP: blood pressure, eGFR: estimated glomerular filtration rate, HbA1c: hemoglobin A1c, HDL-C: high-density lipoprotein, HOMA-β: homeostasis model assessment beta cell function, HOMA-IR: homeostasis model assessment of insulin resistance, K: potassium, LDL-C: low-density lipoprotein, LVEF: left ventricular ejection fraction, Na: sodium, TG: triglycerides, UA: uric acid.
Simple regression analyses to search for relationships between BNP, noradrenaline and plasma glucose
To evaluate the correlations between the levels of BNP, noradrenaline and plasma glucose, we performed simple regression analyses (Supplementary Fig. S1). A positive correlation between noradrenaline and glucose (P < 0.001) was observed only in the ACS subjects, not in the non-ACS subjects, suggesting that sympathetic nervous system activation by ischemic stress during ACS attack increased plasma glucose levels. Although we observed no significant correlations between BNP and glucose in simple regression analyses, plasma glucose levels tended to be lower in the subjects with higher BNP levels than in those with lower levels between some of the groups (bottom panels in Supplementary Fig. S1). The various confounding factors should be eliminated in order to elucidate the precise relationship between plasma BNP and glucose levels in ischemic heart disease (IHD).
Multiple regression analyses to determine the factors associated with plasma glucose and HOMA-IR levels
To assess the independent determinants of the plasma glucose (Table 2) as well as HOMA-IR (Table 3) levels, multiple regression analyses were performed in the ACS and non-ACS subjects. The HbA1c was positively correlated with glucose and HOMA-IR in both subjects, as expected. In the ACS subjects, noradrenaline was positively correlated with glucose (P < 0.001) and HOMA-IR (P < 0.001), whereas BNP was negatively correlated with both parameters (P = 0.003 and P = 0.002, respectively). In contrast, in the non-ACS subjects, noradrenaline had relatively little impact on glucose (P = 0.025) and HOMA-IR (P = 0.013), and no significant correlations were noted between BNP and glucose or HOMA-IR. These contrasting effects of noradrenaline and BNP on glucose and HOMA-IR, which were observed only in ACS subjects, were consistently observed, even after various potentially influential clinical factors (Supplementary Tables S2 and S3) as well as pharmacological therapy (Supplementary Tables S4 and S5) were included as the independent variables. On the other hand, the negative correlations between BNP and glucose or HOMA-IR in the ACS subjects were no longer observed during the remission phase, although the positive correlation between noradrenaline and glucose was still observed (Supplementary Tables S6 and S7).
Table 2.
The multiple regression analyses to identify the clinical factors influencing the plasma glucose level.
| ACS R2 = 0.451 | Non-Standard Coefficient | Standard Regression Coefficient | Test statistic | P-value | 95% CI | VIF | |
|---|---|---|---|---|---|---|---|
| Regression Coefficient | Standard Error | ||||||
| HbA1c | 28.084 | 2.677 | 0.541 | 10.490 | <0.001 | 22.807 to 33.362 | 1.035 |
| BNP | −0.044 | 0.015 | −0.164 | −2.998 | 0.003 | −0.074 to −0.015 | 1.166 |
| Noradrenaline | 0.069 | 0.011 | 0.343 | 6.173 | <0.001 | 0.047 to 0.091 | 1.201 |
| Non-ACS R2 = 0.465 | Non-Standard Coefficient | Standard Regression Coefficient | Test statistic | P-value | 95% CI | VIF | |
| Regression Coefficient | Standard Error | ||||||
| HbA1c | 21.867 | 0.808 | 0.679 | 27.068 | <0.001 | 20.281 to 23.453 | 1.002 |
| BNP | −0.005 | 0.003 | −0.041 | −1.562 | 0.119 | −0.012 to 0.001 | 1.071 |
| Noradrenaline | 0.011 | 0.005 | 0.058 | 2.248 | 0.025 | 0.001 to 0.021 | 1.069 |
R2: adjusted coefficient of determination, CI: confidence interval, VIF: variance inflation factor.
ACS: Acute Coronary Syndrome, BNP: B-type natriuretic peptide, HbA1c: hemoglobin A1c.
Table 3.
