Abstract
Objectives The aim of this study is to report the clinical outcome of extracranial pericranial flaps (ePCF) used for reconstruction of clival dural defects following failure of primary repair.
Design Retrospective review of skull base database.
Setting Academic medical center.
Participants Patients undergoing reconstruction of clival defects with ePCF following endoscopic endonasal surgery (EES).
Main outcome measures Postoperative cerebrospinal fluid (CSF) leak, meningitis, and flap necrosis.
Results Seven patients (five males and two females) who underwent ePCF reconstruction for clival defects following EES were included. All patients (ages 8–64 years) had a postoperative CSF leak due to a failed primary clival reconstruction (five had one, one had two, and one had three failed CSF leak repairs prior to ePCF reconstruction). Nasoseptal and inferior turbinate (lateral nasal wall) flaps were not available for secondary reconstruction due to prior surgeries. The immediate success rate of ePCF for the reconstruction of clival defects in patients with multiple flap failures was 58%. Two patients developed CSF leaks that were successfully repaired endoscopically with the addition of free tissue grafts; one patient had partial flap necrosis that required debridement; none required an additional vascularized flap. Width of the defect, length of the defect, properties of the ePCF, and age did not demonstrate significance ( p > 0.05) for adverse outcome.
Conclusion An ePCF is a reconstructive option for high-risk, large clival defects when other local and regional vascularized flaps are not available or fail. ePCFs can be used for reconstruction of clival defects in all populations, including pediatric patients.
Keywords: chordoma, clival defect, pericranial flap, skull base reconstruction, skull base tumors
Introduction
Restoring the structural integrity of the skull base following endoscopic endonasal surgery (EES) remains one of the major challenges in modern skull base surgery. The guiding principle is to prevent communication between the paranasal sinuses and the intracranial space to minimize the risk for cerebrospinal fluid (CSF) leak, tension pneumocephalus, and meningitis. Multi-layered reconstructions covered by a vascularized flap (pedicled autograft) have yielded the best results. 1 Reconstruction choices are dependent on prior cranial base surgery, the size, shape, and location of the defect, as well as planned adjuvant therapy, individual patient characteristics, and the capabilities of the surgical team. 1
In the sagittal plane, endoscopic endonasal approaches (EEAs) include transfrontal, transcribriform, transplanum, transsellar, transclival, and transodontoid approaches. 2 For most approaches, the nasoseptal flap is the preferred vascularized flap for reconstruction. 3 4 For defects of the anterior cranial base, the extracranial pericranial flap (ePCF) is utilized when the nasoseptal flap is inadequate due to prior surgery with compromise of the vascular pedicle or tumor involvement. For clival defects, the lateral nasal wall flap (inferior turbinate flap) is utilized when a nasoseptal flap in not available or has failed. 1 Even in cases where the vascular pedicle for a nasoseptal flap is compromised, the blood supply for a lateral nasal wall flap is usually intact. When there is failure of primary and secondary reconstructions, regional flaps, such as the temporoparietal flap and palatal flap, are reconstructive options.
The pericranial flap (PCF) has not been promoted as a suitable option in the algorithm for high-flow clival defects, in contrast to the flaps mentioned above. 1 The ePCF is most commonly used for reconstruction of anterior skull base defects, not for dural defects after posterior fossa surgery (transclival and transodontoid approaches). 5 6 7 8 In a radiological study among adult subjects, the average PCF length needed to cover a clival defect was reported to be between 18.5 and 20.4 cm. 8 It has been concluded that PCF may not reach caudally enough to cover clival defects and therefore should not be considered as the primary method for reconstruction. 1 5 8 Alternatively, the temporoparietal fascia flap (TPF) was suggested to be the next best option for the reconstruction of clival defects when the NSF is unavailable, and the defect is too large for an inferior turbinate flap. 5
No data exist in the current medical literature regarding the technical and clinical considerations of using the ePCF for the reconstruction of clival defects. The aim of this paper is to report and critically appraise the clinical outcome of ePCF that were used for reconstruction of clival dural defects. The results and limitations are presented on the basis of our experience with seven cases. Cadaveric dissections, mirroring the surgical situation, allowed for an illustration and refinement of the surgical technique used in the reported cases.
