Abstract
Background
The role of inflammation has been well established in many cancers, including hepatobiliary cancers. Elevated levels of interleukin-6 (IL-6), a pro-inflammatory marker, are associated with poor overall survival (OS) in hepatocellular carcinoma (HCC) patients.
Methods
We performed a study to establish the role of IL-6 as a prognostic biomarker in both HCC and biliary cancer patients and further assessed the impact of IL-6 on pain score and performance status, two parameters that affect the quality of life. We evaluated 91 patients with newly diagnosed unresectable hepatobiliary cancer and compared them with age, gender and BMI matched healthy controls.
Results
We found that IL-6 levels were elevated in hepatobiliary cancer patients compared to healthy controls. Higher levels of IL-6 were associated with poor prognosis, elevated pain scores and poor performance status in patients. Interestingly, we found an association between elevated IL-6 levels and the presence of portal vein thrombosis (PVT) at the time of cancer diagnosis.
Conclusions
This study suggests that IL-6 is an important prognostic biomarker in hepatobiliary cancers, where elevated levels are not only associated with a worse survival but also linked to an inferior quality of life.
Keywords: Hepatobiliary cancer, hepatocellular cancer (HCC), health-related quality of life, biomarker, interleukin-6 (IL-6)
Introduction
Hepatobiliary cancers pose a significant public health problem, both in the United States and globally. In 2017, approximately 40,710 cases were diagnosed with liver and intrahepatic bile duct cancer in the United States with additional 11,740 cases with gallbladder cancer and other biliary tract cancers (1). Of these, approximately 32,750 patients died from liver, gall bladder or other bile duct malignancies (1).
The role of inflammation has been well studied in different cancers including hepatobiliary cancers (2). Interleukin-6 (IL-6) is a pro-inflammatory cytokine that leads to cell proliferation, protection from apoptosis and increased metastatic potential in cancers through different signaling pathways including the PI3 kinase, JAK/STAT, p38 MAP kinase and others (3). Elevation of serum IL-6 has been reported in a wide variety of cancers (4-7). IL-6 has been found to have prognostic significance in different cancers. A study by Jang et al. found that a high level of IL-6 was associated with inferior survival in patients with hepatocellular cancer (HCC) undergoing loco-regional treatment (8). There is limited literature about whether it is a prognostic biomarker in biliary cancers. In our study, we attempted to establish the role of IL-6 as a prognostic biomarker in both HCC and biliary cancers.
Cancer often produces different symptoms like pain, cachexia fatigue, etc., which often lead to poor quality of life in patients. It is believed that in advanced cancer, the homeostasis between pro-inflammatory and anti-inflammatory cytokines is tipped in favor of the former, leading to a state of systemic inflammation. This results in cancer-related symptoms. IL-6 has been found to be associated with cancer-related fatigue and depression in lung cancer (9). Similarly, several other pro-inflammatory cytokines have been studied in the context of cancer-related symptoms. The results, however, have not been consistent due to a heterogeneous patient population, inadequate study designs and inconsistent measurements. We attempted to correlate IL-6 level with ECOG performance status and cancer-related pain in hepatobiliary cancers.
Methods
Study design & patients
Our study was a single center, retrospective study to assess the role of IL-6 as a prognostic biomarker in hepatobiliary cancers. Our study population consisted of 91 patients (62 males, 29 females) with newly diagnosed hepatobiliary cancers (HCC, gallbladder cancer, intra- and extra-hepatic cholangiocarcinoma) between February 2006 and September 2013. Hepatobiliary cancers were diagnosed histologically/cytologically or with hallmark findings demonstrated radiographically. A total of 91 controls were matched for age, gender and BMI, as those factors are known to affect IL-6 levels the most (10-15).
Blood tests were performed on the patients’ serum samples that were stored between February 2006 and September 2013. Serum concentration of IL-6 was determined using a commercial enzyme-linked immunosorbent assay (ELISA) kit. We also attempted to correlate the level of IL-6 with alpha-fetoprotein (AFP), a known prognostic biomarker in hepatobiliary cancers. This study obtained approval from Gastrointestinal Disease Site Research Group (GI DSRG).
Statistical analysis
Associations between baseline covariates and IL6 measurements were assessed using accelerated failure time (AFT) models. These models account for the left-truncation evinced in IL6 measurements below the limit of detection. About 2/3 of the healthy controls had left-truncated IL6 measurements; 1/10 of the HCC cases had left-truncated IL6 measurements. A Weibull distribution well described the distribution of observed IL6 measurements.
