Abstract
Autoimmunity is increasingly recognized as having a central role in essential hypertension. Heat shock proteins (HSPs) are immunodominant molecules with high interspecies homology and autoimmune reactivity directed against HSP70 may play a role in the pathogenesis of hypertension. Autoimmunity to HSP70 may result from molecular mimicry between human HSP and bacterial HSP or, alternatively, as a response to HSP70–peptide complexes generated during cellular stress and delivered to the major histocompatibility complex by antigen‐presenting cells. HSP70 is increased in the circulation and kidney of hypertensive patients, and genetic polymorphisms of HSP70 are associated with essential hypertension. Depending on the route and conditions of administration, HSP70 may induce or suppress immune‐related inflammation. Renal inflammation induced by immunity to HSP70 causes hypertension in laboratory animals, and administration of specific peptide sequences of HSP70 results in a protective anti‐inflammatory response that prevents and corrects salt‐induced hypertension. Potential therapeutic uses of HSP70 in essential hypertension deserve to be investigated.
Linked Articles
This article is part of a themed section on Immune Targets in Hypertension. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v176.12/issuetoc
Abbreviations
- APC
antigen‐presenting cell
- DAMPs
danger‐associated molecular patterns
- HSF
heat shock factor
- HSP
heat shock protein
- isoLGs
isolevuglandins
- l‐NAME
N ω‐nitro‐l‐arginine methyl ester
- MHC I
major histocompatibility complex class I
- MHC II
major histocompatibility complex class II
- PRR
pattern recognition receptor
- SHR
spontaneously hypertensive rat
- Treg
regulatory T‐cell
- TLR
toll‐like receptor
Introduction
Hypertension (defined here as BP ≥ 140/90 mmHg) is the most important contributor to the global burden of disease and causes 9.4 million deaths each year (Lim et al., 2010). Despite important advances in the knowledge and treatment of hypertensive cardiovascular disease, the vast majority of patients with hypertension do not have a recognized aetiology and are grouped as patients with ‘essential’ or ‘primary’ hypertension. A large body of experimental and clinical evidence has established that autoimmune reactivity plays a central role in essential hypertension and potential antigens are being actively investigated (Rodriguez‐Iturbe et al., 2017). In 2004, we (Rodriguez‐Iturbe et al., 2004) raised the possibility that heat shock proteins (HSPs) drive autoimmunity in this condition.
Renal overexpression of HSP70 and high titres of anti‐HSP antibodies have been found in hypertensive cardiovascular disease; however, a causal role of HSP70 in hypertension has not been conclusively established. The aim of this article is to review the observations that support the notion that HSP70 is not an innocent bystander but instead plays a pivotal role in the pathogenesis of high BP.
HSPs are intracellular proteins that respond to cellular stress and act as chaperones of other ‘client’ peptides to which they bind to prevent their irreversible aggregation and promote their correct folding, physiological assembly and intracellular trafficking. The HSPs interact in a complex network of ATP‐independent and ATP‐dependent chaperones that are primarily cytoprotective and are critically involved in the functional preservation of many regulatory pathways. Once their function is accomplished, the HSPs dissociate from their client proteins.
HSPs were initially discovered in the larvae of Drosophila melanogaster exposed to heat (Ritossa, 1962). They constitute 5–10% of the total protein content of the cells and increase markedly in response to physical, metabolic (reactive oxygen and nitrogen species), hypoxic, ischaemia–reperfusion and toxic damage and represent one of the best preserved defence mechanisms in prokaryotic and eukaryotic cells (Hendrick and Hartl, 1993). Overproduction of HSPs results from the interaction of phosphorylated trimmers of heat shock factors (HSFs) with the promoter region or heat shock element of the HSP gene. There are four HSFs of which HSF1 and HSF2 are the most widely expressed, and their activation (phosphorylation) is induced by members of the MAPK subfamilies (ERK1, JNK and p38 protein kinase). Under normal conditions, monomeric HSFs are sequestered in the cytoplasm in complexes with HSP40, HSP70, HSP90 and cytosolic chaperonin‐containing T‐complex 1 ring protein (TCP1), but when stress‐induced denatured proteins increase in the cell they bind to the HSPs, the HSP–HSF complexes are dissociated, and the free HSFs are liberated, phosphorylated and translocated to the nucleus. The intracellular content of HSPs is adjusted to an appropriate amount by transcriptional and poststranscriptional regulatory mechanisms (Kregel, 2002; Gomez‐Pastor et al., 2017).
