Abstract
Background
Hereditary diffuse gastric cancer (HDGC) syndrome results most often from a mutation in the CDH1 tumor suppressor gene and confers a 56–70% lifetime risk of gastric cancer. In asymptomatic carriers HDGC is characterized by multiple foci of signet ring cancer cells (SRCC) throughout the gastric mucosa. Because SRCC foci are less than 1 mm in diameter, and often make up less than 2% of the gastric mucosa, they are undetectable on standard screening endoscopy. International consensus guidelines recommend thorough white light endoscopic gastric mapping with a systematic method for gastric biopsies. Despite a painstaking approach to gastric mapping, retrospective analyses have demonstrated a low rate of detection and poor sensitivity of standard white light endoscopy for detecting occult cancer cells. Therefore, a more sensitive cancer screening tool is needed for these high-risk patients. Confocal endoscopic microscopy (CEM) allows for real-time, in vivo histologic imaging of gastrointestinal mucosa during upper endoscopy. Clinical application has demonstrated the ability of CEM to differentiate normal mucosal cells and cancer cells.
Methods
A phase II clinical trial is currently underway to compare CEM to standard endoscopic gastric mapping in an effort to reduce the false negative detection rate of SRCC in patients diagnosed with HDGC. After an upper endoscopy with gastric mapping is performed, patients will undergo probe-based CEM. The endoscopist will scan the anatomic zones of the stomach in a similar fashion to the systematic gastric mapping approach. Any abnormal areas visualized with the CEM probe will be biopsied and sent for permanent pathologic analysis. The primary endpoint is to determine if CEM affords a higher sensitivity for detection of SRCC in CDH1 germline mutation carriers compared to white light endoscopy with gastric mapping.
Discussion
This novel screening technique is expected to provide greater sensitivity for detecting occult cancer in patients with HDGC, thereby improving cancer risk-assessment and overall cancer care.
Trial registration
ClinicalTrials.gov NCT03648879 (registration date: August 28, 2018, clinicaltrials.gov).
Keywords: CDH1, gastric cancer, confocal endomicroscopy
Introduction
Although gastric adenocarcinoma represents 1.7% of all new cancer cases in the United States, it is the fifth most common cause of cancer death worldwide (1,2). Common risk factors include H. pylori infection, tobacco smoking, and diet. Hereditary causes account for 1–3% of gastric cancer cases globally. The three main heritable forms of gastric cancer are hereditary diffuse gastric cancer (HDGC) syndrome, gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS), and familial intestinal gastric cancer (FIGC) (3). HDGC is caused most often by an inherited autosomal dominant mutation in the CDH1 gene. This genetic mutation imparts a 56–70% lifetime risk of developing diffuse-type gastric cancer, and a 42% risk of developing invasive lobular breast cancer in female carriers (4).
The International Gastric Cancer Linkage Consortium (IGCLC) has developed guidelines to help physicians select patients who should be tested for CDH1 mutation (4). At the time of diagnosis of pathogenic CDH1 mutation, a screening esophagogastroduodenoscopy (EGD) is recommended. Because affected patients often harbor occult, early-stage gastric cancer that is only detectable by histopathology, a risk reducing gastrectomy is recommended after the age of 20, or 5 years earlier than the age of diagnosis of the youngest affected family member. For patients who choose not to proceed with risk-reducing gastrectomy, annual endoscopic surveillance is recommended (5). The IGCLC recommends six biopsies from each anatomical zone of the stomach (antrum, transitional zone, body, fundus, and cardia) and any visible lesion (6). If any biopsies reveal signet ring cancer cells (SRCC) on histopathology then the patient should be advised to undergo therapeutic total gastrectomy (Figure 1).
Figure 1.

Signet ring cancer cells found on biopsy during endoscopy from a patient with CDH1 germline mutation (HE staining, 10×).