The multiple regression analyses to identify the clinical factors influencing HOMA-IR level.
| ACS R2 = 0.098 | Non-Standard Coefficient | Standard Regression Coefficient | Test statistic | P-value | 95% CI | VIF | |
|---|---|---|---|---|---|---|---|
| Regression Coefficient | Standard Error | ||||||
| HbA1c | 1.121 | 0.554 | 0.135 | 2.023 | 0.044 | 0.029 to 2.214 | 1.036 |
| BNP | −0.010 | 0.003 | −0.217 | −3.078 | 0.002 | −0.016 to −0.004 | 1.165 |
| Noradrenaline | 0.009 | 0.002 | 0.276 | 3.858 | <0.001 | 0.004 to 0.013 | 1.198 |
| Non-ACS R2 = 0.066 | Non-Standard Coefficient | Standard Regression Coefficient | Test statistic | P-value | 95% CI | VIF | |
| Regression Coefficient | Standard Error | ||||||
| HbA1c | 0.800 | 0.107 | 0.248 | 7.480 | <0.001 | 0.590 to 1.010 | 1.002 |
| BNP | −0.001 | 0.000 | −0.047 | −1.357 | 0.175 | −0.001 to 0.000 | 1.071 |
| Noradrenaline | 0.002 | 0.001 | 0.086 | 2.495 | 0.013 | 0.000 to 0.003 | 1.070 |
R2: adjusted coefficient of determination, CI: confidence interval, VIF: variance inflation factor.
ACS: Acute Coronary Syndrome, BNP: B-type natriuretic peptide, HbA1c: hemoglobin A1c, HOMA-IR: homeostasis model assessment of insulin resistance.
Concept of the proposed path model
To eliminate the confounding biases and clarify the contribution of noradrenaline and BNP to glucose as well as to HOMA-IR more directly, path models based on a covariance structure analysis were proposed in the ACS, non-ACS (Figs 1 and S2) and ACS-rem groups (Supplementary Fig. S3).
Figure 1.
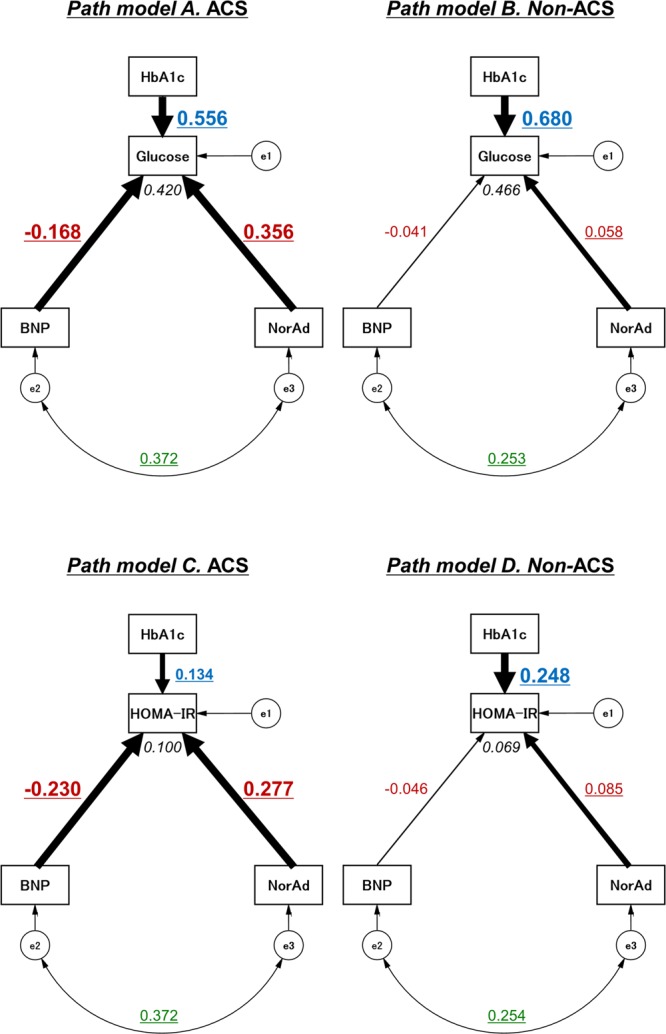
Path diagrams against plasma glucose levels and HOMA-IR levels. Path models theoretically proposed to clarify the contribution of BNP and noradrenaline to glucose as well as to HOMA-IR levels in ACS subjects (n = 216) (Path model A and C, respectively) and in non-ACS subjects (n = 856) (Path model B and D, respectively). Each path has a coefficient showing the standardized coefficient of a regressing independent variable on a dependent variable of the relevant path. These variables indicate standardized regression coefficients (direct effect) [underlined portions indicate remarkable values], squared multiple correlations [narrow italics] and correlations among exogenous variables [green]. ACS = acute coronary syndrome; BNP = B-type natriuretic peptide; e = extraneous variable; HbA1c = hemoglobin A1c; HOMA-IR, homeostasis model assessment of insulin resistance; NorAd = noradrenaline.
