Abstract
Background
Acute adrenal hemorrhages are a rare event compared to other abdominal visceral injuries because of the anatomic localization of the adrenal glands; main causes are trauma and ruptured neoplasms. This manuscript reports on a single center experience of transarterial embolizations of adrenal hemorrhages in emergency setting.
Methods
In this retrospective analysis from 2010 to date, 17 patients (12 men and 5 women, mean age: 59.8 years) presenting with adrenal bleedings were treated by endovascular embolization. The etiology was traumatic in 7 cases, ruptured neoplasm in 8 cases and spontaneous in 2 patients assuming oral anticoagulant therapy. After thin slice contrast enhanced CT, a superselective embolization was conducted with different embolizing agents according to the type of vessel lesion and operator preference.
Results
Technical success rate, considered as interruption of adrenal bleeding detectable at angiography, was 94.1%. Clinical success rate, considered as hemodynamic stability restoration within 24 hours from the procedure, was 82.3%. Vessels involved were the superior adrenal artery in 5 patients, the middle adrenal artery in 8 patients, the inferior adrenal artery in one patient and more than one adrenal artery in 3 patients. No procedure-related major complications occurred and no patients had infarctions, necrosis, abscess formation, or required long-term steroid supplementation.
Conclusions
Acute adrenal hemorrhages can be safely and effectively managed by catheter directed embolizations; the source of bleeding has to be carefully investigated at CT and angiography because adrenal glands present with a wide and complex vascular arterial network.
Keywords: Adrenal gland, hemorrhage, embolization, acute
Introduction
Acute adrenal hemorrhages are a rare event compared to other abdominal visceral injuries because these glands are located in the deep retroperitoneal space in a relatively protected anatomic location (the perirenal space surrounded by fat); main causes of adrenal bleedings are trauma and ruptured neoplasms.
Trans-arterial embolization is a minimally invasive procedure that has already proven to be safe and effective in managing abdominal hemorrhages (1,2); because of the wide and complex vascular network of the adrenal glands, a careful examination of the arterial source of bleeding should always be conducted before releasing the embolizing agents.
Published reports are mainly limited to case reports and small case series (3-6) due the low frequency.
However technological advances in computed tomography (CT) scanning and the widespread use of imaging diagnosis in the clinical practice have raised the detection of adrenal injuries sustained by up to 25% of severely traumatized patients (6-8). Depending on the extent of the injury and patients hemodynamic stability, adrenal hemorrhage is addressed surgically or conservatively.
Aim of this manuscript is to report on a single center experience of transarterial embolizations of adrenal hemorrhages in emergency setting.
Methods
Sample
This is a monocentric retrospective analysis conducted in a tertiary care center focusing on patients treated by endovascular embolization because of adrenal hemorrhages in acute setting.
The institutional ethical committee approved this retrospective review of patients’ medical and imaging record in accordance with the Helsinki Declaration 2013 (9).
The medical electronical records were investigated from 2010 to date searching for hemorrhage embolizations in acute setting; overall 1,126 patients have been identified and 17 of these (12 men and 5 women, mean age: 59.8 years) presented with adrenal bleedings (Table 1) and so were included in this analysis.
Table 1. Patients characteristics: sex, age, etiology of hemorrhages and side of the adrenal gland involved.
| Patient | Sex | Age (years) | Etiology | Side |
|---|---|---|---|---|
| 1 | Male | 66 | Neoplastic | Left |
| 2 | Male | 56 | Traumatic | Left |
| 3 | Male | 60 | Traumatic | Left |
| 4 | Male | 44 | Traumatic | Right |
| 5 | Female | 61 | Neoplastic | Right |
| 6 | Male | 76 | Traumatic | Left |
| 7 | Male | 52 | Traumatic | Right |
| 8 | Male | 60 | Neoplastic | Left |
| 9 | Male | 53 | Neoplastic | Right |
| 10 | Male | 53 | Neoplastic | Right |
| 11 | Female | 59 | Spontaneous | Left |
| 12 | Female | 50 | Traumatic | Right |
| 13 | Female | 72 | Neoplastic | Right |
| 14 | Male | 67 | Neoplastic | Right |
| 15 | Male | 67 | Neoplastic | Right |
| 16 | Female | 83 | Spontaneous | Left |
| 17 | Male | 38 | Traumatic | Bilateral |
In 7 patients the hemorrhage was traumatic (2 of these were iatrogenic after nephrectomy for carcinoma), in 8 patients the bleeding was caused by neoplasms (Figures 1,2) and in 2 patients assuming oral anticoagulant therapy the etiology was spontaneous.
