Abstract
Introduction Surgery has been the standard treatment for Cushing's disease. Currently, the endoscopic endonasal approach (EEA) is the most widely used technique. However, among some endocrinologists and neurosurgeons used to the microscope assisted technique, there are still questions about the effectiveness and safety of transitioning to the EEA. We aim to show our initial experience with such transition.
Method Retrospective review of medical records of patients, who underwent EEA in our center as a first treatment for Cushing's disease, and with a minimum 18 months of follow-up, from March 2004 to March 2014
Results Our cohort had 16 patients (14 females and 2 males), with a mean age of 33.7 years. The mean follow-up was 52.0 months. Magnetic resonance imaging (MRI) identified an adenoma in 93.8% of the patients (56.2% microadenomas and 37.5% macroadenomas). Postoperative cerebrospinal fluid (CSF) leak was observed in two patients (12.5%). No new neurological deficits were present after surgery. The early remission and sustained remission rates after a single procedure were 87.5 and 68.75%, respectively. Weight reduction, improved control of blood pressure, and lower serum glucose levels were documented in 68.75, 60, and 55.5% of patients, respectively, after remission.
Conclusion Despite the need for specialized training, equipment and team building by ENT (Ear, Nose and Throat) and neurosurgery, the transition from microscope assisted pituitary surgery to endoscopic endonasal approach is possible and safe. The clinical outcomes, even in the early years, are similar to the previous microscope assisted treatment, and over time, with greater experience and knowledge, there is a tendency for improvement.
Keywords: neurosurgery, skull base, endocrinology, endoscopy, otolaryngology, pituitary diseases, Cushing's disease, pituitary ACTH hypersecretion
Introduction
Cushing's disease is the most common cause of endogenous Cushing's syndrome, responsible for 62.2% of cases, with a prevalence of 39.1 cases/million and an incidence of 0.7 to 3 cases/million/year. It is more frequently diagnosed during the fourth decade of life, in slightly younger females (mean 30.5 years) than males (mean 37.1 years), with a preference for the female gender (3–5:1). 1 2 3 4 5
The most common clinical features are obesity, diabetes mellitus, hypertension, and facial plethora. Psychiatric changes (depression, emotional lability, anxiety, psychosis, panic attacks, suicidal ideation, and paranoia) and neurocognitive deficits (learning impairment and memory deficits) can also be observed. 2 3 5 If untreated, Cushing's disease is associated a high morbidity rate from cardiac and cerebrovascular events, immunosuppression, osteoporosis, and psychiatric disturbances, leading to a mortality rate 1.9–4.8 times higher than the general population. 2 3
Cushing's disease is caused by a benign monoclonal pituitary corticotroph adenoma that secretes excessive adrenocorticotropic hormone (ACTH), leading to continuous supraphysiological secretion of glucocorticoids from the adrenal glands. Therefore, the circadian variation is lost and there is a negative feedback inhibition on corticotropin releasing hormone (CRH) secretion by the hypothalamus. However, the adenoma itself is relatively resistant to inhibition by endogenous circulating cortisol, causing a suppressed secretion of CRH and elevated levels of ACTH, with high levels of cortisol. 1
After the case series published by Edward R. Laws 3 and Charles B Wilson, 4 showing the viability and success of selective adenomectomy, pituitary surgery is the standard treatment for Cushing's disease. 5 The endoscopic endonasal approach (EEA) for pituitary tumors, using the technique described by Jho and Carrau 6 is used by several groups with excellent results. 7 However, among some endocrinologists and neurosurgeons, used to the microscope assisted technique, there are still questions about the effectiveness and safety of transitioning to the EEA. Ideally, surgery will achieve complete endocrine cure with a selective adenomectomy, while preserving pituitary function. Current series show remission rates between 65 and 85%, with recurrence rates of 10 to 35% in a 10-year period. 7 8 9 10
In this series, we aim to show our initial 10 years of experience with the treatment of Cushing's disease, using EEA in a large tertiary hospital in São Paulo, Brazil to further evaluate the safety and efficacy of this technique.
Patients and Methods
We retrospectively reviewed the medical records of all patients diagnosed with Cushing's disease, currently under treatment at our institution and included only those, who underwent EEA as the initial surgical treatment from January 2005 to March 2014, and who had a minimum of 2 years of follow-up after surgery. Patients, who had had previous surgical treatment for pituitary disease, were excluded from this study.
