Abstract
Objective The aim of this anatomic study is to describe a fully endoscopic lateral orbitotomy extradural approach to the cavernous sinus, posterior, and infratemporal fossae.
Material and Methods Three prefixed latex-injected head specimens (six orbital exposures) were used in the study. Before and after dissection, a computed tomography scan was performed on each cadaver head and a neuronavigation system was used to guide the approach. The extent of bone removal and the area of exposure of the targeted corridor were evaluated with the aid of OsiriX software (Pixmeo, Bernex, Switzerland).
Results The lateral orbital approach offers four main endoscopic extradural routes: the anteromedial, posteromedial, posterior, and inferior. The anteromedial route allows a direct route to the optic canal by removal of the anterior clinoid process, whereas the posteromedial route allows for exposure of the lateral wall of the cavernous sinus. The posterior route is targeted to Meckel's cave and provides access to the posterior cranial fossa by exposure and drilling of the petrous apex, whereas the inferior route gives access to the pterygopalatine and infratemporal fossae by drilling the floor of the middle cranial fossa and the bone between the second and third branches of the trigeminal nerve.
Conclusion The lateral orbitotomy endoscopic approach provides direct access to the cavernous sinus, posterior, and infratemporal fossae. Advantages of the approach include a favorable angle of attack, minimal brain retraction, and the possibility of dissection within the two dural layers of the cavernous sinus without entering its neurovascular compartment.
Keywords: lateral orbital approach, endoscopic surgery, skull base surgery, anatomy
Introduction
Classical anterolateral skull base approaches to lesions located in the middle and posterior cranial base often require quite extensive osteotomies in which the surgeon aims to gain a favorable angle of attack and to decrease brain retraction when targeting deep-seated lesions. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 During the recent years, with the introduction of minimally invasive techniques and with the use of the endoscope as the sole visualization tool, less invasive surgical corridors to the deep skull base compartments were created without sacrificing the said favorable angle of attack. 16 17 18 19 20 In this context, the orbit, a four-sided pyramidal ventral structure situated between the viscerocranium and neurocranium, takes a central role. Indeed, the orbit is a key anatomical area that reveals multiple possibilities for corridors to reach deeper and surgically challenging areas, such as the parasellar area, the cavernous sinus, Meckel's cave, and even the posterior fossa. 17 19 20 21 22 The increased interest in the orbit—an interest manifested in recent anatomical and clinical studies 14 17 23 24 25 —is provoked by the fact that, upon removal of the bony lateral and superior walls of the orbit, a wide ventrolateral route is disclosed, allowing access to these areas of the skull base.
Surgical approaches to the orbit date back to the late 19th century, when Krönlein first described the lateral orbitotomy. 26 Nowadays, this approach has become increasingly popular as a microsurgical corridor to the parasellar area and the lateral wall of the cavernous sinus. 17 23 24 25 Compared with the traditional frontotemporal or orbitozygomatic craniotomy, lateral orbitotomy has the potential advantage of a reduced approach-related morbidity, caused by danger to the frontal branch of the facial nerve, extensive disruption of the temporalis muscle, increased bone loss, exposure of a large portion of the brain, and brain retraction. Also, the lateral orbitotomy approach allows for a favorable and the most anterolateral surgical corridor possible, parallel to the skull base. 16 23 Some of the disadvantages of using the traditional microscopic technique in a lateral orbitotomy for deep-seated skull base lesions are the ones related to the conic shape of the operative field in the depth: a limited visualization area, insufficient illumination, and blind peripheral spots. 17 23 24 25 Nowadays, with the design of new dedicated instrumentation, remarkable developments have occurred in the field of endoscopy, all pioneered in transsphenoidal approaches and, recently, in transorbital surgery, that allow for improved working angles, shorter working distances, and optimal visualization of deep anatomical structures by bringing the target closer to the operative field. 4 16 19 20 27 28 29 30 31 32 In such settings, the endoscopic techniques could expand the boundaries encountered with the traditional microscopic view and widen the possibilities of the traditional lateral orbitotomy approach. Therefore, the aim of the study set forth in this article is to analyze and describe fully endoscopic lateral orbitotomy extradural anatomical routes to the cavernous sinus, posterior, and infratemporal fossae.
Material and Methods
The study was conducted on three prefixed latex-injected head specimens (six orbital exposures) in the Laboratory of Surgical Neuroanatomy (Barcelona, Spain). Dissections were performed with a high-definition endoscopic camera (KARL STORZ, Tuttlingen, Germany), 18 cm in length and 4 mm in diameter, and with 0° and 30° Hopkins rod-lens endoscopes (KARL STORZ). A set of dedicated endoscopic endonasal skull base surgical instruments (KARL STORZ) and an electric drill system with cutting and diamond burs (Midas Rex Legend EHS, Medtronic, Fort Worth, Texas, United States) were used. Each specimen was fixed with an industrially manufactured embalming mixture composed of different percentages of phenol, ethanol, formaldehyde, and glycerin. The arterial system was injected with red latex. Five screws were implanted in the specimen's skull as permanent bone reference markers to allow coregistration with the neuronavigation system.
Before the dissections, computed tomography (CT) scans were performed on each cadaver head by using a multislice helical acquisition protocol (slice thickness, 0.6 mm; gantry angle, 0°). The heads were precisely positioned in the scanner (SOMATOM Sensation 64; Siemens AG, Erlangen, Germany) to obtain a projection perpendicular to the palate. The images were exported in Digital Imaging and Communications in Medicine format and later imported in the OsiriX software (Pixmeo, Bernex, Switzerland) to visually compare the extent of bone removal (pre- and postdissection) with the use of the program's three-dimensional (3D) volumetric rendering functions.
Imaging data were transferred to the laboratory navigation-planning workstation, and point registration was performed. A registration correlation tolerance of 2 mm was considered acceptable. Neuronavigation (Medtronic StealthStation) was used to check superficial landmarks and track boundaries of the surgical approach. The extent of bone removal and the area of exposure to the targeted corridor were visualized by means of the superimposition of pre- and postdissection CT scans and navigation coordinates data with the aid of OsiriX software (Pixmeo).
Results
Ergonomics
The head was positioned as in a standard pterional approach, rotated 30° to the contralateral side and 10° extended, thus taking advantage of gravity to retract the brain, aiding the exposure. To simulate the position in a surgical scenario, surgeons were standing behind the head, allowing for improved freedom of movement, needed for a three- or four-hand technique. The endoscope was dynamically moved freehand and was positioned in the cranial part of the surgical field while two or even three instruments were positioned caudally to the endoscope, thus enhancing the working space and visualization while decreasing tissue trauma from retraction. The angle of visualization obtained in such manner is quite familiar to neurosurgeons because it is similar to the traditional pterional view, which facilitates orientation and the mental representation of the 3D anatomy.
Stepwise Dissection
The procedure began with a transeyelid skin incision following the curvature of a superior eyelid crease, with the direction of the incision running from the midpupillary line to 1 cm behind the lateral orbital ridge, along the zygomatic arch, which is easily recognized 23 24 33 ( Fig. 1 ).
Fig. 1.
