Abstract
Background
The colorectal cancer (CRC) tumorigenesis is driving by genetic alterations leading to changes in protein expression such as p53. The p53 is frequently expressed in CRC and its association with clinicopathological features is still controversial. Moreover, accumulated evidence suggests that both p53 and nuclear exporter protein, exportin 1 (XPO1), are working in reciprocal manner may lead to loss of p53 nuclear localization and enhance cancer progression through hyperactive nuclear export. Accordingly, the present study aimed to evaluate the expression of p53 in CRC Yemeni patients and to explore the association between the p53 and XPO1 coexpression in relation to clinicopathological features.
Methods
A series of 40 formalin fixed paraffin embedded (FFPE) tissue blocks taken from CRC patients that diagnosed as adenocarcinoma were prospectively collected and then analyzed for p53 and XPO1 expression by immunohistochemistry (IHC). The patients and tumor clinicopathological characteristics were retrieved from the histopathology reports and the P value <0.05 were considered statistically significant.
Results
The p53 expression was observed in 60% (24/40) of CRC tumor samples. Significantly, the p53 expression was noted in 72.4% (21/29) of the left side compared to 27.3% (3/11) of the right side colon tumors (P=0.014). Furthermore, p53 expression was positively and significantly correlated with well-but not moderate- or poorly-differentiated tumors (P=0.023). No significant difference was observed between the p53 expression and age, gender and tumor size. Regarding the XPO1 expression, the p53 expression didn’t show an association with XPO1 expression. The coexpression of p53 and XPO1 analysis revealed that 100% (11/11) tumors with negative p53 and positive XPO1 coexpression was noted with lymph node metastasis with significant difference (P=0.003) and more frequently observed in moderate-or poorly- differentiated tumors.
Conclusions
The loss of p53 accompanied with increased XPO1 expressions was associated with the progression of histopathological features of CRC Yemeni patients. Further studies are needed to elucidate the p53 genetic mutations in relation to the XPO1 coexpression in CRC prognosis.
Keywords: Colorectal cancer (CRC), p53, exportin 1 (XPO1), Yemen
Introduction
Colorectal cancer (CRC) is a major health dilemma, rating as the third most frequently diagnosed cancer with higher mortality worldwide (1). The development of CRC is a complex process based on the accumulation of genetic mutations such as tumor suppressor genes, which may lead to disruption of cellular homeostasis (2). TP53 mutation was reported in all types of cancer with different rates, which lead to changes in the p53 protein expression within the cell and may eventually lose its function that involved in the regulation of cell cycle, DNA repair and apoptosis (3). In CRC, the p53 protein expression is frequently observed and its prognostication role and association with clinicopathological features still controversial (4). Recently, Cao et al., 2017, reported that the loss of p53 expression was frequently noted in CRC tumors and was associated with aggressive clinicopathological characteristics such as positive lymph node metastasis and advanced tumor stage (5). Although some studies failed to find any association between the p53 expression and clinicopathological characteristics (6), other studies indicated that the p53 expression was associated with metastatic stage (7) and the p53 protein overexpression may act as an independent predictor of tumor recurrence in CRC (8).
In addition to its function as guardian of the genome, the p53 predominantly works as a transcription factor and its function crucially depends on its nuclear localization, which is regulated, into some extent, by nuclear transport machinery such as exportin 1 (XPO1). The XPO1, also known as chromosomal maintenance 1 (CRM1), is a nuclear export chaperone, mediates the nuclear export of several proteins which are essential for growth regulation and tumor suppression such as p53, leading to cytoplasmic degradation (9). Overexpression of XPO1 is identified in CRC (10) and the accumulated evidence suggests that both p53 and XPO1 are working in reciprocal manner, while the XPO1 gene has a binding site for p53 and its transcription activity may be repressed by p53, the increased activity of XPO1 can cause the p53 mislocalization and dysfunction in cancers (11,12). From the above indications and because of individual marker expression has a limited prognostic value, we aimed to evaluate the association between the expression of p53 and clinicopathological features in CRC Yemeni patents, which is still unknown, and tried to explore the ambiguous relationship between the coexpression of p53 and XPO1 in CRC tissue samples in relation to histopathological features.