We analyzed presenting symptoms, comorbidities, and pituitary function, time to surgery, operative complications, and postoperative improvement, pituitary function, and complications.
All patients underwent EEA following the tenets of surgery defined by Jho and Carrau 6 at our institution by our Skull Base surgery group, with an otolaryngologist and neurosurgeon working together using a 2-surgeons 4-hands technique. The patients were positioned supine with the head tilted left and slightly rotated to the right. Neither somatosensory evoked potentials (SSEP's) nor image guidance were available for any of the cases. Topical vasoconstriction was applied to the nasal cavity. Surgeries before 2009 did not use the nasoseptal flap. After that we routinely used it in the skull base reconstruction. The right middle turbinate was resected and the remaining ones were lateralized. Bilateral posterior and anterior ethmoidectomies and a wide sphenoidectomy were done with a posterior septectomy. The sellar floor was drilled and the dura sharply opened. The tumor was removed with a combination of suction, curretes, dissection, and forceps. Reconstruction was done in a multilayered fashion, using heterologous materials such as, collagen and cellulose hemostatic agents, and fibrin glue, and autologous agents such as, fascia lata, fat graft, and the nasoseptal flap, followed by nasal packing.
After discharge, patients were followed-up with regular returns to our Pituitary Diseases Clinic, undergoing clinical evaluation for signs of recurrence, laboratory assessment for both postoperative hypopituitarism, and signs of recurrence and control of morbidities.
Results
Our final cohort was composed of 16 patients (14 females and 2 males). The age at diagnosis ranged from 16 to 53 years, with a mean age of 33.7 years. The mean follow-up was 52.0 months, ranging from 24 to 94.3 months.
The patients presenting symptoms are summarized in Table 1 and their imaging characteristics on magnetic resonance imaging (MRI) are shown on Table 2 . In nine patients (56.25%) selective adenomectomy was possible, in five (31.25%) patients we could not identify normal pituitary gland and completely emptied the sella (total hypophysectomy) preserving the stalk, and in two (12.5%) a hemihypophysectomy was done.
Table 1. Presenting symptoms and comorbidities in our patients.
| Symptoms/comorbidities | % |
|---|---|
| Weight gain | 93.7 |
| Purple abdominal striae | 62.5 |
| Hyperandrogenism | 62.5 |
| Muscle weakness | 43.7 |
| Hypertension | 93.7 |
| Obesity (body mass index [BMI] > 30 kg/m 2 ) | 68.7 |
| Metabolic syndrome | 56.2 |
| Dyslipidemia | 28.5 |
Table 2. MRI (magnetic resonance imaging) findings in our patients.
| MRI findings | % |
|---|---|
| Microadenoma | 56.2 |
| Macroadenoma | 37.5 |
| Cavernous sinus invasion | 12.5 |
| Normal MRI | 6.2 |
The average time interval between admission of patients to our clinic and surgery was 9.3 months, ranging from 26 days to 35.6 months. The longest interval was due to the initial refusal of the patient to undergo surgery.
In nine patients (56.25%) selective adenomectomy was possible and two (12.5%) underwent hemi-hypophysectomy, because no clear tumor was identified on preoperative imaging or intraoperatively. For five patients (31.25%). we decided alongside endocrinology to perform a total hypophysectomy.
Postoperative cerebrospinal fluid (CSF) leak was observed in two patients (12.5%). Both patients underwent surgical review and closure of the leak. One of the patients presented no further complications and was discharged. One; however, developed meningitis (6,2%) and underwent a prolonged antibiotics course. Epistaxis was observed in two patients (12.5%) and were successfully resolved with bedside tamponade, without the need for surgical review. No new neurological deficits were observed after surgery. Diabetes insipidus (DI) was observed in 7 patients (43.7%), with a mean duration of 10.6 months (2 to 25.9 months). At the end of follow-up, one patient had not recovered from DI. Table 3 summarizes the complications we encountered with this technique.