AFT parameter estimates for dichotomous covariates (i.e., gender) are described in terms of acceleration factors. These estimates describe the ratio of median IL6 measurements (i.e., female vs. male). Model-based estimates for IL6 medians are provided here; these are adjusted for the left-truncation. Differences in overall survival (OS) were assessed using AFT methods as well. These results were supplemented with Kaplan-Meier curves and assessed by the Log-rank test. In the present setting, the estimated model coefficients describe the ratio of median IL6 measurements (generally case vs. control). The corresponding null hypothesis is that the acceleration factor =1.
The modeled associations were also described using common summary statistics. While these statistics are generally informative, their interpretation may be clouded in the case of the left-truncated IL6 measurements. Dot plots and scatter plots were used for visual comparison of the IL6 measurements between baseline subgroups. P values less than 0.05 were considered statistically significant, with no adjustments to control the overall type 1 error rate. All data analyses were generated using SAS/STAT software, Version 9.4. Copyright 2012, SAS Institute Inc. SAS is a registered trademark of SAS Institute Inc., Cary, NC, USA.
Multivariable AFT modeling assessed the effect of IL6 on OS, adjusting for the main effect of potential confounding factors identified in the earlier results (AFP, cirrhosis, uncontrolled pain, thrombosis). These factors were previously shown to be associated with IL6. The AFT modeling results were corroborated with a multivariable cox proportional hazards model adjusted for the same covariates.
Results
Baseline characteristics
A total of 91 study subjects with unresectable hepatobiliary cancers and 91 healthy controls with no history of hepatobiliary cancers were included in the study. The clinical characteristics of these patients are listed in Table 1. Both case and controls had the same proportion of males (68.1%) and females (31.9%), with appropriately matched age groups. The median level of serum IL-6 was found to be elevated in cases compared to controls.
Table 1. Baseline characteristics of cases and controls.
| Characteristics | Cases (n=91) | Control (n=91) |
|---|---|---|
| Gender (male/female, %) | 62 (68.1)/29 (31.9) | 62 (68.1)/29 (31.9) |
| Median age (years) | 59 [24–86] | 59 [23–85] |
| Race (White/others, %) | 78 (85.7)/13 (14.3) | 88 (96.7)/3 (3.3) |
| Median BMI | 28.3 (17.3–49.7) | 27.4 (18.2–48.8) |
| Cirrhosis (yes/no, %) | 27 (29.7)/64 (70.3) | 0 (0)/87 (100) |
| Median IL-6 level | 17.2 (3.1–140.4) | 3.1 (3.1–37.6) |
Table 2 further breaks down hepatobiliary cancers into HCC and gallbladder/biliary cancer groups. From the group of 91 hepatobiliary patients, 80 patients had HCC, while 11 had gallbladder and biliary cancer. Median age at diagnosis was 59 years in both groups. Cases were also assessed for pain concerns and performance status to further aid in the quality of life measures.
Table 2. Hepatobiliary cancer patients’ characteristics, classification, and markers.
| Characteristics | Liver (n=80) | Gallbladder/biliary (n=11) |
|---|---|---|
| Gender (male/female, %) | 59 (73.8)/21 (26.3) | 3 (27.3)/8 (72.7) |
| Median age (years) | 59 [24–86] | 59 [42–71] |
| Race (White/others, %) | 68 (85.0)/12 (15.0) | 10 (90.9)/1 (9.1) |
| Median BMI | 28.3 (17.3–49.7) | 30 (20.7–37.8) |
| Cirrhosis (yes/no, %) | 27 (33.8)/53 (66.2) | 0 (0)/11 (100) |
| Median pain rating | 2 (0–9) | 4 (0–7) |
| Uncontrolled pain (yes/no, %) | 50 (62.5)/30 (37.5) | 577 (63.6)/4 (36.4) |
| Treatment (%) | ||
| None | 19 (23.8) | 0 (0) |
| Monotherapy | 48 (60.0) | 5 (45.5) |
| Combination therapy | 13 (16.3) | 6 (54.5) |
| Median IL-6 (pg/mL) | 17.7 (3.1–140.4) | 13.5 (3.1–33.6) |
| ECOG (%) | ||
| 0 | 25 (31.3) | 3 (27.3) |
| 1 | 36 (45) | 7 (63.6) |
| 2 | 14 (17.5) | 1 (9.1) |
| 3 | 5 (6.3) | 0 (0) |
IL-6 levels are elevated in hepatobiliary cancer patients
We assessed the level of IL-6 levels in patients with hepatobiliary cancer in comparison to matched controls from our databank from individuals with no evidence of cancer. As reflected in Table 1, patients with hepatobiliary cancer were found to have elevated IL-6 levels with a median value of 17.2 in comparison to 3.1 in patients with no history of cancer (Figure 1, P<0.01).
Figure 1.
