Abstract
Antibiotics have been categorized as emerging pollutants due to their indiscriminate usage, continuous input and persistence in various environmental matrices even at lower concentrations. Cephalosporins are the broad-spectrum antibiotics of β-lactam family. Owing to its enormous production and consumption, it is reported as the second most prescribed antibiotic classes in Europe. The cephalosporin wastewater contains toxic organic compounds, inorganic salts, and active pharmaceutical ingredients (API) which pose a potential threat to the organisms in the environment. Therefore, removal of cephalosporin antibiotics from the environment has become mandatory as it contributes to increase in the level of chemical oxygen demand (COD), causing toxicity of the effluent and production of cephalosporin-resistant microbes. So far, several processes have been reported for degradation/removal of cephalosporins from the environment. A number of individual studies have been published within the last decade covering the various aspects of antibiotics. However, a detailed compilation on cephalosporin antibiotics as an emerging environmental contaminant is still lacking. Hence, the present review intends to highlight the current ecological scenario with respect to distribution, toxicity, degradation, various remediation technologies, and the regulatory aspects concerning cephalosporins. The latest successful technologies for cephalosporin degradation/removal discussed in this review will help researchers for a better understanding of the nature and persistence of cephalosporins in the environment along with the risks associated with their existence. The research thrust discussed in this review will also evoke new technologies to be attempted by the future researchers to develop sustainable options to remediate cephalosporin-contaminated environments.
Keywords: β-lactam antibiotics, Cephalosporin, Environment, Pharmaceuticals, Wastewater
Introduction
Antibiotic pollution has posed a major global threat (Kumar et al. 2019). The antibiotics and their metabolites in the aquatic environment are exerting a negative impact on all the organisms. The easy migration of antibiotics in drinking water causes serious drug resistance which brings the environmental risk in view of residual antibiotics released into the ecosystem (Szymańska et al. 2019). Water and soil pollution influence the structure and function of ecosystem vitally (Dabrowska et al. 2018). The main sources of water pollution with antibiotics are drug-manufacturing industry, animal wastes from livestock farming and human wastes from hospitals and domestic activities (Feier et al. 2018). The trace amount of antibiotics in the aquatic system is a great challenge for the assessment of water quality due to their toxic effect on non-target organisms (Vasiliadou et al. 2018). Occurrence of antibiotics has been reported in hospital wastewaters (Baranchesme and Munir 2018), WWTP biosolids, surface waters and ground waters (Zhang et al. 2018a), drinking water, sediments and biota (Williams and Kookana 2018). The possible strategies for remediation of antibiotics include adsorption, hydrolysis, thermolysis, bio-electrochemical methods, oxidation processes, photolysis, use of polyethylenimine cross-linked nanofiltration membranes, microbial and enzymatic processes (Ahmed et al. 2015; Nigam et al. 2017; Kumar et al. 2019; Yan et al. 2019; Dangi et al. 2018; Feier et al. 2018; Zhao et al. 2018).
Cephalosporin is a widely used class of β-lactam group of antibiotics for the treatment and prevention of bacterial diseases by disrupting the peptidoglycan layer synthesis in the cell walls of both Gram-positive and Gram-negative bacteria (Magdaleno et al. 2015). In terms of quantity, cephalosporin accounts for 50–70% of the total antibiotics used by humans (Selvi et al. 2015a, b). Large-scale production and consumption of cephalosporin antibiotics has been reported worldwide (Mirzaei et al. 2018). Cephalosporin is considered as the second most prescribed antibiotic classes in Europe (Estrada et al. 2012). In the year 2007, the emission amount of cephalosporins was reported as 85.9 t in Turkey (Turkdogan and Yetilmezsoy 2009).
The wastewater containing cephalosporin antibiotics contains active pharmaceutical ingredients (API), inorganic salts and toxic organic compounds, thus leading to a high risk to biological survival in the environment (Yang et al. 2016; Guo and Chen 2015). Cephalosporin wastewater can be treated by chemical processes (Serna-Galvis et al. 2017), physio-chemical processes (Zavareh and Eghbalazar 2017), biological processes (Sundararaman and Saravanane 2010) and combined processes (Gadipelly et al. 2014). Recently, the performance and microbial community of an expanded granular sludge bed reactor for the treatment of cephalosporin wastewater has also been reported by Li et al. (2019). However, it has become a great challenge to remediate cephalosporin wastewater effectively with an ensured bio-safety measure.
Within the last decade, a number of general review articles covering different aspects of various antibiotics such as input sources, occurrence, fate, effects, detection and remediation techniques, degradation, etc., have been published (Kummerer 2009; Homem and Santos 2011; Gadipelly et al. 2014; Ahmed et al. 2015; Caracciolo et al. 2015; Feier et al. 2018; Williams and Kookana 2018; Al-Maadheed et al. 2019; Yan et al. 2019; Charuaud et al. 2019; Szymanska et al. 2019; Kumar et al. 2019). Apart from clinical aspects, there are no reviews on cephalosporins covering their environmental existence, toxicity and remediation processes, in particular. Therefore, the present review addresses the current state of knowledge concerning the bioavailability, ecological distribution, degradation, remediation processes and toxicity of cephalosporin antibiotics, a specific class of emerging pollutant in recent years. This review is compiled based on the information collected from various databases such as Google scholar, Google books, ScienceDirect, PubMed, Directory of Open Access Journals (DOAJ), Science open Access, Web of Science, etc., We have compiled almost more than a decade of research reports until March 2019. Apart from the above said aspects, the regulatory aspects concerning cephalosporins degradation/removal are also included in this article. We believe that this article will surely help future researchers for a better understanding of the nature and persistence of cephalosporins in the environment along with the risks associated with their existence.
Usage
Cephalosporins are considered as a highly important class of drugs that belongs to β-lactam group of antibiotics. According to United Kingdom’s National Health Scheme, cephalosporins account for almost one-third of all antibiotics prescribed by physicians (Talaro and Chess 2008). They act as potent antimicrobial agents and their glory have been maintained among medicinal chemists for over 60 years. Hu and Zhu (2016) reported on Acremonium chrysogenum, a filamentous fungus, as an important industrial microorganism. Cephalosporin C (CPC), one of the fungal metabolite is a major resource for 7-amino cephalosporanic acid (7-ACA) production. It is considered to be an industrially important precursor/intermediate that can be used in the production of many first-line cephalosporins antibiotics. Cephalosporins have obvious advantages compared to first-discovered penicillin, as they are more resistant to penicillinase and most effective against several penicillin-resistant strains. Moreover, reports are less on incidence of antagonistic effects of cephalosporins compared to penicillin antibiotics and other anti-bacterial agents. Therefore, they exist as the most widely used anti-bacterial drugs in clinical practice.
The clinical consumption and the production output of cephalosporins are quite enormous owing to their strong bactericidal action and broad anti-bacterial spectrum. The production output of cephalosporins is reported to increase very year due to the expanding market demand worldwide (Adriaenssens et al. 2012). Cephalosporins are used for treating acute pneumonia, respiratory and urinary tract infections, bones and joints, skin, soft tissues, and bloodstream-related infections. Cefotaxime and cefoperazone are extensively used in pre- and post-operative chemotherapy related to abdominal, orthopaedic, cardiac, pelvic, oesophageal, pulmonary and vascular surgery infections (Gerald et al. 1990). The Indian pharmaceutical market has ranked cephalosporins and combinations, broad-spectrum penicillins and fluoroquinolones as the top three classes of therapeutics. The global cephalosporin market was valued at $77,764 million in 2016, and is estimated to reach $199,754 million by 2023, growing at a CAGR (compound annual growth rate) of 14.4% from 2017 to 2023 (Kaul and Srivastava 2017). Orchid Chemicals and Pharmaceuticals Ltd is the largest cephalosporin manufacturers in India with 12% share in the cephalosporin market worldwide. AurobindoPharma also produces different cephalosporins available in the market, including its first launch of a fourth generation injectable broad-spectrum cephalosporin antibiotic, cefpirome in India (Bhattacharyya and Sen 2006). The oral cephalosporins in the Indian market are available in various brands at cheaper price that makes their prescription quite common among physicians as well as economically viable option for the patients (Karve and Paul 2016). In addition, massive usage of cephalosporin antimicrobials in animal husbandry was also reported as a major cause for selection and transmission of antibiotic resistance that eventually enters into the food chain (Abraham et al. 2015; Lalak et al. 2016).
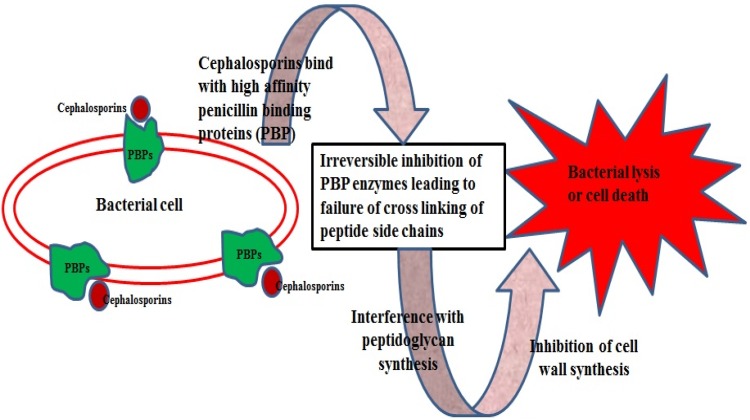
Table 1 summarizes few examples of cephalosporin antibiotics of different generations, their activity and treatments for which they are being used. They act effectively against Gram-negative bacteria, but show some reduced activity against Gram-positive bacteria, and show enhanced resistance to β-lactamases. However, substitution of new molecules to the penam and cephem nuclei led to the synthesis of semi-synthetic cephalosporin antibiotics compounds with a higher activity against Gram-negative and -positive microorganisms (García-Estrada and Martin 2011). Cephalosporin antibiotics have the same mechanism of action as other β-lactam antibiotics, but are less susceptible to β-lactamases (Fig. 1).