Table 3. Complication rates in our patients.
| Complications | % |
|---|---|
| Cerebrospinal fluid (CSF) leak | 12.5 |
| Epistaxis | 12.5 |
| Meningitis | 6.2 |
| Transient Diabetes insipidus (DI) | 43.7 |
| Permanent DI | 7.2 |
Patient Follow-up
According to the patients', endocrine response to surgery, we divided them into two initial groups. The early remission group (clinical or laboratory evidence of adrenal insufficiency) was comprised of 14 patients (87.5%). Eleven early remission patients (78.5%) required corticosteroids replacement therapy after discharge, for a mean period of 18 months (5–39.4 months).
The second group that was comprised of two patients (12.5%), showed no clinical or laboratory signs of adrenal insufficiency. Both underwent a second EEA and one patient went into remission after the second intervention, while the second patient required adjuvant radiosurgery, and up to the end of follow-up had not gone into remission.
In the early remission group, recurrent disease was observed in three patients. The time to relapse was 24, 84.2 and 89 months (mean 65.7 months). All three underwent a second EEA, with 2 of them going into remission after. One patient remained with elevated cortisol levels after a second EEA, and received adjuvant radiosurgery, after which he went into remission. A graphic of the distribution of our patients is represented in Fig. 1 .
Fig. 1.
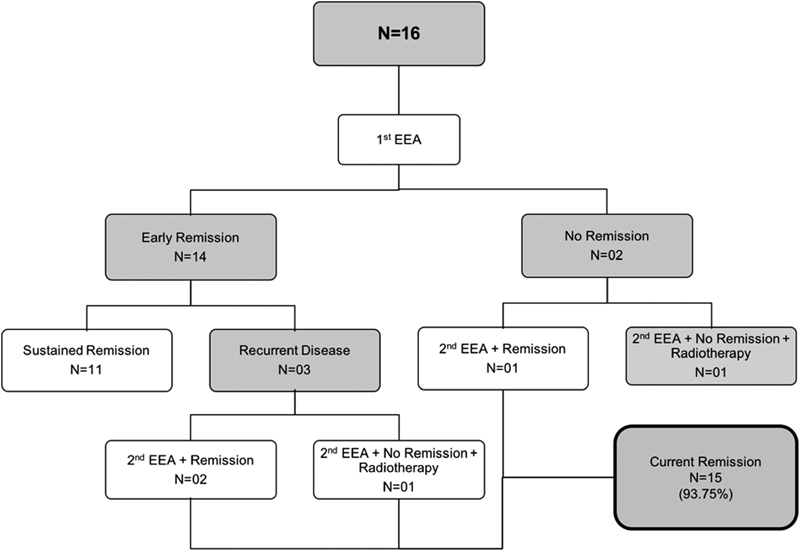
Flow chart showing the distribution of our patients at the end of follow-up.
Panhypopituitarism was observed in 3 patients (18.7%). The main comorbidities identified among our patient were obesity, hypertension, and diabetes, which showed improvement in 68.7, 60 and 55.5%, respectively, after surgery.
Discussion
We encountered in our practice a higher-than-usual rate macroadenomas (37.5%), which many times have destroyed the gland, requiring us to empty the sella in a total hypophysectomy, despite our efforts to preserve gland function. The incidence of CSF leak was greatly reduced after 2010, when we routinely started using the vascularized nasoseptal flap for skull base reconstruction. 8 11 12 Despite the technical difficulty, caused by the larger-than-average tumors, this shows that the complication rate of our initial EEA series is comparable to the microscopic rates of complication and disease control. Since early and sustained remission rates after a single procedure were 87.5 and 68.75%, respectively, in agreement with current studies of more experienced groups that report early and sustained remission rates of 70 to 98% and 50 to 98%, respectively. 8 9 10 11 12
The endoscopic transsphenoidal approach has many advantages over the traditional microsurgical approach: (1) neither a sublabial incision or nasal speculum are required; (2) the endoscope offers a panoramic view of the sella and parasellar structures; (3) avoids the use of intraoperative fluoroscopy, by providing better anatomical identification landmarks; (4) allows the insertion of the endoscope into the sellar and suprasellar region, thereby allowing the surgeon to reach areas inaccessible with microsurgical technique. 9 12 While some argue that endoscopic vision may make it difficult to distinguish between the tumor and normal pituitary, the current high definition, endoscopes, and cameras available have resolved this issue offering excellent visualization.
Conclusion
The EEA is a safe and effective technique for the treatment of Cushing's disease, with remission rates and complications similar to those reported by groups with extensive experience with microsurgical technique. The improved lighting, wider view angle, and the ability to explore the sella, and adjacent regions are obvious advantages of the endoscopic technique.