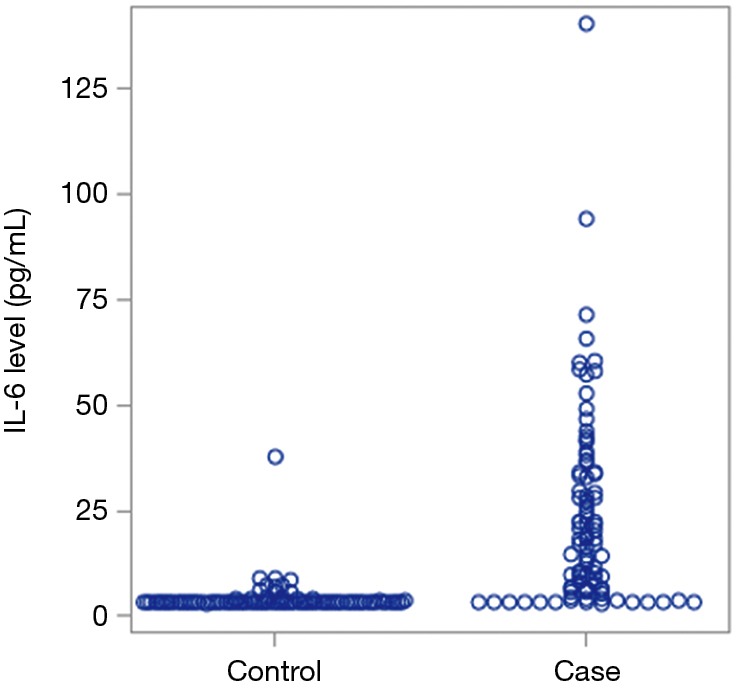
Comparing IL-6 levels between patients with no history of cancer vs. hepatobiliary cancer patients [3.1 (3.1–37.6) vs. 17.2 (3.1–140.4) respectively, P<0.01]. IL-6, interleukin-6.
IL-6 levels are affected by pain score and performance status
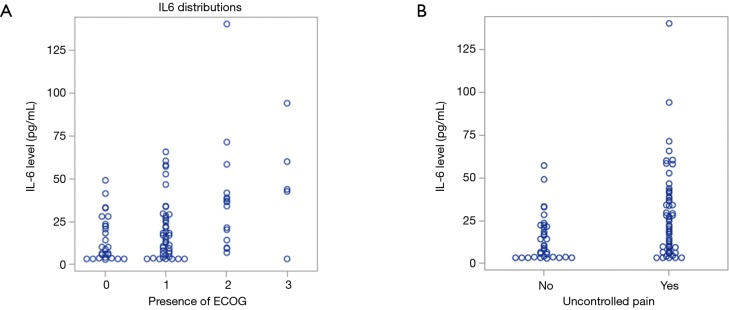
Quality of life is a broad term that includes various aspects, ranging from performance status, adequate pain control to carry out daily activities, human interaction with loved ones and much more. Worsening of Eastern Cooperative Oncology Group (ECOG) performance status was associated with higher levels of IL-6 (Figure 2A). In conjunction with that, patients with uncontrollable pain also had higher levels of IL-6 (Figure 2B). Uncontrolled pain was defined as any score more than 0, on a scale of 0 to 10. A combination of poor performance status and uncontrolled pain can be seen as a surrogate of poor quality of life in patients. This was from charted pain scores in nursing assessment documents.
Figure 2.
Increased IL-6 level and poorer quality of life. (A) Increasing levels of IL-6 observed in worse performance status (higher ECOG patients) (P=0.0009); (B) higher level of IL-6 associated with uncontrolled pain (P<0.01). ECOG, Eastern Cooperative Oncology Group; IL-6, interleukin-6.
Higher IL-6 level is associated with worsened OS
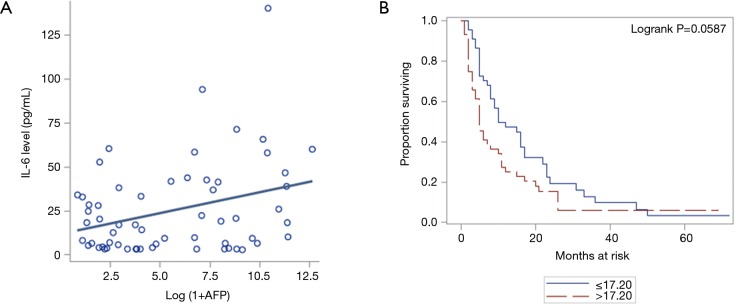
AFP is a well-established tumor marker in HCC. A higher level of AFP is associated with worsening OS (16). We observed that increasing AFP is associated with higher values of IL-6 (Figure 3A, P=0.0146). Besides, we found that IL-6 ≤17.20 is associated with better OS in comparison to the patients with IL-6 >17.20 (Figure 3B) although it was not statistically significant (P=0.0587).