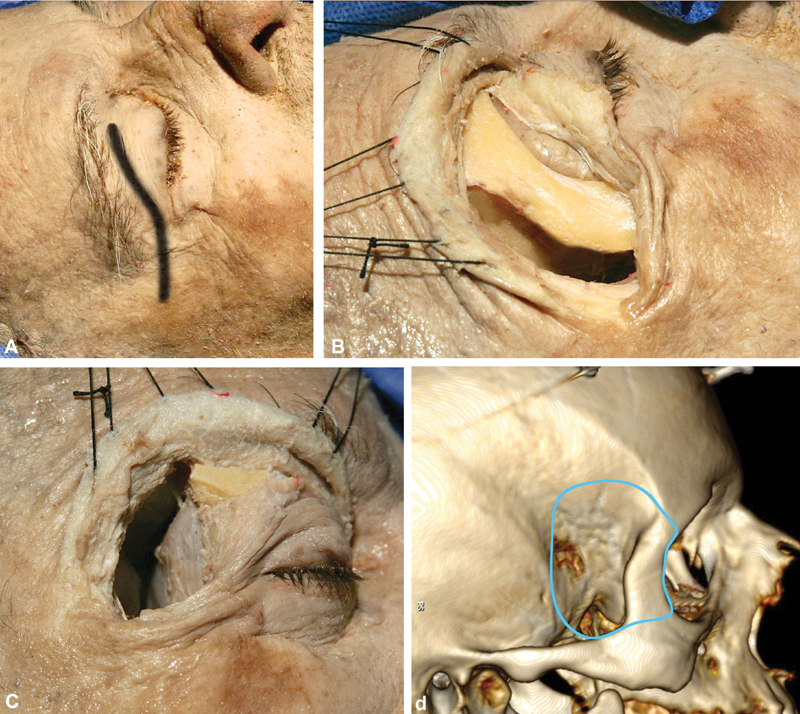
Macroscopic part of the approach. ( A ) The skin incision is through the eyelid, starting from the midpupillary line and extending 1 cm behind the lateral orbital ridge, along the zygomatic arch. ( B ) Exposure of the lateral orbital rim in strict subperiosteal fashion. The lateral orbital rim is removed by drilling of the sphenoid ridge and exposure of the frontal, temporal dura and periorbita, followed by two osteotomies, using an oscillating saw, just above the frontozygomatic suture superiorly and at the level of the zygomatic arch. ( C ) Macroscopic view of the surgical field after the osteotomies and after the bone removal extending to the pterion is completed. The frontal, temporal lobes, as well as the periorbital, are visualized. ( D ) Three-dimensional reconstruction using the OsiriX software (version 5.8.1, Pixmeo, Bernex, Switzerland), presenting the limits of the craniotomy (the visualized screws are the markers for the neuronavigation).
One of the possible complications during the soft-tissue dissection is injury to the frontal branch of the facial nerve. 34 35 36 37 38 39 40 However, according to the literature, there is a safe zone for the facial nerve in the area that is 2.5 cm posterior to the lateral canthus of the eye. 36 37 41 Therefore, if the dissection does not pass this limit, the danger posed by possible damage to the frontal branch of the facial nerve is kept to a minimum.
The anatomy of the transpalpebral eyelid approach has been explained in detail elsewhere. 23 In brief, the superior eyelid has two lamellae that are divided by the orbital septum, which starts from the superior orbital rim and is continuous with the frontalis muscle aponeurosis superiorly and the periorbita. The anterior lamella consists of the skin and orbicularis oculi muscle, and the posterior lamella extends from the levator aponeurosis, the Muller's muscle, and the palpebral conjunctiva. 23 When the skin incision is made, care should be taken not to perforate the septum. A plane between the orbicularis oculi and the orbital septum is developed superiorly and laterally until the orbital rim is identified. 23 The periosteum is divided sharply, and subperiosteal dissection, starting from the frontal and zygomatic bone, is performed until the zygomaticotemporal foramen and supraorbital foramen/notch are exposed inferiorly and superiorly, respectively. The temporalis muscle fascia is incised along the posterior edge of the orbital rim, and the anterior part of the temporalis muscle is detached from the bone, exposing the lateral wall of the orbit, the greater sphenoid wing, and the pterional region. The periorbita is then dissected from the bone with a sharp dissector, starting from the frontozygomatic suture and continuing medially to the supraorbital notch and inferiorly toward the inferior orbital fissure. A dissector can be safely passed through the inferior orbital fissure, as the latter contains only fibrous and adipose tissue. 5 42 Keeping the periorbita intact is a key maneuver of this step of the dissection.
The lateral orbitotomy is performed from the frontozygomatic suture to the zygomatic angle and the inferior orbital fissure with the aid of a high-speed drill ( Fig. 1 ). At this point, the sphenoid ridge represents the key landmark on the lateral skull surface, located below the usual placement of the MacCarty keyhole 5 14 42 43 with comparative exposure. 44 It can be used as a guide to the bone drilling, exposing the frontal and temporal dura and the periorbita upon its removal. The sphenoid ridge is drilled until the meningo-orbital band is completely exposed. 45 46 The inferior limit of the bone drilling is the inferior orbital fissure. Laterally, the sphenoid ridge approximates the pterion at the sphenosquamosal suture, which, in the depth, corresponds to the anterior sylvian point of the brain—a natural widening of the arachnoid. 47 After the craniotomy is performed, the pterion marks the posterior limit of bone removal.
The removal of the lateral orbital wall and sphenoid ridge allows exposure of the periorbita and the temporal and frontal dura. At this point, the dissection continues under endoscopic guidance (0° endoscope) ( Fig. 2 ). The dura of the frontal and temporal lobes is dissected from the inner bone until the superior orbital fissure is reached. Along the lateral border of the superior orbital fissure, the meningo-orbital band 46 48 is identified. It connects the frontotemporal basal dura to the periorbita and is also described in the literature as the frontotemporal dural fold 49 or dural bridge 50 ( Fig. 3 ). This is the key dural structure, from which the two dural layers (the periosteal dura and dura propria of the most cranial aspect of the lateral wall of the cavernous sinus) can be easily and safely separated. At this point, the meningo-orbital band is sharply dissected and divided, allowing subsequent extradural exposure of the cavernous sinus. 17 46 Surgically speaking, this space is very important, because it offers access to the inner layer of the lateral wall of the cavernous sinus, without the need to enter the venous compartment of the sinus itself. Even more importantly, it provides a “natural” access without manipulation of the cavernous sinus' cranial nerves. 17 25 49 51 52 53 54 55 56 This technique creates a virtually bloodless surgical plane, as reported in the clinical setting by Fukuda et al. 46
Fig. 2.
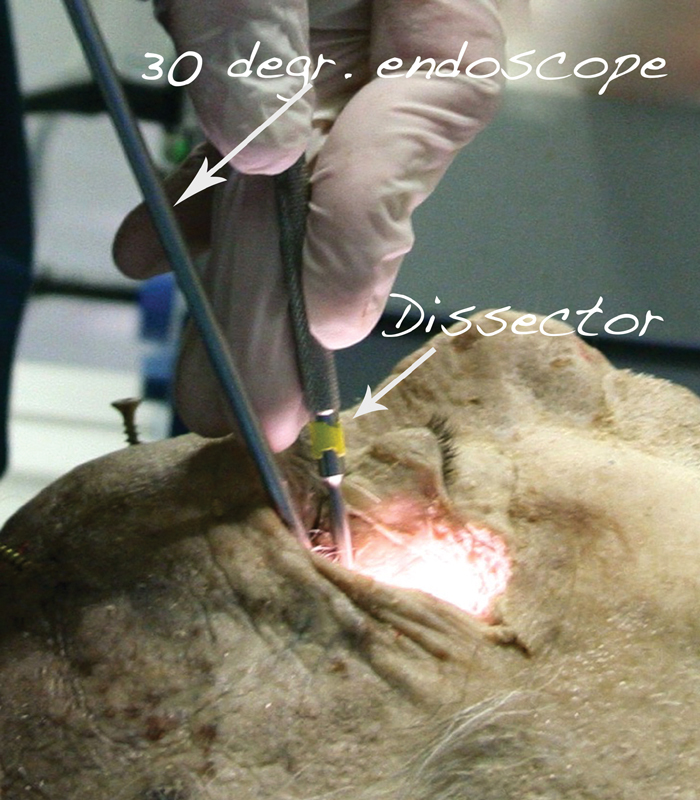
Position of head, endoscope, and instruments in the laboratory settings.
Fig. 3.
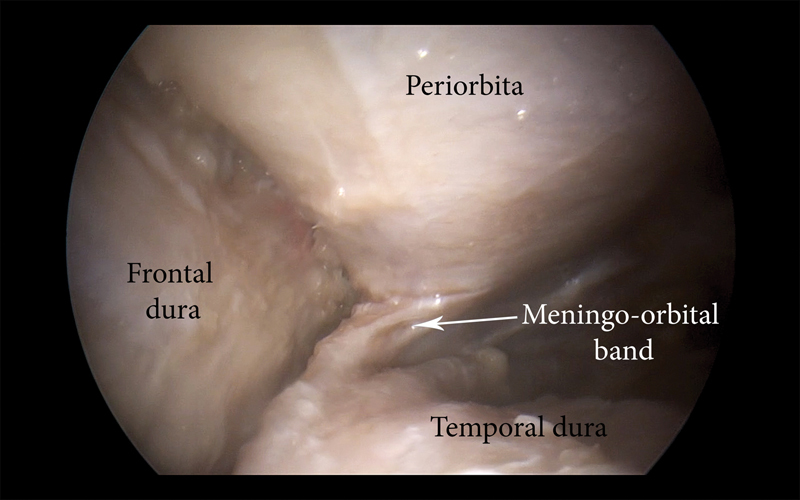
Right lateral orbital approach. Endoscopic view of the right meningo-orbital band.