Table 1.
Few examples of cephalosporin antibiotics
| Generation | Derivatives (brand names) | Description | Treatment | References |
|---|---|---|---|---|
| First | Cefazolin (ancef, kefzol), cefadroxil (duricef), cephalexin (keflex) | Gram-positive: has activity against penicillinase-producing, methicillin-susceptible, staphylococci and streptococci. Gram-negative: has moderate activity against Proteus mirabilis, Escherichia coli, and Klebsiella pneumoniae | Upper and lower respiratory tract infections, uncomplicated urinary tract infections | Gerald et al. (1990) |
| Second | Cefaclor (keflor), cefuroxime (zinacef), cefoxitin (mefoxin) | Gram-positive: has less activity than first-generation. Gram-negative: has greater activity than first-generation | Upper respiratory tract infections, skin and soft tissue infections, urinary tract infections | Gerald et al. (1990) |
| Third | Cefoperazone (cefobid), cefotaxime (claforan), cefdinir (omnicef) | Gram-positive: has decreased activity against Gram-positive organisms. Gram-negative: have a broad spectrum of activity and more increased activity than previous generations | Gram-negative mediated meningitis, complicated urinary tract infections and osteomyelitis | Eric (2000) |
| Fourth | Cefepime (maxipime), cefpirome (cefrom), cefozopran (firstcin) | Gram-positive: have extended-spectrum activity as first-generation cephalosporins. Gram-negative: exist as zwitter ions that can penetrate the outer membrane of Gram-negative bacteria. They also have a greater resistance to β-lactamases than the third-generation cephalosporins | Used in treating meningitis | Kosinski and Joseph (2007) |
| Fifth | Ceftobiprole, ceftaroline, ceftolozane | Gram-negative: strongly active against Pseudomonas sp. and appears to be less susceptible to resistance development | Complicated abdominal and urinary tract infections | Craig and Andes (2013) |
Fig. 1.
Mode of action of cephalosporins
Classification
Extensive research on cephalosporin drug molecules has produced four generations of cephalosporins, which are categorized based on the time/period of their discovery and antimicrobial properties (El-Shaboury et al. 2007). Since the introduction of first cephalosporin in the year 1964, four generations of cephalosporins have been introduced into clinical application in these 50 years of study (Bryskier 2000; Hwu et al. 2003; Singh 2004; Macheboeuf et al. 2007). Based on the comprehensive research study of cephalosporin’s structure and activity relationship, fifth generation of cephalosporins (ceftobiprole and ceftaroline) has been launched. Evaluation of human clinical trials and safety concerns in terms of drug impurities (based on ICH guidelines) are under study (Butler and Cooper 2011; Alsante et al. 2014).
Based on generation, cephalosporin antibiotics will differ in their antimicrobial spectrum, metabolism, β-lactamase stability, absorption, and side-effects. For instance, first-generation cephalosporins have narrow or limited spectrum than the third, fourth or fifth generation that has broader spectrum activity (Page 2007).
Occurrence of cephalosporins in wastewater
Cephalosporin wastewater mainly contains residual intermediates, organic solvents, other reactants, and less amounts of unrecycled products, most of which are heterocyclic macromolecular compounds (Lefebvre et al. 2014; Kaya et al. 2017). It also contains a variety of toxic organic compounds which pose a potential threat to the organisms in the environment (Ng et al. 2014, 2015). The presence of cephalosporin in aqueous matrices was found to contribute to a very high COD of the effluent which proportionately increases the cost of the remediation. Cephalosporin wastewater can be treated by physical, chemical, biological and combined processes (Li et al. 2019).
Cephalosporin-manufacturing pharmaceutical industries often suffer from improper treatment and disposal due to the presence of cephalosporins at high concentration. There are research reports on the occurrence of cephalosporins in aqueous environments, viz., surface and ground water, municipal and hospital wastewater (Saravanane and Sundararaman 2009; Collado et al. 2013). The concentration of cefdinir, a third-generation cephalosporin in a pharmaceutical wastewater was reported in the range of 125–175 mg/L (Selvi et al. 2014). Likewise, ceftazidime, an important third-generation cephalosporin, prescribed for treating serious community-acquired and nosocomial infections in humans, was found at a concentration of 5.0 μg/L of ceftazidime in hospital wastewater after effluent treatment (Thai et al. 2018). Yu et al. (2016) studied the distribution and persistence of seven cephalosporins viz., cefradine (CF), cefotaxime (CTX), cefoxitin (CFX), cephalexin (CEF), ceftriaxone (CRO), cefazolin (CZO), and cefuroxime (CXM) in the cephalosporin-producing wastewater. The mean concentration of the seven cephalosporins was reported as 24.38 μg/L in cephalosporin-producing wastewater due to their poor biodegradability (Yu et al. 2016).
A solid phase extraction and ultra-performance liquid chromatography (UPLC)-tandem mass spectrometry (MS) method were used for rapid and reliable detection for cephalosporins in the effluent. The average concentration of seven cephalosporins was in the range of 12.85–141.55 μg/L and 0.05–24.38 μg/L, respectively. The quantification limit ranged from 27.5 to 131.8 ng/L with a recovery percentage of 73% in influent and 102% in effluents. Despite the high cephalosporin removal efficiency of 78.8–99.7%, the discharge of cephalosporin per day was reported up to 1.9 kg. Employing high temperature and light was found to accelerate the degradation rates of the tested cephalosporins. Among the four tested cephalosporins, CXM showed high persistence in the effluent. However, the degradation of cephalosporin in wastewater was not possible under normal temperature.
Duan (2009) reported that effluent containing cephalosporin released from the production unit will be highly bio-toxic and tough to degrade. This could possibly cause a great threat to human beings and the environment. This type of wastewater having varying pH contains high level of complex components viz., soluble solids, colloidal solids, organic substances, with high amount of suspended matter and biological toxic substances of non-biodegradable and bacteriostatic antibiotics. Priya and Philip (2015) and Li et al. (2015) stated that the biologically treated cephalosporin-containing pharmaceutical effluent is a complex industrial effluent generated by cephalosporin production units. Therefore, being toxic and refractory, cephalosporin-containing wastewater requires more advanced treatment methods.
Toxicity of cephalosporins
Pharmaceutical compounds are designed to be biologically active even at low doses which may pose a significant ecotoxicological threat to the aquatic organisms even at environmental relevant concentrations (Caracciolo et al. 2015). The release of the cephalosporin antibiotics in the environment is considered as most significant because of the excretion of a huge percentage of unchanged antibiotics as API that remains bioactive in soil–water ecosystems, thus causing great harm to the human beings and the environment (Khadka and Pokhrel 2013). Cojocel et al. (1988) reported on the nephrotoxic potential of cephaloridine, cephalothin, cefotiam, cefoperazone, cefoxitin, cefazolin and cefotaxime, the derivatives of first, second, and third-generation cephalosporins. Higher environmental toxicity in aqueous environment created by photo-transformation or photodegradation of cephalosporin antibiotics was reported by Wang and Lin (2012). Cephalosporin-resistant microorganisms were reported to cause serious public health problems leading to complications in treating microbial infections and causes imbalances in microbial ecosystem (Subbiah et al. 2012; Kronenberg et al. 2013; Daoud et al. 2017). The toxic effect of 7-ACA, one of the toxic, functional group of cephalosporins was studied with embryos of Zebra fishes. Their increased toxicity showed variable abnormal phenotypes during the organogenesis process in aqueous medium. Phenotypical variations include, abnormal notochord development, cardiovascular, abdomen, cranial, nerve deformities and pigment formation (Zhang et al. 2013, 2015). A similar study by Zhang et al. (2010) also reported on toxic effects of C3 and C7 substituents of cephalosporins, which are far more toxic than 7-ACA. These functional groups were found to exhibit toxic effects either independently or synergistically.
Another toxicity study on N-methylthiotetrazole (MTT) ring, a C3 substituent of cephalosporin was found to cause vitamin K deficiency disorders such as haemorrhage and hypo-prothrombinemia and inhibition of aldehyde dehydrogenase (Antabuse-like effect). Likewise, the –COO group at C7 position was found to cause platelet dysfunction (Katukuri et al. 2016). Until today, various cephalosporin-related safety concerns remain unresolved, due to lack of understanding of the toxicity mechanism. Therefore, knowledge on the detailed elucidation of mechanisms of toxicity, finding the responsible genes or proteins coding for toxicity, and screening the drug using novel biomarkers becomes imperative. Han et al. (2018) conducted transcriptomics-based systematic toxicity evaluation of cephalosporins in zebrafish embryos. The results showed that the transcriptomics can be a useful tool in determining the structure–toxicity relationship of the drug, organ toxicity, and improving the drug safety assessments.
Recently, the toxic effect of a novel cephalosporin, cefatrizine amidine sodium, and its potential impurities were evaluated by Qian et al. (2018) in Zebra fish embryos. The results of toxicity tests showed that structurally similar cephalosporins might have almost similar toxic effects. Cephalosporins compounds with smaller TPSA (topological polarity surface area) values are more likely to cause severe toxic effects, which may be due to increased passive absorption capacity. Though the C3 and C7 substituents were reported to be highly toxic, thiazole ring at C3 position and α-phenylglycine and 2-amino-5-thiazolyl groups at C7 position were found to be less toxic than the other functional groups. However, toxic functional groups associated with 7-ADCA were found to exert entirely different toxic effects via different pathways. The toxic effects investigations were studied based on computational calculations and structure–toxicity relationship. It was concluded that the teratogenic effect observed in Zebra fish embryos was due to the triazine ring at C3 position of cefatrizine amidine.