Methods
Patients and tissue samples
Total of 40 formalin fixed paraffin embedded (FFPE) tissue blocks from CRC patients diagnosed as adenocarcinoma were collected prospectively from the National Oncology Center (NOC), Sana’a, Republic of Yemen, after the informed consent taken from each patient. The study was approved by the Ethics Committee of the Ministry of Public Health and Population, the National Health and Medical Research Committee (NHMRC) (Reference: B2/10-2017). The patients’ age, gender and other tumor clinicopathological characteristics were retrieved from the patient’s histopathology report with the following inclusion criteria. Tumor stages were classified according to the TNM staging system, the American Joint Committee of Cancer (AJCC). The size of tumor was taken as the maximum diameter of the tumor in centimeters. The locations of the tumors were grouped into right (beginning from the cecum extending to the hepatic flexure and transverse colon) or left (starting from splenic flexure extending to the sigmoid colon and rectum). Binary system of lymph node (LN) metastasis as positive (N1) or negative (N0) was used. Paraffin tissue blocks representing the diagnosed tumor cells with adjacent normal epithelium were cut for hematoxylin and eosin (H&E) and selected for further immunohistochemistry (IHC) staining.
Immunohistochemical analysis
Consecutive 4-µm sections were cut from the FFPE tissue blocks adhered onto positive charge slides and deparaffinized sections bring to phosphate buffered saline (PBS) at pH 7.4. For antigen retrieval, the slides were placed in a container containing citric acid (pH 6.0) and heated in a microwave oven for 15 minutes at maximum power and then 3% H2O2 in methanol was used to block endogenous peroxidase activity. The tissue sections incubated overnight at 4 °C with monoclonal antibodies against p53 (dilution 1:500, DO-7; Cell Marque) and XPO1/CRM1 (dilution 1:400, sc-74454; Santa Cruz Biotechnology). Then, the antibodies were amplified by HiDef Detection™ HRP Polymer detection system (Cell Marque) for 10 minutes for each step. The color was developed with the chromogen substrate, liquid diaminobenzidine (DAB, Cell Marque), and the sections counterstained with hematoxylin (13). Sections of colon cancer known as positive for p53 and XPO1 expression were used as positive controls. Negative control also used in every IHC batch experiment.
Each section was evaluated by two independent investigators according to the following criteria. Nuclear expression of the p53 protein was evaluated and classified as negative (<10% of tumor cells) or positive (≥10% of tumor cells) according to the percentage of 500-tumor cells count (14). For the XPO1 expression, we used immunoreactive score (IRS) system according to the formula; the index of immunoreactive score (IRS) = (percentage of positive cells score) × (staining intensity score). Accordingly, the percentage of positive cells (nuclear and/or cytoplasmic) scored as 0 (0%), 1 (<10%), 2 (11–50%), 3 (51–80%), or 4 (>80%); while the intensity of staining scored as 0 (negative), 1 (weak), 2 (moderate), or 3 (strong). The resulting IRS values between 0–12 were further divided in to 0 (no staining) or 1–3 (weak) which considered as negative, while 4–8 (moderate) or 9–12 (strong) were considered as positive XPO1 expression in CRC tumors (15).
Statistical analysis
The associations between the p53 expression and the clinicopathological variables as well as the XPO1 expression were analyzed using Chi-square and Fisher’s exact tests. Spearman rank test was also used. The SPSS 18.0 software was used for data analysis and the P values <0.05 was considered statistically significant difference.
Results
Association between the p53 expression and clinicopathological features
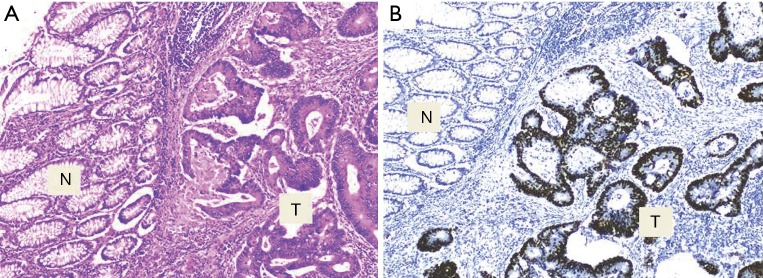
The p53 expression was observed in 60% (24/40) of CRC tissue samples while no immunostaining was seen in the adjacent normal epithelial colon cells (Figure 1). The association between the p53 expression and clinicopathological characteristics of the patients’ tumors is summarized in Table 1. Briefly, all of age, gender and tumor size groups didn’t show significant difference by the p53 expression. Significantly, the p53 positive expression was noted in 72.4% (21/29) of the left side colon tumors compared to 27.3% (3/11) in the right side (P=0.014), and according to the evaluated tumor differentiation, a significant positive correlation of p53 expression was observed with well- but not moderate- or poorly-differentiated tumors (P=0.023). Most of positive p53 expression, 66.7% (16/24), was correlated to well differentiate tumors. Even though the frequency of the positive p53 expression was similar in both positive and negative lymph node metastasis tumors (12/24 of each), the negative p53 expression with 75% (12/16) were observed in tumors with positive lymph node metastasis.