Endoscopic Routes
From this point on, depending on the working angle, four endoscopic routes can be described—anteromedial, posteromedial, posterior ( Fig. 4A ), and inferior ( Fig. 4B )—each targeted to a different skull base region: the optic nerve, the lateral wall of the cavernous sinus and Meckel's cave posteriorly, the petrous apex, and the pterygopalatine and infratemporal fossae. Dissection is then continued with the use of a 30° endoscope, which allows a superb and angled visualization during the targeted dissection in the deep aspect of the surgical field.
Fig. 4.
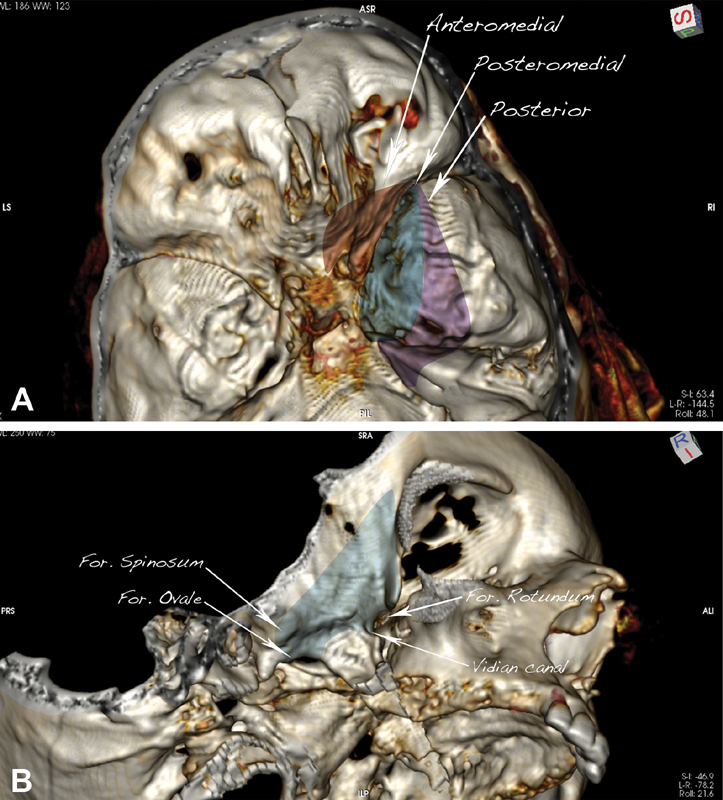
The surgical routes described in the study. ( A ) Anteromedial corridor (dark red), targeted to the optic canal; posteromedial (dark blue), targeted to the lateral cavernous sinus; posterior (purple), targeted to Meckel's cave and the petrous apex. ( B ) Inferior corridor (light blue), targeted to the pterygopalatine and infratemporal fossae.
 Anteromedial route
, targeted to the anterior clinoid process (ACP), optic foramen, optic nerve, and clinoid segment of the internal carotid artery (ICA) (
Fig. 5A
–
C
)
Anteromedial route
, targeted to the anterior clinoid process (ACP), optic foramen, optic nerve, and clinoid segment of the internal carotid artery (ICA) (
Fig. 5A
–
C
)
Fig. 5.
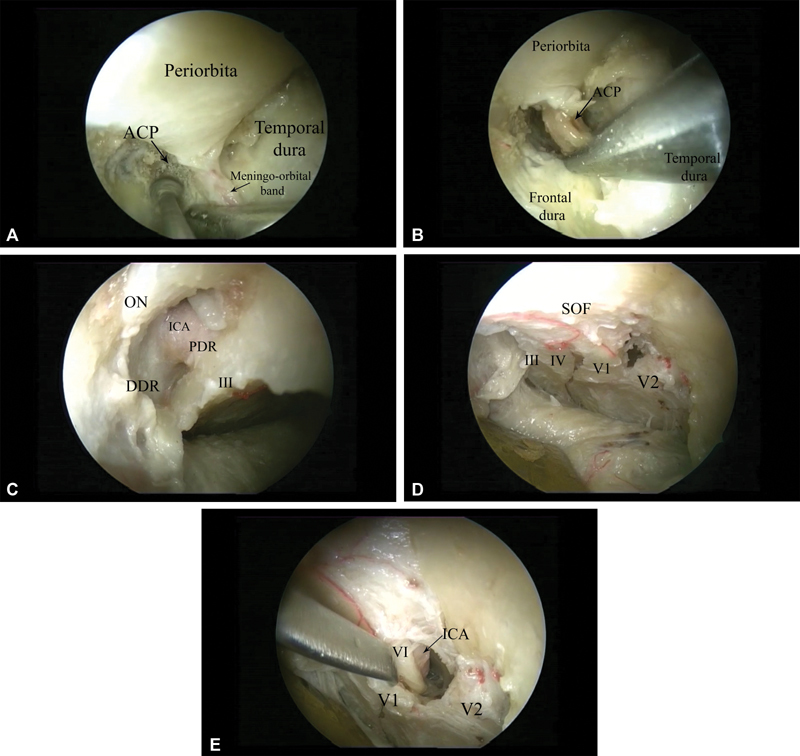
Anatomical structures exposed through the different corridors (right side) ( A ) Drilling of the anterior clinoid process (ACP) using the anteromedial route; ( B ) removal of the anterior clinoid process with pituitary rongeur. ( C ) Endoscopic view via anteromedial route after removal of the anterior clinoid process and decompression of the optic nerve. The third nerve and the clinoid segment of the ICA, together with the proximal and distal dural rings as well as the optic nerve, are visualized. ( D ) Posteromedial route, exposing the lateral wall of the cavernous sinus. The III, IV, V1, and V2 nerves are seen. ( E ) The V1 nerve is elevated, and the abducens nerve, which is located medial to it, overlying the cavernous carotid artery, is exposed. Abbreviations: ACP, anterior clinoid process; DDR, distal dural ring; ICA, internal carotid artery; III, cranial nerve III; PDR, proximal dural ring; SOF, superior orbital fissure.
After dividing the meningo-orbital band, the surgeon peels off the dura propria until the cortical bone of the lesser sphenoid wing and the ACP are exposed. Anterior clinoidectomy is then performed with a diamond drill, starting with the unroofing of the optic canal, thus allowing optic nerve decompression superiorly. Next, the ACP is hollowed out with a small diamond burr under continuous irrigation ( Fig. 5A ). After sufficient spongious bone is removed from the ACP, leaving only cortical bone, the ACP is dissected circumferentially and is detached from the optic strut ( Fig. 5B ). This maneuver allows for the removal of the ACP. At that point, the optic nerve and the clinoid segment of the ICA, as well as the proximal and distal dural rings, are exposed, and the optic canal is opened widely ( Fig. 5C ).
 Posteromedial route
, targeted to the lateral wall of the cavernous sinus (
Fig. 4A
)
Posteromedial route
, targeted to the lateral wall of the cavernous sinus (
Fig. 4A
)
From the lateral part of the superior orbital fissure in a posteromedial direction, an extradural, straightforward route to the lateral wall of the cavernous sinus is completely revealed. The key landmark for surgical dissection is the meningo-orbital band ( Figs. 3 and 5A ). Upon its division and with the dissection kept posteriorly, a clear surgical plane between the dura of the temporal lobe and dura propria of the lateral wall of the cavernous sinus is created. 49 51 52 55 57 Keeping the sharp dissection in a posteromedial direction, this route enables both extradural exposure of the entire lateral wall of the cavernous sinus and visualization of the third and fourth cranial nerves as well as the first and second division of the fifth cranial nerve. The abducens nerve runs within the cavernous sinus. Therefore, if the dura propria of the cavernous sinus is opened and the V1 nerve is gently displaced, medial to it, within the cavernous sinus, the abducens nerve is exposed in strict relationship with the intracavernous segment of the ICA ( Fig. 6A–C ).
Fig. 6.