Cephalosporin resistance
Antibiotic resistance is a significant and a serious public health issue. Indiscriminate and inappropriate usage of antibiotics in pets, livestock, and humans has resulted in the development of ARB. The occurrence of ARB might develop due to continuous and long-term exposure to environments containing antibiotics low concentrations from ng/L to μg/L in surface and wastewater (Kumar and Pal 2018). The following mechanisms were reported to cause antibiotic resistance in bacteria, viz. (1) structural alterations of the antimicrobial target, (2) complete or partial loss/reduction of affinity towards antibiotics, (3) reduced antimicrobial dosages, (4) porin mutations leading to decreased permeability or an exit increase by the pumping out by an efflux transporter, (5) the presence of enzymes that partially or totally destroys the antimicrobial agents, and (6) the development of an alternative metabolic (evasion mechanism) pathway involving precursors (Livermore 1995; Walsh 2000; Harbottle et al. 2006).
Resistance to β-lactams is reported as one of the major global health concern. The most common resistance mechanism is through expression of β-lactamases (Blas), a set of enzymes that cleaves the β-lactam ring, due to which the antibiotics lose its activity (Drawz and Bonomo 2010; Lalak et al. 2016). Many bacteria of extended-spectrum β-lactamase (ESBL) producers group were reported to exhibit resistance to various antibiotics of β-lactam group (Shaikh et al. 2015). Generally, a group of bacteria that are resistant to third-generation cephalosporin derivatives, viz., cefotaxime and ceftazidime are termed as strong ESBL producers. This may be due to the effective activity of the third-generation cephalosporins against the Gram-negative bacterium. Their resistance is mainly attributed to various types of ESBL produced by the bacterium and/or plasmid-mediated AmpC β-lactamases (PABL). Ferjani et al. (2017) reported on the incidence and distribution of both ESBL and PABL in E. coli strains isolated from the intestinal microflora of healthy Tunisian children. The GI tracts of the healthy children served as an important reservoir for ESBL-producing E. coli. They concluded on maintenance of the hygienic school environment and sensible use of antibiotics which will help in avoiding this serious clinical condition. According to USFDA, new rules have been implemented to use ceftiofur, a third-generation cephalosporin derivative in food animals due to the increasing prevalence of third-generation-resistant enteric bacteria (Subbiah et al. 2012).
Oliveira et al. (2015) also reported on the clinical outcomes and the risk factors of third-generation-resistant Enterobacteriaceae present in samples collected from patients upon hospital admission. Thai et al. (2016) devised and synthesized a fluorogenic substrate of β-lactamases as a probe, which was able to detect resistance to the third-generation oxyimino-cephalosporin-derived antibiotics such as cefotaxime and ceftazidime. In particular, the probe could identify the ceftazidime resistance in bacteria that was not detectable using conventional pH-sensing materials, indicating the practical utility of the probe. Lalak et al. (2016) reported on phenotypic and genotypic characterization of commensal E. coli along with gut flora, producing cephalosporinases of healthy slaughter animals. The results confirmed the existence and possible spread of cephalosporin-resistant E. coli in farm animals via IncX1 plasmids to other bacterium including animal and human pathogens. The genetic characterization studies too indicated the ecological aspects of selection and distribution of cephalosporin-resistance gene in E. coli isolated from food-producing farm animals. Mo et al. (2016) too reported similar studies on the incidence of cephalosporin-resistant E. coli in Norwegian broiler flocks. Parent flocks (13.8%) and broiler flocks (22.5%) were detected with cephalosporin-resistant E. coli. Their findings also highlighted the high level of biosecurity implementation with restricted entry to many people into the hatchery during production cycles. Manageiro et al. (2017) also reported on similar findings of diverse resistance mechanisms in commensal E. coli strains collected from turkeys, broilers and flocks. They highlighted on the resistance mechanism involving genes such as, the mcr-1 gene against colistin, a novel blaCTX-M-166-variant gene against third-generation cephalosporins and the blaESAC, an unusual gene found in food-producing farm animals. Recently, Zhang et al. (2018a) studied the genetic basis of the resistance mechanisms of the extended-spectrum cephalosporins in fecal and clinical Enterobacteriaceae isolated from dogs in Canada.
Remediation of cephalosporins
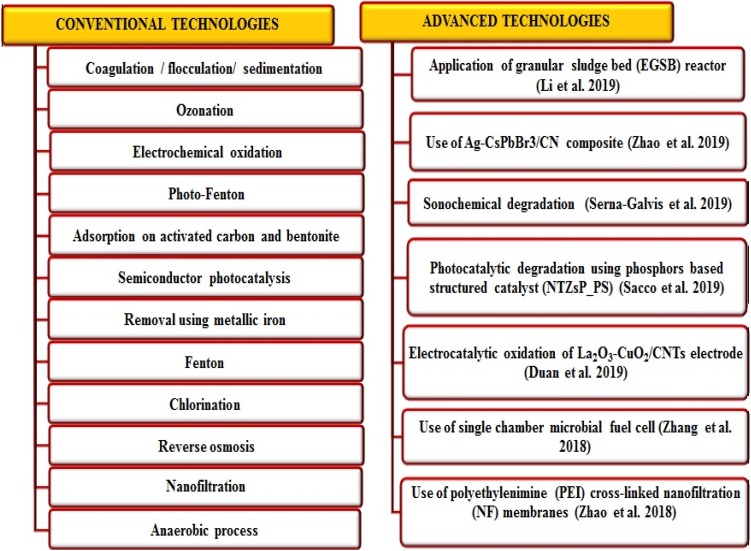
Antibiotics have been detected globally in almost all environmental matrices which infer on poor remediation by conventional treatment methodologies. However, various physico-chemical methods including few conventional and advanced technologies are in practice to degrade or remove antibiotics from pharmaceutical wastewaters (Fig. 2). But, they do have certain limitations which include high operating cost, intensive labour, inefficiency of treating highly polluted wastewater and requirement of expensive equipment for remediation processes (Homem and Santos 2011). Thus, to overcome these limitations, bioremediation can be a novel approach as a less expensive and an eco-friendly alternative to the conventional treatment methods reported so far. It is a promising method to degrade active pharmaceutical ingredients with additional safety compared to conventional methods (Dąbrowska et al. 2018).
Fig. 2.
Various conventional and advanced technologies employed for antibiotic remediation
Adsorption process is one of the most effective, economical and efficient method for pollutant remediation. It has numerous advantages, including cost effectiveness, ease of operation, applicability in batch and continuous processes both, possibility of regeneration and reusability (Watkinson et al. 2007; Delgado et al. 2012). Reports on cephalosporin adsorption using various adsorbents like activated carbon, ion-exchange resins, dead biomass, nanoparticles, etc., are available (Liu et al. 2011; Vasiliu et al. 2011; Fakhri and Adami 2014; Selvi and Das 2015a). However, these adsorbents encounter several drawbacks such as high cost, limited success, sludge generation, production of secondary (toxic) compounds, etc. The potential use of magnesium oxide (MgO) nanoparticles as an adsorbent towards adsorption cefixime and cephalexin was investigated (Fakhri and Adami 2014). They investigated on various adsorption parameters such as high pH, decreased temperature, increased contact time and adsorbent dosage which were found to favour maximum cephalosporin removal. Guo and Chen (2015) reported on a combined system of using freshwater green alga (Chlorella pyrenoidosa) and activated sludge to remove cephalexin, cefradine, cefixime and ceftazidime. The total removal rates were found to be 94.9, 89.9, 100, and 89.7%, respectively. Similarly, Guo et al. (2016) evaluated the applicability of lipid-accumulating microalgae strains, namely, Chlorella sp. Cha-01, Mychonastes sp. YL-02, and Chlamydomonas sp. Tai-03 to remove 7-ACA from wastewater with simultaneous production of biofuel. They reported on three main mechanisms such as adsorption, hydrolysis and photolysis reactions which were responsible for 7-ACA removal. This demonstration was considered promising for the dual purpose of antibiotic remediation and biofuel production.
Cai et al. (2017) reported on the performance of a physical treatment using microwave (MW) for disintegration and degradation of cephalosporin mycelial drug and residual cephalosporin antibiotics. This result showed effective disintegration of the mycelial drug and almost complete (99.9%) degradation of the residual cephalosporins, which suggested the effective utilization of this method for practical applications. Another novel treatment method of oxidation-driven sonoelectro-chemical catalysis process using a nano-coated electrode was reported to treat non-biodegradable and toxic cephalosporin-containing pharmaceutical wastewater effectively (Yang et al. 2014).
Recently, a new practical procedure was reported to remove antibiotic contamination, particularly cephalosporins using mushrooms (Dąbrowska et al. 2018). The fruiting bodies and their mycelia of two edible mushrooms viz. Imleria badia and Lentinula edodes from liquid in vitro cultures were chosen. The study was conducted at different time intervals by testing the possibility and speed of antibiotic myco-remediation using cefuroxime axetil in different doses. The degraded products in biomass were identified using UPLC/MS analysis and a probable degradation pathway also proposed I. badia and L. edodes mycelia took short time duration to remove the cefuroxime axetil from the medium. It was concluded that myco-remediation can be used as an alternative tool for remediation of cephalosporins contamination.