Unfortunately, screening endoscopy with gastric biopsies does not guarantee early detection of cancer in patients with HDGC. CDH1 is a tumor suppressor gene, and due to the second hit hypothesis, patients with a CDH1 mutation are more likely to develop non-contiguous islands of intramucosal SRCC (3,7). Early-stage gastric adenocarcinomas detected in affected patients are characterized by a diffuse-type histology of SRCC, often with multiple foci located within the lamina propria of the gastric mucosa. SRCC foci can make up less than 2% of the gastric mucosa and each focus is very often less than 1 mm in greatest diameter (8). This makes detection of early gastric cancer in patients with a CDH1 mutation very difficult. A retrospective study of 23 patients with a CDH1 mutation demonstrated that EGD is not an adequate screening modality. Of the 23 patients in this study, only 2 were found to have SRCC foci on EGD with biopsy. All 23 patients underwent a prophylactic total gastrectomy. On final pathology, 22/23 patients were found to have foci of SRCC. This means that screening EGD only had a 9% detection rate of early gastric cancer (9).
Our own experience evaluating patients with HDGC suggests that although comprehensive gastric mapping with biopsies may be superior at detecting early-stage cancer when compared to standard endoscopy, the overall detection rate remains too low to recommend surveillance over risk-reducing gastrectomy with confidence. A recent analysis of 54 patients with predisposition to gastric cancer was performed at our institution. Of these patients, 40 (74%) had a primary genetic abnormality predisposing to gastric malignancy with the remainder having a strong family history of gastric cancer. Subjects were screened through standard EGD with random biopsies or the gastric mapping protocol developed by Yao (10). Of the 56 EGDs performed, 17 (30%) were done via gastric mapping and 39 (70%) via standard EGD with targeted biopsies of abnormalities and random biopsies from the antrum and body. SRCC were identified in 4/17 (24%) patients undergoing gastric mapping, and in none (0/39 patients) undergoing standard EGD. Twenty-two of these patients subsequently underwent a risk-reducing gastrectomy. Four of these 22 (18%) patients had SRCC foci and had previously undergone gastric mapping. Of the 18 patients who underwent standard endoscopy followed by gastrectomy, 8 (44%) cases of SRCC were identified that had not been found on standard EGD. It should be noted that alternative visual enhancement to white light endoscopy has also been studied, including narrow band imaging, chromoendoscopy, and utilization of Congo red and indigo-carmine dyes (11,12). However, none of these enhancements have led to increased rates of cancer detection.
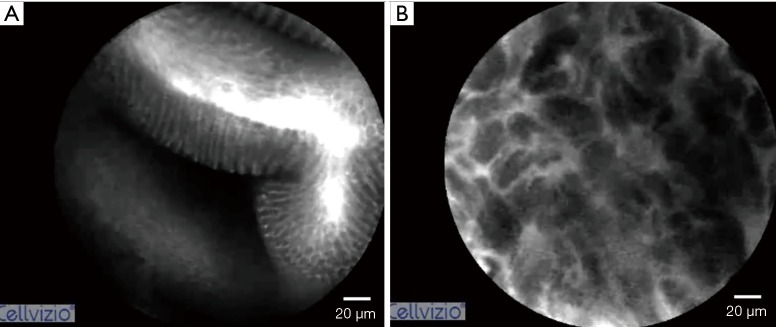
Confocal endoscopic microscopy (CEM) provides in vivo histologic images of the gastrointestinal mucosa through use of a standard endoscope. CEM utilizes tissue autofluorescence or the aid of an intravenous contrast agent to highlight vasculature, lamina propria, intracellular spaces, and the architecture of each cell (13). Kakeji et al. used CEM to examine gastric mucosa in the ex vivo and in vivo setting. In both models, irregular nuclear size and shape were used to differentiate between normal mucosal cells and cancer cells (Figure 2). CEM findings were correlated with histopathologic images (14). Kitabatake et al. also used CEM in vivo and found that the confocal images of gastric cancer differed from normal mucosa, which was also confirmed with H&E staining (15).
Figure 2.
In vivo confocal endomicroscopy image of (A) normal stomach and (B) gastric cancer. Images courtesy of Mauna Kea Technologies and Dr. Changqing Li, Institute Shandong University, Qilu Hospital, Shandong, China.