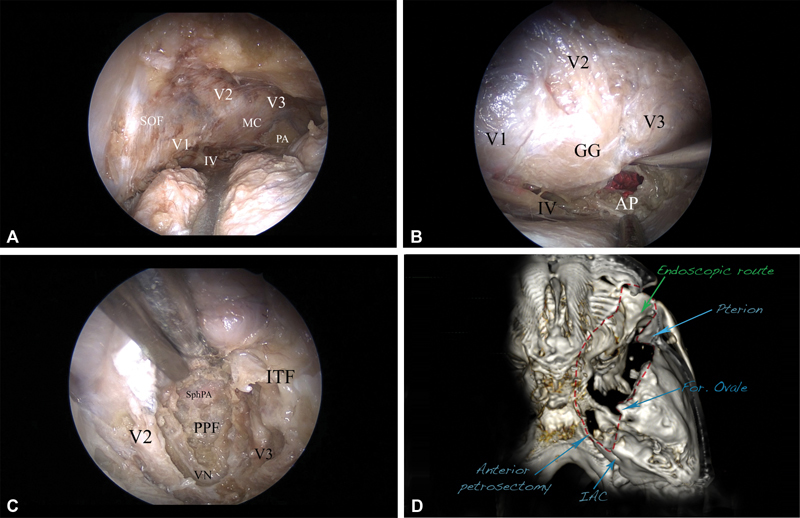
The posterolateral route and exposure to the posterior fossa, right side. ( A ) The whole cavernous sinus is seen, including the Meckel's cave (MC), V1, V2, V3, and IV cranial nerves, as well as the petrous apex (PA). ( B ) The mandibular division of the trigeminal nerve is elevated, and the anterior petrosectomy (AP) is done, exposing the corridor of the posterior fossa. The dura is incised, and the pons, as well as the trigeminal nerve, is seen. ( C ) The inferior route (light blue, Fig. 4B ), exposing the pterygopalatine fossa (PPF) and the infratemporal fossa (ITF). The bone between V2 and V3, as well as the floor of the middle cranial fossa, is removed, completely unroofing V2 and V3 and thus opening access to the PPF and the ITF. The vidian nerve (VN), as well as the vidian canal, are revealed. The sphenopalatine artery (SphPA) is seen in the PPF. ( D ) Postoperative computed tomography (CT) scan-based three-dimensional (3D) reconstruction presenting the area of bone removal (red dashed line), corresponding to the exposure to the posterior fossa (anterior petrosectomy) and to the infratemporal fossa (temporal fossa floor removal). 3D reconstruction using the OsiriX software (version 5.8.1, Pixmeo, Bernex, Switzerland). Abbreviations: AP, anterior petrosectomy; ITF, infratemporal fossa; MC Meckel's cave; PA, petrous apex; PPF, pterygopalatine fossa; SphPA, sphenopalatine artery; VN, vidian nerve.
 Posterior route
, targeted to Meckel's cave and the petrous apex (
Fig. 4A
)
Posterior route
, targeted to Meckel's cave and the petrous apex (
Fig. 4A
)
From the superior orbital fissure, in a posterolateral direction, the greater sphenoid wing forms the floor of the middle cranial fossa. When the endoscopic extradural dissection between the temporal lobe dura and dura propria of the cavernous sinus is targeted in the posterolateral direction, the second and third divisions of the fifth cranial nerve, Meckel's cave, foramen ovale, and foramen spinosum are gradually exposed. According to Kawase et al, 58 59 60 the posterior cranial fossa could be entered once the bone between the V3, arcuate eminence, and greater superficial petrosal nerve is drilled. Dedicated endoscopic instrumentation, as well as 30° endoscopic angled visualization, allows for drilling the petrous apex ( Fig. 6A , B ). The dissection is guided at every step by the neuronavigation system, which helps the orientation and the extent of bone drilling.
 Inferior route
, targeted to the infratemporal and pterygopalatine fossae (
Fig. 4B
)
Inferior route
, targeted to the infratemporal and pterygopalatine fossae (
Fig. 4B
)
Drilling the middle fossa floor under neuronavigation control allows surgical access to the pterygopalatine and infratemporal fossae. Dissection is performed with а 30° endoscope, repositioned to the superolateral border of the lateral orbital craniotomy and targeted inferiorly ( Fig. 2 ). The repositioning creates an oblique angle of view, allowing the exact visual trajectory needed to drill the middle fossa floor. Drilling of the bone between V2 and V3 provides the access needed to enter the pterygopalatine fossa, with visualization of the sphenopalatine artery. Unroofing of the foramen rotundum allows for mobilization of the V2 nerve and its decompression in clinical settings. The vidian canal is situated inferolateral to V2. When drilling of the bone is extended between V2 and V3, the vidian nerve and canal are also identified ( Fig. 6C ). Following the vidian nerve posteriorly leads to the petrous segment of the ICA, as already described in the literature. 61 Unroofing the V3 nerve and further drilling of the middle fossa floor allows access to the infratemporal fossa. The mandibular division of the trigeminal nerve can be followed into its endo- and exocranial course.
A CT scan is performed after each dissection, revealing the extent of bone removal and the endoscopic corridors to the posterior and infratemporal fossae ( Fig. 6D ).
Discussion
In recent years, endoscopic (in particular, endonasal) techniques have revolutionized skull base surgery by allowing access to difficult skull base areas, such as the clival and petroclival regions, odontoid process, and pterygopalatine and infratemporal fossae, that have been traditionally approached by extensive transcranial surgeries requiring significant soft-tissue dissections and bone removal. 27 29 31 32 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 Newer methods of reconstruction of the skull base after the surgery have significantly decreased postoperative complications related to cerebrospinal fluid leaks and have even allowed the further expansion of indications of endoscopic endonasal surgery. 29 76 77 78 79 80 81 82 83 84 Nonetheless, despite these advances, the transsphenoidal perspective still has important limitations, in particular in relation to the lateral wall of the cavernous sinus and to the middle cranial fossa, which represent difficult-to-reach areas via a ventral pathway, mainly because of the unsafe crossing of important neurovascular structures.
The lateral orbital approach offers a different angle of attack to reach the middle cranial fossa and the lateral wall of the cavernous sinus, with possible extensions to the posterior, infratemporal, and pterygopalatine fossae. The space provided by using classical microsurgical techniques is relatively limited and requires advanced microsurgical skills. The limitations could be avoided with the use of endoscopic techniques, as demonstrated in this article.
Lateral orbitotomy, done by removing the lateral orbital wall, was first described in 1889 by Krönlein 26 and was used for intraorbital tumor pathology. 17 An important structure that is part of the lateral orbital wall is the sphenoid ridge, which represents a lateral extension of the posterior aspect of the lesser sphenoid wing. Yasargil referred to the sphenoid ridge as “the key landmark” in the approach to the circle of Willis. 85 On the external skull surface (in the temporal fossa), the sphenoid ridge projects over the greater sphenoid wing. 44 Laterally, the sphenoid ridge approximates the pterion at the sphenosquamosal suture, with this region referred to as the anterior sylvian point. 47 Intuitively, the sphenoid ridge is a straightforward keyhole to multiple skull base areas. 44 In the lateral orbital approach, when the sphenoid ridge is completely removed, it exposes the anatomic structures that are usually revealed via frontotemporal craniotomy and its modifications ( Figs. 5 and 6 ). More recently, other authors have used the lateral orbital approach in anatomical studies and small clinical series to reach intracranial structures such as the cavernous sinus lesions in the middle fossa, to excise cranio-orbital meningiomas to achieve optic nerve decompression, 17 24 25 and even to treat middle cerebral artery aneurysms. 86 The potential advantage of such a minimally invasive approach is that it requires only a cosmetic skin incision and results in less soft-tissue trauma and less manipulation of the temporalis muscle. 17 23 24 25
In these published surgical series, however, microscopic techniques are used, with the aforementioned limitation when working in a narrow corridor. In a very well-conducted anatomical study and illustrative case report, Altay et al 17 demonstrated that the entire cavernous sinus can be exposed by means of lateral orbitotomy, drilling the greater sphenoid wing, and performing optic nerve decompression through anterior clinoidectomy. More recently, Ulutas et al 25 demonstrated that using the same approach described by Altay et al, but changing the orientation of the microscope, could achieve control over critical neurovascular structures not only to the cavernous sinus but also to the superior orbital fissure and orbital apex. Nevertheless, the authors stress the limitations of this approach using microscopic techniques through a small area created by lateral orbitotomy and recommend practice in cadaveric dissection before attempting an actual surgical case, to become familiarized with the complex anatomy that is required through this surgical corridor. 25 A larger series, using lateral orbitotomy for middle fossa meningiomas, is that of Mariniello et al, 24 describing the treatment of sphenoid wing meningiomas without significant optic nerve compression or cavernous sinus invasion. Recently, Mandel et al 86 published a series of 25 cases with unruptured middle cerebral artery aneurysms managed successfully through lateral orbitotomy and eyelid skin incision. The author describes the approach as a safe alternative to pterional craniotomy in selected cases and one that has yielded excellent cosmetic results.