Methods
This study was performed at the University of Pittsburgh School of Medicine and University of Pittsburgh Medical Center (UPMC) and was approved by the University of Pittsburgh Institutional Review Board (IRB# PRO14080496).
Patient Selection
Seven patients (5 males and 2 females, ages 8–64 years) who underwent PCF reconstruction for clival defects due to EES between June 2010 and November 2016 were included ( Table 1 ). No patients were excluded.
Table 1. Clinical features of the patients, their clival dural defects, and PCF.
| Patient | Age | Primary diagnosis | Prior treatments | Number of prior unsuccessful CSF leak surgeries | Indication for PCF | Prior radiation therapy |
Frontal sinus surgery | Lumbar drain | PCF side | Width of clival dural defect (cm) a | Length of the clival dural defect (cm) a | Level of the inferior border of the clival defect | Estimated length of PCF needed (cm) a b |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 14 | Recurrent Chordoma | Chordoma surgery, CSF leak surgery | 1 | CSF leak (failed graft) | Yes | N/A | Yes | Right | 2,15 | 1,99 | Inferior border of C1 | 17.74 |
| 2 | 22 | Recurrent Chordoma | Chordoma surgery, CSF leak surgery, RT | 1 | CSF leak (necrotic ITF) | Yes | Draf-II | Yes | Right | 1,77 | 3,28 | Inferior one third of clivus | 18.99 |
| 3 | 8 | Primary Chordoma | CSF leak surgery | 2 | CSF leak (necrotic ITF, TPF) | No | N/A | No | Bilateral | 3,01 | 3,62 | Inferior border of C1 | 17.89 |
| 4 | 52 | Recurrent Chordoma | Chordoma surgery, CSF leak surgery, RT | 1 | CSF leak (necrotic ITF) | Yes | Draf-III | Yes | Right | 2,45 | 4,19 | Inferior one third of clivus | 18.34 |
| 5 | 64 | Recurrent chordoma | Chordoma surgery, CSF leak surgery, RT | 1 | CSF leak (failed ITF) | Yes | Draf-III | No | Right | 1,94 | 3,23 | Inferior one third of clivus | 20.59 |
| 6 | 15 | Primary chordoma | CSF leak surgery | 3 | CSF leak (failed ITF) | No | Draf-III | Yes | Bilateral | 3,42 | 4,82 | Inferior one third of clivus | 20.54 |
| 7 | 60 | Primary chordoma | CSF leak surgery | 1 | CSF leak (necrotic NSF, ITF) | No | Draf-II | Yes | Left | 2,95 | 5,45 | Inferior one third of clivus | 20.50 |
Abbreviations: CSF, cerebrospinal fluid; ITF, inferior turbinate flap; PCF, pericranial flaps; NSF, nasoseptal flap; RT, radiation therapy; TPF, temporoparietal flap.
Measurements were done on preoperative CT scans and sagittal and axial sections.
From nasion to the most inferior part of the anticipated defect + external pedicle length + 3 cm. 8
Instrumentation and Procedure
Clinical data were retrieved from the UPMC Center for Cranial Base Surgery database and included age, primary diagnosis, past medical and surgical history, prior radiotherapy or chemotherapy, indications for surgery, prior reconstructions, and indications for PCF reconstruction. Intraoperative data included region and size of the clival defect, degree of CSF leak (high or low flow), laterality of PCF, and extent of sinus surgery required for flap introduction (ethmoid and frontal). Postoperative data included use of a lumbar drain, CSF leak, flap necrosis, and complications, such as meningitis or frontal sinusitis and mucocele.
Using axial and sagittal sections of the preoperative computed tomography (CT) scan, the anticipated length of the pericranial flap was calculated by drawing a line along the skull base from the nasion to the top of the defect, added to the distance from the top to the bottom of the defect. As previously described, an additional 3 cm was added to the measurement to calculate the length of the PCF needed ( Table 2 ). 8 Additionally, the largest size of the clival defect was measured on axial and sagittal sections of CT scan ( Table 2 ).
