Abstract
Gastrostomy is commonly used to provide enteral nutrition when patient require a nutrition support due to not enough oral eating. Gastrostomy tube can lead to many complications; squamous cell carcinoma (SCC) is an extremely rare complication of the site of gastrostomy, it was described after several years of enteral nutrition or as part of a metastasis of head and neck tumors. We describe the case of a 60-year-old man heart-liver transplanted for hereditary amyloidosis. He required the setting of a gastrostomy-tube for enteral feeding and developed after only 18 months a SCC on the site of gastrostomy confirmed in the histologic report. The increased risk of SCC in transplant patients is due to immunosuppressive therapies, even though everolimus could reduce this risk. The pose of a gastrostomy is responsible of a chronic cutaneous inflammation, which is another risk factor for SCC. In these immunocompromised patients, gastrostomy or other chronic skin injury requires special monitoring, especially if the wound does not heal.
Keywords: Gastrostomy tube, squamous cell carcinoma (SCC), everolimus, transplant patient
Introduction
Gastrostomy tube placement is a commonly method to provide the enteral nutrition when oral eating is not possible or not sufficient. Gastrostomy tube is usually placed endoscopically [percutaneous endoscopic gastrostomy (PEG)]. The reported rates of complications varied from 16% to 70% (1,2). The majority of these complications are minors, included peristomal wound infection, stomal leakage, buried bumper, gastric ulcer, ileus, fistulous tracts and inadvertent removal (3). In rare cases, tumor implantation at gastrostomy site has been reported, especially in patients with head and neck cancer (4). Furthermore, primary squamous cell carcinoma (SCC) of the peristomal skin of a gastrostomy tube was previously reported in two cases in the literature (5,6). We described in this report the first case of SCC in peristomal skin of a gastrostomy in a transplanted patient.
Case presentation
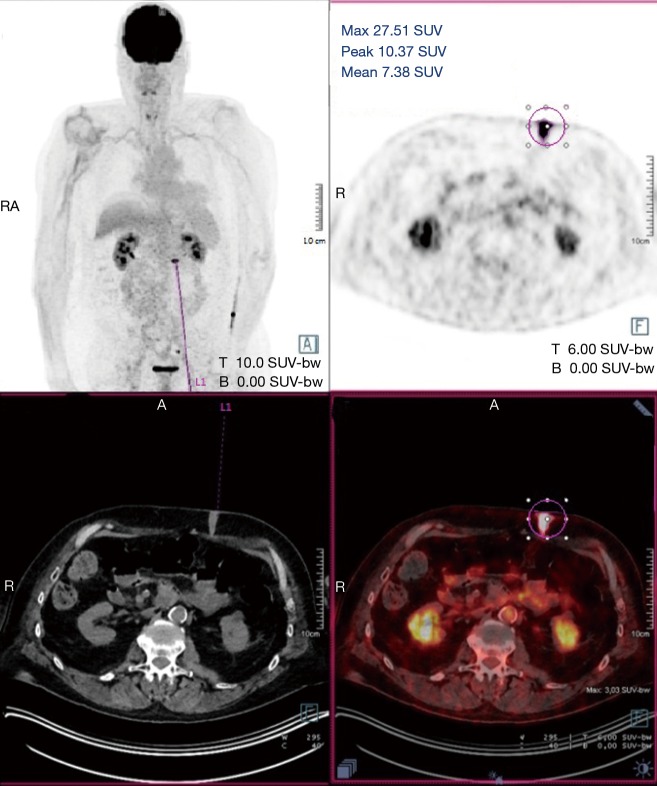