Both of the aforementioned studies by Altay et al 17 and Ulutas et al 25 used a small transverse linear skin incision extending from the lateral epicanthus laterally down to the lateral orbital ridge. In contrast, we prefer the superior eyelid incision described in detail by Aziz et al 23 and used in Mariniello et al 24 and Mandel et al. 86 We believe that this incision, combined with lateral orbitotomy, provides significantly more space in which to maneuver the surgical instruments and reduces their collision with the endoscope. Moreover, such an incision, according to real clinical series, produces very good cosmetic results and is barely visible when the patient opens his or her eye. 23
In our cadaveric study, we were able to expose the entire cavernous sinus and Meckel's cave, perform optic nerve decompression by anterior clinoidectomy and opening of the optic foramen, reach the petrous apex and perform an anterior petrosectomy to the posterior cranial fossa, and drill the floor of the middle cranial fossa and expose the infratemporal and pterygopalatine fossa. We believe that all this was made possible because of the superior visualization that the endoscope (0° and, especially, 30°) can provide, along with dedicated endoscopic instruments.
A minimally invasive approach has been defined by Kassam et al 29 as “access and visualization through the narrowest practical corridor providing maximum effective action at the target with minimal disruption of normal tissues.” In working along the narrowed surgical routes from the orbit to the middle and posterior skull base through lateral orbitotomy, the endoscope provides improved illumination and magnification and, at the same time, requires only narrow working channels, unlike the wide-angle space that is need for open or microscopic visualization. Thus, a freehand endoscopic technique with dedicated instrumentation allows the targeted dissection to take place through smaller and less disruptive surgical corridors and, at the same time, reveals a wider and closer operative field.
In recent years, another endoscopic technique—namely, ventral endoscopic transorbital surgery—has gained more and more popularity. According to some authors, the various approaches taken by this technique allows access not only to the contents of the orbit but also to the intracranial compartment, including the anterior cranial fossa, middle fossa, and lateral cavernous sinus. 20 A growing number of publications describe how the technique allows a multiportal, multiangled approach to lesions of the skull base that are extremely difficult to reach, even with expanded transnasal endoscopic procedures. 16 18 19 28 48 87 88 89 90 The transorbital approaches have the cosmetic advantage of having limited or no skin incision and eliminate the danger to the frontal branch of the facial nerve, as may occur in a standard frontotemporal approach. One of the major disadvantages of ventral transorbital approaches is the unfamiliar perspective of the anatomy of this region as seen from a ventral viewpoint. 48 For this reason, extensive practice in dissection, as well as further anatomical studies, is needed to gain more experience with the ventral transorbital approaches. Another disadvantage of purely ventral transorbital approaches is globe retraction, which might be one of the major factors limiting adequate transorbital access to the lateral cavernous sinus and Meckel's cave. According to some authors, 10 mm of globe retraction at the lateral orbital rim is safe and not associated with negative ophthalmologic outcomes. 19
Nevertheless, removal of the lateral orbital wall gains space for instrument maneuverability, avoids collision of the instruments with the endoscope, and might offer advantages to prevent mechanical or ischemic damage of intraorbital structures in clinical settings. Removal of the lateral orbital rim and wall, with subsequent proper reconstruction, as shown in clinical series, is associated with excellent cosmetic outcomes. 23 24 Moreover, the approach provides wider working space, limited orbital retraction, and the possibility of having more working corridors ( Fig. 4A , B ), and even switching to the ventral transorbital route, during surgery if necessary. Furthermore, the lateral orbital approach is versatile, allowing not only optic nerve decompression and cavernous sinus and Meckel's cave exposure, but also routes to the posterior infratemporal and pterygopalatine fossae.
The main differences between the ventral transorbital approaches and the lateral orbital approach described here are the head positioning, the amount of bone removal, and the surgical perspective. In the lateral orbital approach, the head positioning and surgical perspective are already familiar to neurosurgeons because they are the same as in traditional anterolateral neurosurgical approaches (pterional, anterior petrosectomy). Therefore, the learning curve is likely to be less steep. In the ventral transorbital approach, the head positioning and surgical perspective are the ones used in transsphenoidal surgery ( Table 1 ). Hence, the anatomical view of intracranial structures is unfamiliar to most neurosurgeons and requires additional practice in dissection.
Table 1. Comparison between the lateral orbital and the transorbital endoscopic approaches.
| Lateral orbital approach | Transorbital approach | |
|---|---|---|
| Positioning | Behind the head and the anatomical exposure is very close to the one that neurosurgeons are trained from the classical pterional approach | Lateral or in front of the head, which is a positioning very close to the one from the transsphenoidal surgery (potentially steeper learning curve) |
| Skin incision | Eyelid incision in the upper eye crease | Eyelid incision in the upper eye crease/No skin incision |
| Working space | Removal of the lateral orbital rim provides a very wide working field with minimal orbital manipulation; minimal disruption of the temporalis muscle is required | Narrow due to the bony border of the lateral orbital rim; increased eye bulb compression and higher chance for approach related morbidity, especially for lengthy procedures |
| Surgical trajectories | The approach provides possibilities for multiple surgical trajectories including just by switching surgical position (from behind the head to anterior to the head), to have the same working corridor as the transorbital approach (surgical perspective anterior to the frontal and temporal poles) | Anterior working corridor |
| Route to infratemporal and pterygopalatine fossae | By drilling of the floor of the middle fossa the lateral orbital approach offers a straightforward route to the infratemporal and pterygopalatine fossae | Possible surgical corridors, but limited maneuverability |
| Corridor to the posterior cranial fossa | Possible trough anterior petrosectomy drilling. The surgical perspective is the same as the one used in classical skull base interventions | Possible, but the surgical perspective and the visualization of the anatomy requires a steeper learning curve to get a proper orientation |
The transorbital and lateral orbital approaches are two newly described endoscopic routes that can be studied and compared one with each other for clinical advantages, disadvantages, indications, and complications. In examining the lateral orbital approach, this study has borne useful information, but there are, however, several limitations to consider. First, the cadaver specimens used in the study allow detailed anatomic description during the step-by-step dissection, as well as offering an evaluation of the surgical exposure and maneuverability involved, but they cannot replicate the clinical environment and the challenges faced in clinical settings. As a result, there is a need for further investigation into these two approaches and their outcomes in selected patients.
Conclusion
The lateral orbital endoscopic approach offers four minimally invasive corridors: anteromedial, posteromedial, posterior, and inferior. The anteromedial corridor is targeted to the ACP and the optic nerve. The posteromedial corridor is targeted to lateral wall of the cavernous sinus. The posterior corridor is targeted to Meckel's cave and the posterior cranial fossa. The inferior corridor is targeted to the infratemporal and pterygopalatine fossae. The anatomical descriptive study presented in this article reveals the possibilities for minimally invasive corridors that allow endoscopic techniques along with dedicated instrumentation. Further studies are needed to explore the advantages and disadvantages of this approach and its clinical implications.
Funding Statement
Funding None.
Footnotes
Conflict of Interest All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers' bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements) or nonfinancial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
This article does not contain any studies with human participants performed by any of the authors.