Figure 3.
Increased IL-6 level is associated with worse overall survival. (A) Higher level of IL-6 associated with higher levels of AFP (P=0.0146); (B) higher level of IL-6 associated with poor overall survival (P=0.0587). IL-6, interleukin-6.
IL-6 levels vary based on demographics
Examining whether IL-6 levels vary by gender and race, we observed a higher level of IL-6 in male patients (P=0.5). Also, in comparison to other races, Caucasian patients had higher levels of IL-6 (P=0.85). However, both these outcomes were not statistically significant.
IL-6 levels in patients with portal venous thrombosis
We evaluated serum levels of IL-6 in the presence and absence of thrombosis given that is thought to be in part due to inflammation. An increase in IL-6 is associated with an increased risk of thrombosis, and this association was found to be statistically significant (Figure 4, P=0.03).
Figure 4.
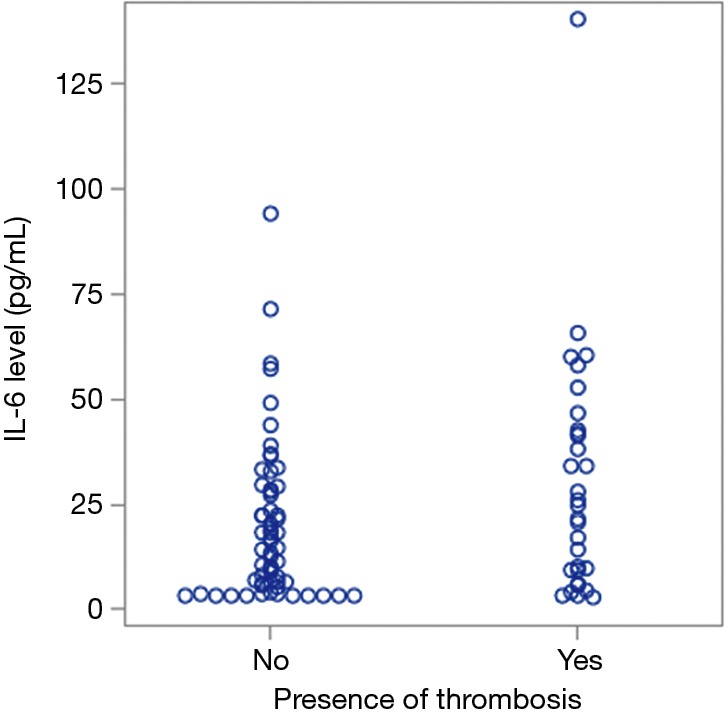
IL-6 levels are higher in patients with thrombosis (P=0.03). IL-6, interleukin-6.
Discussion
For decades, AFP has been the most well-established biomarker in HCC patients. Serum AFP levels trend down after birth, and they tend to remain low throughout adulthood (17). For early screening of HCC, AFP is used in conjunction with imaging studies like ultrasound or computed tomographic (CT) scanning. In addition to that, AFP has a valuable role in postoperative surveillance for HCC recurrence (18,19). AFP has also been established as a prognostic biomarker in HCC; with higher AFP levels associated with early recurrence after definitive treatment generally leading to reduced OS (20-23).
Zhang et al. and Schraiber Ldos et al. showed that AFP level might predict recurrence-free survival (RFS) following treatment but does not contribute towards OS (23,24). On the other hand, Silva et al. tried to establish the prognostic utility of baseline serum AFP values in HCC patients and showed that increased AFP level independently predicts poor OS (18). Since the available data on AFP as a prognostic biomarker is controversial, it is essential to establish additional biomarkers that can aid in prognosis. These additional markers may also help in monitoring treatment response and implement management plans appropriately. It has been an active area of research and studies have explored CpG island methylator phenotype (CIMP), osteopontin (OPN), matrix metalloproteinase 7 (MMP7), pregnancy-specific glycoprotein 9 (PSG9), methylation level of long interspersed nucleotide element-1 (LINE-1), T-cell immunoglobulin and mucin domain-containing molecule-3, IL-6 and C-reactive protein as prognostic biomarkers in HCC patients (8,25-28).
Chronic inflammation significantly contributes to cancer development and progression (29). This inflammation cascade further stimulates Th1 and Th2 responses (29). Th2 response is comprised of pro-inflammatory cytokines like IL-4, IL-6, and IL-10. In another study, IL-6 was found to be elevated in HCC patients undergoing loco-regional treatment and was associated with poor prognosis (8). Additionally, this concept of chronic inflammation contributing to progression of cancer has been explored in other cancers as well. IL-6, along with other inflammatory markers were found to be elevated in ovarian cancer, colon cancer, and many others (30-32). Recent studies have also confirmed that IL-6 is associated with tumor growth, metastasis and drug resistance (33-36). From a treatment perspective, it was observed that down-regulating the effects of IL-6 has further helped in potentiating the response of chemotherapy (30). Recently, the REACH 2 study confirmed that ramucirumab, an anti-anigiogenic therapy, is beneficial in HCC patients with high AFP (37).