A 60-year-old man who underwent a combined heart-liver transplantation in 2013 for hereditary amyloidosis, developed several infectious complications after transplantation leading to severe malnutrition. In 2016, due to his nutritional status, a PEG tube was placed for enteral feeding. After 18 months, the gastrostomy tube was removed because the patient’s nutritional status has improved. Few months later, he presented in our department for persistent gastrostomy site leakage with local skin infections. Despite a local care therapy, the gastrocutaneous fistula persisted which impacted on quality of life (Figure 1). Because the conservative approach failed, we planned to perform a surgical excision of the gastrostomy site. The immunosuppressive regimen was changed by switching everolimus for tacrolimus to avoid surgical complications related to tissue healing. Two months after stopping Everolimus, the granulation considerably increased associated with a poor general condition. Computed tomography revealed a tumoral process at the expense of the gastrostomy site associated with a gastrocutaneous fistula. Gastric endoscopy was performed and not found a gastric invasion. Positron emission tomography scan revealed an isolated high metabolism of the tumor (standard uptake value =10.4) with no other anomaly (Figure 2). We performed a surgical resection of the tumor and surrounding abdominal wall and submitted for histological analysis. No sign of local extension to adjacent organs was found during the procedure. The postoperative course was uneventful and the patient was discharged in postoperative day 7. The pathological report of the specimen revealed a 5.5-centimeter well-differentiated squamous cell skin carcinoma (SCC) with tumor-free margins (Figure 3). Everolimus treatment was resumed after surgery with close follow-up.
Figure 1.
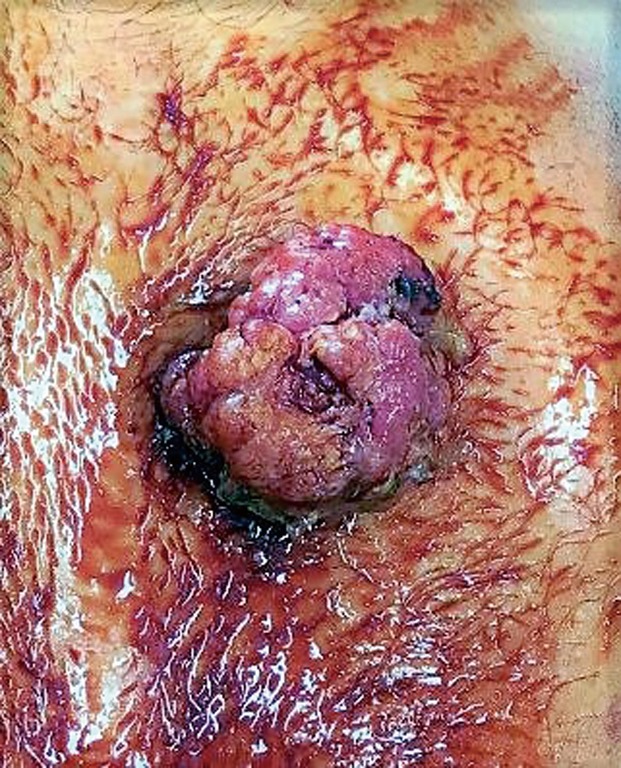
Macroscopic view of gastrostomy site at time of surgery.
Figure 2.
Positron emission tomography scan with hypermetabolism in the gastrostomy site.
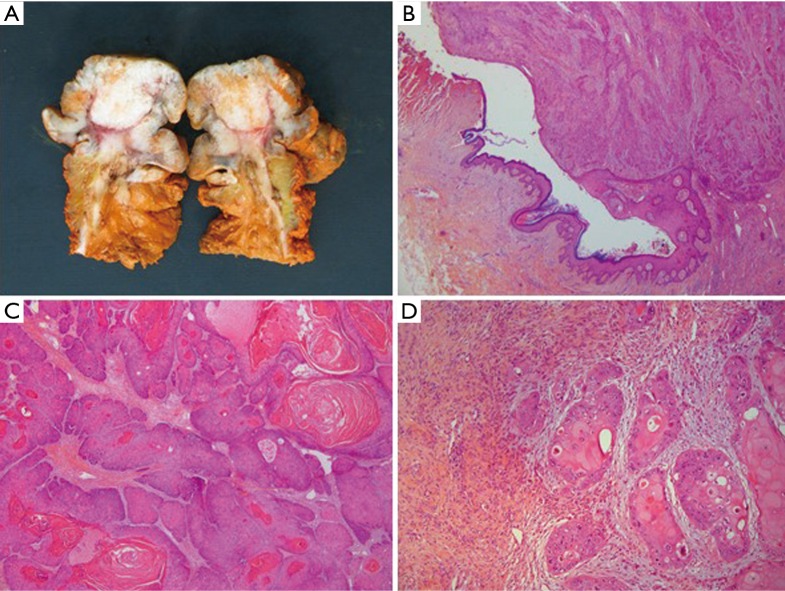
Figure 3.