A natural history protocol for the longitudinal study of patients with HDGC is open at the National Institutes of Health (NIH) Clinical Center in Bethesda, Maryland. This unique setting allows clinicians and scientists to perform cutting-edge cancer research while also providing expert clinical care to patients and families affected by HDGC. The large cohort of affected patients and families participating in our longitudinal study undoubtedly will help transform discovery into healthcare advances for patients with both hereditary and sporadic forms of gastric cancer.
A prospective, phase II clinical trial is now open in our center for patients with HDGC to determine if CEM affords a higher level of sensitivity for detection of SRCC compared to the current method of gastric mapping. A secondary goal of the current study is to define the false negative rate of SRCC detection by CEM in those patients who choose to undergo risk-reducing total gastrectomy. As part of the unique environment at NIH for pioneering research, we are able to study the stomachs of patients who have had total gastrectomy by maintaining whole organ viability ex vivo for further drug testing and development. Such correlative studies based on our clinical study of improved detection of early-stage gastric cancer are expected to improve our understanding of the drivers of carcinogenesis in patients with HDGC.
Methods
Design
Prospective, single arm phase II study of CEM for detection of intramucosal SRCC foci. All patients will be enrolled at the NIH Clinical Center in Bethesda, Maryland (NCT03648879). This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute (NCI).
Eligibility
Eligible patients must be ≥18 years old and physiologically able to undergo an EGD. Confirmation of a pathogenic, or likely pathogenic, CDH1 germline mutation or a germline mutation previously associated with HDGC (e.g., CTNNA1) is required.
Exclusion
Patients with concurrent illness or comorbidities for which EGD is contraindicated are excluded. Examples of such comorbidities include thrombocytopenia, contraindication to anesthesia, unstable angina or recent myocardial infarction, known bleeding disorder, or current use of therapeutic anticoagulation medication.
Intervention
Prior to endoscopy patients will receive the contrast agent fluorescein if no known allergy is documented. Patients will undergo upper endoscopy with gastric mapping as outlined by IGCLC. In addition, patients will undergo probe-based CEM. The Cellvizio 100 Series system with the Confocal® GastroFlex MiniprobesTM (Mauna Kea Technologies) will be utilized in all cases. The probe is passed through the operating channel of a standard endoscope. The endoscopist will scan the anatomic zones of the stomach in a similar fashion to the systematic gastric mapping approach. Any abnormal areas visualized with the CEM probe will be biopsied and sent for permanent pathologic analysis. Patients who proceed to risk reducing total gastrectomy will contribute to secondary endpoints. The CEM technology will be used to evaluate the stomach ex vivo prior to submission for permanent pathologic analysis.
Study endpoints
The primary endpoint is to determine if CEM affords a higher sensitivity for detection of SRCC in CDH1 germline mutation carriers compared to white light endoscopy with gastric mapping. The secondary endpoint is to define the false negative rate of CEM detection of SRCC in patients who choose to undergo prophylactic total gastrectomy with permanent pathologic analysis. This study also supports research at the NCI in the areas of gastric cancer pathogenesis and mechanisms of metastasis.
Sample size calculation
To demonstrate if there would be an improvement between the historical detection rate of 4/17 (24%) with gastric mapping at our institution and a detection rate which could be consistent with 50% using the CEM approach, we will enroll 24 evaluable patients, with an accrual ceiling of 27 to allow for unevaluable patients. If there are 9/24 (38%) patients whose signet ring foci can be detected using CEM, this would demonstrate statistically greater detection than the historical figure as well as being consistent with a desirable 50% detection rate based on evaluation of 90% confidence bounds around 9/24. Accrual is expected to occur over 12–18 months.
Future management of patients
Outcomes may affect future management of patients. If SRCC are found on biopsy, it will be recommended to the patient that they undergo a risk reducing gastrectomy.
Discussion
HDGC syndrome is most often due to a mutation in the CDH1 tumor suppressor gene, resulting in a 56–70% lifetime risk of gastric cancer. HDGC is characterized by multiple foci of intramucosal SRCC throughout the gastric mucosa. Because these foci are less than 1 mm in diameter and can make up less than 2% of the gastric mucosa, they are often undetectable on standard white light screening endoscopy with gastric biopsies. The NIH is currently conducting a prospective, single-arm phase II study of CEM for detection of intramucosal SRCC. This novel screening technique is expected to provide greater sensitivity for detecting occult cancer in patients with HDGC, thereby improving cancer risk-assessment and overall cancer care.