The theoretical path model was created by positioning the levels of BNP and noradrenaline in parallel with consideration of the correlation between these two factors. The association between two factors was linked by two-way arrows. HbA1c and (where indicated) various other clinical parameters were also included as potential factors affecting plasma glucose levels and HOMA-IR. The paths between variables were drawn from independent variables to dependent variables (cause-and-effect relationships) with directional arrows for every regression model.
Results of the path model
The precise results of the path models are shown in Tables 4 and S8 (ACS and non-ACS subjects), and Supplementary Table S9 (ACS-rem subjects). Consistent with the results from the multiple regression analyses (Tables 2,3 and S6,S7), HbA1c was positively correlated with plasma glucose as well as HOMA-IR levels in all subjects (Figs 1 and S3). Furthermore, BNP and noradrenaline were positively correlated with each other in both ACS and non-ACS subjects (Fig. 1). The exploratory factor analysis with consideration of the positive correlation between BNP and noradrenaline revealed that, in ACS subjects, high noradrenaline levels were a significant cause of increases in glucose levels (Path model A, β = 0.356, P < 0.001) and HOMA-IR (Path model C, β = 0.277, P < 0.001), whereas high BNP levels were a significant cause of decreases in glucose levels (Path model A, β = −0.168, P = 0.003) and HOMA-IR (Path model C, β = −0.230, P = 0.001). Of note, these contrasting influences of noradrenaline and BNP on glucose and HOMA-IR were observed only in ACS subjects; noradrenaline had relatively little impact on glucose (Path model B, β = 0.058, P = 0.024) or HOMA-IR levels (Path model D, β = 0.085, P = 0.013), and BNP no longer affected them (Path models B and D, respectively) in non-ACS subjects. Likewise, these findings of the inverse effects of noradrenaline and BNP on glucose and HOMA-IR, which were observed only in ACS subjects, were consistently observed, even after various potentially influential clinical factors were included in each path model (Path models E-H, Supplementary Fig. S2 and Supplementary Table S8). On the other hand, consistent with the results from the multiple regression analyses (Supplementary Tables S6 and S7), BNP no longer had a significant impact on the glucose or HOMA-IR levels, even during the remission phase, although noradrenaline still increased glucose but not HOMA-IR levels (Supplementary Fig. S3 and Supplementary Table S9).
Table 4.
The results of path model based on covariance structure analyses.
| Clinical Factor | Estimate | Standard error | Test statistic | P-value | ||
|---|---|---|---|---|---|---|
| Path model (A) | ||||||
| Glucose (R2 = 0.420) | ← | BNP | −0.044 | 0.015 | −3.001 | 0.003 |
| ← | Noradrenaline | 0.069 | 0.011 | 6.339 | <0.001 | |
| ← | HbA1c | 27.945 | 2.618 | 10.675 | <0.001 | |
| Path model (B) | ||||||
| Glucose (R2 = 0.466) | ← | BNP | −0.005 | 0.003 | −1.582 | 0.114 |
| ← | Noradrenaline | 0.011 | 0.005 | 2.253 | 0.024 | |
| ← | HbA1c | 21.875 | 0.804 | 27.212 | <0.001 | |
| Path model (C) | ||||||
| HOMA-IR (R2 = 0.100) | ← | BNP | −0.010 | 0.003 | −3.275 | 0.001 |
| ← | Noradrenaline | 0.009 | 0.002 | 3.932 | <0.001 | |
| ← | HbA1c | 1.113 | 0.540 | 2.059 | 0.039 | |
| Path model (D) | ||||||
| HOMA-IR (R2 = 0.069) | ← | BNP | −0.001 | 0.000 | −1.345 | 0.179 |
| ← | Noradrenaline | 0.002 | 0.001 | 2.495 | 0.013 | |
| ← | HbA1c | 0.798 | 0.106 | 7.501 | <0.001 | |
The results (direct effect) of the path model theoretically proposed analysis to identify the clinical factors influencing the plasma glucose levels or HOMA-IR using ACS subjects (Path models A and C) and using non-ACS subjects (Path models B and D) (see Fig. 1).
R2: squared multiple correlations.
BNP: B-type natriuretic peptide, HbA1c: hemoglobin A1c, HOMA-IR: homeostasis model assessment of insulin resistance.
To visualize the relationships among pairs of estimands, bivariate marginal posterior distributions are shown (Fig. 2). The negative regulations of BNP in contrast to the positive regulations of noradrenaline for plasma glucose (upper panels) as well as HOMA-IR (lower panels) levels were more pronounced in ACS subjects (left panels) than in non-ACS (right panels) or ACS-rem subjects (Supplementary Fig. S4).
Figure 2.