References
- 1.Al-Mefty O. Supraorbital-pterional approach to skull base lesions. Neurosurgery. 1987;21(04):474–477. doi: 10.1227/00006123-198710000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Hakuba A, Liu S, Nishimura S. The orbitozygomatic infratemporal approach: a new surgical technique. Surg Neurol. 1986;26(03):271–276. doi: 10.1016/0090-3019(86)90161-8. [DOI] [PubMed] [Google Scholar]
- 3.Ikeda K, Yamashita J, Hashimoto M, Futami K. Orbitozygomatic temporopolar approach for a high basilar tip aneurysm associated with a short intracranial internal carotid artery: a new surgical approach. Neurosurgery. 1991;28(01):105–110. doi: 10.1097/00006123-199101000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Jane J A, Park T S, Pobereskin L H, Winn H R, Butler A B. The supraorbital approach: technical note. Neurosurgery. 1982;11(04):537–542. [PubMed] [Google Scholar]
- 5.Aziz K M, Froelich S C, Cohen P L, Sanan A, Keller J T, van Loveren H R. The one-piece orbitozygomatic approach: the MacCarty burr hole and the inferior orbital fissure as keys to technique and application. Acta Neurochir (Wien) 2002;144(01):15–24. doi: 10.1007/s701-002-8270-1. [DOI] [PubMed] [Google Scholar]
- 6.Balasingam V, Noguchi A, McMenomey S O, Delashaw J B., Jr Modified osteoplastic orbitozygomatic craniotomy. Technical note. J Neurosurg. 2005;102(05):940–944. doi: 10.3171/jns.2005.102.5.0940. [DOI] [PubMed] [Google Scholar]
- 7.Chanda A, Nanda A. Anatomical study of the orbitozygomatic transsellar-transcavernous-transclinoidal approach to the basilar artery bifurcation. J Neurosurg. 2002;97(01):151–160. doi: 10.3171/jns.2002.97.1.0151. [DOI] [PubMed] [Google Scholar]
- 8.Figueiredo E G, Deshmukh P, Zabramski J Met al. Quantitative anatomic study of three surgical approaches to the anterior communicating artery complex Neurosurgery 200556(2, Suppl):397–405., discussion 397–405 [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez L F, Crawford N R, Horgan M A, Deshmukh P, Zabramski J M, Spetzler R F.Working area and angle of attack in three cranial base approaches: pterional, orbitozygomatic, and maxillary extension of the orbitozygomatic approach Neurosurgery 20025003550–555., discussion 555–557 [PubMed] [Google Scholar]
- 10.Hsu F P, Clatterbuck R E, Spetzler R F.Orbitozygomatic approach to basilar apex aneurysms Neurosurgery 200556(1, Suppl):172–177., discussion 172–177 [DOI] [PubMed] [Google Scholar]
- 11.Schwartz M S, Anderson G J, Horgan M A, Kellogg J X, McMenomey S O, Delashaw J B., Jr Quantification of increased exposure resulting from orbital rim and orbitozygomatic osteotomy via the frontotemporal transsylvian approach. J Neurosurg. 1999;91(06):1020–1026. doi: 10.3171/jns.1999.91.6.1020. [DOI] [PubMed] [Google Scholar]
- 12.Sekhar L N, Kalia K K, Yonas H, Wright D C, Ching H.Cranial base approaches to intracranial aneurysms in the subarachnoid space Neurosurgery 19943503472–481., discussion 481–483 [DOI] [PubMed] [Google Scholar]
- 13.Sindou M, Emery E, Acevedo G, Ben-David U. Respective indications for orbital rim, zygomatic arch and orbito-zygomatic osteotomies in the surgical approach to central skull base lesions. Critical, retrospective review in 146 cases. Acta Neurochir (Wien) 2001;143(10):967–975. doi: 10.1007/s007010170001. [DOI] [PubMed] [Google Scholar]
- 14.Tanriover N, Ulm A J, Rhoton A L, Jr, Kawashima M, Yoshioka N, Lewis S B.One-piece versus two-piece orbitozygomatic craniotomy: quantitative and qualitative considerations Neurosurgery 2006580402ONS-229–ONS-237., discussion ONS-237 [DOI] [PubMed] [Google Scholar]
- 15.Youssef A S, Willard L, Downes A et al. The frontotemporal-orbitozygomatic approach: reconstructive technique and outcome. Acta Neurochir (Wien) 2012;154(07):1275–1283. doi: 10.1007/s00701-012-1370-9. [DOI] [PubMed] [Google Scholar]
- 16.Locatelli D, Pozzi F, Turri-Zanoni M et al. Transorbital endoscopic approaches to the skull base: current concepts and future perspectives. J Neurosurg Sci. 2016;60(04):514–525. [PubMed] [Google Scholar]
- 17.Altay T, Patel B C, Couldwell W T. Lateral orbital wall approach to the cavernous sinus. J Neurosurg. 2012;116(04):755–763. doi: 10.3171/2011.12.JNS111251. [DOI] [PubMed] [Google Scholar]
- 18.Balakrishnan K, Moe K S. Applications and outcomes of orbital and transorbital endoscopic surgery. Otolaryngol Head Neck Surg. 2011;144(05):815–820. doi: 10.1177/0194599810397285. [DOI] [PubMed] [Google Scholar]
- 19.Bly R A, Ramakrishna R, Ferreira M, Moe K S. Lateral transorbital neuroendoscopic approach to the lateral cavernous sinus. J Neurol Surg B Skull Base. 2014;75(01):11–17. doi: 10.1055/s-0033-1353363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramakrishna R, Kim L J, Bly R A, Moe K, Ferreira M., Jr Transorbital neuroendoscopic surgery for the treatment of skull base lesions. J Clin Neurosci. 2016;24:99–104. doi: 10.1016/j.jocn.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arai H, Sato K, Katsuta T, Rhoton A L., JrLateral approach to intraorbital lesions: anatomic and surgical considerations Neurosurgery 199639061157–1162., discussion 1162–1163 [DOI] [PubMed] [Google Scholar]
- 22.Rhoton A L., JrThe orbit Neurosurgery 200251(4, Suppl):S303–S334. [PubMed] [Google Scholar]
- 23.Abdel Aziz K M, Bhatia S, Tantawy M Het al. Minimally invasive transpalpebral “eyelid” approach to the anterior cranial base Neurosurgery 201169(2, Suppl Operative):ons195–ons206., discussion 206–207 [DOI] [PubMed] [Google Scholar]
- 24.Mariniello G, Maiuri F, de Divitiis Eet al. Lateral orbitotomy for removal of sphenoid wing meningiomas invading the orbit Neurosurgery 201066(6, Suppl Operative):287–292., discussion 292 [DOI] [PubMed] [Google Scholar]
- 25.Ulutas M, Boyacı S, Akakın A, Kılıç T, Aksoy K. Surgical anatomy of the cavernous sinus, superior orbital fissure, and orbital apex via a lateral orbitotomy approach: a cadaveric anatomical study. Acta Neurochir (Wien) 2016;158(11):2135–2148. doi: 10.1007/s00701-016-2940-z. [DOI] [PubMed] [Google Scholar]
- 26.Lyson T, Sieskiewicz A, Rogowski M, Mariak Z. Endoscopic lateral orbitotomy. Acta Neurochir (Wien) 2014;156(10):1897–1900. doi: 10.1007/s00701-014-2205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciporen J N, Moe K S, Ramanathan D et al. Multiportal endoscopic approaches to the central skull base: a cadaveric study. World Neurosurg. 2010;73(06):705–712. doi: 10.1016/j.wneu.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 28.Ferrari M, Schreiber A, Mattavelli D et al. The inferolateral transorbital endoscopic approach: a preclinical anatomical study. World Neurosurg. 2016;90:403–413. doi: 10.1016/j.wneu.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 29.Kassam A B, Prevedello D M, Carrau R L et al. Endoscopic endonasal skull base surgery: analysis of complications in the authors' initial 800 patients. J Neurosurg. 2011;114(06):1544–1568. doi: 10.3171/2010.10.JNS09406. [DOI] [PubMed] [Google Scholar]