Increased and decreased circulating levels of HSPs have been found in a large number of disease and non‐disease conditions (Qui et al., 2015) and have been implicated in cancer, transplantation rejection, atherosclerosis, Alzheimer's disease, diabetes, arthritis, multiple sclerosis, asthma, neurodegeneration (Huntington's chorea and Parkinson's disease), cerebral and myocardial ischaemia, Mesoamerican (dehydration induced) nephropathy and immunity to infectious agents. Our own studies have focused on the role that autoimmune reactivity directed against HSP70 may be playing in the pathogenesis of hypertension.
HSP70 and immune reactivity
HSPs are classified by their molecular weight and grouped in families. HSP70 and other members of the HSPA (HSP70) family have an N‐terminal ATPase domain and a C‐terminal domain that binds hydrophobic regions in polypeptides. Repeated binding and dissociation is required for the refolding that takes place until hydrophobic regions in the polypeptide are no longer exposed. In addition to the chaperone function, HSPs, and specifically HSP70, are immunodominant molecules capable of stimulating as well as suppressing inflammation (van Eden et al., 2012; Calderwood et al., 2016).
Their ability to induce self‐immune reactivity may be part of natural autoimmunity and also may be associated with pathological autoimmune conditions. Anti‐HSP antibodies, particularly anti‐HSP60 and anti‐HSP70, have been demonstrated in umbilical cord and remain at constant levels independently of infections (Menoret et al., 2000), suggestive that these anti‐HSP autoantibodies may have a role in the normal immune system. Disease‐related HSP autoimmunity is considered by some to be a consequence of overriding the protection of natural immunity as a result of a phenotypical change in natural autoantibodies or caused by the increment in anti‐HSP reactivity induced by genetic or environmental causes (van Eden et al., 2005; Cohen, 2013).
The HSPs are intracellular proteins, but they are also present in extracellular locations relevant to their capacity to induce autoimmune responses. In fact, HSP70 and low titres of anti‐HSP70 antibodies are found in the peripheral circulation in normal individuals (Pockley et al., 1998; Srivastava et al., 2016). The mechanisms that result in the extracellular translocation of HSP70 include not only cellular damage but also extrusion in exosomes and ectosomes (Lancaster and Febbraio, 2005; Calderwood, 2007), as well as an association with secretory lysosomes (Mambula and Calderwood, 2006) and possibly an interaction with lipids in cell membranes (Nickel and Seedorf, 2008).
The predisposition of HSPs to induce autoimmunity is critically associated with their interspecies homology. Molecular mimicry means there is potential for cross‐immune reactivity between the HSPs in invading microorganisms and the corresponding HSP in the host and, thereby, the generation of unintentional autoimmune responses directed to human HSP. Epitopes of Mycobacterium leprae and Mycobacterium tuberculosis HSP70 are recognized by T‐cell clones (van Eden et al., 1988; Janson et al., 1991) that are capable of inducing inflammatory diseases (Van Eden et al., 1985). In addition to molecular mimicry, autoimmunity directed to HSP70 may also be the result of epitope spreading after immune reactivity is generated by novel HSP70–peptide epitopes produced during cellular injury.