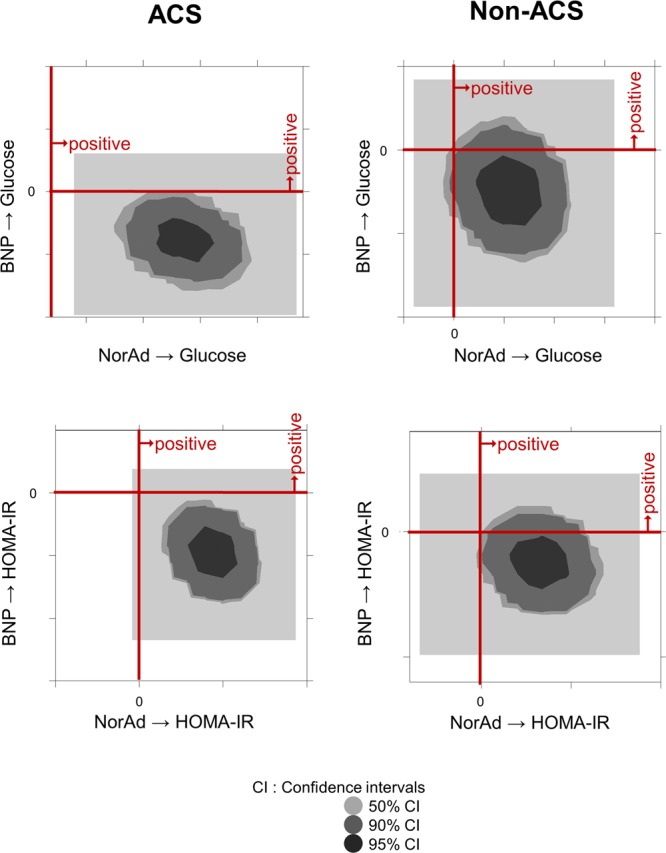
Bayesian estimation. Bivariate marginal posterior distributions are shown in order to help visualize the relationships among pairs of estimands. The credible region (indicated as CI) is conceptually similar to a bivariate confidence region that is familiar to most data analysts acquainted with classical statistical inference methods.
Discussion
In addition to the classical actions of NPs on cardiovascular systems, such as hemodynamic regulation, recent studies have indicated that NPs also regulate energy balance and glucose homeostasis10–12,15,16,19. Using covariance structure analyses, the present study showed that noradrenaline increases plasma glucose levels (possibly supplying energy substrate), while BNP decreases glucose levels (possibly promoting glucose utilization) and substantially improves IR in ACS subjects but not in non-ACS or ACS-rem subjects. These findings suggest that BNP and noradrenaline act cooperatively to facilitate glucose utilization during an ACS attack, but those collaborative actions become ambiguous during the stable ischemic phase.
Although NPs perform various actions during an ACS attack, the direct effects of NPs on energy metabolism have not yet been clearly proven in humans, because many neurohumoral factors are influenced by one another and contribute to the pathogenesis of cardiovascular diseases. Therefore, the collaborative actions of BNP and noradrenaline on glucose metabolism may be hidden by or become ambiguous due to other confounding factors. The covariance structure analysis used in the present study can be performed based on the cofounding biases and is useful for inferring cause-and-effect relationships5,20–25. We successfully proposed a path model based on a covariance structure analysis in order to clarify the causative role of either BNP or noradrenaline in the regulation of plasma glucose and HOMA-IR levels with consideration of the inherent correlation between BNP and noradrenaline themselves. In fact, no significant correlation was observed between BNP and glucose in our simple regression analysis. However, when using a covariance structure analysis, which eliminates potential cofounding factors, the negative correlations between these two factors were clearly delineated only in the ACS subjects (Path models A and C, E and G), with a significant positive correlation preserved between BNP and noradrenaline in both ACS and non-ACS subjects (Figs 1 and S2).
A complex interaction of counter-regulatory hormones, such as catecholamines, cortisol and cytokines can cause the development of stress induced hyperglycemia5,7. The derangement of these neurohumoral factors antagonizes the action of insulin, promotes lipolysis, and increases circulating free fatty acid levels, all of which can ultimately lead to high hepatic glucose output and IR1,5,7. We recently reported that IR actually increases during ACS attack and suggested that there is an insulin-independent mechanism to promote glucose metabolism in order to cope with the critical ischemic condition5,26.