Figure 1.
Expressions of p53 in tumor cells and adjacent normal colon epithelium with H&E staining (A) and IHC staining (B). Tumor areas [T] and adjacent normal epithelium [N] at magnification 100×.
Table 1. Association between the p53 expression, clinicopathological features and XPO1 expression.
| Clinicopathological features and XPO1 | Total No. (%) | p53 expression | P | |
|---|---|---|---|---|
| Positive | Negative | |||
| Age | 0.604 | |||
| <50 years | 22 (55.0) | 14 | 8 | |
| ≥50 years | 18 (45.0) | 10 | 8 | |
| Gender | 0.436 | |||
| Female | 22 (55.0) | 12 | 10 | |
| Male | 18 (45.0) | 12 | 6 | |
| Tumor location | 0.014 | |||
| Right | 11 (27.5) | 3 | 8 | |
| Left | 29 (72.5) | 21 | 8 | |
| Tumor size | 0.329 | |||
| ≤5 cm | 14 (35.0) | 10 | 4 | |
| >5 cm | 26 (65.0) | 14 | 12 | |
| Tumor stage | 0.148 | |||
| I–II | 11 (27.5) | 9 | 2 | |
| III–IV | 29 (72.5) | 15 | 14 | |
| Lymph node metastasis | 0.114 | |||
| Positive | 24 (60.0) | 12 | 12 | |
| Negative | 16 (40.0) | 12 | 4 | |
| Tumor differentiation | 0.023 | |||
| Well | 19 (47.5) | 16 | 3 | |
| Moderate | 7 (17.5) | 1 | 6 | |
| Poorly | 14 (35.0) | 7 | 7 | |
| XPO1 expression | 0.728 | |||
| Positive | 29 (72.5) | 18 | 11 | |
| Negative | 11 (27.5) | 6 | 5 | |
Coexpression of p53 and XPO1 in CRC
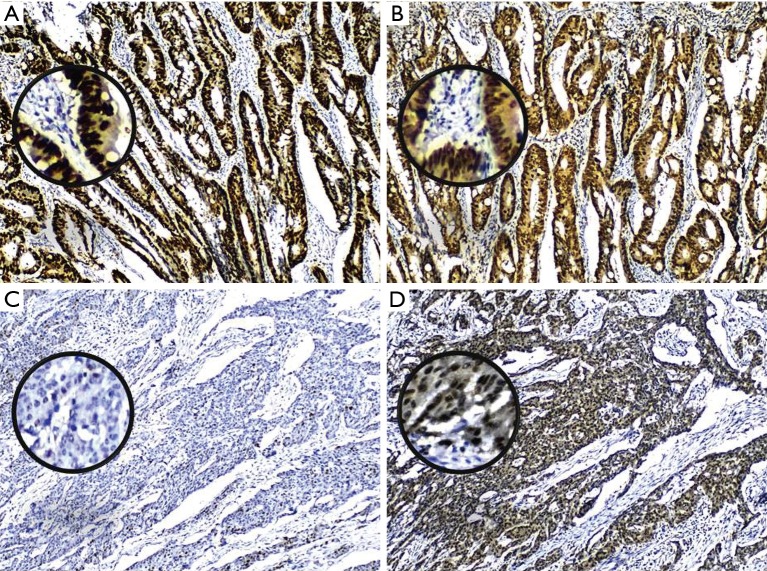
The positive expression of XPO1 was identified in 72.5% of the CRC tumors. Although there was no association between the expression of p53 and XPO1 (P=0.728), the frequency of negative p53 expression was more than doubled in tumors with XPO1 positivity (68.75%, 11/16) compared to the negative one (31.25%, 5/16) (Table 1). Further evaluation of the p53 and XPO1 coexpression in relation to clinicopathological characteristics revealed that the tumors with negative p53 expression and an increase of XPO1 expression were observed as moderate-or poorly-differentiated while combined positive of p53 and XPO1 expression was predominantly observed in well differentiated tumors (Table 2, Figure 2). Furthermore, 100% (11/11) tumors with negative p53 and positive XPO1 expression was associated and identified with positive lymph node metastasis with significant difference (P=0.003) (Table 3). Other clinicopathological characteristics such as age, gender and tumor location didn’t show a significant difference according to the p53 and XPO1 coexpression.