In addition, we found that IL-6 marker was elevated in males in comparison to females. The reason why males tend to develop HCC more often than females, was studied elegantly in a DEN-induced mouse model of HCC and it was found that IL-6 mediated inflammation in Kupfer cells mediated via a co-adapter protein Myd 88, which is more frequently seen in males (14). Therefore we explored, IL-6 as a biomarker to study further and provide a rationale so it may be explored further as a therapeutic target.
In our study, we found that IL-6 level was elevated in patients with hepatobiliary cancers in comparison to healthy subjects. Jang et al. saw that IL-6 level correlates with tumor size, stage, and aggressiveness (8); however, their study included patients with HCC undergoing loco-regional treatment. In contrast, we examined patients with unresectable hepatobiliary cancers and showed that elevated IL-6 correlates with poor prognosis. This suggests that IL-6 may be associated with unique biologic mechanisms that lead to poor survival in such patients. In one study, IL-6 was found to decrease apoptosis in HCC cells and contributed to metastases (38-41).
This is the first study we are aware of to find that IL-6 is associated with elevated pain score and poor performance status. These two parameters are important criteria contributing to the quality of life questionnaire in the European Organization for Research and Treatment of Cancer (EORTC) (42). These data would imply, therefore, that higher levels of IL-6 in hepatobiliary cancer patients are associated with poor quality of life in the absence of formal QOL assessment. However, if validated along with QOL assessment it could potentially serve as a surrogate for QOL and indicates the possible role of IL-6 levels guiding early intervention focused on symptom management, such as timely involvement of palliative care services.
Amongst patients with hepatobiliary cancers, we did not find differences in IL-6 levels between males and females. As IL-6 expression is suppressed by estrogen (43) and our female patients were predominantly post-menopausal, this might be the reason why there was no gender disparity detected in our study. Our study was underpowered to detect any difference amongst IL-6 levels in different racial groups as our population was mainly Caucasian (91.2%). IL-6 levels directly correlated with AFP in our study, suggestive of a complementary role for this cytokine. While this has been proposed before in literature, our study was unique to highlight a potential independent role for IL-6 in prognostication of patients with hepatobiliary cancers after controlling for AFP levels (44-46).
Portal vein thrombosis (PVT) is an independent adverse prognostic factor in patients with HCC (47-49). Given that inflammatory driven pathways may be one of the mechanisms for the development of thrombosis (50), it has been shown that in patients with cirrhosis, empiric treatment with enoxaparin prevented PVT and patients receiving the enoxaparin demonstrated significantly lower levels of IL-6 throughout follow-up as compared to the controls (51). Our study showed a confirmatory association with presence of PVT at the time of diagnosis of cancer and elevated IL-6 levels. It is unclear why there was not a higher incidence of other venous thromboembolism. We found 34.1% of patients with PVT, which is very similar to the previously reported incidence of 20–65% in several studies (52-56). One explanation might be that the association signified increased aggressiveness of the cancer and direct invasion into the PV rather than generalized activation of the coagulation cascade, and auto-anticoagulation due to advanced cirrhosis. Along with this other mechanism of action have been described contributing to the increasing incidence of PVT, ranging from platelet dysfunction, endothelial dysfunction increased level of von-Willebrand factor contributing to hemodynamic instability (57).
Conclusions
In summary, our study demonstrated that IL-6 is an important prognostic biomarker in hepatobiliary cancers. Elevated IL-6 is not only associated with an inferior survival in such patients but possibly also associated with a poorer quality of life. Given the moderate sample size in our study, larger studies and prospective validation of our findings may aid in patient selection and better prognostication. Our results also suggest that clinicians should assess QOL and consider an early palliative care referral for patients with an elevated serum IL-6. Finally, with available therapies targeting transforming growth factor (TGF) beta and recent success of ramucirumab in HCC patients with high levels of AFP, therapeutic interventions directed against IL-6 are becoming increasingly important. Therefore, studies investigating the role of IL-6 as a potential therapeutic target may have value in hepatobiliary cancers.
Acknowledgements
None.
Ethical Statement: The study was approved by Gastrointestinal Disease Site Research Group (GI DSRG).
Footnotes
Conflicts of Interest: Presented at GI ASCO in January 2017 (http://ascopubs.org/doi/10.1200/jco.2015.33.3_suppl.257).