Zhang et al. (2018b) reported the application of the single-chamber microbial fuel cell (MFC) for the treatment of cefazolin sodium (CFZS)-contaminated wastewater. The single-chamber MFCs having graphite-felt bioanode and an activated carbon air–cathode showed high removal of CFZS. MFC with thick CFZS-acclimatized anodic biofilms exhibited CFZS high tolerance up to 450 mg/L. The duration of CFZS acclimatization and thickness of biofilms were important for the development of high-CFZS tolerance. The removal rate of 1.2–6.8 mg/L/h for CFZS was generated using single-cell MFC without any change of existing electricity production. Therefore, the use of single-chamber MFC could serve as a promising bio-electrochemical system for the remediation of CFZS from wastewaters in a cost-effective manner.
Removal of cephalosporin antibiotic (cefadroxil) was performed using polyethylenimine (PEI) cross-linked nanofiltration (NF) membranes (Zhao et al. 2018). Such membranes were prepared by cross-linking modification of P84 co-polyimide precursor membranes using polyethylenimine (PEI) with various molecular weights. This work provided a concept of separation behaviour of a positively charged NF membrane for removal of antibiotics at various pH.
A pilot-scale expanded granular sludge bed (EGSB) reactor having diameter 0.5 m, height 4.9 m and effective volume 0.92 m3 was designed and used for the treatment of cephalosporin-contaminated wastewater (Li et al. 2019). Methano bacterium and Methanom assiliicoccus were predominant archaea in the granular sludge for each of the organic loading rates (OLR). This work was mainly focused on the microbial research of cephalosporin wastewater. The biogas production, microbial community at different OLRs and reactor’s treatment performance were studied. The ESGB reactor showed better performance after 414 d of operation with the COD removal efficiency of 72%. Among the three pathways, hydrogenotrophic and methylotrophic pathways were the main methanogenic types, and acetoclastic pathway was reported as the secondary type.
Degradation of cephalosporins
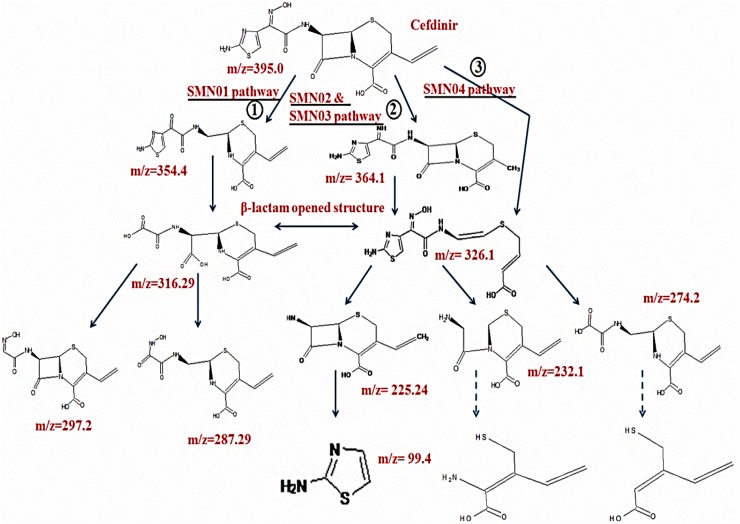
Few studies have been reported on the use of bacteria viz., P. putida and P. fluorescens (Krishnan et al. 2012), Bacteroids sp. and Bacillus sp. (Wagner et al. 2011) and mixed bacterial population (Alexy et al. 2004) as a remediation agent for cephalosporin derivatives degradation. Wang and Lin (2012) reported on photodegradation of cefazolin (CFZ), cephapirin (CFP), cephalexin (CFX) and cephradine (CFD). Jiang et al. (2010) demonstrated direct photolysis of cefradine, cefepime and cefuroxime by as a primary process to eliminate cephalosporin present in the surface lake water, and biodegradation to eliminate cephalosporins in the sediment. Biodegradation studies based on the performance of Ustilago sp. SMN03, and Candida sp. SMN04, two novel yeast strains isolated from cephalosporin-containing pharmaceutical effluent was also reported for degradation of cefdinir (Selvi et al. 2014, 2015a). They reported as 81% and 84% of cefdinir degradation within 6 d by both the isolates individually. Experiments were carried out under optimized parameters (pH, shaking speed, temperature, an inoculum dosage and an initial cefdinir concentration) using both conventional and RSM-based approaches. Six novel intermediates of cefdinir degradation with possible pathways of degradation were proposed (Fig. 3). Opening or deactivating of the β-lactam ring was regarded as one of the important steps in β-lactam antibiotic degradation. An additional study on the involvement of yeast enzymes in cefdinir degradation was also reported.
Fig. 3.
Three different degradation pathways of cefdinir, a third-generation cephalosporin by the yeast isolates (Selvi et al. 2014, 2015a, b)
A case study was conducted in the cephalosporin-manufacturing drug company in Chennai, Tamil Nadu, India. Biotransformation of cephalosporin was demonstrated using cephalosporin-degrading cultures as an inoculum source in an anaerobic fluidized bed reactor. The reactor was operated in a continuous mode with a periodic addition (bioaugmentation) of acclimatized cells from an off-line enriched-reactor, till a maximum removal efficiency of 81% was achieved with a maximum cephalosporin concentration of 175 mg/L at 3 h, hydraulic retention time (Saravanane and Lavanya 2006). They concluded on implementation of biotransformation process as a potential, cost-effective and eco-friendly alternative for cephalosporin removal from the environment.
Cephalosporin-containing wastewater was processed by various bio-chemical-based treatment methods such as Sequencing Batch Reactor Activated Sludge Process (SBR), Up-flow Anaerobic Sludge Bed (UASB), biological activated carbon process (BAC), and hydrolytic acidification. Among all the treatments, hydrolytic acidification was found to enhance the biodegradability characteristics of the effluent effectively. Similarly, the BAC process involving aerobic microbes efficiently eliminated the chroma to 40 and COD to less than 100 mg/L, thus confining the permissible discharge limit standards of “Integrated wastewater discharge standards” (Duan 2009). According to Collado et al. (2013), conventional biological based wastewater remediation is the main technology used for treating cephalosporin-containing pharmaceutical effluent. However, Yang et al. (2016) reported that biological treatments cannot treat cephalosporin pharmaceutical effluent up to permissible levels of discharge standards (GB 21903-2008) in China. A batch mode study on cefdinir degradation in real pharmaceutical wastewater was demonstrated using immobilized and yeast biofilm of Candida sp. SMN04 on various single and hybrid matrices. Maximum degradation of 96.6 and 92.2% was noted within 48 h. Similar degradation involving continuous-flow column studies were also reported (Selvi et al. 2015b).
A novel nano-bio hybrid system for enhanced cephalosporin degradation by combining yeast species and nanoparticles was also reported (Selvi and Das 2015b, 2016). An integrated system of n-MgO (magnesium oxide nanoparticles) and n-Fe0 (zero-valent iron nanoparticles) coated on Candida sp. SMN04 was used for cefdinir-enhanced degradation. An optimized concentration of nanoparticles was used for coating the yeast. This integrated nano-bio system was found to show 88% cefdinir degradation within 2.5 d.
Antibiotics undergo degradation by various mechanisms, namely, hydrolysis, group transfer and redox mechanisms (Wright 2005). Of these, hydrolysis is a clinically important degradation mode and mostly applied to many antibiotic compounds particularly β-lactam antibiotics. The role of degradative enzymes, identification of the degraded products and the knowledge of degradation kinetics are very crucial in understanding the complete degradation mechanisms of cephalosporin antibiotics. This information also assists in designing scaling up processes. β-Lactamase enzyme plays an important role in hydrolysis of cephalosporin antibiotics (Brites et al. 2013). Various other researchers have also reported on the role of β-lactamases enzyme towards cephalosporin degradation (Wagner et al. 2011; Popa et al. 2014). Other enzymes, namely, Cyt P450, manganese peroxidase, NADPH reductase and amylase were reported to catalyze wide range of reactions of drug metabolism and also in the synthesis of fine chemicals (Urlacher and Eiben 2006; Yalchin and Corbaci 2013). Involvement of the activities of degradative enzymes, namely, ESBL, NADPH reductase, Cyt P450, amylase, laccase and manganese peroxidase were studied during cefdinir degradation (Selvi et al. 2015a). Among all, ESBL was found to be responsible for the opening of the β-lactam ring, one of the important steps in the cefdinir degradation process.
Sonochemical degradation of cephalosporins (cephalexin and cephadroxyl) along with other classes of antibiotics has been reported (Serna-Galvis et al. 2019). This study showed that the application of ultrasonic waves caused degradation of antibiotics from diverse classes of antibiotics and the process of sonodegradation is strongly dependent on the chemical structure of the pollutant. The elucidation of primary transformation products of cephalosporins indicated that sonogenerated hydroxyl radical attacked β-lactam ring and modified the cephalosporin cores from β-lactam antibiotics.
Zhao et al. (2019) reported the use of Ag–CsPbBr3/CN composite to degrade 7-aminocephalosporanic acid (7-ACA) under visible light irradiation. The 7% Ag–CsPbBr3/CN catalyst showed superior photocatalytic activity with an approximate degradation of 92.79%, thus breaking down 7-ACA to CO2, H2O and other small molecules within 140 min. A possible mechanism for 7-ACA degradation over Ag–CsPbBr3/CN composite was proposed based on various tests such as adsorption test, Brunauer–Emmett–Teller (BET) measurement, UV–Vis diffuse reflectance spectra (DRS), photoluminescence spectra (PL), transient photocurrent response and electrochemical impedance spectroscopy (EIS) measurement. In addition, a hypothetical pathway for 7-ACA degradation was proposed based on the liquid chromatography–mass spectroscopy (LC–MS) analysis for better understanding of the degradation process. This study opened up a new insight for the use of Ag–CsPbBr3 as an effective photocatalyst to degrade cephalosporin antibiotics.