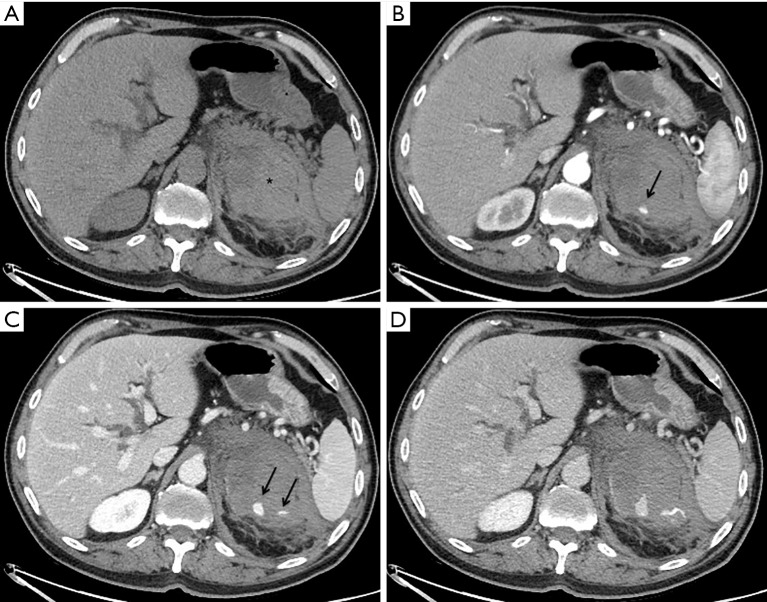
Figure 1.
Patient 1, a 66 years old male with neoplastic bleeding from lung metastasis of the left adrenal gland. Axial CT in basal (A), arterial (B), venous (C) and delayed (D) phases detecting a large hematoma (asterisk) with multiple foci of contrast agent extravasation (black arrows) increasing during the scan, sign of active bleeding.
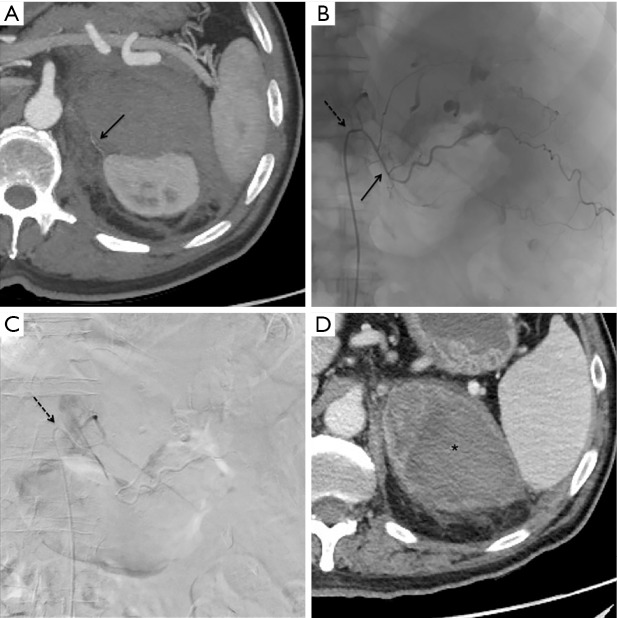
Figure 2.
Patient 1, same patient of Figure 1. Axial maximum intensity projection post-processing CT reconstruction (A): the superior adrenal artery is appreciable (black arrow). Procedural fluoroscopy (B) showing a 5 Fr Cobra catheter (dotted black arrow) at the origin of the left phrenic artery and a 2.7 Fr microcatheter (continuous black arrow) at the origin of superior adrenal artery: similar to CT (Figure 1), multiple foci of contrast agent extravasation are evident and so 300–500 micron microparticles were adopted as embolizing agent. Post embolization digital subtraction angiography (C) after contrast injection from the 5 Fr Cobra catheter (dotted black arrow), revealing no more extravasation in the embolized area. CT scan of control in arterial phase 24 hours after the embolization procedure (D) detecting left adrenal hematoma (black asterisk) without signs of active bleeding.