Table 2. Outcome of reconstruction of clival defects with PCF.
| Patients | Postoperative frontal sinusitis | Postoperative meningitis | Postoperative CSF leak | Postoperative flap necrosis | Outcome | Management |
|---|---|---|---|---|---|---|
| 1 | No | No | No | No | Successful | |
| 2 | No | No | No | No | Successful | |
| 3 | No | No | No | No | Successful | |
| 4 | No | Yes | Yes | Yes (partial) | Failed (necrotic) | Fascia lata and fat grafts |
| 5 | Yes (right side) | No | Yes | No | Failed (CSF leak) | Fascia lata and fat graft |
| 6 | Yes (right side) | Yes | Yes | No | Failed (CSF leak) | Stitch viable PCF up to nasopharynx + fat graft |
| 7 | Yes (left side) | No | No | No | Successful |
Abbreviations: CSF, cerebrospinal fluid; PCF, pericranial flap.
The surgical procedure for an ePCF warrants additional endonasal surgery to transfer the PCF to the clivus. This includes a total anterior and posterior ethmoidectomy on one side with removal of all mucosa on the anterior cranial base and medial orbital wall and an extended endoscopic frontal sinusotomy (Draf-II or III). A bicoronal scalp incision is made over the vertex of the cranium to optimize cosmesis. Posteriorly, the scalp can be dissected in a subgaleal plane to extend the length of the pericranial flap. The scalp is then elevated deep to the periosteum to the level of the supraorbital rims. Care is taken to preserve the temporal branches of the facial nerve bilaterally. The periosteum is elevated from the nasal bones at the level of the nasofrontal junction. A drill is used to create a transverse opening 1.5 to 2.0 cm wide at the level of the nasion below the frontal sinuses. The vertical dimension must be sufficient to prevent compression of the flap pedicle (>1.0 cm). The pericranial flap is then dissected from the scalp in a subgaleal plane, pedicled on one or both supraorbital and supratrochlear vessels. The flap pedicle is carefully narrowed to provide a greater arc of rotation without injury to the vascular supply. The pericranial flap is then transposed into the nasal cavity through the bony window at the nasion and advanced endoscopically along the skull base to the clival defect. Even a unilateral flap is more than adequate to cover the width of the skull base.
Outcome Measures
Clinical, intraoperative and postoperative data of patients were assessed for the reconstruction of clival defects with ePCF. Reconstruction of the clival defect with an ePCF was considered successful if the patient was free of any of the following: postoperative CSF leak, meningitis, or flap necrosis. Anticipated radiologically measured length of the PCF and size of the clival defect were compared with outcomes. Regarding the surgical procedure, special considerations, possibilities and limitations of using a PCF for clival defects were explored with cadaver dissections.
Statistical Analysis
Statistical analysis was made using computer software (SPSS version 17.0, SPSS Inc. Chicago, Illinois, United States). Chi-squared ( χ 2 ) and Fisher's exact tests were used for the comparison of categorical data while Wilcoxon and Mann–Whitney U tests were used for the analysis of non-parametric variables based on the distribution pattern of the data. Data were expressed as “mean (standard deviation [SD])” and “median (interquartile range [IQR])” where appropriate. Statistical significance was established at p < 0.05.
Results
The mean age of patients was 34 (8–64) years, whereas median age was 22 (IQR: 46) years. Mean follow-up was 23.6 (1–77) months. Clinical properties of patients, such as age, primary diagnosis, primary surgery, approach, prior radiation therapy (RT), indication, and decision making behind the use of an ePCF are shown in Table 1 .
The primary diagnosis was chordoma (four recurrent and three primary) in all patients who underwent endoscopic endonasal transclival surgery. Prior to reconstruction with ePCF, all patients failed repair of postoperative CSF leaks following primary surgery (five had one, one had two, and one had three failed CSF leak revisions after primary reconstruction failure). The indication for ePCF reconstruction was multiple recurrent CSF leaks (range 2–4) in all patients ( Table 1 ). CSF leak was due to failed grafts in one, failed viable flaps in two, and necrotic flaps in four cases. Neither NSF nor inferior turbinate flap was available in any of the cases due to prior surgeries at the time of ePCF reconstruction. Four patients had preoperative RT prior to ePCF reconstruction. One of the patients had chronic kidney disease and renal transplantation (Patient #5) which likely had an impact on wound healing.