- 30.Koutourousiou M, Gardner P A, Stefko S T et al. Combined endoscopic endonasal transorbital approach with transconjunctival-medial orbitotomy for excisional biopsy of the optic nerve: technical note. J Neurol Surg Rep. 2012;73(01):52–56. doi: 10.1055/s-0032-1323156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muto J, Prevedello D M, Ditzel Filho L F et al. Comparative analysis of the anterior transpetrosal approach with the endoscopic endonasal approach to the petroclival region. J Neurosurg. 2016;125(05):1171–1186. doi: 10.3171/2015.8.JNS15302. [DOI] [PubMed] [Google Scholar]
- 32.Dallan I, Castelnuovo P, de Notaris M et al. Endoscopic endonasal anatomy of superior orbital fissure and orbital apex regions: critical considerations for clinical applications. Eur Arch Otorhinolaryngol. 2013;270(05):1643–1649. doi: 10.1007/s00405-012-2281-3. [DOI] [PubMed] [Google Scholar]
- 33.Andaluz N, Romano A, Reddy L V, Zuccarello M. Eyelid approach to the anterior cranial base. J Neurosurg. 2008;109(02):341–346. doi: 10.3171/JNS/2008/109/8/0341. [DOI] [PubMed] [Google Scholar]
- 34.Ammirati M, Spallone A, Ma J, Cheatham M, Becker D.An anatomicosurgical study of the temporal branch of the facial nerve Neurosurgery 199333061038–1043., discussion 1044 [DOI] [PubMed] [Google Scholar]
- 35.Coscarella E, Vishteh A G, Spetzler R F, Seoane E, Zabramski J M. Subfascial and submuscular methods of temporal muscle dissection and their relationship to the frontal branch of the facial nerve. Technical note. J Neurosurg. 2000;92(05):877–880. doi: 10.3171/jns.2000.92.5.0877. [DOI] [PubMed] [Google Scholar]
- 36.Krayenbühl N, Isolan G R, Hafez A, Yaşargil M G.The relationship of the fronto-temporal branches of the facial nerve to the fascias of the temporal region: a literature review applied to practical anatomical dissection Neurosurg Rev 200730018–15., discussion 15 [DOI] [PubMed] [Google Scholar]
- 37.Schmidt B L, Pogrel M A, Hakim-Faal Z. The course of the temporal branch of the facial nerve in the periorbital region. J Oral Maxillofac Surg. 2001;59(02):178–184. doi: 10.1053/joms.2001.18271. [DOI] [PubMed] [Google Scholar]
- 38.Spiriev T, Ebner F H, Hirt B et al. Fronto-temporal branch of facial nerve within the interfascial fat pad: is the interfascial dissection really safe? Acta Neurochir (Wien) 2016;158(03):527–532. doi: 10.1007/s00701-016-2711-x. [DOI] [PubMed] [Google Scholar]
- 39.Spiriev T, Poulsgaard L, Fugleholm K. Techniques for preservation of the frontotemporal branch of facial nerve during orbitozygomatic approaches. J Neurol Surg B Skull Base. 2015;76(03):189–194. doi: 10.1055/s-0034-1396599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yaşargil M G, Reichman M V, Kubik S. Preservation of the frontotemporal branch of the facial nerve using the interfascial temporalis flap for pterional craniotomy. Technical article. J Neurosurg. 1987;67(03):463–466. doi: 10.3171/jns.1987.67.3.0463. [DOI] [PubMed] [Google Scholar]
- 41.Mori K, Osada H, Yamamoto T, Nakao Y, Maeda M. Pterional keyhole approach to middle cerebral artery aneurysms through an outer canthal skin incision. Minim Invasive Neurosurg. 2007;50(04):195–201. doi: 10.1055/s-2007-985837. [DOI] [PubMed] [Google Scholar]
- 42.Shimizu S, Tanriover N, Rhoton A L, Jr, Yoshioka N, Fujii K.MacCarty keyhole and inferior orbital fissure in orbitozygomatic craniotomy Neurosurgery 200557(1, Suppl):152–159., discussion 152–159 [DOI] [PubMed] [Google Scholar]
- 43.Tubbs R S, Loukas M, Shoja M M, Cohen-Gadol A A.Refined and simplified surgical landmarks for the MacCarty keyhole and orbitozygomatic craniotomy Neurosurgery 201066(6, Suppl Operative):230–233. [DOI] [PubMed] [Google Scholar]
- 44.Spiriev T, Poulsgaard L, Fugleholm K. One piece orbitozygomatic approach based on the sphenoid ridge keyhole: anatomical study. J Neurol Surg B Skull Base. 2016;77(03):199–206. doi: 10.1055/s-0035-1564590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bayassi S. Meningo-orbital fold (MOF) as a guiding point in extradural approach to the anterior clinoid process [in Polish] Neurol Neurochir Pol. 2005;39(01):49–55. [PubMed] [Google Scholar]
- 46.Fukuda H, Evins A I, Burrell J C, Iwasaki K, Stieg P E, Bernardo A. The meningo-orbital band: microsurgical anatomy and surgical detachment of the membranous structures through a frontotemporal craniotomy with removal of the anterior clinoid process. J Neurol Surg B Skull Base. 2014;75(02):125–132. doi: 10.1055/s-0033-1359302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ribas G C, Ribas E C, Rodrigues C J.The anterior sylvian point and the suprasylvian operculum Neurosurg Focus 200518(6B, 6b):E2. [PubMed] [Google Scholar]
- 48.Dallan I, Di Somma A, Prats-Galino A et al. Endoscopic transorbital route to the cavernous sinus through the meningo-orbital band: a descriptive anatomical study. J Neurosurg. 2017;127(03):622–629. doi: 10.3171/2016.8.JNS16465. [DOI] [PubMed] [Google Scholar]
- 49.Coscarella E, Başkaya M K, Morcos J J.An alternative extradural exposure to the anterior clinoid process: the superior orbital fissure as a surgical corridor Neurosurgery 20035301162–166., discussion 166–167 [DOI] [PubMed] [Google Scholar]
- 50.Ammirati M, Bernardo A. Anatomical study of the superior orbital fissure as seen during a pterional approach. J Neurosurg. 2007;106(01):151–156. doi: 10.3171/jns.2007.106.1.151. [DOI] [PubMed] [Google Scholar]
- 51.Dolenc V. Wien, New York: Springer-Verlag; 2013. Anatomy and Surgery of the Cavernous Sinus. [Google Scholar]
- 52.Dolenc V. Wien, New York: Springer-Verlag; 2003. Microsurgical Anatomy and Surgery of the Central Skull Base. [Google Scholar]
- 53.Day J D.Cranial base surgical techniques for large sphenocavernous meningiomas: technical note Neurosurgery 20004603754–759., discussion 759–760 [DOI] [PubMed] [Google Scholar]
- 54.Goel A. The extradural approach to lesions involving the cavernous sinus. Br J Neurosurg. 1997;11(02):134–138. doi: 10.1080/02688699746483. [DOI] [PubMed] [Google Scholar]
- 55.Kawase T, van Loveren H, Keller J T, Tew J M.Meningeal architecture of the cavernous sinus: clinical and surgical implications Neurosurgery 19963903527–534., discussion 534–536 [DOI] [PubMed] [Google Scholar]
- 56.Shi X, Han H, Zhao J, Zhou C. Microsurgical anatomy of the superior orbital fissure. Clin Anat. 2007;20(04):362–366. doi: 10.1002/ca.20391. [DOI] [PubMed] [Google Scholar]
- 57.Kinzel A, Spangenberg P, Lutz Set al. Microsurgical and histological identification and definition of an interdural incision zone in the dorsolateral cavernous sinus Acta Neurochir (Wien) 2015157081359–1367., discussion 1367 [DOI] [PubMed] [Google Scholar]
- 58.Kawase T, Toya S, Shiobara R, Mine T. Transpetrosal approach for aneurysms of the lower basilar artery. J Neurosurg. 1985;63(06):857–861. doi: 10.3171/jns.1985.63.6.0857. [DOI] [PubMed] [Google Scholar]
- 59.Kawase T, Shiobara R, Toya S.Anterior transpetrosal-transtentorial approach for sphenopetroclival meningiomas: surgical method and results in 10 patients Neurosurgery 19912806869–875., discussion 875–876 [PubMed] [Google Scholar]
- 60.Kawase T, Shiobara R, Toya S.Middle fossa transpetrosal-transtentorial approaches for petroclival meningiomas. Selective pyramid resection and radicality Acta Neurochir (Wien) 1994129(3-4):113–120. [DOI] [PubMed] [Google Scholar]