Acknowledgements
This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute.
Ethical Statement: All participants are consented through a written consent form as part of clinical protocols approved by the National Institutes of Health, National Cancer Institute Institutional Review Board. The reference number is 385122 and our protocol was cleared by the National Cancer Institute Deputy Ethics Counselor.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Cancer Stat Facts: Stomach Cancer. Surveillance, Epidemiology, and End Results Program. Accessed August 2018. Available online: https://seer.cancer.gov/statfacts/html/stomach.html
- 2.Cancer. World Health Organization. Accessed August 2018. Available online: http://www.who.int/news-room/fact-sheets/detail/cancer
- 3.Oliveira C, Pinheiro H, Figueiredo J, et al. Familial gastric cancer: genetic susceptibility, pathology, and implications for management. Lancet Oncol 2015;16:e60-70. 10.1016/S1470-2045(14)71016-2 [DOI] [PubMed] [Google Scholar]
- 4.van der Post RS, Vogelaar IP, Manders P, et al. Accuracy of Hereditary Diffuse Gastric Cancer Testing Criteria and Outcomes in Patients With a Germline Mutation in CDH1. Gastroenterology 2015;149:897-906.e19. 10.1053/j.gastro.2015.06.003 [DOI] [PubMed] [Google Scholar]
- 5.Moreira L, Castells A. Surveillance of patients with hereditary gastrointestinal cancer syndromes. Best Pract Res Clin Gastroenterol 2016;30:923-35. 10.1016/j.bpg.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 6.Fitzgerald RC, Hardwick R, Huntsman D, et al. Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research. J Med Genet 2010;47:436-44. 10.1136/jmg.2009.074237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pećina-Slaus N. Tumor suppressor gene E-cadherin and its role in normal and malignant cells. Cancer Cell Int 2003;3:17. 10.1186/1475-2867-3-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huntsman DG, Carneiro F, Lewis FR, et al. Early gastric cancer in young, asymptomatic carriers of germ-line E-cadherin mutations. N Engl J Med 2001;344:1904-9. 10.1056/NEJM200106213442504 [DOI] [PubMed] [Google Scholar]
- 9.Hebbard PC, Macmillan A, Huntsman D, et al. Prophylactic total gastrectomy (PTG) for hereditary diffuse gastric cancer (HDGC): the Newfoundland experience with 23 patients. Ann Surg Oncol 2009;16:1890-5. 10.1245/s10434-009-0471-z [DOI] [PubMed] [Google Scholar]
- 10.Yao K. The endoscopic diagnosis of early gastric cancer. Ann Gastroenterol 2013;26:11-22. [PMC free article] [PubMed] [Google Scholar]
- 11.Syngal S, Brand RE, Church JM, et al. ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol 2015;110:223-62; quiz 263. 10.1038/ajg.2014.435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Post RS, Vogelaar IP, Carneiro F, et al. Hereditary diffuse gastric cancer: updated clinical guidelines with an emphasis on germline CDH1 mutation carriers. J Med Genet 2015;52:361-74. 10.1136/jmedgenet-2015-103094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunbar KB, Canto MD. Confocal endomicroscopy. Tech Gastrointest Endosc 2010;12:90-9. 10.1016/j.tgie.2010.02.010 [DOI] [Google Scholar]
- 14.Kakeji Y, Yamaguchi S, Yoshida D, et al. Development and assessment of morphologic criteria for diagnosing gastric cancer using confocal endomicroscopy: an ex vivo and in vivo study. Endoscopy 2006;38:886-90. 10.1055/s-2006-944735 [DOI] [PubMed] [Google Scholar]
- 15.Kitabatake S, Niwa Y, Miyahara R, et al. Confocal endomicroscopy for the diagnosis of gastric cancer in vivo. Endoscopy 2006;38:1110-4. 10.1055/s-2006-944855 [DOI] [PubMed] [Google Scholar]