Photocatalytic degradation of ceftriaxone, a third-generation β-lactam antibiotic was reported under UV and solar light irradiation in the presence of phosphor-based structured catalyst—(Nitrogen-doped TiO2 coupled with ZnS blue phosphors) immobilized on macroscopic polystyrene (NTZsP_PS) (Sacco et al. 2019). The photocatalytic activity was tested both in a laboratory condition using UV light and in outdoor experiments under natural sunlight. Ceftriaxone was removed successfully (100%) with structured photocatalysts NTZsP_PS under UV irradiation and showed good stability after several reuse cycles both in distilled water and real wastewater. Under the natural sunlight condition, it was found that less than 2 kJ/L of Qlight was sufficient to totally degrade ceftriaxone. Based on the results, it was concluded that photocatalytic technology based on a phosphor-structured photocatalyst can be considered as a valuable process for the degradation of antibiotics from water and wastewater.
The application of La2O3–CuO2/CNTs electrode with excellent electrocatalytic oxidation ability was tested for removal of ceftazidime from aqueous solution (Duan et al. 2019). The electrode was synthesized by solvo-thermal method and calcination in nitrogen atmosphere. The degradation of ceftazidime was studied by modified electrode through optimization of five parameters viz. pH, electrolyte concentration, current density, electrode space, and volume of solution. La2O3–CuO2/CNTs electrodes exhibited good recycling and reuse features with 90% electrochemical degradation efficiency towards 1 mg/L of ceftazidime within 30 min. The degraded products and routes of ceftazidime degradation were deduced by LC–MS analysis using modified electrode. The results confirmed the efficiency of La2O3–CuO2/CNTs electrode towards degradation of ceftazidime into smaller organic molecules. Serna-Galvis et al. (2017) also reported on electrochemical treatment using of three different antibiotic classes (cephalosporins penicillins, and fluoroquinolones) Ti/IrO2 anode and a Zr cathode. At the end of the process, more than 90% of the initial concentration of antibiotics was found to be decreased and they too elucidated electro-generated active chlorine was the main degradation route of antibiotics.
Regulatory aspect
There is widespread concern regarding the environmental emission of antibiotics through pharmaceutical wastewater, hospital effluent, animal husbandry, etc., which promotes the development of antibiotic resistance and constitutes a major threat to the public health. Another important issue associated with antibiotic occurrence can be the cause of the disappearance or inhibition of organic matter and chemical degrading microorganisms in soil, water and sewage treatment plants. Governments are now recognizing that antibiotic resistance is a priority health concern and ought to be regulated (Caracciolo et al. 2015). Occurrence of deaths due to antibiotic resistance has been reported as 58,000 in India, 25,000 in EU and 23,000 in USA (Laxminarayan et al. 2013). The minimization of overuse of antibiotic has been suggested by the Centre for Disease Dynamics, Economics & Policy report (CDDEP 2015). In the USA, the national action plan has been made to prevent the occurrence of antibiotic-resistance bacteria (House 2015). Consequently, the European Union (EU) also recommends the prudent use of antibiotics both in human and veterinaries by setting Maximum Residue Limits (MRL) of antibiotics, thus, controlling the emerging antibiotic resistance being passed into the food chain (EFSA European food safety authority and antimicrobial resistance 2011). Southeast Asian countries also started working in the same direction and have presented various strategies in controlling antibiotic resistance (WHO World Health Organization 2015). Moreover, European Food Safety Authority (EFSA) in association with the European Centre for Disease control (ECDC) and European Medicines Agency (EMEA) is also working together to combat the issues of antibiotic resistance. ECDC is organizing the European Antibiotic Awareness Day on 18th November of every year to promote awareness on antibiotic resistance and its associated public threat (EFSA 2011). Though, antibiotic ban is used as defensive attempt, it will not be a right solution, as it could lead to serious public health consequences (Phillip et al. 2004). Therefore, legislation should be set considering the negative impact of antibiotic banning too.
Conclusion
Antibiotics are the emerging pollutants of global concern due to the development of antibiotic resistance. They have been detected worldwide in environmental matrices indicating their ineffective removal from water and wastewater. Cephalosporins make up the largest share of human and animal use antibiotics in most of the countries and account for approximately 50–70% usage. Pharmaceutical compounds like cephalosporins with high availability and concentration can be scrutinized for their prospective application to serve as primary indicators of the overall pharmaceutical load. Despite several reports on the antibiotic degradation/removal, there is a research thrust to develop more novel strategies towards successful cephalosporin remediation. To the best of our knowledge, there is a lack of review papers describing the literature updates on cephalosporin antibiotics as an environmental contaminant. Therefore, this article summarizes the updated information on cephalosporin antibiotics, their usage, persistence, detection, remediation, and degradation pathways until today. We strongly believe that the proposed article will enrich the existing literature base and will be helpful for the researchers to remediate cephalosporins from the ecosystem. Currently, the potential impacts of the transformation products of cephalosporins on human and animal health are largely unknown and need to be deeply investigated. There is a scientific gap on the occurrence and fate of cephalosporins in natural waters and tap water which need to be studied. Above all, implication of strict environmental standards by regulatory agencies and responsible mode of discharge by potential antibiotic sources will surely combat the threat of antibiotic pollution and transmission of antibiotic resistance, thus by controlling possible ecological risks.
Acknowledgements
The authors would like to thank VIT, Vellore, Thiruvalluvar University, Vellore and PSG college of Technology, Coimbatore for providing laboratory facility.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Abraham S, Jordan D, Wonga HS, Johnson JR, Toleman MA, Wakeham DL, Gordon DM, Turnidge JD, Mollinger JL, Gibson JS, Trott DJ. First detection of extended-spectrum cephalosporin- and fluoroquinolone-resistant Escherichia coli in Australian food-producing animals. J Glob Antimicrob Resist. 2015;3:273–277. doi: 10.1016/j.jgar.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Adriaenssens N, Coenen S, Versporten A, Muller A, Minalu G, Faes C, Vankerckhoven V, Aerts M, Hens N, Molenberghs G, Goossens H. European surveillance of antimicrobial consumption (ESAC): outpatient antibiotic use in Europe (1997–2009) J Antimicrob Chemother. 2012;66:3–12. doi: 10.1093/jac/dkr453. [DOI] [PubMed] [Google Scholar]
- Ahmed MB, Zhou JL, Ngo HH, Guo W. Adsorptive removal of antibiotics from water and wastewater: progress and challenges. Sci Total Environ. 2015;532:112–126. doi: 10.1016/j.scitotenv.2015.05.130. [DOI] [PubMed] [Google Scholar]
- Alexy R, Kümpel T, Kümmerer K. Assessment of degradation of 18 antibiotics in the closed bottle test. Chemosphere. 2004;57:505–512. doi: 10.1016/j.chemosphere.2004.06.024. [DOI] [PubMed] [Google Scholar]
- Al-Maadheed S, Ipek G, Aishah BA, Latiff BS. Antibiotics in hospital effluent and domestic wastewater treatment plants in Doha, Qatar. J Water Process Eng. 2019;28:60–68. [Google Scholar]
- Alsante KM, Huynh-Ba KC, Baertschi SW. Recent trends in product development and regulatory issues on impurities in active pharmaceutical ingredient (API) and drug products. Part 2: safety considerations of impurities in pharmaceutical products and surveying the impurity landscape. AAPS Pharm Sci Tech. 2014;15(1):237–251. doi: 10.1208/s12249-013-0061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranchesme F, Munir M. Strategies to combat antibiotic resistance in the wastewater treatment plants. Front Microbiol. 2018;8:2603. doi: 10.3389/fmicb.2017.02603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya BK, Sen SK. Antibiotics business: a glimpse indian. J Biotechnol. 2006;5:471–476. [Google Scholar]