Regarding intraoperative findings, all patients had large posterior fossa defects following initial surgery and presented with high-flow CSF leaks. ePCF was right-sided in four, left-sided in one, and bilateral in two patients ( Table 2 ). Unilateral ethmoidectomy was performed in all cases on the side of the harvested flap. Two children had an undeveloped frontal sinus, but the remaining five patients needed a frontal sinusotomy, which was extended Draf-II in two and Draf-III in three. A lumbar drain was placed in five cases.
Mean width and length of the clival defect were 2.52 cm (1.77–3.42) and 3.79 cm (1.99–5.45), respectively ( Table 1 ). The lowest level of the defect was the inferior clivus in five and the inferior border of the first cervical vertebrae in two patients. The mean anticipated length of the PCF based on radiographic analysis was 19.23 cm (17.74–20.59).
Postoperatively, three (42%) patients had failure of ePCF reconstruction, one due to CSF leak, one due to meningitis and CSF leak, and one due to partial flap necrosis (distal part), meningitis and CSF leak ( Tables 1 and 2 ). Flap survival rate was 86%, whereas success of first repair was 58%. Other postoperative complications included deep venous thrombosis in one and hydrocephalus in three patients. Flap failures were successfully managed by fascia lata and fat grafts in two patients. Nasopharyngeal mucosa was sutured to the viable PCF in one case for the management of CSF leak. None of the patients had clinically significant frontal sinusitis symptoms. However, three patients (two with Draf-III and one with Draf-II) had frontal sinus opacification on the side of the PCF on postoperative magnetic resonance imaging (MRI) scans. There were no frontal mucoceles. Three patients (#3, #6, and #7) received postoperative RT after healing was complete.
Mean width of the clival defect in patients with a successful or failed ePCF was 2.47 cm (SD: 0.60) and 2.60 cm (SD: 0.75), respectively ( p = 0.72). Mean length of the clival defect in patients with a successful or failed ePCF was 3.58 cm (SD: 1.42) and 4.08 cm (SD: 0.80), respectively ( p = 0.72). Mean width of the clival defect in patients with a unilateral or bilateral PCF was 2.25 cm (SD: 0.46) and 3.21 cm (SD: 0.28), respectively ( p = 0.05). Mean length of the clival defect in patients with a unilateral or bilateral PCF was 3.62 cm (SD: 1.28) and 4.22 cm (SD: 0.84), respectively ( p = 0.43). Mean estimated length of PCF was 18.78 cm (SD: 1.27) and 19.83 cm (SD: 1.26) in successful and failed reconstructions, respectively ( p = 0.15). Neither the anticipated flap length nor the lowest level of clival defect had a statistically significant effect on flap outcome ( p = 0.18). Mean age was 26 (SD: 23.38) and 43.67 (SD: 25.54) years in patients with successful and failed PCF, respectively ( p = 0.28).
Discussion
The reconstructive algorithm for large dural defects associated with a transclival approach is a multi-layered reconstruction including vascularized tissue. Our preferred technique is inlay and onlay collagen and fascial grafts with adipose tissue graft and a vascularized flap. The inclusion of adipose tissue interposed between the fascia and vascularized flap has been shown to prevent pontine herniation through the defect. 9 Local vascularized flaps are utilized as the first option. These include the nasoseptal flap and lateral nasal wall flap, both dependent on the sphenopalatine artery. 10 When a local flap is not available or has failed, a second option is to utilize regional vascularized flaps. The temporoparietal fascial flap is most frequently employed and is based on the superficial temporal artery. Transposition of the flap requires the creation of a tunnel from the infratemporal skull base across the pterygopalatine space to the clival region. 5 Limitations of this flap include difficulty of harvest, distance from the surgical field (long pedicle), morbidity of additional dissection, and limited flap dimensions. The temporoparietal fascial flap is most suitable for reconstruction of middle and inferior clival defects. Clinical experience with the palatal flap is limited. 5
Traditionally, the ePCF has not been advocated in the reconstruction algorithm for high-flow clival defects. In patients who have had local flap failures or wound healing problems, reconstruction of a clival defect with ePCF should be considered. Relative advantages of the ePCF include accessibility, ease of dissection, reliable blood supply, large dimensions (length and width), and limited morbidity with good cosmesis ( Fig. 1 ). The bilateral blood supply allows the design of a unilateral flap, preserving the contralateral flap for future reconstructive needs. Additionally, vascularization of anteriorly based PCF was found to be superior to laterally based flaps in a recent experimental study, suggesting a more reliable and durable reconstruction. 11 An extracranial PCF minimizes the morbidity of PCF reconstruction by avoiding a craniotomy. There is also potential for minimally invasive endoscopic harvest. 12
Fig. 1.