Table 2. CRC-differentiated tumors and coexpression of p53 and XPO1.
| P53 and XPO1 coexpression | Tumor differentiation | ||
|---|---|---|---|
| Well | Moderate | Poorly | |
| P53+/XPO+ | 12 | 1 | 5 |
| P53+/XPO− | 4 | 0 | 2 |
| P53−/XPO+ | 1 | 5 | 5 |
| P53−/XPO− | 2 | 1 | 2 |
| Total | 19 | 7 | 14 |
CRC, colorectal cancer.
Figure 2.
CRC tumor differentiation with p53 and XPO1 coexpression. Positive immunostaining coexpression of both p53 (A) and XPO1 (B) in well differentiated CRC tumors, while p53expression loss (C) is accompanied with increase XPO1 expression (D) in poorly differentiated tumors, at magnification 100×. CRC, colorectal cancer.
Table 3. Association between the coexpression of p53 and XPO1 in lymph node metastasis.
| P53/XPO1 coexpression | Lymph node metastasis | Total | P | |
|---|---|---|---|---|
| Positive | Negative | |||
| P53 positive | 0.640 | |||
| XPO1 positive | 8 | 10 | 18 | |
| XPO1 negative | 4 | 2 | 6 | |
| P53 negative | 0.003 | |||
| XPO1 positive | 11 | 0 | 11 | |
| XPO1 negative | 1 | 4 | 5 | |
Discussion
Although the molecular and cellular biomarker trends make the CRC more understandable, CRC still harvests the lives of many of its victims around the world with different rates. In Yemen, 17.1% of total cancers were raised in the digestive system and one-third of them were observed as CRC (16). The early onset of CRC in Yemeni people in their thirties and forties with high frequency at the left sided colon tumors (17) urged to evaluate the biology of CRC among these patients which is still poorly understood.
In spite the association between the p53 expression and reliable prognostic clinicopathological characteristics such as age, tumor location, tumor grade as well as lymph node metastasis had been studied in many nations, revealed controversial results; such association still unknown in CRC Yemeni patients. Moreover, the ambiguous relationship of p53 with the XPO1 nuclear cytoplasmic transporter persuaded us to evaluate their coexpression association with histopathological characteristics in CRC tissue samples.
Published literature reveals that the rates of the p53 expression in CRC range from 27% to 76% (18), while in our series the p53 positivity was detected in 60%. However, the frequency of p53 expression among Yemeni patients was far from that reported in China (19) and Turkey (20) but close to the prevalence reported in Saudi Arabia (21) and Iran (22) with 43%, 82.1%, 57.5%, and 59% respectively. These differences may attribute to ethnic and technicality (4). No p53 immunostaining was detected in the adjacent normal epithelial colon cells supporting the hypothesis of that the CRC carcinogenesis occurs through nonfunctional mutated p53 protein.
The assumption of CRC pathogenesis variation between the left and right side was significantly implied in our findings as higher proportion of p53 positivity in the left colon tumors compared to the right side which congruent with many studies (23,24).
In our series, most of the patients’ tumors with positive lymph node metastasis were poorly differentiated indicating that these aspects of aggressive tumor behavior (25) may be a marker of poor prognosis in this study. Analysis of the p53 expression revealed that the p53 expression was strongly associated with well differentiated tumors while some interesting high frequency of the p53 loss expression was predominated in moderate- or poorly-differentiated tumors as well as in positive lymph node metastatic tumors. Therefore, there was a strong trend of p53 loss related to more aggressive tumors behaviors than overexpression among CRC patients’ tumors. These aspects were in concordance and supported by Cao et al., 2017, who reported that the loss of p53 expression level was associated with aggressive clinicopathological characteristics such as lymph node and distant metastasis as well as with advanced tumor stages in patients with colon cancer (5). Trends of the p53 loss expression with worsening histological grade were also noted by Rambau et al. (26) while the in vivo study by Schwitalla et al. strongly suggested that the loss of p53 triggers the NF-κB activation and epithelial-mesenchymal transition occurrence leading to colonic tumor invasion and lymph node metastasis (27).