Among those subjects with neoplastic hemorrhages, 5 presented with adrenal metastasis (4 from lung cancers and 1 from hepatocarcinoma) and 3 were primitive neoplasms (2 pheochromocytoma and 1 adenocarcinoma).
Bleeding involved in 7 patients the left adrenal gland, in 9 patients the right adrenal gland and in 1 patient it was bilateral; among those subjects with traumatic not iatrogenic etiology (Figure 3), the right adrenal gland was involved twice as well as the left gland while in one case both glands bled. Patients with left side involvement presented other traumatic lesions even on the right side of the body, while patients with right involvement had lesions only on the same body side.
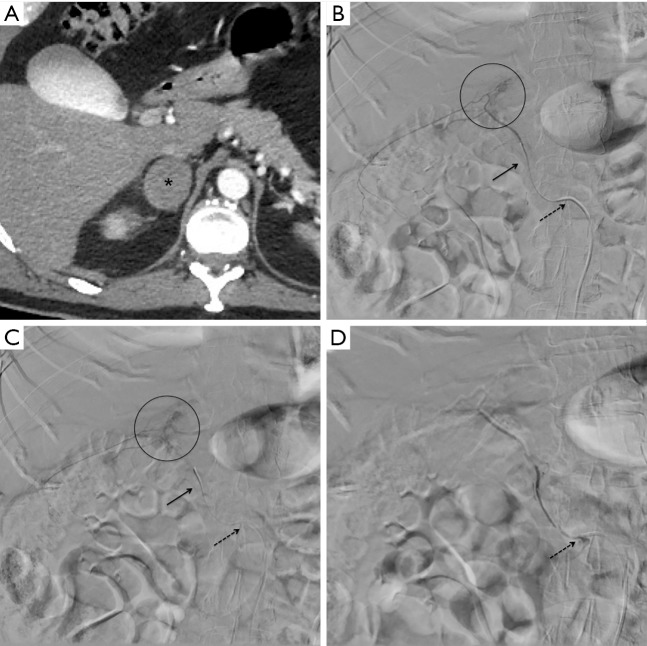
Figure 3.
Patient 7, a 52 years old male with traumatic bleeding of the right adrenal gland after a motorbike accident. Axial CT in arterial phase (A) detecting a hematoma with contextual multiple spots of contrast agent extravasation (black asterisk) in correspondence of the right adrenal gland. Procedural digital subtraction angiography (B,C) showing a 5 Fr Cobra catheter (dotted black arrow) at the origin of the right renal artery from where originates the middle adrenal artery; a 2.7 Fr microcatheter (continuous black arrow) reaches the distal segment of the right middle adrenal artery and the contrast agent injection confirms the multiple spot extravasation findings (black circle), as CT; Gelfoam was adopted as embolizing agent. Post embolization digital subtraction angiography (D) after contrast injection from the 5 Fr Cobra catheter (dotted black arrow), revealing no more extravasation in the embolized area.
Technique
Urgent embolization was required because of hemoglobin fall and hemodynamic instability due to hemorrhage.
A diagnosis of active bleeding was performed at contrast enhanced CT before each procedure; active bleeding was detected in correspondence of adrenal glands.
The adrenal arteries were analyzed before the procedure with post-processing CT maximum intensity projections and multiplanar reconstructions in arterial phases (slice thickness 0.6 mm) to plan the procedural strategy.
An aortic angiography as well as a renal arteriography were performed in all cases.
A 5 Fr femoral introducer was positioned in the right femoral artery under US-guidance; the aortic angiography was conducted with a 5 Fr Pig-Tail (contrast agent injection parameters: volume 20 mL, flow 20 mL/s) while phrenic, renal and adrenal arteries originating directly from the aorta were investigated through 5 Fr diagnostic catheters with different curve tips (most commonly Shepherd or Cobra1 Cook Medical® or Merit Medical®).
Superselective angiography of the adrenal arteries was obtained by positioning a 2.7 Fr microcatheter (Terumo® Progreat) at the origin of the vessel revealing the vascular injuries.
Different embolizing agents were adopted according to the type of vessel lesion and operator preference.
In case of persistent hemodynamic instability or further decrease in hemoglobin values after embolization, another CT was performed in order to detect possible bleeding recurrence.
Statistical analysis
All data analyses were performed in a MATLAB environment (MathWorks, Inc, Natick, Massachusetts, USA).