Of importance, the T‐cells that are reactive to fragments of HSPs, most notably HSP60 and HSP70, are capable of inducing a protective, anti‐inflammatory regulatory T‐cell (Treg) response. Experimental models of arthritis, type 1 diabetes, atherosclerosis and experimental allergic encephalitis have been shown to be protected by the administration of microbial HSPs (Wendling et al., 2000; van Eden et al., 2005).
HSP70 in adaptive immunity
Exogenous and membrane‐bound antigens are presented by major histocompatibility complex class II (MHC II) molecules in antigen‐presenting cells (APCs), in association with co‐stimulatory signals to CD4+ lymphocytes that are activated and generate a helper pro‐inflammatory immune response. Endogenous and viral antigens are presented by MHC I to CD8+ lymphocytes that generate a cytotoxic immune response. However, elution studies have also demonstrated that a number of antigenic peptides in MHC II molecules are proteins from the cytoplasm, membrane and nucleus and are, therefore, delivered to this location by cross‐presentation pathways that include chaperone‐mediated traffic. HSP70 has been found to protect cytoplasmic autoantigens from degradation and present them to MHC II molecules (Auger et al., 1996; Zhou et al., 2005). HSP70 is also a participant in chaperone‐mediated autophagy (Deffit and Blum, 2015) and in the internalization of receptor–ligand complexes mediated by one of its client proteins: claritin (Stricher et al., 2013).
In addition to playing a role in the traffic of endogenous antigens to the MHC I and MHC II molecules, HSPs may also chaperone client exogenous antigens and facilitate their cross‐presentation to the MHC I in APCs. In this manner, HSPs may be instrumental in CD8+‐generated responses to exogenous antigens (Ichiyanagi et al., 2010; Imai et al., 2011; Murshid et al., 2012). Therefore, HSPs are involved in the access of antigens to both MHC I and MHC II molecules in APCs and may be responsible for simultaneous activation of both CD4+ and CD8+ T‐cells, a phenomenon known as dendritic cell ‘licensing’ (Smith et al., 2004).
HSP70 in innate immunity
The activation of innate immunity is the initial, non‐specific response to pathogen‐associated molecular patterns (PAMPs) and danger‐associated molecular patterns (DAMPs) that when interacting with pattern recognition receptors (PRRs) activate transcription factors and induces the production of proinflammatory cytokines. The best studied PRRs are the toll‐like receptors (TLRs). HSP70 utilizes TLR2 and TLR4 to transduce its pro‐inflammatory signal (Asea et al., 2002). However, the direct interaction between HSPs and TLRs has yet to be conclusively proven, and it is likely that the activation of innate immune mechanisms by HSP70 may involve binding to scavenger receptors in endothelial cells that cooperate with TLRs in the downstream activation of the innate immune response (Gong et al., 2009; Murshid et al., 2016). HSP70 may act as a DAMP using TLR4‐initiated pathways to stimulate innate immunity (Asea et al., 2002; Calderwood et al., 2012); however, many of the HSP70‐induced responses suppress inflammation (van Eden et al., 2012).
While there is compelling evidence for the participation of HSPs in autoimmune reactivity, some studies have demonstrated that endotoxin contamination of the HSP preparation plays a role in the cytokine generation that HSP70 induces in macrophages (Gao and Tsan, 2003) as well as in the activation of APCs (Bausinger et al., 2002) and in the stimulation of antibody responses induced by peptide–HSP70 complexes (Marincek et al., 2008). Therefore, under certain conditions, HSP70 may require collaboration with endotoxin for the responses it induces in the immune system.