The signaling complexity and diverse roles of the p53 protein in cellular regulation may be lost either through the nuclear mislocalization or gene mutation leading to increase of its oncogenic properties in cancer (28). Therefore, the clarification of the association between p53 and other proteins expression such as XPO1 in tissue samples may help to define the prognostic value of p53 in CRC. Previously, a report indicated that while the XPO1 gene expression is regulated negatively by p53 (11), the XPO1induces loss of the p53 function through nuclear-cytoplasmic transport and then degradation through MDM2-mediated ubiquitination (9).
Although our findings showed that there was no significant association between the p53 and XPO1 expression, an inverse relationship was noted between the loss of p53 and increase of XPO1 expressions. Accordingly, the trends of p53 loss expression accompanied with increased intensity of the XPO1 expression conceded to evaluate their coexpression in relation to histopathological features. The results showed almost all the tumors with negative p53 expression and increases of the XPO1 expression were moderate- or poorly-differentiated tumors. In this study, most of the patients’ tumors with worsening histological grade were positive for lymph node metastasis, which spurred us to study the association between these aspects of aggressive tumor behavior and the p53/XPO1 coexpression. Consistently, the in vitro studies showed that the XPO1 inhibitor increases the expression and nuclear localization of p53 through the inhibition of p53 nuclear export by the XPO1 (29,30), which indicate that the XPO1 may has an indirect effect on cancer behavior through p53. In our study, further interesting finding was that all tumors with loss p53 expression and an increase of the XPO1 expression were significantly observed with positive lymph node metastasis and mainly seen in TNM advance stage (III and IV) tumors. The XPO1 activity may abrogate the function of p53 by inhibiting its access to the nucleus through nucleo-cytoplasmic transport mechanism, which may lead to decrease the p53 activity and tumor progression (31). Cytoplasmic sequestration of mutant or wild-type p53 has been reported in a variety of tumors including CRC (32). Moreover, Sun et al. reported that the cytoplasmic expression of p53 but not nuclear was associated with poor survival in CRC patients (33). On the other hand, an association between the p53 overexpression and better survival of CRC patients has been reported occasionally (34,35). Taken together, the loss of the nuclear p53 expression accompanied with increase XPO1 expression may be considered as a factor of aggressive histopathological features among patients in this study. Therefore, further investigations are needed considering molecular defining of functional p53 and its prognostic role in relation to XPO1 status in CRC patients.
Conclusions
As Yemeni patients with CRC, the loss of p53 in combination with increased of XPO1 expression was associated with aggressive histopathological features with poorly differentiated tumors and lymph node metastasis. Further studies are needed to elucidate the TP53 mutations in relation to XPO1 coexpression in CRC prognosis.
Acknowledgements
We would like to thank the patients and staff at the National Oncology Center, Yemen and the staff at the Pathobiology Department, Faculty of Science, Mahidol University, for their sincerely helping and support during perform this study.