In the meantime, increasing attention is being paid to the possibility that NPs stimulate triglyceride lipolysis, induce adipose tissue browning and activate the thermogenic program10,13,15,16,27–29. Furthermore, NPs also promote muscle mitochondrial biogenesis and fat oxidation12, all of which lead to protection against glucose intolerance and improve IR. In fact, we found in the present study that BNP substantially improves IR induced by noradrenaline (or other counter-regulatory neurohumoral factors against insulin), particularly during ACS attack (Fig. 1). A recent study by Coué et al. proposed biphasic effects of NPs on adipose tissues19: NPs first enhance the glucose uptake through the activation of the insulin-independent but guanylyl cyclase-A (GC-A)–cyclic GMP (cGMP)-PKG-Akt-dependent pathway and subsequently promote lipolysis through the same GC-A-cGMP-PKG pathway, as reported previously13,16,29. They also reported that the activation of the NP receptor-cGMP pathway in skeletal muscle reduces lipotoxicity and improves the sensitivity of insulin-Akt signaling in IR model mice, although these are relatively chronic effects11. The activation of Akt signaling, a major downstream target of insulin, by NPs was also previously reported by others30,31. Interestingly, those studies showed that NPs induce Akt signaling activation in cardiomyocytes and exert cardioprotective effects. Previous studies also suggested that NPs improve the glucose metabolism and IR via AMPK signaling pathway in failing heart tissues29,32. Meanwhile, we and others reported that NPs reduce oxidative stress and inflammatory activity both in vitro and in vivo, leading to an improvement of IR33–35. Finally, two studies investigated the direct effects of NPs on the regulation of the cardiac glucose metabolism by showing that A-type NP increases myocardial glucose uptake during hypoxia through the upregulation of glucose transporters 1 and 418,36. Thus, in the present study, it is possible that BNP also promoted the uptake of glucose and its metabolism in the myocardium during an ACS attack directly and/or indirectly through the decrease in IR and the improvement of insulin sensitivity. Although the underlying mechanisms by which NPs reduce the glucose level only during the acute phase of an ACS attack remain incompletely understood, the present study at least shows that BNP is able to induce temporary shifts in the whole-body response to glucose and insulin during ACS. Future research is warranted to further explore the mechanistic link between NP signaling and glucose metabolism (such as via a direct effect on skeletal/cardiac muscle, adipose tissue or the liver).
The sample size was somewhat small in the present study. However, a covariance structure analysis as well as a conventional multiple regression analysis consistently showed the contrasting influences of noradrenaline and BNP on glucose and HOMA-IR only in the ACS subjects, with comparable standard regression coefficients. Furthermore, only a limited number of patients actually showed significantly increased BNP levels during ACS in the present study. However, we applied a Bayesian SEM using a program embedded in the IBM SPSS AMOS software program (version 25.0; Amos Development Corporation). Although SEM applies the robust-weighted least-square approach, the Bayesian SEM implements the Gibbs sampler algorithm. Bayesianism permits uncertainty despite little information being available, and Bayesian approaches allow us to incorporate background knowledge into the analyses. We believe that additional testing with Bayesian SEM would be rationalized and helpful for reassessing our data from a completely different angle of statistics. The selected two-dimensional contour line was used in this study because it was easily visualized (Figs 2 and S4).
There is another limitation of this study. Although all patients with ACS in the present study fulfilled the diagnostic criteria, in some cases, the clinical conditions of cardiac catheterization were slightly different between acute myocardial infarction and unstable angina pectoris. Namely, short-term stabilization with medical treatment, such as the use of anti-angina agents, was preferentially performed prior to emergent catheterization in some (but not all) cases, particularly in cases of unstable angina. Thus, the clinical and pathophysiological conditions at the time of emergent catheterization in patients with unstable angina were more diverse than those in patients with acute myocardial infarction, for which coronary angiography tended to be performed in time-sensitive situations. Accordingly, covariance structure analyses using path models A or C revealed that high BNP was a significant cause of the more pronounced decreases that were observed in the glucose and HOMA-IR levels of patients with acute myocardial infarction (path from BNP to glucose; β = −0.224, P = 0.004 and to HOMA-IR; β = −0.266, P = 0.012), whereas the finding that high noradrenaline was a significant cause of the consistent increases in both parameters (path from noradrenaline to glucose; β = 0.306, P < 0.001 and to HOMA-IR; β = 0.217, P = 0.04). These data reinforce the hypothesis that collaborative activities of BNP and noradrenaline are involved in glucose utilization, particularly during the acute phase of an ACS attack.
The chronic persistent action of NPs has been shown to induce adipose tissue browning, lipolysis and thermogenesis as well as muscle mitochondrial biogenesis, which leads to the reduction of IR and the improvement of insulin sensitivity. However, we showed in the present study that the acute action of BNP, in collaboration with noradrenaline, promotes glucose utilization with substantially improved IR in patients with ACS. These findings highlight a previously underappreciated role of NPs in glucose metabolism, which overwhelms the increased IR during ACS attack. The administration of agents that increase circulatory natriuretic peptides levels during the acute phase of ACS may provide therapeutic benefits37.