Pathological analysis: macroscopic and microscopic sections. (A) Photograph of the surgical specimen after median incision. Orange ink has been applied to evaluate the margin; (B) magnification at the edge of the lesion showing neoplastic cells arising from normal epidermis (HE staining, ×100 magnification); (C) keratinizing well-differentiated invasive squamous cell carcinoma (HE staining, ×200 magnification); (D) deep invasion into the wall of the gastrostomy (HE staining, ×200 magnification).
Discussion
SCC is a common form of skin cancer that develops in the squamous cells. It’s also considered as the most common cutaneous malignancies (7) and the major cause of morbidity after organ transplantation (8). Furthermore, the incidence of SCC is significantly increased in organ transplant recipients, especially in patients with heart or lung transplants (9). The main risk factors of SCC include sun chronic exposure, viral infections (human papillomaviruses), male sex, older age and smoking (10). Chronic cutaneous inflammation is also recognized as one important player in the development of SCC (11). Thus, the peristomal skin of gastrostomy is usually characterized by a local skin irritation due to gastric secretions leading to chronic inflammation.
Two similar cases have been reported, describing a primary SCC of peristomal skin of gastrostomy. Oh et al. reported the case of a 24-year-old man with a seizure disorder requiring enteral nutrition by gastrostomy since his second year of life. He developed a large amount of granulation tissue around the gastrostomy site, treated for 9 months with nitrate and local care. Excision of this granulation revealed a SCC (5). The second case concerned a 73-year-old man who suffered esophageal chemical burns after swallowing sodium hypochlorite 50 years earlier that was initially managed with esophageal exclusion and placement of a gastrostomy for enteral feeding. He presented with an exophytic and painful mass of the skin adjacent to his gastrostomy site. Biopsy of the mass revealed a SCC (6). These two cases had similarities and concerned patients with a prolonged gastrostomy use (respectively 22 and 50 years). Unlike these cases, our patient developed a SCC after only 2 years of enteral nutrition by gastrostomy tube. Moreover, in the current case, the granulation significantly increased 2 months after stopping Everolimus treatment. To our knowledge, this is the first report of a primary SCC arising from a gastrostomy tube site in a transplant patient.
After organ transplantation, immunosuppressive therapy is necessary to prevent rejection but also increases the risk of skin cancer formation. For example, cyclosporine promotes the tumor growth via VEGF-mediated angiogenesis and inhibits DNA repair after ultraviolet-induced skin damage. Furthermore, other immunosuppressive medication, such as azathioprine, sensitizes DNA to ultraviolet-A radiation and increase the risk of malignant transformation (7).
It was noteworthy that tumor progression was correlated with the use of everolimus. It is one of the mammalian target of rapamycin (mTOR) inhibitors with an antitumoral effect (12). The mTOR pathway is a major mechanism of cellular growth and homeostasis and is associated with risk reduction in de novo malignancy. mTOR inhibitors have also been associated with a decreased risk of developing non-melanoma skin cancers in comparison to calcineurin inhibitors (7,13). In our case, we hypothesize that development of SCC was attributed to a chronic inflammation in the skin surrounding the gastrostomy site in an immunocompromised patient after interruption of Everolimus treatment.