Ethical Statement: The study was approved by the National Health and Medical Research Committee (NHMRC), the Ethics Committee of the Ministry of Public Health and Population, Republic of Yemen (Reference: B2/10-2017). Informed consent was obtained from all patients for being included in the study.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Bhandari A, Woodhouse M, Gupta S. Colorectal cancer is a leading cause of cancer incidence and mortality among adults younger than 50 years in the USA: a SEER-based analysis with comparison to other young-onset cancers. J Investig Med 2017;65:311-5. 10.1136/jim-2016-000229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dow LE, O'Rourke KP, Simon J, et al. Apc Restoration Promotes Cellular Differentiation and Reestablishes Crypt Homeostasis in Colorectal Cancer. Cell 2015;161:1539-52. 10.1016/j.cell.2015.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Worthley DL, Whitehall VL, Spring KJ, et al. Colorectal carcinogenesis: road maps to cancer. World J Gastroenterol 2007;13:3784-91. 10.3748/wjg.v13.i28.3784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munro AJ, Lain S, Lane DP. P53 abnormalities and outcomes in colorectal cancer: a systematic review. Br J Cancer 2005;92:434-44. 10.1038/sj.bjc.6602358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao DZ, Ou XL, Yu T. The association of p53 expression levels with clinicopathological features and prognosis of patients with colon cancer following surgery. Oncol Lett 2017;13:3538-46. 10.3892/ol.2017.5929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwon HC, Kim SH, Oh SY, et al. Clinicopathological significance of p53, hypoxia-inducible factor 1alpha, and vascular endothelial growth factor expression in colorectal cancer. Anticancer Res 2010;30:4163-8. [PubMed] [Google Scholar]
- 7.Resnick MB, Routhier J, Konkin T, et al. Epidermal growth factor receptor, c-MET, beta-catenin, and p53 expression as prognostic indicators in stage II colon cancer: a tissue microarray study. Clin Cancer Res 2004;10:3069-75. 10.1158/1078-0432.CCR-03-0462 [DOI] [PubMed] [Google Scholar]
- 8.Liang JT, Huang KC, Jeng YM, et al. Microvessel density, cyclo-oxygenase 2 expression, K-ras mutation and p53 overexpression in colonic cancer. Br J Surg 2004;91:355-61. 10.1002/bjs.4447 [DOI] [PubMed] [Google Scholar]
- 9.Freedman DA, Levine AJ. Nuclear export is required for degradation of endogenous p53 by MDM2 and human papillomavirus E6. Mol Cell Biol 1998;18:7288-93. 10.1128/MCB.18.12.7288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shintani M, Tashiro A, Sangawa A, et al. Expression of chromosomal regional maintenance protein-1 may be associated with subcellular survivin expression in human gastric and colorectal carcinoma. Oncol Lett 2016;12:4630-4. 10.3892/ol.2016.5220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golomb L, Bublik DR, Wilder S, et al. Importin 7 and exportin 1 link c-Myc and p53 to regulation of ribosomal biogenesis. Mol Cell 2012;45:222-32. 10.1016/j.molcel.2011.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan DS, Bedard PL, Kuruvilla J, et al. Promising SINEs for embargoing nuclear-cytoplasmic export as an anticancer strategy. Cancer Discov 2014;4:527-37. 10.1158/2159-8290.CD-13-1005 [DOI] [PubMed] [Google Scholar]
- 13.Allegra CJ, Paik S, Colangelo LH, et al. Prognostic value of thymidylate synthase, Ki-67, and p53 in patients with Dukes' B and C colon cancer: a National Cancer Institute-National Surgical Adjuvant Breast and Bowel Project collaborative study. J Clin Oncol 2003;21:241-50. 10.1200/JCO.2003.05.044 [DOI] [PubMed] [Google Scholar]
- 14.Zlobec I, Steele R, Michel RP, et al. Scoring of p53, VEGF, Bcl-2 and APAF-1 immunohistochemistry and interobserver reliability in colorectal cancer. Mod Pathol 2006;19:1236-42. 10.1038/modpathol.3800642 [DOI] [PubMed] [Google Scholar]
- 15.Noske A, Weichert W, Niesporek S, et al. Expression of the nuclear export protein chromosomal region maintenance/exportin 1/Xpo1 is a prognostic factor in human ovarian cancer. Cancer 2008;112:1733-43. 10.1002/cncr.23354 [DOI] [PubMed] [Google Scholar]
- 16.Basaleem HO, Al-Sakkaf KA. Colorectal cancer among Yemeni patients. Characteristics and trends. Saudi Med J 2004;25:1002-5. [PubMed] [Google Scholar]