Results
Technical success, considered as interruption of adrenal bleeding detectable at angiography, was 94.1% (16 out 17 patients); 1 patient required a second embolization after 5 hours because of bleeding relapse.
Clinical success, considered as hemodynamic stability restoration within 24 hours from the procedure, was obtained in 82.3% (14 out of 17 patients); 3 patients died during the recovery, 2 because of associated major traumatic injuries and 1 because of advanced oncological disease.
The vessels involved were the superior adrenal artery in 5 patients, the middle adrenal artery in 8 patients, the inferior adrenal artery in 1 patient and more than one adrenal artery in 3 patients (Table 2).
Table 2. Adrenal arteries embolized, appearances of hemorrhages at angiography and embolizing agents adopted.
| Patient | Adrenal artery | Angiographic sign | Embolizing agent |
|---|---|---|---|
| 1 | Superior | Extraluminal blush | Microparticles |
| 2 | Superior/middle | Extraluminal blush | Glue |
| 3 | Middle | Extraluminal blush | Glue |
| 4 | Middle | Pseudoaneurysm | Glue |
| 5 | Superior | Extraluminal blush | Microparticles |
| 6 | Middle | Extraluminal blush | Glue/coils/microparticles |
| 7 | Middle | Extraluminal blush | Gelfoam |
| 8 | Middle | Disomogeneous impregnation | Glue |
| 9 | Middle | Disomogeneous impregnation | Microparticles/coils |
| 10 | Superior | Disomogeneous impregnation | Coils |
| 11 | Middle | Extraluminal blush | Microparticles |
| 12 | Superior | Extraluminal blush | Microparticles/gelfoam |
| 13 | Superior/middle | Extraluminal blush | Microparticles/glue |
| 14 | Inferior | Extraluminal blush | Coils |
| 15 | Middle/inferior | Extraluminal blush | Microparticles/coils |
| 16 | Superior | Extraluminal blush | Gelfoam |
| 17 | Middle | Extraluminal blush | Microparticles |
The superior adrenal artery originated from the aorta [1], the phrenic artery [5] and the hepatic artery [1]; the middle adrenal artery originated from the aorta [7] and the renal artery [4]; the inferior adrenal artery originated from the renal artery [2].
In 13 cases the angiographic bleeding appearance was extraluminal contrast agent blush, in 3 it was a disomogeneous hematoma impregnation and in 1 it was a pseudoaneurysm.
The embolizing agents adopted were: glue in 4 cases, microparticles (ranging 300–500 or 500–700 micron) in 4 cases, gelfoam in 2 cases, coils in 2 cases and more than one of the abovementioned agents in 5 cases.
According to CIRSE classification system (10) for complication related to interventional procedure, we reported 2 groin hematoma self-limiting with compressive bandage (grade II) without sequelae.
No patients had infarctions, necrosis, abscess formation, or required long-term steroid supplementation.
Discussion
Transarterial adrenal embolization has been used for different aims: oncologic applications for palliation, such as pain relief, reduction of tumor bulk, and preoperative reduction of tumor vascularity; treatment of endocrinological diseases as hyperaldosteronism; emergency procedures for hemostasis of retroperitoneal hemorrhage caused by ruptured tumors, traumatic injuries and adrenal artery aneurysms (11).
The successful outcome of adrenal embolization requires first of all the proper interpretation of adrenal arteries anatomy. Arterial flow to each gland is usually supported by 3 arteries: superior, middle and inferior adrenal arteries which origin mostly from the phrenic artery, the aorta and the renal artery respectively. Other possible origins involve celiac axis, intercostal arteries and gonadal arteries (3).
Each adrenal artery divides into 10–50 small branches; these penetrate the visceral capsule and form a complex vascular plexus (12,13) which in case of injuries generate massive hemorrhages. On the other hand this vascular network allows to avoid gland infarction after transarterial embolization.
Because of this complex arterial supply, in order to accurately plan the embolization, it is crucial to deeply investigate the source of bleeding at contrast-enhanced CT with thin slice and post-processing reconstructions. Furthermore, in traumatic hemorrhages, CT allows to detect eventual associated vascular injuries.