- 61.Osawa S, Rhoton A L, Jr, Seker A, Shimizu S, Fujii K, Kassam A B.Microsurgical and endoscopic anatomy of the vidian canal Neurosurgery 2009640502385–411., discussion 411–412 [DOI] [PubMed] [Google Scholar]
- 62.de Lara D, Ditzel Filho L F, Prevedello D Met al. Endonasal endoscopic approaches to the paramedian skull base World Neurosurg 201482(6, Suppl):S121–S129. [DOI] [PubMed] [Google Scholar]
- 63.Tsirbas A, Kazim M, Close L. Endoscopic approach to orbital apex lesions. Ophthal Plast Reconstr Surg. 2005;21(04):271–275. doi: 10.1097/01.iop.0000169254.44642.3d. [DOI] [PubMed] [Google Scholar]
- 64.Cappabianca P, Cavallo L M, Esposito I, Solari D. Pittsburgh: Karger; 2008. Sellar-Tuberculum approach. [Google Scholar]
- 65.Cavallo L M, Prevedello D M, Solari D et al. Extended endoscopic endonasal transsphenoidal approach for residual or recurrent craniopharyngiomas. J Neurosurg. 2009;111(03):578–589. doi: 10.3171/2009.2.JNS081026. [DOI] [PubMed] [Google Scholar]
- 66.Cavallo L M, Messina A, Gardner P et al. Extended endoscopic endonasal approach to the pterygopalatine fossa: anatomical study and clinical considerations. Neurosurg Focus. 2005;19(01):E5. [PubMed] [Google Scholar]
- 67.Fernandez-Miranda J C, Gardner P A, Prevedello D M, Kassam A B.Expanded endonasal approach for olfactory groove meningioma Acta Neurochir (Wien) 200915103287–288., author reply 289–290 [DOI] [PubMed] [Google Scholar]
- 68.Fernandez-Miranda J C, Prevedello D M, Gardner P, Carrau R, Snyderman C H, Kassam A B.Endonasal endoscopic pituitary surgery: is it a matter of fashion? Acta Neurochir (Wien) 2010152081281–1282., author reply 1282 [DOI] [PubMed] [Google Scholar]
- 69.Fortes F S, Carrau R L, Snyderman C H et al. Transpterygoid transposition of a temporoparietal fascia flap: a new method for skull base reconstruction after endoscopic expanded endonasal approaches. Laryngoscope. 2007;117(06):970–976. doi: 10.1097/MLG.0b013e3180471482. [DOI] [PubMed] [Google Scholar]
- 70.Gardner P A, Prevedello D M, Kassam A B, Snyderman C H, Carrau R L, Mintz A H. The evolution of the endonasal approach for craniopharyngiomas. J Neurosurg. 2008;108(05):1043–1047. doi: 10.3171/JNS/2008/108/5/1043. [DOI] [PubMed] [Google Scholar]
- 71.Kassam A, Snyderman C, Carrau R. An evolving paradigm to the ventral skull base. Skull Base. 2004;14 01:xx. [Google Scholar]
- 72.Kassam A, Thomas A J, Snyderman Cet al. Fully endoscopic expanded endonasal approach treating skull base lesions in pediatric patients J Neurosurg 2007106(2, Suppl)75–86. [DOI] [PubMed] [Google Scholar]
- 73.Kassam A B, Gardner P, Snyderman C, Mintz A, Carrau R. Expanded endonasal approach: fully endoscopic, completely transnasal approach to the middle third of the clivus, petrous bone, middle cranial fossa, and infratemporal fossa. Neurosurg Focus. 2005;19(01):E6. [PubMed] [Google Scholar]
- 74.Kassam A B, Vescan A D, Carrau R L et al. Expanded endonasal approach: vidian canal as a landmark to the petrous internal carotid artery. J Neurosurg. 2008;108(01):177–183. doi: 10.3171/JNS/2008/108/01/0177. [DOI] [PubMed] [Google Scholar]
- 75.Morera V A, Fernandez-Miranda J C, Prevedello D Met al. “Far-medial” expanded endonasal approach to the inferior third of the clivus: the transcondylar and transjugular tubercle approaches Neurosurgery 201066(6, Suppl Operative):211–219., discussion 219–220 [DOI] [PubMed] [Google Scholar]
- 76.Hadad G, Bassagasteguy L, Carrau R L et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope. 2006;116(10):1882–1886. doi: 10.1097/01.mlg.0000234933.37779.e4. [DOI] [PubMed] [Google Scholar]
- 77.Kassam A B, Thomas A, Carrau R Let al. Endoscopic reconstruction of the cranial base using a pedicled nasoseptal flap Neurosurgery 2008630101ONS44–ONS52., discussion ONS52–ONS53 [DOI] [PubMed] [Google Scholar]
- 78.Oliver C L, Hackman T G, Carrau R L et al. Palatal flap modifications allow pedicled reconstruction of the skull base. Laryngoscope. 2008;118(12):2102–2106. doi: 10.1097/MLG.0b013e318184e719. [DOI] [PubMed] [Google Scholar]
- 79.Prevedello D M, Barges-Coll J, Fernandez-Miranda J C et al. Middle turbinate flap for skull base reconstruction: cadaveric feasibility study. Laryngoscope. 2009;119(11):2094–2098. doi: 10.1002/lary.20226. [DOI] [PubMed] [Google Scholar]
- 80.Leng L Z, Brown S, Anand V K, Schwartz T H.“Gasket-seal” watertight closure in minimal-access endoscopic cranial base surgery Neurosurgery 2008620502E342–E343., discussion E343 [DOI] [PubMed] [Google Scholar]
- 81.Cappabianca P, Cavallo L M, Valente Vet al. Sellar repair with fibrin sealant and collagen fleece after endoscopic endonasal transsphenoidal surgery Surg Neurol 20046203227–233., discussion 233 [DOI] [PubMed] [Google Scholar]
- 82.Spaziante R, de Divitiis E, Cappabianca P, Zona G. Philadelphia: WB Saunders; 2005. Repair of the sella turcica after transsphenoidal surgery; pp. 390–408. [Google Scholar]
- 83.Esposito F, Dusick J R, Fatemi N, Kelly D F.Graded repair of cranial base defects and cerebrospinal fluid leaks in transsphenoidal surgery Neurosurgery 2007600402295–303., discussion 303–304 [DOI] [PubMed] [Google Scholar]
- 84.Nyquist G G, Anand V K, Mehra S, Kacker A, Schwartz T H. Endoscopic endonasal repair of anterior skull base non-traumatic cerebrospinal fluid leaks, meningoceles, and encephaloceles. J Neurosurg. 2010;113(05):961–966. doi: 10.3171/2009.10.JNS08986. [DOI] [PubMed] [Google Scholar]
- 85.Altay T, Couldwell W T.The frontotemporal (pterional) approach: an historical perspective Neurosurgery 20127102481–491., discussion 491–492 [DOI] [PubMed] [Google Scholar]
- 86.Mandel M, Figueiredo E G, Mandel S A, Tutihashi R, Teixeira M J. Minimally invasive transpalpebral endoscopic-assisted amygdalohippocampectomy. Oper Neurosurg (Hagerstown) 2017;13(01):2–14. doi: 10.1227/NEU.0000000000001179. [DOI] [PubMed] [Google Scholar]
- 87.Di Somma A, Cavallo L M, de Notaris M et al. Endoscopic endonasal medial-to-lateral and transorbital lateral-to-medial optic nerve decompression: an anatomical study with surgical implications. J Neurosurg. 2017;127(01):199–208. doi: 10.3171/2016.8.JNS16566. [DOI] [PubMed] [Google Scholar]
- 88.Koppe M, Gleizal A, Orset E, Bachelet J T, Jouanneau E, Rougeot A. Superior eyelid crease approach for transobital neuroendoscopic surgery of the anterior cranial fossa. J Craniofac Surg. 2013;24(05):1616–1621. doi: 10.1097/SCS.0b013e3182a2d635. [DOI] [PubMed] [Google Scholar]
- 89.Dallan I, Castelnuovo P, Locatelli D et al. Multiportal combined transorbital transnasal endoscopic approach for the management of selected skull base lesions: preliminary experience. World Neurosurg. 2015;84(01):97–107. doi: 10.1016/j.wneu.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 90.Dallan I, Locatelli D, Turri-Zanoni M et al. Transorbital endoscopic assisted resection of a superior orbital fissure cavernous haemangioma: a technical case report. Eur Arch Otorhinolaryngol. 2015;272(12):3851–3856. doi: 10.1007/s00405-015-3556-2. [DOI] [PubMed] [Google Scholar]