Methods
Study patients
Patients with IHD who underwent cardiac catheterization in our institution for the evaluation of coronary organic lesions from May 2015 to March 2018 were included in this study.
IHD was diagnosed based on symptoms, electrocardiography, blood sampling and the coronary artery morphology20. Organic lesions producing ≥75% luminal stenosis of the coronary arteries on coronary angiography were defined based on the modified American Heart Association coronary tree segment classification. Patients with coronary spastic angina were also included. ACS was defined as the presence of myocardial infarction (MI) or unstable angina pectoris, as described in detail previously5,26. All patients with ACS underwent cardiac catheterization within 24 hours of the onset. Patients were excluded if they were receiving or beginning to receive dialysis, were in cardiogenic shock (Killip grade IV) or had been treated with insulin. Based on these selection criteria, 1072 consecutive cases, including 216 cases with ACS (112 with MI), were enrolled in the present study (Table 1). Among ACS subjects, biochemical data after acute-phase treatment were available in 91 cases (the data were collected mostly at the time of discharge), defined as ACS-rem.
The ethics committee of The Jikei University School of Medicine approved the study protocol (24–355[7121]), and we complied with the routine ethical regulations of our institution. All patients provided their written informed consent before undergoing the procedure, and all clinical investigations were conducted in accordance with the principles expressed in the Declaration of Helsinki. According to our routine ethical regulations, we also posted a notice about the study design and contact information at a public location in our institution.
Data collection
The clinical characteristics were collected retrospectively from the hospital medical records. We collected blood samples and hemodynamic data during cardiac catheterization. Plasma and serum biochemical analyses, including the levels of glucose, BNP, and noradrenaline, were performed in a central laboratory of our hospital during the study period5,20. Some of the patients had comorbid cardiovascular diseases, such as valvular disease, arrhythmia, cardiomyopathy and other conditions. Hypertension, diabetes mellitus (DM) and dyslipidemia were defined as described previously5,20. HOMA-IR and the estimated glomerular filtration rate (eGFR) were calculated as described previously5,20,26. The left ventricular ejection fraction (LVEF) was measured at the time of left ventriculography5,20.
Statistical analyses
Continuous variables are expressed as the mean ± standard deviation. To compare the clinical characteristics between the ACS and non-ACS subjects, the statistical analyses were performed using the chi-square test for categorial variables and the Mann-Whitney U test for continuous variables. The correlation between the plasma glucose level, BNP and noradrenaline in each group was investigated by a simple regression analysis and expressed as Spearman’s correlation coefficient. Plasma glucose levels were compared among four groups divided by the indicated BNP levels with the Mann-Whitney U test. A multiple regression analysis was performed to compare multiple values. The above-mentioned statistical analyses were performed using the SPSS Statistics software program (version 23.0, SPSS Inc., Chicago, IL, USA). P-values of < 0.05 were considered to indicate statistical significance.
A path analysis based on a covariance structure analysis was used to elucidate the contribution of BNP and noradrenaline to the plasma glucose level and HOMA-IR, respectively, in patients with and without ACS. This analysis compares the power among multiple independent variables that confound each other, and specifically identifies probable causal effects on either the plasma glucose level or HOMA-IR5,20–25. The causality model defines some hierarchical regression models between clinical factors and plasma glucose level or HOMA-IR. For every regression, the total variance in the dependent variable is theorized to be caused by either independent variables that are included in the model or by extraneous variables (e)5,20–25. The path analysis was performed using the IBM SPSS AMOS software program (version 25; Amos Development Corporation, Meadville, PA, USA). The obtained structural equation models (SEMs) were tested and confirmed at a significance level of P < 0.05.
In addition, we applied a Bayesian SEM using a program embedded in the IBM SPSS AMOS software program (version 25.0; Amos Development Corporation). The frequency polygon was described with the marginal posterior distributions of the estimands. The selected two-dimensional contour line was used in this study because it was easily visualized.
Supplementary information
Acknowledgements
The authors are grateful to Ms. Kumiko Nishiyama for providing assistance in the data collection. This work was supported in part by grants-in-aid for the Ministry of Education, Culture, Sports, Science and Technology [JP17K09531] (to T. Nagoshi).
Author Contributions
G.U., T.N., M.Y. conceived and designed the study, and wrote the manuscript. A.Y., Y.I., Y.T., H.K., K.O., K.M. and T.O. contributed to acquisition of data and edited the manuscript. S.I., T.T. and M.K. analyzed and interpreted the data. All authors read and approved the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Goki Uno and Tomohisa Nagoshi contributed equally.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-44216-0.