Accordingly to literature data, in this sample the right adrenal gland has been involved more often than the left one in traumatic hemorrhages. Bleedings occur more often on the right side because of its anatomical location, especially in blunt abdominal trauma (6). Proposed mechanisms of the right adrenal gland are direct compression of the adrenal gland between the spine and the liver or an acute rise in intra-adrenal venous pressure due to compression of the inferior vena cava (7,8). Alternatively hemorrhage may be secondary to deceleration forces that result in shearing of the small vessels that perforate the adrenal capsule.
About neoplastic hemorrhages, rapid tumor growth has been postulated to result in elevated intracapsular pressure that leads to capsular tear and hemorrhage. Massive retroperitoneal bleeding can origin from spontaneous rupture of adrenal tumors, as pheochromocytoma, myelolipoma, metastases, and an adrenal cortical neoplasm (3).
Pheochromocytoma is the most common primary adrenal tumor to cause massive bleeding. It accounted for approximately 48% of hemorrhagic adrenal masses in a review of 133 reported cases (14); this can be explained by massive release of catecholamines with associated vasoconstriction, tumoral necrosis and hemorrhage (15).
Concerning metastasis, the most frequent site of primary tumors resulting in hemorrhagic adrenal metastases is the lung (16,17); this data is in accordance with the one observed in the present study.
Because of the rarity of adrenal hemorrhages, till today no consensus has been reached on the management of this scenario. Several authors have advised aggressive management and early intervention (18-20) while in contrast an increasing number of studies have reported that conservative management can be adopted as well (17,21). In the center where the study has been conducted, the indication for embolization is mainly based on the clinical conditions of the patient: in case of hemodynamic instability with CT signs of arterial bleeding, transarterial embolization are performed.
Concerning the choice of the embolizing agent, there is no evidence in literature of the superiority of a specific one; actually it depends on operator preference, availability and type of vascular injury. Frequently, multiple agents are employed even if microparticles are the most adopted because of their distalization in the small peripheral adrenal branches. Based on the reported experience, use of a microcatheter is mandatory considering the low caliper of adrenal arteries and the possibility of spasms.
Overall, among all the embolization performed in our department because of acute bleeding, adrenal hemorrhages represented 1.5%; this value is comparable to the data reported in literature (20) by Liao et al. referring adrenal gland involved in 1.7% of all the thoraco-abdominal trauma. However it should be considered that with the rising employment of CT scan in trauma centers, this value will probably increase and consequently even its endovascular management because of the frequent associated thoraco-abdominal injuries.
This study presents multiple limitations: first this is a retrospective analysis and therefore bias selections should be considered; then the number of patients is small, however this is due to low frequency of the scenario and nevertheless this is the widest case-series of acute adrenal bleeding embolizations reported in literature based on our knowledge.
In conclusion, acute adrenal hemorrhages can be safely and effectively managed by catheter directed embolizations; anatomical examination of the arterial supply to these glands has to be carefully examined because multiple vessels can be involved with vascular variants. In case of trauma, associated multiple bleeding sources with thoraco-abdominal localizations should be expected.
Acknowledgements
None.
Ethical Statement: The institutional ethical committee approved this retrospective review of patients’ medical and imaging record in accordance with the Helsinki Declaration 2013. Participant gave informed consent before taking part to this study.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Velmahos GC, Chahwan S, Falabella A, et al. Angiographic embolization for intraperitoneal and retroperitoneal injuries. World J Surg 2000;24:539-45. 10.1007/s002689910087 [DOI] [PubMed] [Google Scholar]