- Brites LM, Oliveira LM, Barboza M. Kinetic study on cephamycin C degradation. Appl Biochem Biotechnol. 2013;171:2121–2128. doi: 10.1007/s12010-013-0502-x. [DOI] [PubMed] [Google Scholar]
- Bryskier A. Cephems: fifty years of continuous research. J Antibiot (Tokyo) 2000;53:1028–1037. doi: 10.7164/antibiotics.53.1028. [DOI] [PubMed] [Google Scholar]
- Butler M, Cooper AM. Antibiotics in the clinical pipeline in 2011. J antibiot. 2011;64(6):413–425. doi: 10.1038/ja.2011.44. [DOI] [PubMed] [Google Scholar]
- Cai C, Liu H, Wang B. Performance of microwave treatment for disintegration of cephalosporin mycelial dreg (CMD) and degradation of residual cephalosporin antibiotics. J Hazard Mater. 2017;331:265–272. doi: 10.1016/j.jhazmat.2017.02.034. [DOI] [PubMed] [Google Scholar]
- Caracciolo BA, Topp E, Grenni P. Pharmaceuticals in the environment: biodegradation and effects on natural microbial communities. A review. J Pharma Biomed Anal. 2015;106:25–36. doi: 10.1016/j.jpba.2014.11.040. [DOI] [PubMed] [Google Scholar]
- CDDEP Centre for Disease Dynamics, Economics and Policy . State of the world antibiotics. Washington: CDDEP; 2015. [Google Scholar]
- Charuaud L, Emilie J, Anne J, Marie-Florence T, Barbara LB. Veterinary pharmaceutical residues from natural water to tap water: sales, occurrence and fate. J Hazard Mater. 2019;361:169–186. doi: 10.1016/j.jhazmat.2018.08.075. [DOI] [PubMed] [Google Scholar]
- Cojocel C, Gottsche U, Tolle K-L, Baumann K. Nephrotoxic potential of first, second and third generation cephalosporins. Arch Toxicol. 1988;62:458–462. doi: 10.1007/BF00288350. [DOI] [PubMed] [Google Scholar]
- Collado S, Quero D, Laca A, Diaz M. Efficiency and sensitivity of the wet oxidation/biological steps in coupled pharmaceutical wastewater treatment. Chem Eng J. 2013;234:484–490. [Google Scholar]
- Craig WA, Andes DR. In vivo activities of ceftolozane, a new cephalosporin, with and without tazobactam against Pseudomonas aeruginosa and Enterobacteriaceae, including strains with extended-spectrum β-lactamases, in the thighs of neutropenic mice. Antimicrob Agent Chemother. 2013;57(4):1577–1582. doi: 10.1128/AAC.01590-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dąbrowska M, Muszyńska B, Starek M, Żmudzki P, Opoka W. Degradation pathway of cephalosporin antibiotics by in vitro cultures of Lentinula edodes and Imleria badia. Internat Biodeterior Biodegrad. 2018;127:104–112. [Google Scholar]
- Dangi AK, Sharma B, Hill RT, Shukla P. Recent advances in applied microbiology. Singapore: Springer; 2018. Bioremediation of through microbes; applications and perspectives; pp. 259–273. [Google Scholar]
- Daoud Z, Salem-Sokhn E, Dahdouh E, Irani J, Matar GM, Doron S. Resistance and clonality in Escherichia coli and Klebsiella spp. and relationship with antibiotic consumption in major Lebanese hospitals. J Global Antimicrob Resist. 2017;11:45–51. doi: 10.1016/j.jgar.2017.07.011. [DOI] [PubMed] [Google Scholar]
- Delgado LF, Charles P, Glucina K, Morlay C. The removal of endocrine disrupting compounds, pharmaceutically activated compounds and cyanobacterial toxins during drinking water preparation using activated carbon—a review. Sci Total Environ. 2012;435–436:509–525. doi: 10.1016/j.scitotenv.2012.07.046. [DOI] [PubMed] [Google Scholar]
- Drawz SM, Bonomo RA. Three decades of beta-lactamase inhibitors. Clin Microbiol Rev. 2010;23(1):160–201. doi: 10.1128/CMR.00037-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H. Study on the treatment process of wastewater from cephalosporin production. J Sustain Dev. 2009;2:133–136. [Google Scholar]
- Duan P, Xiaoming Y, Geli H, Jie W, Zhirong S, Xiang H. La2O3-CuO2/CNTs electrode with excellent electrocatalytic oxidation ability for ceftazidime removal from aqueous solution. Coll Surf A. 2019;569:119–128. [Google Scholar]
- EFSA european food safety authority; antimicrobial resistance (2011). Available at http://www.efsa.europa.eu/en/topics/topic/amr.htm
- El-Shaboury SR, Saleh GA, Mohamed FA, Rageh AH. Analysis of cephalosporin antibiotics. J Pharm Biomed. 2007;45:1–19. doi: 10.1016/j.jpba.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Eric MS. In: The antimicrobial drugs. Pratt WB, editor. Oxford, USA: Oxford University Press; 2000. [Google Scholar]
- Estrada AL, Li YY, Wang A. Biodegradability enhancement of wastewater containing cefalexin by means of the electro–fenton oxidation process. J Hazard Mater. 2012;227–228:41–48. doi: 10.1016/j.jhazmat.2012.04.079. [DOI] [PubMed] [Google Scholar]
- Fakhri A, Adami S. Adsorption and thermodynamic study of cephalosporins antibiotics from aqueous solution onto MgO nanoparticles. J Taiwan Ins Chem Eng. 2014;45:1001–1006. [Google Scholar]
- Feier B, Anca F, Cecilia C, Robert S. Electrochemical detection and removal of pharmaceuticals in waste waters. Curr Opin Electrochem. 2018;11:1–11. [Google Scholar]
- Ferjani S, Saidani M, Hamzaoui Z, Alonso CA, Torres C, Maamar E, Slim AF, Boutiba BBI. Community fecal carriage of broad-spectrum cephalosporin-resistant Escherichia coli in Tunisian children. Diag Microbiol Infect Dis. 2017;87:188–192. doi: 10.1016/j.diagmicrobio.2016.03.008. [DOI] [PubMed] [Google Scholar]
- Gadipelly C, González AP, Yadav GD, IIbáñez OR, Rathod VK, Marathe KV. Pharmaceutical industry wastewater: review of the technologies for water treatment and reuse. Ind Eng Chem Res. 2014;53:11571–11592. [Google Scholar]
- García-Estrada C, Martin J. Penicillins and cephalosporins. Compr Biotechnol. 2011;3:255–268. [Google Scholar]
- Gerald LM, Merl AC, Goodman LS, Gilman A. Penicillins, cephalosporin and other β-lactam antibiotics. Goodman and Gilmans, the pharmacological basis of therapeutics. New York: Pargamon Press; 1990. pp. 1065–1097. [Google Scholar]
- Guo R, Chen J. Application of alga-activated sludge combined system (AASCS) as a novel treatment to remove cephalosporins. Chem Eng J. 2015;260:550–556. [Google Scholar]
- Guo WQ, Zheng HS, Li S, Du JS, Feng XC, Yin RL, Wu QL, Ren NQ, Chang JS. Removal of cephalosporin antibiotics 7-ACA from wastewater during the cultivation of lipid-accumulating microalgae. Bioresour Technol. 2016;221:284–290. doi: 10.1016/j.biortech.2016.09.036. [DOI] [PubMed] [Google Scholar]
- Han Y, Zhang J, Chang Q. A systematic toxicity evaluation of cephalosporins via transcriptomics in zebrafish and in silico ADMET studies. Food Chem Toxicol. 2018;116:264–271. doi: 10.1016/j.fct.2018.04.046. [DOI] [PubMed] [Google Scholar]
- Harbottle H, Thakur S, Zhao S, White DG. Genetics of antimicrobial resistance. Anim Biotechnol. 2006;17:111–124. doi: 10.1080/10495390600957092. [DOI] [PubMed] [Google Scholar]
- Homem V, Santos L. Degradation and removal methods of antibiotics from aqueous matrices. J Environ Manag. 2011;92:2304–2347. doi: 10.1016/j.jenvman.2011.05.023. [DOI] [PubMed] [Google Scholar]
- House W (2015) National action plan for combating antibiotics resistant bacteria. Washington, USA
- Hu Y, Zhu B. Study on genetic engineering of Acremonium chrysogenum, the cephalosporin C producer. Syn Syst Biotechnol. 2016;1:143–149. doi: 10.1016/j.synbio.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwu JR, Ethiraj KS, Hakimelahi GH. Biological activity of some monocyclic and bicyclic beta-lactams with specified functional groups. Mini Rev Med Chem. 2003;3:305–313. doi: 10.2174/1389557033488132. [DOI] [PubMed] [Google Scholar]
- Jiang M, Wang L, Ji R. Biotic and abiotic degradation of four cephalosporin antibiotics in a lake surface water and sediment. Chemosphere. 2010;80:1399–1405. doi: 10.1016/j.chemosphere.2010.05.048. [DOI] [PubMed] [Google Scholar]
- Karve AV, Paul KB. Economic analysis of oral cephalosporins in the Indian market. Int J Res Med Sci. 2016;4:4143–4149. [Google Scholar]
- Katukuri GR, Maddala RN, Ramamoorthi K, Hande M. Cefoperazone induced gastrointestinal bleeding. J Clin Diagn Res. 2016;10:10–11. doi: 10.7860/JCDR/2016/19694.8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul P, Srivastava A (2017) Pharmaceuticals, market research report, cephalosporin market by generation. Global opportunity analysis and industry forecast 2017–2023. Allied Mark Res. https://www.alliedmarketresearch.com/cephalosporin-market
- Kaya Y, Bacaksiz AM, Bayrak H, Gonder ZB, Vergili I, Hasar H, Yilmaz G. Treatment of chemical synthesis-based pharmaceutical wastewater in an ozonation anaerobic membrane bioreactor (AnMBR) system. Chem Eng J. 2017;322:293–301. [Google Scholar]
- Khadka DB, Pokhrel B. Antibiotics polluting environment; multilayer threats. Food Wave. 2013;1:17–27. [Google Scholar]