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 2.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883-9. 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson C, Han Y, Hughart N, et al. Interleukin-6 and its receptor, key players in hepatobiliary inflammation and cancer. Transl Gastrointest Cancer 2012;1:58-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu CW, Wang SR, Chao MF, et al. Serum interleukin-6 levels reflect disease status of gastric cancer. Am J Gastroenterol 1996;91:1417-22. [PubMed] [Google Scholar]
- 5.Drachenberg DE, Elgamal AA, Rowbotham R, et al. Circulating levels of interleukin-6 in patients with hormone refractory prostate cancer. Prostate 1999;41:127-33. [DOI] [PubMed] [Google Scholar]
- 6.Yanagawa H, Sone S, Takahashi Y, et al. Serum levels of interleukin 6 in patients with lung cancer. Br J Cancer 1995;71:1095-8. 10.1038/bjc.1995.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goydos JS, Brumfield AM, Frezza E, et al. Marked elevation of serum interleukin-6 in patients with cholangiocarcinoma: validation of utility as a clinical marker. Ann Surg 1998;227:398-404. 10.1097/00000658-199803000-00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jang JW, Oh BS, Kwon JH, et al. Serum interleukin-6 and C-reactive protein as a prognostic indicator in hepatocellular carcinoma. Cytokine 2012;60:686-93. 10.1016/j.cyto.2012.07.017 [DOI] [PubMed] [Google Scholar]
- 9.Paulsen Ø, Laird B, Aass N, et al. The relationship between pro-inflammatory cytokines and pain, appetite and fatigue in patients with advanced cancer. PLoS One 2017;12:e0177620. 10.1371/journal.pone.0177620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris TB, Ferrucci L, Tracy RP, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med 1999;106:506-12. 10.1016/S0002-9343(99)00066-2 [DOI] [PubMed] [Google Scholar]
- 11.Mohamed-Ali V, Goodrick S, Rawesh A, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab 1997;82:4196-200. [DOI] [PubMed] [Google Scholar]
- 12.Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, et al. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci 2003;100:9090-5. 10.1073/pnas.1531903100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kern PA, Ranganathan S, Li C, et al. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab 2001;280:E745-51. 10.1152/ajpendo.2001.280.5.E745 [DOI] [PubMed] [Google Scholar]
- 14.Naugler WE, Sakurai T, Kim S, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 2007;317:121-4. 10.1126/science.1140485 [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa H, Maeda S, Yoshida H, et al. Serum IL-6 levels and the risk for hepatocarcinogenesis in chronic hepatitis C patients: an analysis based on gender differences. Int J Cancer 2009;125:2264-9. 10.1002/ijc.24720 [DOI] [PubMed] [Google Scholar]
- 16.Bai DS, Zhang C, Chen P, et al. The prognostic correlation of AFP level at diagnosis with pathological grade, progression, and survival of patients with hepatocellular carcinoma. Sci Rep 2017;7:12870. 10.1038/s41598-017-12834-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spangenberg HC, Thimme R, Blum HE. Serum markers of hepatocellular carcinoma. Semin Liver Dis 2006;26:385-90. 10.1055/s-2006-951606 [DOI] [PubMed] [Google Scholar]
- 18.Silva JP, Gorman RA, Berger NG, et al. The prognostic utility of baseline alpha-fetoprotein for hepatocellular carcinoma patients. J Surg Oncol 2017;116:831-40. 10.1002/jso.24742 [DOI] [PubMed] [Google Scholar]
- 19.Zhao YJ, Ju Q, Li GC. Tumor markers for hepatocellular carcinoma. Mol Clin Oncol 2013;1:593-8. 10.3892/mco.2013.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Debruyne EN, Delanghe JR. Diagnosing and monitoring hepatocellular carcinoma with alpha-fetoprotein: New aspects and applications. Clin Chim Acta 2008;395:19-26. 10.1016/j.cca.2008.05.010 [DOI] [PubMed] [Google Scholar]
- 21.Blank S, Wang Q, Fiel MI, et al. Assessing prognostic significance of preoperative alpha-fetoprotein in Hepatitis B-associated hepatocellular carcinoma: Normal is not the new normal. Ann Surg Oncol 2014;21:986-94. 10.1245/s10434-013-3357-z [DOI] [PubMed] [Google Scholar]