- 2.Ierardi AM, Iacobellis F, Carrafiello G, et al. Vascular Emergencies of the Retroperitoneum: Recent Advances in MDCT and Interventional Radiology. In: Patlas M, Katz D, Scaglione M. editors. 2018 MDCT and MR Imaging of Acute Abdomen. Cham: Springer International Publishing; 2018;151-77. [Google Scholar]
- 3.Fowler AM, Burda JF, Kim SK. Adrenal Artery Embolization: Anatomy, Indications, and Technical Considerations. AJR 2013;201:190-201. 10.2214/AJR.12.9507 [DOI] [PubMed] [Google Scholar]
- 4.Igwilo OC, Sulkowski RJ, Shah MR, et al. Embolization of traumatic adrenal hemorrhage. J Trauma 1999;47:1153-5. 10.1097/00005373-199912000-00032 [DOI] [PubMed] [Google Scholar]
- 5.Dinc H, Simsek A, Ozyavuz R, et al. Endovascular treatment of massive retroperitoneal haemorrhage due to inferior adrenal artery injury. Acta Radiol 2002;43:326-8. 10.1034/j.1600-0455.2002.430316.x [DOI] [PubMed] [Google Scholar]
- 6.Ikeda O, Urata J, Araki Y, et al. Acute adrenal hemorrhage after blunt trauma. Abdominal Imaging 2007; 32:248-52. 10.1007/s00261-006-9046-7 [DOI] [PubMed] [Google Scholar]
- 7.Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 38-1984. Hypertension and an adrenal mass after a vehicular accident. Massachusetts General Hospital Case Records, case 38-1984. N Engl J Med 1984;311:783-90. [DOI] [PubMed] [Google Scholar]
- 8.Wilms G, Marchal G, Baert A, et al. CT and ultrasound features of post-traumatic adrenal hemorrhage. J Comput Assist Tomogr 1987;11:112-5. 10.1097/00004728-198701000-00022 [DOI] [PubMed] [Google Scholar]
- 9.World Medical Association Declaration of Helsinky: ethical principles for medical research involving human subjects. JAMA 2013;310:2191-4. 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 10.Filippiadis DK, Binkert C, Pellerin O, et al. Cirse quality assurance document and standards for classification of complications: the cirse classification system. Cardiovasc Intervent Radiol 2017;40:1141-6. 10.1007/s00270-017-1703-4 [DOI] [PubMed] [Google Scholar]
- 11.Ierardi AM, Petrillo M, Patella F, et al. Interventional radiology of the adrenal glands: current status. Gland Surg 2018;7:147-65. 10.21037/gs.2018.01.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bookstein JJ. The roles of angiography in adrenal disease. In: Abrams HL. editor. Abrams angiography: vascular and interventional radiology. Boston, MA: Little, Brown, 1983:1395-424. [Google Scholar]
- 13.Dobbie JW, Mackay AM, Symington T. The structure and functional zonation of the human adrenal cortex. In: James VH, Landon J. editors. The investigation of hypothalamic-pituitary-adrenal function. London, UK: Cambridge University Press, 1968:103-11. [Google Scholar]
- 14.Marti JL, Millet J, Sosa JA, et al. Spontaneous adrenal hemorrhage with associated masses: etiology and management in 6 cases and a review of 133 reported cases. World J Surg 2012; 36:75–82. 10.1007/s00268-011-1338-6 [DOI] [PubMed] [Google Scholar]
- 15.Habib M, Tarazi I, Batta M. Arterial embolization for ruptured adrenal pheochromocytoma. Curr Oncol 2010;17:65-70. 10.3747/co.v17i6.597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lam KY, Lo CY. Metastatic tumours of the adrenal glands: a 30-year experience in a teaching hospital. Clin Endocrinol (Oxf) 2002;56:95-101. 10.1046/j.0300-0664.2001.01435.x [DOI] [PubMed] [Google Scholar]
- 17.Ambika S, Melton A, Lee D, et al. Massive retroperitoneal adrenal hemorrhage secondary to lung cancer metastasis treated by adrenal artery embolization. Clin Lung Cancer 2009;10:E1-4. 10.3816/CLC.2009.n.053 [DOI] [PubMed] [Google Scholar]
- 18.Udobi KF, Childs EW. Adrenal crisis after traumatic bilateral adrenal hemorrhage. J Trauma 2001;51:597-600. 10.1097/00005373-200109000-00036 [DOI] [PubMed] [Google Scholar]
- 19.Stawicki SP, Hoey BA, Grossman MD, et al. Adrenal gland trauma is associated with high injury severity and mortality. Curr Surg 2003;60:431-6. 10.1016/S0149-7944(02)00796-1 [DOI] [PubMed] [Google Scholar]
- 20.Liao CH, Lin KJ, Fu CY, et al. Adrenal Gland Trauma: Is Extravasation an Absolute Indication for Intervention? World J Surg 2015;39:1312-9. 10.1007/s00268-015-2953-4 [DOI] [PubMed] [Google Scholar]
- 21.Lee YS, Jeong JJ, Nam KH, et al. Adrenal injury following blunt abdominal trauma. World J Surg 2010;34:1971-4. 10.1007/s00268-010-0537-x [DOI] [PubMed] [Google Scholar]