References
- 1.Nagoshi, T., Yoshimura, M., Rosano, G. M., Lopaschuk, G. D. & Mochizuki, S. Optimization of Cardiac Metabolism in Heart Failure. Curr Pharm Des17, 3846–3853, BSP/CPD/E-Pub/000724 (2011). [DOI] [PMC free article] [PubMed]
- 2.Kashiwagi Y, et al. Expression of SGLT1 in Human Hearts and Impairment of Cardiac Glucose Uptake by Phlorizin during Ischemia-Reperfusion Injury in Mice. PLOS One. 2015;10:e0130605. doi: 10.1371/journal.pone.0130605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shao D, Tian R. Glucose Transporters in Cardiac Metabolism and Hypertrophy. Compr Physiol. 2015;6:331–351. doi: 10.1002/cphy.c150016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Horst IC. Acute coronary syndromes: Early metabolic modulation–a solution for MI? Nat Rev Cardiol. 2012;9:377–378. doi: 10.1038/nrcardio.2012.75. [DOI] [PubMed] [Google Scholar]
- 5.Ito S, et al. Possible increase in insulin resistance and concealed glucose-coupled potassium-lowering mechanisms during acute coronary syndrome documented by covariance structure analysis. PLOS One. 2017;12:e0176435. doi: 10.1371/journal.pone.0176435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramachandran A, et al. High incidence of glucose intolerance in Asian-Indian subjects with acute coronary syndrome. Diabetes Care. 2005;28:2492–2496. doi: 10.2337/diacare.28.10.2492. [DOI] [PubMed] [Google Scholar]
- 7.Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet. 2009;373:1798–1807. doi: 10.1016/S0140-6736(09)60553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yasue H, et al. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation. 1994;90:195–203. doi: 10.1161/01.CIR.90.1.195. [DOI] [PubMed] [Google Scholar]
- 9.Brunner-La Rocca HP, Kaye DM, Woods RL, Hastings J, Esler MD. Effects of intravenous brain natriuretic peptide on regional sympathetic activity in patients with chronic heart failure as compared with healthy control subjects. J Am Coll Cardiol. 2001;37:1221–1227. doi: 10.1016/s0735-1097(01)01172-x. [DOI] [PubMed] [Google Scholar]
- 10.Moro C, Lafontan M. Natriuretic peptides and cGMP signaling control of energy homeostasis. Am J Physiol Heart Circ Physiol. 2013;304:H358–368. doi: 10.1152/ajpheart.00704.2012. [DOI] [PubMed] [Google Scholar]
- 11.Coue M, et al. Defective Natriuretic Peptide Receptor Signaling in Skeletal Muscle Links Obesity to Type 2 Diabetes. Diabetes. 2015;64:4033–4045. doi: 10.2337/db15-0305. [DOI] [PubMed] [Google Scholar]
- 12.Miyashita K, et al. Natriuretic peptides/cGMP/cGMP-dependent protein kinase cascades promote muscle mitochondrial biogenesis and prevent obesity. Diabetes. 2009;58:2880–2892. doi: 10.2337/db09-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu W, et al. Enhancing natriuretic peptide signaling in adipose tissue, but not in muscle, protects against diet-induced obesity and insulin resistance. Sci Signal. 2017;10:eaam6870. doi: 10.1126/scisignal.aam6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jordan J, Birkenfeld AL, Melander O, Moro C. Natriuretic Peptides in Cardiovascular and Metabolic Crosstalk: Implications for Hypertension Management. Hypertension. 2018;72:270–276. doi: 10.1161/HYPERTENSIONAHA.118.11081. [DOI] [PubMed] [Google Scholar]
- 15.Kimura H, et al. The thermogenic actions of natriuretic peptide in brown adipocytes: The direct measurement of the intracellular temperature using a fluorescent thermoprobe. Sci Rep. 2017;7:12978. doi: 10.1038/s41598-017-13563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bordicchia M, et al. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122:1022–1036. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heinisch BB, et al. B-type natriuretic peptide (BNP) affects the initial response to intravenous glucose: a randomised placebo-controlled cross-over study in healthy men. Diabetologia. 2012;55:1400–1405. doi: 10.1007/s00125-011-2392-1. [DOI] [PubMed] [Google Scholar]
- 18.Kudoh A, Katagai H, Takazawa T. Atrial natriuretic peptide increases glucose uptake during hypoxia in cardiomyocytes. J Cardiovasc Pharmacol. 2002;40:601–610. doi: 10.1097/00005344-200210000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Coue M, et al. Natriuretic peptides promote glucose uptake in a cGMP-dependent manner in human adipocytes. Sci Rep. 2018;8:1097. doi: 10.1038/s41598-018-19619-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka Y, et al. Close linkage between serum uric acid and cardiac dysfunction in patients with ischemic heart disease according to covariance structure analysis. Sci Rep. 2017;7:2519. doi: 10.1038/s41598-017-02707-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinoshita K, et al. Potent influence of obesity on suppression of plasma B-type natriuretic peptide levels in patients with acute heart failure: An approach using covariance structure analysis. Int J Cardiol. 2016;215:283–290. doi: 10.1016/j.ijcard.2016.04.111. [DOI] [PubMed] [Google Scholar]