- 22.Pinato DJ, Karamanakos G, Arizumi T, et al. Dynamic changes of the inflammation-based index predict mortality following chemoembolisation for hepatocellular carcinoma: A prospective study. Aliment Pharmacol Ther 2014;40:1270-81. 10.1111/apt.12992 [DOI] [PubMed] [Google Scholar]
- 23.Schraiber Ldos S, de Mattos AA, Zanotelli ML, et al. Alpha-fetoprotein level predicts recurrence after transplantation in hepatocellular carcinoma. Medicine (Baltimore) 2016;95:e2478. 10.1097/MD.0000000000002478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, Ge NL, Chen Y, et al. Long-term outcomes and prognostic analysis of radiofrequency ablation for small hepatocellular carcinoma: 10-year follow-up in Chinese patients. Med Oncol 2015;32:77. 10.1007/s12032-015-0532-z [DOI] [PubMed] [Google Scholar]
- 25.Rong W, Zhang Y, Yang L, et al. Post-surgical resection prognostic value of combined OPN, MMP7, and PSG9 plasma biomarkers in hepatocellular carcinoma. Front Med 2019;13:250-8. 10.1007/s11684-018-0632-1 [DOI] [PubMed] [Google Scholar]
- 26.Wang Q, Wang G, Liu C, et al. Prognostic value of CpG island methylator phenotype among hepatocellular carcinoma patients: A systematic review and meta-analysis. Int J Surg 2018;54:92-9. 10.1016/j.ijsu.2018.04.033 [DOI] [PubMed] [Google Scholar]
- 27.Miyata T, Yamashita YI, Baba Y, et al. Prognostic value of LINE-1 methylation level in 321 patients with primary liver cancer including hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Oncotarget 2018;9:20795-806. 10.18632/oncotarget.25124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li F, Li N, Sang J, et al. Highly elevated soluble Tim-3 levels correlate with increased hepatocellular carcinoma risk and poor survival of hepatocellular carcinoma patients in chronic hepatitis B virus infection. Cancer Manag Res 2018;10:941-51. 10.2147/CMAR.S162478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan TT, Coussens LM. Humoral immunity, inflammation and cancer. Curr Opin Immunol 2007;19:209-16. 10.1016/j.coi.2007.01.001 [DOI] [PubMed] [Google Scholar]
- 30.Li S, Tian J, Zhang H, et al. Down-regulating IL-6/GP130 targets improved the anti-tumor effects of 5-fluorouracil in colon cancer. Apoptosis 2018;23:356-74. 10.1007/s10495-018-1460-0 [DOI] [PubMed] [Google Scholar]
- 31.Luo JH, Zhang CY, Lu CY, et al. Serum expression level of cytokine and chemokine correlates with progression of human ovarian cancer. Eur J Gynaecol Oncol 2017;38:33-9. [PubMed] [Google Scholar]
- 32.Andersen BL, Goyal NG, Weiss DM, et al. Cells, cytokines, chemokines, and cancer stress: A biobehavioral study of patients with chronic lymphocytic leukemia. Cancer 2018;124:3240-8. 10.1002/cncr.31538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klein C, Wüstefeld T, Assmus U, et al. The IL-6-gp130-STAT3 pathway in hepatocytes triggers liver protection in T cell-mediated liver injury. J Clin Invest 2005;115:860-9. 10.1172/JCI23640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pop VV, Seicean A, Lupan I, et al. IL-6 roles - Molecular pathway and clinical implication in pancreatic cancer - A systemic review. Immunology Letters 2017;181:45-50. 10.1016/j.imlet.2016.11.010 [DOI] [PubMed] [Google Scholar]
- 35.Bharti R, Dey G, Mandal M. Cancer development, chemoresistance, epithelial to mesenchymal transition and stem cells: A snapshot of IL-6 mediated involvement. Cancer Letters 2016;375:51-61. 10.1016/j.canlet.2016.02.048 [DOI] [PubMed] [Google Scholar]
- 36.Cohen S, Bruchim I, Graiver D, et al. Platinum-resistance in ovarian cancer cells is mediated by IL-6 secretion via the increased expression of its target cIAP-2. J Mol Med (Berl) 2013;91:357-68. 10.1007/s00109-012-0946-4 [DOI] [PubMed] [Google Scholar]
- 37.Zhu AX, Galle PR, Kudo M, et al. A study of ramucirumab (LY3009806) versus placebo in patients with hepatocellular carcinoma and elevated baseline alpha-fetoprotein (REACH-2). J Clin Oncol 2018;36:TPS538 10.1200/JCO.2018.36.4_suppl.TPS538 [DOI] [Google Scholar]
- 38.Reichner JS, Mulligan JA, Spisni R, et al. Effect of IL-6 overexpression on the metastatic potential of rat hepatocellular carcinoma cells. Ann Surg Oncol 1998;5:279-86. 10.1007/BF02303786 [DOI] [PubMed] [Google Scholar]