In conclusion, the development of carcinoma in a gastrostomy site is rare but may be increased in organ transplant recipients. This is attributed to the immunosuppressive drugs and significantly increase morbidity and mortality. We must take special attention to the risk of SCC in these immunocompromised patients by regular monitoring the entire skin coating and especially the various sites of prolonged cutaneous trauma.
Acknowledgements
None.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Blomberg J, Lagergren J, Martin L, et al. Complications after percutaneous endoscopic gastrostomy in a prospective study. Scand J Gastroenterol 2012;47:737-42. 10.3109/00365521.2012.654404 [DOI] [PubMed] [Google Scholar]
- 2.Keung EZ, Liu X, Nuzhad A, et al. In-hospital and long-term outcomes after percutaneous endoscopic gastrostomy in patients with malignancy. J Am Coll Surg 2012;215:777-86. 10.1016/j.jamcollsurg.2012.08.013 [DOI] [PubMed] [Google Scholar]
- 3.Lynch CR, Fang JC. Prevention and management of complications of percutaneous endoscopic gastrostomy (PEG) tubes. Pract Gastroenterol 2004;28:66-76. [Google Scholar]
- 4.Fung E, Strosberg DS, Jones EL, et al. Incidence of abdominal wall metastases following percutaneous endoscopic gastrostomy placement in patients with head and neck cancer. Surg Endosc 2017;31:3623-7. 10.1007/s00464-016-5394-8 [DOI] [PubMed] [Google Scholar]
- 5.Oh PS, Gill KZ, Lynch LJ, et al. Primary squamous cell carcinoma arising at a gastrostomy tube site. J Pediatr Surg 2011;46:756-8. 10.1016/j.jpedsurg.2011.01.018 [DOI] [PubMed] [Google Scholar]
- 6.Cerdán Santacruz C, Díaz Del Arco C, Rubio Herrera MÁ, et al. Squamous Cell Carcinoma of the Peristomal Skin of a Gastrostomy: Case Study. J Wound Ostomy Continence Nurs 2017;44:384-6. 10.1097/WON.0000000000000345 [DOI] [PubMed] [Google Scholar]
- 7.Chockalingam R, Downing C, Tyring SK. Cutaneous Squamous Cell Carcinomas in Organ Transplant Recipients. J Clin Med 2015;4:1229-39. 10.3390/jcm4061229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adami J, Gäbel H, Lindelöf B, et al. Cancer risk following organ transplantation: a nationwide cohort study in Sweden. Br J Cancer 2003;89:1221-7. 10.1038/sj.bjc.6601219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krynitz B, Edgren G, Lindelöf B, et al. Risk of skin cancer and other malignancies in kidney, liver, heart and lung transplant recipients 1970 to 2008--a Swedish population-based study. Int J Cancer 2013;132:1429-38. 10.1002/ijc.27765 [DOI] [PubMed] [Google Scholar]
- 10.Dreno B. Skin cancers after transplantation. Nephrol Dial Transplant 2003;18:1052-8. 10.1093/ndt/gfg023 [DOI] [PubMed] [Google Scholar]
- 11.Gomes M, Teixeira AL, Coelho A, et al. The role of inflammation in lung cancer. Adv Exp Med Biol 2014;816:1-23. 10.1007/978-3-0348-0837-8_1 [DOI] [PubMed] [Google Scholar]
- 12.Dumortier J, Dharancy S, Calmus Y, et al. Use of everolimus in liver transplantation: The French experience. Transplant Rev (Orlando) 2016;30:161-70. 10.1016/j.trre.2015.12.003 [DOI] [PubMed] [Google Scholar]
- 13.Jung JW, Overgaard NH, Burke MT, et al. Does the nature of residual immune function explain the differential risk of non-melanoma skin cancer development in immunosuppressed organ transplant recipients? Int J Cancer 2016;138:281-92. 10.1002/ijc.29450 [DOI] [PubMed] [Google Scholar]