- Kosinski MA, Joseph WS. Update on the treatment of diabetic foot infections. Clin Podiatr Med Surg. 2007;24(3):383–396. doi: 10.1016/j.cpm.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Kronenberg A, Hilty M, Endimiani A, Muhlemann K. Temporal trends of extended-spectrum cephalosporin-resistant Escherichia coli and Klebsiella pneumonia isolates in in-and outpatients in Switzerland, 2004 to 2011. Euro Surveil. 2013;18:1–10. [PubMed] [Google Scholar]
- Krishnan S, Roach B, Kasinathan K, Annamalai P, Nooruddin T, Gunasekaran M (2012) Studies on the biodegradation of cephalosporin drugs in pharmaceutical effluent using Pseudomonas putida and Pseudomonas fluorescence. Conference proceedings Botany Colombus OH July 7–11
- Kumar A, Pal D. Antibiotic resistance and wastewater: correlation, impact and critical human health challenges. J Environ Chem Eng. 2018;6:52–58. [Google Scholar]
- Kumar M, Jaiswal S, Sodhi KK, Shree P, Singh DK, Agrawal PK, Shukla P. Antibiotics bioremediation: perspectives on its ecotoxicity and resistance. Environ Intern. 2019;124:448–461. doi: 10.1016/j.envint.2018.12.065. [DOI] [PubMed] [Google Scholar]
- Kummerer K. Antibiotics in the aquatic environment—a review—part I. Chemosphere. 2009;75:417–434. doi: 10.1016/j.chemosphere.2008.11.086. [DOI] [PubMed] [Google Scholar]
- Lalak A, Wasyl D, Zaja M, Skarzynska M, Hoszowski A, Samcik I, Wozniakowski G, Szulowski K. Mechanisms of cephalosporin resistance in indicator Escherichia coli isolated from food animals. Vet Microbiol. 2016;194:69–73. doi: 10.1016/j.vetmic.2016.01.023. [DOI] [PubMed] [Google Scholar]
- Laxminarayan R, Dues A, Wattal C, Zaidi AK, et al. Antibiotic resistance—the need for global solutions. Lancet Infect Dis. 2013;13(12):1057–1098. doi: 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- Lefebvre O, Shi X, Wu CH, Ng HY. Biological treatment of pharmaceutical wastewater from the antibiotics industry. Water Sci Technol. 2014;69(4):855–861. doi: 10.2166/wst.2013.729. [DOI] [PubMed] [Google Scholar]
- Li C, Chen J, Wang J, Ma Z, Han P, Luan Y, Lu A. Occurrence of antibiotics in soils and manures from greenhouse vegetable production bases of Beijing, China and an associated risk assessment. Sci Total Environ. 2015;521–522:101–107. doi: 10.1016/j.scitotenv.2015.03.070. [DOI] [PubMed] [Google Scholar]
- Li Z, Hu Y, Liu C, Shen J, Wu J, Li H, Wang K, Zuo J. Performance and microbial community of an expanded granular sludge bed reactor in the treatment of cephalosporin wastewater. Bioresour Technol. 2019;275:94–100. doi: 10.1016/j.biortech.2018.12.003. [DOI] [PubMed] [Google Scholar]
- Liu H, Liu W, Zhang J, Zhang C, Ren L, Li Y. Removal of cephalexin from aqueous solutions by original and Cu(II)/Fe(III) impregnated activated carbons developed from lotus stalks. Kinetics and equilibrium studies. J Hazard Mater. 2011;185:1528–1535. doi: 10.1016/j.jhazmat.2010.10.081. [DOI] [PubMed] [Google Scholar]
- Livermore DM. β-Lactamases in laboratory and clinical resistance. Clin Microbiol Rev. 1995;8:557–584. doi: 10.1128/cmr.8.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macheboeuf P, Fischer DS, Jr Brown, Zervosen TA, Luxen A, Joris B, Dessen A, Schofield CJ. Structural and mechanistic basis of penicillin-binding protein inhibition by lactivicins. Nat Chem Biol. 2007;3:565–569. doi: 10.1038/nchembio.2007.21. [DOI] [PubMed] [Google Scholar]
- Magdaleno A, Saenz ME, Juarez AB, Moretton J. Effects of six antibiotics and their binary mixtures on growth of Pseudokirchneriella subcapitata. Ecotoxicol Environ Saf. 2015;113:72–78. doi: 10.1016/j.ecoenv.2014.11.021. [DOI] [PubMed] [Google Scholar]
- Manageiro V, Clementec L, Graça R, Correiac I, Albuquerque T, Ferreiraa E, Caniça M. New insights into resistance to colistin and third-generation cephalosporins of Escherichia coli in poultry, Portugal: novel blaCTX-M-166 and blaESAC genes. Intern J Food Microbiol. 2017;263:67–73. doi: 10.1016/j.ijfoodmicro.2017.10.007. [DOI] [PubMed] [Google Scholar]
- Mirzaei RM, Yunesia S, Nasseri M, Gholami E, Jalilzadeh S, Shoeibi A, Mesdaghinia A. Occurrence and fate of most prescribed antibiotics in different water environments of Tehran, Iran. Sci Total Environ. 2018;619–620:446–459. doi: 10.1016/j.scitotenv.2017.07.272. [DOI] [PubMed] [Google Scholar]
- Mo SS, Kristoffersen AB, Sunde M, Nødtvedt A, Norwegian AN. Risk factors for occurrence of cephalosporin-resistant Escherichia coli in Norwegian broiler flocks. Prev Vet Med. 2016;130:112–118. doi: 10.1016/j.prevetmed.2016.06.011. [DOI] [PubMed] [Google Scholar]
- Ng KK, Shi X, Tang MKY, Ng HY. A novel application of anaerobic bioentrapped membrane reactor for the treatment of chemical synthesis-based pharmaceutical waste water. Sep Purif Technol. 2014;132:634–643. [Google Scholar]
- Ng KK, Shi X, Ng HY. Evaluation of system performance and microbial communities of a bioaugmented anaerobic membrane bioreactor treating pharmaceutical wastewater. Water Res. 2015;81:311–324. doi: 10.1016/j.watres.2015.05.033. [DOI] [PubMed] [Google Scholar]
- Nigam VK, Arfi T, Kumar V, Shukla P. Bioengineering of nitrilases towards its use as green catalyst: applications and perspectives. Ind J Microbiol. 2017;57(2):131–138. doi: 10.1007/s12088-017-0645-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira MC, Oliveira CR, Goncalves KV, Santos MS, Tardelli AC, Nobre VA. Enterobacteriaceae resistant to third generation cephalosporins upon hospital admission: risk factors and clinical outcomes. Braz J Infect Dis. 2015;19(3):239–245. doi: 10.1016/j.bjid.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page MG. Emerging cephalosporins. Expert Opin Emer Drugs. 2007;12:511–524. doi: 10.1517/14728214.12.4.511. [DOI] [PubMed] [Google Scholar]
- Phillip I, Casewell M, Cox T, DeGroot B, Friis C, Jones R, Nightingale C, Preston R, Waddel J. Does the use of antibiotic in food animals pose a risk to human health? A critical review of published data. J Antimicrob Chemother. 2004;53:28–32. doi: 10.1093/jac/dkg483. [DOI] [PubMed] [Google Scholar]
- Popa C, Favier L, Dinica R, Semrany S, Djelal H, Amrane A, Bahrim G. Potential of newly isolated wild Streptomyces strains as agents for the biodegradation of a recalcitrant pharmaceutical, carbamazepine. Environ Technol. 2014;35:3082–3091. doi: 10.1080/09593330.2014.931468. [DOI] [PubMed] [Google Scholar]
- Priya VS, Philip L. Treatment of volatile organic compounds in pharmaceutical wastewater using submerged aerated biological filter. Chem Eng. 2015;J266:309–319. [Google Scholar]
- Qian J, Hana Y, Li J, Zhang J, Hu C. Toxic effect prediction of cefatirizine amidine sodium and its impurities by structure-toxicity relationship of cephalosporins. Toxicol In Vitro. 2018;46:137–147. doi: 10.1016/j.tiv.2017.09.021. [DOI] [PubMed] [Google Scholar]
- Sacco O, Vincenzo V, Luigi R, Diana S. Intensification of ceftriaxone degradation under UV and solar light irradiation in presence of phosphors based structured catalyst. Chem Eng Process–Process Intensif. 2019;137:12–21. [Google Scholar]
- Saravanane R, Lavanya M. Water environment federation (WEF) workshop. WEFTEC. 2006;06:1739–1746. [Google Scholar]
- Saravanane R, Sundararaman S. Effect of loading rate and HRT on the removal of cephalosporin and their intermediates during the operation of a membrane bioreactor treating pharmaceutical wastewater. Environ Technol. 2009;30:1017–1022. doi: 10.1080/09593330903032865. [DOI] [PubMed] [Google Scholar]
- Selvi A, Das N. Remediation of cefdinir from aqueous solution using pretreated dead yeast Candida sp. SMN04 as potential adsorbent: an equilibrium, kinetics and thermodynamic studies. Der Pharm Lettre. 2015;7:74–81. [Google Scholar]
- Selvi A, Das N. Nano-bio hybrid system for enhanced degradation of cefdinir using Candida sp. SMN04 coated with zero-valent iron nanoparticles. J App Pharm Sci. 2015;6(09):9–017. [Google Scholar]
- Selvi A, Das N. Degradation of Cefdinir by Candida sp. SMN04 and MgO nanoparticles-An integrated (nano-bio) approach. Environ Prog Sustain Energy. 2016;35(3):706–714. [Google Scholar]
- Selvi A, Salam JA, Das N. Biodegradation of cefdinir by a novel yeast strain, Ustilago sp. SMN03 isolated from pharmaceutical wastewater. World J Microbiol Biotechnol. 2014;30:2839–2850. doi: 10.1007/s11274-014-1710-4. [DOI] [PubMed] [Google Scholar]
- Selvi A, Das D, Das N. Potentiality of yeast Candida sp. SMN04 for degradation of cefdinir, a cephalosporin antibiotic: kinetics, enzyme analysis and biodegradation pathway. Environ Technol. 2015;36:3112–3124. doi: 10.1080/09593330.2015.1054318. [DOI] [PubMed] [Google Scholar]