- 39.Kovalovich K, Li W, DeAngelis R, et al. Interleukin-6 Protects against Fas-mediated Death by Establishing a Critical Level of Anti-apoptotic Hepatic Proteins FLIP, Bcl-2, and Bcl-xL. J Biol Chem 2001;276:26605-13. 10.1074/jbc.M100740200 [DOI] [PubMed] [Google Scholar]
- 40.Wang CQ, Sun HT, Gao XM, et al. Interleukin-6 enhances cancer stemness and promotes metastasis of hepatocellular carcinoma via up-regulating osteopontin expression. Am J Cancer Res 2016;6:1873-89. [PMC free article] [PubMed] [Google Scholar]
- 41.El-Folly RF, El-Kabarity RH, Arafa NA. Assessment of the role of interleukin-6 in diagnosis of hepatocellular carcinoma. Egypt J Immunol 2010;17:11-22. [PubMed] [Google Scholar]
- 42.Scott NW, Fayers PM, Aaronson NK, et al. EORTC QLQ-C30 Reference Values. Available online: https://www.eortc.org/app/uploads/sites/2/2018/02/reference_values_manual2008.pdf
- 43.Jiang XP, Yang DC, Elliott RL, et al. Reduction in serum IL-6 after vacination of breast cancer patients with tumour-associated antigens is related to estrogen receptor status. Cytokine 2000;12:458-65. 10.1006/cyto.1999.0591 [DOI] [PubMed] [Google Scholar]
- 44.Cheng KS, Tang HL, Chou FT, et al. Cytokine evaluation in liver cirrhosis and hepatocellular carcinoma. Hepatogastroenterology 2009;56:1105-10. [PubMed] [Google Scholar]
- 45.Hsia CY, Huo TI, Chiang SY, et al. Evaluation of interleukin-6, interleukin-10 and human hepatocyte growth factor as tumor markers for hepatocellular carcinoma. Eur J Surg Oncol 2007;33:208-12. 10.1016/j.ejso.2006.10.036 [DOI] [PubMed] [Google Scholar]
- 46.Wong VWS, Yu J, Cheng ASL, et al. High serum interleukin-6 level predicts future hepatocellular carcinoma development in patients with chronic hepatitis B. Int J Cancer 2009;124:2766-70. 10.1002/ijc.24281 [DOI] [PubMed] [Google Scholar]
- 47.Schöniger-Hekele M, Müller C, Kutilek M, et al. Hepatocellular carcinoma in Central Europe: prognostic features and survival. Gut 2001;48:103-9. 10.1136/gut.48.1.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woo HY, Heo J. New perspectives on the management of hepatocellular carcinoma with portal vein thrombosis. Clin Mol Hepatol 2015;21:115-21. 10.3350/cmh.2015.21.2.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marrero JA, Fontana RJ, Barrat A, et al. Prognosis of hepatocellular carcinoma: Comparison of 7 staging systems in an American cohort. Hepatology 2005;41:707-16. 10.1002/hep.20636 [DOI] [PubMed] [Google Scholar]
- 50.Fox EA, Kahn SR. The relationship between inflammation and venous thrombosis. A systematic review of clinical studies. Thromb Haemost 2005;94:362-5. [DOI] [PubMed] [Google Scholar]
- 51.Villa E, Cammà C, Marietta M, et al. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology 2012;143:1253-60. 10.1053/j.gastro.2012.07.018 [DOI] [PubMed] [Google Scholar]
- 52.Connolly GC, Chen R, Hyrien O, et al. Incidence, risk factors and consequences of portal vein and systemic thromboses in hepatocellular carcinoma. Thromb Res 2008;122:299-306. 10.1016/j.thromres.2007.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rabe C, Pilz T, Klostermann C, et al. Clinical characteristics and outcome of a cohort of 101 patients with hepatocellular carcinoma. World J Gastroenterol 2001;7:208-15. 10.3748/wjg.v7.i2.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schoniger-Hekele M, Muller C, Kutilek M, et al. Hepatocellular carcinoma in Austria: Aetiological and clinical characteristics at presentation. Eur J Gastroenterol Hepatol 2000;12:941-8. 10.1097/00042737-200012080-00015 [DOI] [PubMed] [Google Scholar]
- 55.Pirisi M, Avellini C, Fabris C, et al. Portal vein thrombosis in hepatocellular carcinoma: Age and sex distribution in an autopsy study. J Cancer Res Clin Oncol 1998;124:397-400. 10.1007/s004320050189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ikai I, Itai Y, Okita K, et al. Report of the 15th follow-up survey of primary liver cancer. Hepatol Res 2004;28:21-9. 10.1016/j.hepres.2003.08.002 [DOI] [PubMed] [Google Scholar]
- 57.Castelino DJ, Salem HH. Natural anticoagulants and the liver. J Gastroenterol Hepatol 1997;12:77-83. 10.1111/j.1440-1746.1997.tb00351.x [DOI] [PubMed] [Google Scholar]