- 22.Tsutsumi J, et al. Manifold implications of obesity in ischemic heart disease among Japanese patients according to covariance structure analysis: Low reactivity of B-type natriuretic peptide as an intervening risk factor. Plos One. 2017;12:e0177327. doi: 10.1371/journal.pone.0177327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukumoto R, et al. Conflicting relationship between age-dependent disorders, valvular heart disease and coronary artery disease by covariance structure analysis: Possible contribution of natriuretic peptide. Plos One. 2017;12:e0181206. doi: 10.1371/journal.pone.0181206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogawa K, et al. Parallel comparison of risk factors between progression of organic stenosis in the coronary arteries and onset of acute coronary syndrome by covariance structure analysis. Plos One. 2017;12:e0173898. doi: 10.1371/journal.pone.0173898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugawa S, Masuda I, Kato K, Yoshimura M. Increased Levels of Cardiac Troponin I in Subjects with Extremely Low B-type Natriuretic Peptide Levels. Sci Rep. 2018;8:5120. doi: 10.1038/s41598-018-23441-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sekiyama H, et al. Transient decrease in serum potassium level during ischemic attack of acute coronary syndrome: Paradoxical contribution of plasma glucose level and glycohemoglobin. Cardiovasc Diabetol. 2013;12:4. doi: 10.1186/1475-2840-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang TJ. The natriuretic peptides and fat metabolism. N Engl J Med. 2012;367:377–378. doi: 10.1056/NEJMcibr1204796. [DOI] [PubMed] [Google Scholar]
- 28.Collins S. A heart-adipose tissue connection in the regulation of energy metabolism. Nat Rev Endocrinol. 2014;10:157–163. doi: 10.1038/nrendo.2013.234. [DOI] [PubMed] [Google Scholar]
- 29.Plante E, et al. Treatment with brain natriuretic peptide prevents the development of cardiac dysfunction in obese diabetic db/db mice. Diabetologia. 2014;57:1257–1267. doi: 10.1007/s00125-014-3201-4. [DOI] [PubMed] [Google Scholar]
- 30.Kato T, et al. Atrial natriuretic peptide promotes cardiomyocyte survival by cGMP-dependent nuclear accumulation of zyxin and Akt. J Clin Invest. 2005;115:2716–2730. doi: 10.1172/JCI24280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Breivik L, et al. B-type natriuretic peptide expression and cardioprotection is regulated by Akt dependent signaling at early reperfusion. Peptides. 2015;66:43–50. doi: 10.1016/j.peptides.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Tsukamoto O, et al. Natriuretic peptides enhance the production of adiponectin in human adipocytes and in patients with chronic heart failure. J Am Coll Cardiol. 2009;53:2070–2077. doi: 10.1016/j.jacc.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 33.Shono M, et al. Predominant effect of A-type natriuretic peptide on reduction of oxidative stress during the treatment of patients with heart failure. Circ J. 2007;71:1040–1046. doi: 10.1253/circj.71.1040. [DOI] [PubMed] [Google Scholar]
- 34.Moro C, et al. Atrial natriuretic peptide inhibits the production of adipokines and cytokines linked to inflammation and insulin resistance in human subcutaneous adipose tissue. Diabetologia. 2007;50:1038–1047. doi: 10.1007/s00125-007-0614-3. [DOI] [PubMed] [Google Scholar]
- 35.Coue M, Moro C. Natriuretic peptide control of energy balance and glucose homeostasis. Biochimie. 2016;124:84–91. doi: 10.1016/j.biochi.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 36.Sosa V, Carbo R, Guarner V. Participation of glucose transporters on atrial natriuretic peptide-induced glucose uptake by adult and neonatal cardiomyocytes under oxygenation and hypoxia. Eur J Pharmacol. 2007;568:83–88. doi: 10.1016/j.ejphar.2007.04.040. [DOI] [PubMed] [Google Scholar]
- 37.Kitakaze M, et al. Human atrial natriuretic peptide and nicorandil as adjuncts to reperfusion treatment for acute myocardial infarction (J-WIND): two randomised trials. Lancet. 2007;370:1483–1493. doi: 10.1016/S0140-6736(07)61634-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