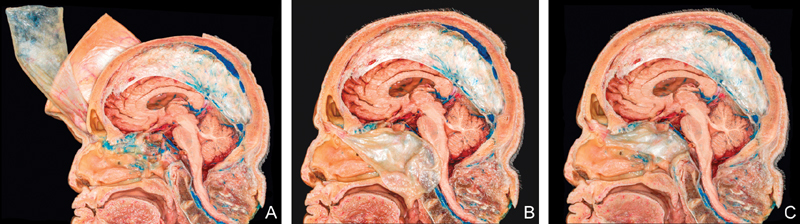
Cadaveric dissections of extracranial pericranial flap (sagittal view). ( A ) Elevated flap with osteotomy at nasion. ( B ) Flap is passed through the bony window below the frontal sinus and along the roof of the ethmoidectomy defect. Note the large dimensions of the flap. ( C ) The flap is placed over the clival defect.
Graft materials such as fascia lata and fat are among the available materials, which may be used to close postoperative CSF leaks and deserve to be discussed. A suitable option for limited CSF leaks without a large necrotic flap or graft is grafting with fascia lata. However, in our particular patient group, four patients had large necrotic flaps, which warranted debridement and flap reconstruction to cover vital structures such as the brainstem and large vessels. Furthermore, adjuvant RT may be a reason to select flaps over grafts. Additionally, all seven patients had at least one unsuccessful repair of the CSF leak, either with grafts or with flaps prior to using the ePCF.
In comparison to other sites, clival defects have the highest risk of postoperative CSF leak following EES. 13 Contributing factors include high CSF flow, proximity of critical vascular structures, dead space, and increased postoperative CSF pressure due to dependent location and patient factors (obesity). In a randomized clinical trial, the use of lumbar spinal drainage was shown to dramatically reduce the risk of postoperative CSF leak and should be employed for all clival dural defects over 1 cm 2 (Zwagerman NT, Wang EW, Shin S, et al. Does lumbar drainage reduce postoperative cerebrospinal fluid leak after endoscopic endonasal skull base surgery? A prospective, randomized controlled trial. Unpublished data).
In a previous review, overall success rate for endoscopic reconstruction of large dural defects was reported as 88.5%. 14 They concluded that reconstruction with a vascular flap significantly increases the success rate. 14 Information regarding the success rate of secondary or tertiary flaps for reconstruction of high-flow, large clival defects was not reported. Contrary to their findings, defect size in our study was not correlated with the success rate of PCF in the reconstruction of clival defects. All the defects in our series were large, however, and the PCF was tailored to provide complete coverage of the defect.
Estimated length of the pericranial flap can be measured on CT scan based on the lowest level of the clival defect 8 15 and is helpful in planning the scalp incision and posterior/retrograde dissection to harvest additional length. The estimated length of the flap, however, had no effect on success of the PCF in our series. In all cases, the PCF was able to reach the inferior limit of the defect ( Figs. 2 and 3 ). It is important to avoid errors that may compromise the vascularity of the flap: atraumatic dissection of pericranial layer from galea, preserve branches of supraorbital vessels, avoid torsion or compression of vascular pedicle, and avoid desiccation of flap.
Fig. 2.