- 17.Al-Samawi A, Aulaqi SM. Histopathological Profile of Colorectal Cancer in Yemen - An Eight Years Retrospective Study. Yemeni J Med Sci 2013;7:20-6. [Google Scholar]
- 18.Tejpar S, Bertagnolli M, Bosman F, et al. Prognostic and predictive biomarkers in resected colon cancer: current status and future perspectives for integrating genomics into biomarker discovery. Oncologist 2010;15:390-404. 10.1634/theoncologist.2009-0233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao DP, Ding XW, Peng JP, et al. Prognostic significance of bcl-2 and p53 expression in colorectal carcinoma. J Zhejiang Univ Sci B 2005;6:1163-9. 10.1631/jzus.2005.B1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erhan Y, Korkut MA, Kara E, et al. Value of p53 protein expression and its relationship with short-term prognosis in colorectal cancer. Ann Saudi Med 2002;22:377-80. 10.5144/0256-4947.2002.377 [DOI] [PubMed] [Google Scholar]
- 21.Al-Kuraya KS, Bavi PP, Ezzat AA, et al. Colorectal carcinoma from Saudi Arabia. Analysis of MLH-1, MSH-2 and p53 genes by immunohistochemistry and tissue microarray analysis. Saudi Med J 2006;27:323-8. [PubMed] [Google Scholar]
- 22.Ghavam-Nasiri MR, Rezaei E, Ghafarzadegan K, et al. Expression of p53 in colorectal carcinoma: correlation with clinicopathologic features. Arch Iran Med 2007;10:38-42. [PubMed] [Google Scholar]
- 23.Paluszkiewicz P, Berbec H, Pawlowska-Wakowicz B, et al. p53 protein accumulation in colorectal cancer tissue has prognostic value only in left-sided colon tumours. Cancer Detect Prev 2004;28:252-9. 10.1016/j.cdp.2004.05.001 [DOI] [PubMed] [Google Scholar]
- 24.Kapiteijn E, Liefers GJ, Los LC, et al. Mechanisms of oncogenesis in colon versus rectal cancer. J Pathol 2001;195:171-8. 10.1002/path.918 [DOI] [PubMed] [Google Scholar]
- 25.Chang GJ, Rodriguez-Bigas MA, Skibber JM, et al. Lymph node evaluation and survival after curative resection of colon cancer: systematic review. J Natl Cancer Inst 2007;99:433-41. 10.1093/jnci/djk092 [DOI] [PubMed] [Google Scholar]
- 26.Rambau PF, Odida M, Wabinga H. p53 expression in colorectal carcinoma in relation to histopathological features in Ugandan patients. Afr Health Sci 2008;8:234-8. [PMC free article] [PubMed] [Google Scholar]
- 27.Schwitalla S, Ziegler PK, Horst D, et al. Loss of p53 in enterocytes generates an inflammatory microenvironment enabling invasion and lymph node metastasis of carcinogen-induced colorectal tumors. Cancer Cell 2013;23:93-106. 10.1016/j.ccr.2012.11.014 [DOI] [PubMed] [Google Scholar]
- 28.Muller PA, Vousden KH. p53 mutations in cancer. Nat Cell Biol 2013;15:2-8. 10.1038/ncb2641 [DOI] [PubMed] [Google Scholar]
- 29.Abdul Razak AR, Mau-Soerensen M, Gabrail NY, et al. First-in-Class, First-in-Human Phase I Study of Selinexor, a Selective Inhibitor of Nuclear Export, in Patients With Advanced Solid Tumors. J Clin Oncol 2016;34:4142-50. 10.1200/JCO.2015.65.3949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang J, Bill MA, Young GS, et al. Novel small molecule XPO1/CRM1 inhibitors induce nuclear accumulation of TP53, phosphorylated MAPK and apoptosis in human melanoma cells. PLoS One 2014;9:e102983. 10.1371/journal.pone.0102983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stommel JM, Marchenko ND, Jimenez GS, et al. A leucine-rich nuclear export signal in the p53 tetramerization domain: regulation of subcellular localization and p53 activity by NES masking. EMBO J 1999;18:1660-72. 10.1093/emboj/18.6.1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jansson A, Gentile M, Sun XF. p53 Mutations are present in colorectal cancer with cytoplasmic p53 accumulation. Int J Cancer 2001;92:338-41. 10.1002/ijc.1189 [DOI] [PubMed] [Google Scholar]
- 33.Sun XF, Carstensen JM, Stål O, et al. Prognostic significance of cytoplasmic p53 oncoprotein in colorectal adenocarcinoma. Lancet 1992;340:1369-73. 10.1016/0140-6736(92)92558-W [DOI] [PubMed] [Google Scholar]
- 34.Lan YT, Chang SC, Li AF, et al. p53 protein accumulation as a prognostic marker in sporadic colorectal cancer. Int J Colorectal Dis 2007;22:499-506. 10.1007/s00384-006-0194-6 [DOI] [PubMed] [Google Scholar]
- 35.Melincovici CS, Mihu CM, Mărginean M, et al. The prognostic significance of p53, Bax, Bcl-2 and cyclin E protein overexpression in colon cancer - an immunohistochemical study using the tissue microarray technique. Rom J Morphol Embryol 2016;57:81-9. [PubMed] [Google Scholar]