- Selvi A, Banerjee M, Das N. Degradation of cefdinir from pharmaceutical wastewater using immobilized Candida sp. SMN04 and biofilm formed on gravels. J App Pharm Sci. 2015;5:73–79. [Google Scholar]
- Serna-Galvis EA, Berrio-Perlaza KE, Torres-Palma RA. Electrochemical treatment of penicillin, cephalosporin, and fluoroquinolone antibiotics via active chlorine: evaluation of antimicrobial activity, toxicity, matrix, and their correlation with the degradation pathways. Environ Sci Pollut Res. 2017;24(30):23771–23782. doi: 10.1007/s11356-017-9985-2. [DOI] [PubMed] [Google Scholar]
- Serna-Galvis EA, Montoya-Rodríguez Diana, Isaza-Pineda Laura, Ibáñez María, Hernández Félix, Moncayo-Lasso Alejandro, Torres-Palma Ricardo A. Sonochemical degradation of antibiotics from representative classes—considerations on structural effects, initial transformation products, antimicrobial activity and matrix. Ultrasound Sonochemistry. 2019;50:157–165. doi: 10.1016/j.ultsonch.2018.09.012. [DOI] [PubMed] [Google Scholar]
- Shaikh S, Fatima J, Shakil S, Rizvi SM, Kamal MA. Antibiotic resistance and extended spectrum beta-lactamases: types, epidemiology and treatment. Saudi J Biol Sci. 2015;22(1):62–64. doi: 10.1016/j.sjbs.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh GS. Beta-lactams in the new millennium. Part-II: cephems, oxacephems, penams and sulbactam. Mini-Rev Med Chem. 2004;4:93–109. doi: 10.2174/1389557043487547. [DOI] [PubMed] [Google Scholar]
- Subbiah M, Shah DH, Besser TE, Ullman JL, Call DR. Urine from treated cattle drives selection for cephalosporin resistant Escherichia coli in soil. PLoS One. 2012;7(11):48919. doi: 10.1371/journal.pone.0048919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundararaman S, Saravanane R. Effect of loading rate and HRT on the removal of cephalosporin and their intermediates during the operation of a membrane bioreactor treating pharmaceutical wastewater. Water Sci Technol. 2010;61(7):1907–1914. doi: 10.2166/wst.2010.881. [DOI] [PubMed] [Google Scholar]
- Szymańska U, Marek W, Ireneusz S, Jarosław K, Gabriela W, Mateusz KW. Presence of antibiotics in the aquatic environment in Europe and their analytical monitoring: recent trends and perspectives. Microchem J. 2019;147:729–740. [Google Scholar]
- Talaro KP, Chess B. Foundations in microbiology. 8. New York: McGraw Hill; 2008. [Google Scholar]
- Thai HBD, Kyung YJ, Park BS, Joon PY, Min SJ, RoAhn D. A fluorogenic substrate of beta-lactamases and its potential as a probe to detect the bacteria resistant to the third-generation oxyimino-cephalosporins. Biosen Bioelectron. 2016;77:1026–1031. doi: 10.1016/j.bios.2015.10.081. [DOI] [PubMed] [Google Scholar]
- Thai PK, Xuan L, Ngan V, Hong P, Thi P, Quang N, Dang NTT, Kieu N, et al. Occurrence of antibiotic residues and antibiotic-resistant bacteria in effluents of pharmaceutical manufacturers and other sources around. Sci Total Environ. 2018;645:393–400. doi: 10.1016/j.scitotenv.2018.07.126. [DOI] [PubMed] [Google Scholar]
- Turkdogan FI, Yetilmezsoy K. Appraisal of potential environment risks associated with human antibiotics consumption in Turkey. J Hazard Mater. 2009;166:297–308. doi: 10.1016/j.jhazmat.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Urlacher VB, Eiben S. Cytochrome P450 monooxygenases: perspectives for synthetic application. Trends Biotechnol. 2006;24:324–330. doi: 10.1016/j.tibtech.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Vasiliadou IA, Molina R, Martinez F, Melero JA, et al. Toxicity assessment of pharmaceutical compounds on mixed culture from activated sludge using respirometric technique: the role of microbial community structure. Sci Total Environ. 2018;630:809–819. doi: 10.1016/j.scitotenv.2018.02.095. [DOI] [PubMed] [Google Scholar]
- Vasiliu S, Bunia I, Racovita S, Neagu V. Adsorption of cefotaxime sodium salt on polymer coated ion exchange resin microparticles: kinetics, equilibrium and thermodynamic studies. Carbohydr Polym. 2011;85:376–387. [Google Scholar]
- Wagner RD, Johnson SJ, Cerniglia CE, Erickson BD. Bovine intestinal bacteria inactivate and degrade ceftiofur and ceftriaxone with multiple β-lactamases. Antimicrobiol Agents Chemother. 2011;11:4990–4998. doi: 10.1128/AAC.00008-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C. Molecular mechanisms that confer antibacterial drug resistance. Nature. 2000;406:775–781. doi: 10.1038/35021219. [DOI] [PubMed] [Google Scholar]
- Wang XH, Lin AY. Phototransformation of cephalosporin antibiotics in an aqueous environment results in higher toxicity. Environ Sci Technol. 2012;46:12417–12426. doi: 10.1021/es301929e. [DOI] [PubMed] [Google Scholar]
- Watkinson AJ, Murby EJ, Costanzo SD. Removal of antibiotics in conventional and advanced wastewater treatment: implications for environmental discharge and wastewater recycling. Water Res. 2007;41:4164–4176. doi: 10.1016/j.watres.2007.04.005. [DOI] [PubMed] [Google Scholar]
- WHO World Health Organization . Antimicrobial resistance: draft global action plan on antimicrobial resistance. Geneva, Switzerland: WHO Press; 2015. [Google Scholar]
- Williams M, Kookana RS. Fate and behavior of environmental contaminants arising from Health care. Health Care Environ Contam. 2018;11:21. [Google Scholar]
- Wright GD. Bacterial resistance to antibiotics: enzymatic degradation and modification. Adv Drug Deliv Rev. 2005;57(10):1451–1470. doi: 10.1016/j.addr.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Yalchin HT, Corbaci C. Isolation and characterization of amylase producing yeasts and improvement of amylase production. Turk J Biochem. 2013;38:101–108. [Google Scholar]
- Yan W, Yong X, Weid Y, Rui D, Shuhu W, Feng Z. The effect of bioelectrochemical systems on antibiotics removal and antibiotic resistance genes: a review. Chem Eng J. 2019;358:1421–1437. [Google Scholar]
- Yang B, Zuo J, Gan L, Yu X, Liu F, Tang X, Wang Y. Advanced treatment of cephalosporin pharmaceutical wastewater by nano-coated electrode and perforated electrode. J Environ Sci Heal A Tox Hazard Subst Environ Eng. 2014;49(11):1258–1264. doi: 10.1080/10934529.2014.910044. [DOI] [PubMed] [Google Scholar]
- Yang B, Zuo J, Li P, Wang K, Yu X, Zhang M. Effective ultrasound electrochemical degradation of biological toxicity and refractory cephalosporin pharmaceutical wastewater. Chem Eng J. 2016;287:30–37. [Google Scholar]
- Yu X, Tang X, Zuo J, Zhang M, Chen L, Li Z. Distribution and persistence of cephalosporins in cephalosporin producing wastewater using SPE and UPLC–MS/MS method. Sci Total Environ. 2016;569–570:23–30. doi: 10.1016/j.scitotenv.2016.06.113. [DOI] [PubMed] [Google Scholar]
- Zavareh S, Eghbalazar T. Efficient and selective removal of cefixime form aqueous solution by a modified bionanocomposite. J Environ Chem Eng. 2017;5(4):3337–3347. [Google Scholar]
- Zhang J, Meng J, Li Y, Hu C. Investigation of the toxic functional group of cephalosporins by zebrafish embryo toxicity test. Arch Pharm (Weinh) 2010;343:553–560. doi: 10.1002/ardp.201000005. [DOI] [PubMed] [Google Scholar]
- Zhang J, Qian J, Tong J, Zhang D, Hu C. Toxic effects of cephalosporins with specific functional groups as indicated by zebrafish embryo toxicity testing. Chem Res Toxicol. 2013;26:1168–1181. doi: 10.1021/tx400089y. [DOI] [PubMed] [Google Scholar]
- Zhang F, Qin W, Zhang JP, Hu CQ. Antibiotic toxicity and absorption in zebrafish using liquid chromatography-tandem mass spectrometry. PLoS One. 2015;10:0124805. doi: 10.1371/journal.pone.0124805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang PLC, Shen X, Chalmers G, Reid-Smith RJ, Slavic D, Dick H, Boerlin P. Prevalence and mechanisms of extended-spectrum cephalosporin resistance in clinical and fecal Enterobacteriaceae isolates from dogs in Ontario Canada. Vet Microbiol. 2018;213:82–88. doi: 10.1016/j.vetmic.2017.11.020. [DOI] [PubMed] [Google Scholar]
- Zhang E, Qingling Yu, Zhai Wenjing, Wang Feng, Scott Keith. High tolerance of and removal of cefazolin sodium in single-chamber microbial fuel cells operation. Bioresour Technol. 2018;249:76–81. doi: 10.1016/j.biortech.2017.10.005. [DOI] [PubMed] [Google Scholar]
- Zhao S, Chaoyi B, Yaxuan Y, Weihua Z, James E, Peng W. Removal of antibiotics using polyethylenimine cross-linked nanofiltration membranes: relating membrane performance to surface charge characteristics. Chem Eng J. 2018;335:101–109. [Google Scholar]
- Zhao Y, Yongbo W, Xuhua L, Huanxian S, Cunjin W, Jun F, Xiaoyun H, Enzhou L. Enhanced photocatalytic activity of Ag-CsPbBr 3/CN composite for broad spectrum photocatalytic degradation of cephalosporin antibiotics 7-ACA. App Catal B Environ. 2019;247:57–69. [Google Scholar]