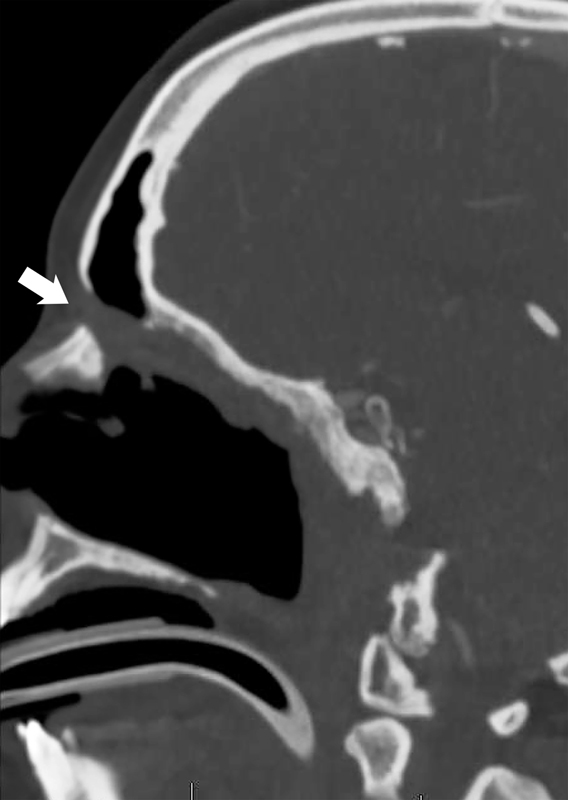
Postoperative parasagittal view of CT scan of patient #2. The extracranial pericranial flap passes through the osteotomy at the nasion (arrow) and along the roof of the ethmoidectomy cavity to reach the clival defect. Drainage of the frontal sinus is maintained by displacing the flap pedicle to the ipsilateral side. CT, computed tomography.
Fig. 3.
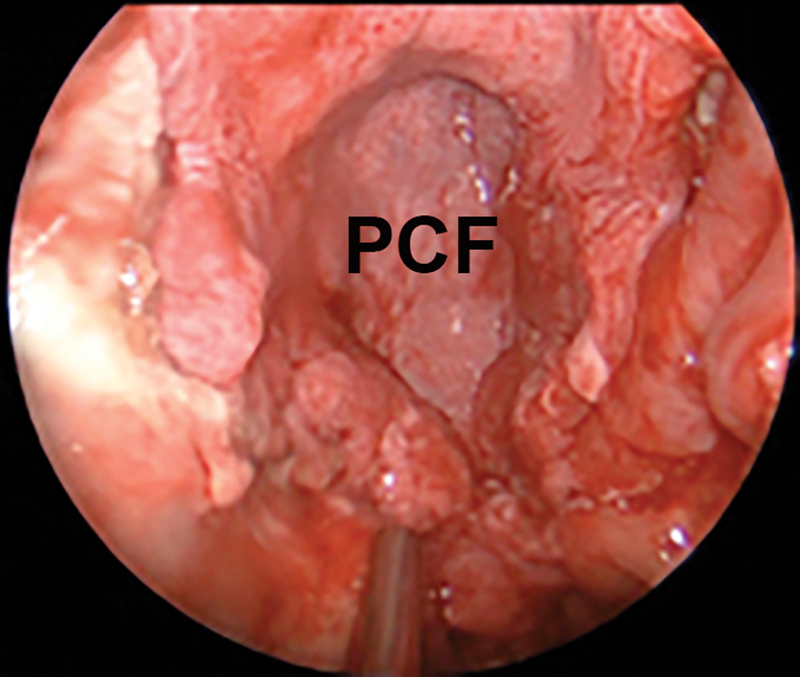
Intraoperative picture of extracranial PCF covering clival defect. PCF, pericranial flap.
There are some special considerations for selection of PCF reconstruction for clival defects ( Table 3 ). To have adequate length, care must be taken during the bicoronal incision so that the pericranium is not divided. 16 Localization and size of the nasionectomy is important in such flaps. Postoperative flap edema may compress the vascular pedicle if the osteotomy is too small. Excessive torsion of the pedicle may also compromise its survival. 17 There are different techniques defined for transfrontal transfer of the flap. 8 18 19 The size of the opening should be at least 10 × 15 mm. Localization of the opening has been described as low (e.g., nasionectomy) or high (e.g., mailbox slot technique). 18 19 By following the general principles of facial aesthetics and scar formation, the bone opening should preferably be on a concave surface rather than on a convex one. Furthermore, one should avoid the thinnest skin over medial canthi. 20 Softening the transition of the surfaces by drilling the sharp edges of the opening limits acute angulation of the pedicle.