In our series of 16 patients, we showed satisfactory results, comparable to those reported in the literature by the most experienced groups. The expectation is that our results become progressively better as we increase our experience.
Disclosures
Funding source: no funding was obtained for this study.
Financial disclosures: none of the author has any financial relationships to disclose.
Submission Statement
The contents of this manuscript have not been copyrighted or published previously.
An abstract version of this data was presented as a poster at the 28th Annual North American Skull Base Society Meeting in February 16–18, 2018.
IRB Review Statement/Ethics Guidelines
This study was conducted in accordance to the local ethics committee guidelines.
References
- 1.Lonser R R, Nieman L, Oldfield E H. Cushing's disease: pathobiology, diagnosis, and management. J Neurosurg. 2017;126(02):404–417. doi: 10.3171/2016.1.JNS152119. [DOI] [PubMed] [Google Scholar]
- 2.Vilar L, Naves L A, Freitas M DC et al. Síndrome de cushing endógena: características clínico-laboratoriais em 73 casos. Arq bras endocrinol metab. 2007;51(04):566–574. doi: 10.1590/s0004-27302007000400010. [DOI] [PubMed] [Google Scholar]
- 3.Ebersold M J, Quast L M, Laws E R, Jr, Scheithauer B, Randall R V. Long-term results in transsphenoidal removal of nonfunctioning pituitary adenomas. J Neurosurg. 1986;64(05):713–719. doi: 10.3171/jns.1986.64.5.0713. [DOI] [PubMed] [Google Scholar]
- 4.Wilson C B, Dempsey L C. Transsphenoidal microsurgical removal of 250 pituitary adenomas. J Neurosurg. 1978;48(01):13–22. doi: 10.3171/jns.1978.48.1.0013. [DOI] [PubMed] [Google Scholar]
- 5.Mehta G U, Lonser R R, Oldfield E H. The history of pituitary surgery for Cushing disease. J Neurosurg. 2012;116(02):261–268. doi: 10.3171/2011.8.JNS102005. [DOI] [PubMed] [Google Scholar]
- 6.Jho H D, Carrau R L. Endoscopy assisted transsphenoidal surgery for pituitary adenoma. Technical note. Acta Neurochir (Wien) 1996;138(12):1416–1425. doi: 10.1007/BF01411120. [DOI] [PubMed] [Google Scholar]
- 7.Dehdashti A R, Ganna A, Karabatsou K, Gentili F.Pure endoscopic endonasal approach for pituitary adenomas: early surgical results in 200 patients and comparison with previous microsurgical series Neurosurgery 200862051006–1015., discussion 1015–1017 [DOI] [PubMed] [Google Scholar]
- 8.Dolci R LL, Miyake M M, Tateno D A et al. Postoperative otorhinolaryngologic complications in transnasal endoscopic surgery to access the skull base. Rev Bras Otorrinolaringol (Engl Ed) 2017;83(03):349–355. doi: 10.1016/j.bjorl.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Starke R M, Reames D L, Chen C J, Laws E R, Jane J A., JrEndoscopic transsphenoidal surgery for cushing disease: techniques, outcomes, and predictors of remission Neurosurgery 20137202240–247., discussion 247 [DOI] [PubMed] [Google Scholar]
- 10.Netea-Maier R T, van Lindert E J, den Heijer M et al. Transsphenoidal pituitary surgery via the endoscopic technique: results in 35 consecutive patients with Cushing's disease. Eur J Endocrinol. 2006;154(05):675–684. doi: 10.1530/eje.1.02133. [DOI] [PubMed] [Google Scholar]
- 11.Jagannathan J, Kanter A S, Sheehan J P, Jane J A, Jr, Laws E R., JrBenign brain tumors: sellar/parasellar tumors Neurol Clin 200725041231–1249., xi [DOI] [PubMed] [Google Scholar]
- 12.Santos A R, Fonseca Neto R M, Veiga J C et al. Endoscopic endonasal transsphenoidal approach for pituitary adenomas: technical aspects and report of casuistic. Arq Neuropsiquiatr. 2010;68(04):608–612. doi: 10.1590/s0004-282x2010000400024. [DOI] [PubMed] [Google Scholar]