Table 3. Special considerations in the selection of PCF reconstruction of clival dural defects.
| Considerations | Descriptions |
|---|---|
| Are there any better reconstruction options? | Nasoseptal and inferior turbinate flaps should be unavailable. |
| What is the most appropriate length of PCF for clival defects? | Since clivus is an extended application of PCF, dissection must be directed posteriorly from the bicoronal incision, to be able to harvest the longest possible flap. |
| Which incision should be used to harvest a PCF? | Since this is an extended application of the PCF, bicoronal incision may be a better choice. |
| Bilaterally or unilaterally based | Unilaterally based flap should be preferred since it is easier to transfer through ethmoidectomy cavity and has a wider angle of rotation. Bilaterally based flaps are reserved for wider defects. |
| When should it be harvested, in the beginning or at the end of surgery? | Harvest after scalp elevation is preferred since the vascular supply is better visualized. |
| What should be the lateral and medial border of the PCF and its pedicle? | Medial border should not pass the midline. Lateral border is the superior temporal line. |
| Where is the most suitable place to create bony window to transfer PCF into nasal cavity? | Just inferior to glabella at nasofrontal junction, at least 10 mm height and 15 mm width to protect pedicle from compression. |
| What are the additional surgeries necessary to transfer PCF to the clivus? | Total ethmoidectomy should be on the ipsilateral side with the flap. The mucosa should be removed to expose bone. Either an extended Draf-II or Draf-III frontal sinusotomy can be performed in unilaterally based flaps, whereas Draf-III must be performed in bilaterally based flaps. |
Abbreviation: PCF, pericranial flaps.
The extent of the frontal sinusotomy is debatable in that both Draf-II and Draf-III frontal sinusotomies have been performed in different techniques. There is a potential risk of obstructing the drainage of frontal sinuses during intranasal transfer of PCF. Either an extended Draf-II or Draf-III frontal sinusotomy can be performed in unilaterally based flaps, whereas Draf-III must be performed in bilaterally based flaps. There was no difference for frontal sinus complications in this study; however, the small number of patients prevents any certain conclusions.
Conclusion
This is the first study which presents clinical outcome of PCF used as a secondary or tertiary flap for clival defects. The extracranial PCF is a valuable reconstructive option for patients who have failed other reconstructive techniques, or when other options are not available. The PCF is applicable to all patient populations, including pediatric patients. Neither clival defect size nor estimated length of the flap had an effect on the success of PCF reconstruction as employed in our series.
Acknowledgments
No funding support.
Footnotes
Conflicts of Interest The authors have no conflicts of interest to disclose.
References
- 1.Patel M R, Stadler M E, Snyderman C H et al. How to choose? Endoscopic skull base reconstructive options and limitations. Skull Base. 2010;20(06):397–404. doi: 10.1055/s-0030-1253573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kassam A, Thomas A J, Snyderman Cet al. Fully endoscopic expanded endonasal approach treating skull base lesions in pediatric patients J Neurosurg 2007106(2, Suppl):75–86. [DOI] [PubMed] [Google Scholar]
- 3.Hadad G, Bassagasteguy L, Carrau R L et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope. 2006;116(10):1882–1886. doi: 10.1097/01.mlg.0000234933.37779.e4. [DOI] [PubMed] [Google Scholar]
- 4.Kassam A B, Thomas A, Carrau R Let al. Endoscopic reconstruction of the cranial base using a pedicled nasoseptal flap Neurosurgery 2008630101ONS44–ONS52., discussion ONS52–ONS53 [DOI] [PubMed] [Google Scholar]
- 5.Patel M R, Taylor R J, Hackman T G et al. Beyond the nasoseptal flap: outcomes and pearls with secondary flaps in endoscopic endonasal skull base reconstruction. Laryngoscope. 2014;124(04):846–852. doi: 10.1002/lary.24319. [DOI] [PubMed] [Google Scholar]
- 6.Kim G G, Hang A X, Mitchell C A, Zanation A M. Pedicled extranasal flaps in skull base reconstruction. Adv Otorhinolaryngol. 2013;74:71–80. doi: 10.1159/000342282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Safavi-Abbasi S, Komune N, Archer J B et al. Surgical anatomy and utility of pedicled vascularized tissue flaps for multilayered repair of skull base defects. J Neurosurg. 2016;125(02):419–430. doi: 10.3171/2015.5.JNS15529. [DOI] [PubMed] [Google Scholar]
- 8.Patel M R, Shah R N, Snyderman C Het al. Pericranial flap for endoscopic anterior skull-base reconstruction: clinical outcomes and radioanatomic analysis of preoperative planning Neurosurgery 20106603506–512., discussion 512 [DOI] [PubMed] [Google Scholar]
- 9.Sigler A C, D'Anza B, Lobo B C, Woodard T D, Recinos P F, Sindwani R. Endoscopic skull base reconstruction: an evolution of materials and methods. Otolaryngol Clin North Am. 2017;50(03):643–653. doi: 10.1016/j.otc.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 10.Kassam A, Snyderman C H, Mintz A, Gardner P, Carrau R L. Expanded endonasal approach: the rostrocaudal axis. Part I. Crista galli to the sella turcica. Neurosurg Focus. 2005;19(01):E3. [PubMed] [Google Scholar]
- 11.Yano T, Okazaki M, Tanaka K, Tsunoda A, Aoyagi M, Kishimoto S. Use of intraoperative fluorescent indocyanine green angiography for real-time vascular evaluation of pericranial flaps. Ann Plast Surg. 2016;76(02):198–204. doi: 10.1097/SAP.0000000000000519. [DOI] [PubMed] [Google Scholar]
- 12.Georgantopoulou A, Hodgkinson P D, Gerber C J. Cranial-base surgery: a reconstructive algorithm. Br J Plast Surg. 2003;56(01):10–13. doi: 10.1016/s0007-1226(03)00013-4. [DOI] [PubMed] [Google Scholar]
- 13.Gruss C L, Al Komser M, Aghi M K et al. Risk factors for cerebrospinal leak after endoscopic skull base reconstruction with nasoseptal flap. Otolaryngol Head Neck Surg. 2014;151(03):516–521. doi: 10.1177/0194599814536688. [DOI] [PubMed] [Google Scholar]
- 14.Harvey R J, Parmar P, Sacks R, Zanation A M. Endoscopic skull base reconstruction of large dural defects: a systematic review of published evidence. Laryngoscope. 2012;122(02):452–459. doi: 10.1002/lary.22475. [DOI] [PubMed] [Google Scholar]
- 15.Klatt-Cromwell C N, Thorp B D, Del Signore A G, Ebert C S, Ewend M G, Zanation A M. Reconstruction of skull base defects. Otolaryngol Clin North Am. 2016;49(01):107–117. doi: 10.1016/j.otc.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Tang I P, Carrau R L, Otto B A et al. Technical nuances of commonly used vascularised flaps for skull base reconstruction. J Laryngol Otol. 2015;129(08):752–761. doi: 10.1017/S002221511500167X. [DOI] [PubMed] [Google Scholar]
- 17.Velasco-Torres H S, Gómez Amador J L, Feinholz S R. Mass effect due to hypertrophic pericranial flap in the reconstruction of dural defect. World Neurosurg. 2015;84(06):2.077E14–2.077E17. doi: 10.1016/j.wneu.2015.07.070. [DOI] [PubMed] [Google Scholar]
- 18.Zanation A M, Snyderman C H, Carrau R L, Kassam A B, Gardner P A, Prevedello D M. Minimally invasive endoscopic pericranial flap: a new method for endonasal skull base reconstruction. Laryngoscope. 2009;119(01):13–18. doi: 10.1002/lary.20022. [DOI] [PubMed] [Google Scholar]
- 19.Majer J, Herman P, Verillaud B. “Mailbox Slot” pericranial flap for endoscopic skull base reconstruction. Laryngoscope. 2016;126(08):1736–1738. doi: 10.1002/lary.25686. [DOI] [PubMed] [Google Scholar]
- 20.Chopra K, Calva D, Sosin M et al. A comprehensive examination of topographic thickness of skin in the human face. Aesthet Surg J. 2015;35(08):1007–1013. doi: 10.1093/asj/sjv079. [DOI] [PubMed] [Google Scholar]