HSP70 overexpression and hypertension
An increasingly recognized feature in hypertension is the presence of immune cell infiltration in the kidneys and arterial walls. While early studies suggested these inflammatory changes might be occurring secondary to hypertensive damage, recent studies suggest the inflammatory response is responsible for impairing the excretion of sodium by the kidney (impaired pressure natriuresis) and contributes to the impaired vasodilator response of small blood vessels (impaired endothelial vasodilatation). Inflammation in these target organs and hypertonicity of the sympathetic nervous system are key elements in the pathophysiology of hypertension. A large body of research during the last decade has established the pivotal role of innate and adaptive immunity in the generation of the inflammatory process that underpins the elevation of BP (Rodriguez‐Iturbe et al., 2017). The postulated role of HSP70 in the pathogenesis of essential hypertension rests on its potential to act as an autoantigen that triggers persistent inflammation in the kidney, vasculature and the CNS. While HSP70 blood levels are not significantly related to risk factors of cardiovascular disease (including hypertension) (Dhingra et al., 2006), there is ample evidence that HSP70 is overproduced in hypertension. High BP induced by a variety of experimental methods results in levels of expression of the HSP70 gene in the aorta that are directly related to the changes in BP (Xu et al., 1995) In experimental hypertension, increased HSP70 protein has been identified in adventitial areas of the arteries and in the kidney (Figure 1) and interestingly in the spontaneously hypertensive rat (SHR) the expression of HSP72 predates the development of hypertension (Bravo et al., 2003; Rodriguez‐Iturbe et al., 2017). Patients with essential hypertension have increased levels of HSP70 and HSP70 mRNA in the peripheral circulation (Srivastava et al., 2016), and their lymphocytes have an augmented expression of the HSP70 gene under heat stress (Kunes et al., 1992).
Figure 1.
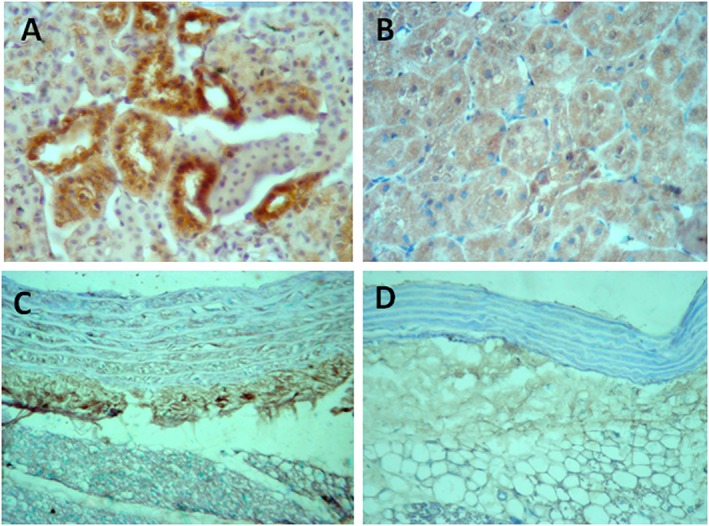
Immunoperoxidase staining for HSP70 in the kidney (A, B) and in adventitial areas of the aorta (C, D). Overexpression in the renal proximal tubules and in periaortic tissue and infiltrating cells of rats with hypertension induced by inhibition of NOS with l‐NAME (A, C) contrasts with the negative staining in placebo‐treated control normotensive rats (B, D) (original magnification ×400).
The association of HSP70 with other potential antigens in hypertension
The identification of antigens that play a role in hypertensive cardiovascular disease is a subject of intense investigation. Independently of its self‐antigenic potential, HSP70 plays a critical role in the complex immune response induced by other antigens. Firstly, the antigenic strength of peptides is increased by their binding to HSPs; this adjuvant characteristic is the basis of the administration of cancer‐related peptides in association with HSP70 (Calderwood et al., 2016). In addition, the activation of CD4 helper cells (Th1, Th2, T17 and Treg) and CD8 cytotoxic responses that are characteristic of hypertension (Trott et al., 2014; Rodriguez‐Iturbe et al., 2017) suggests a correspondent relationship between extra‐ and intracellular antigenic peptides with HSPs (Binder, 2014).
Among the peptides of pathological relevance are the protein–protein and protein–DNA adducts formed by the binding of lysine residues to γ‐ketoaldehydes (isolevuglandins and isoketals) generated via the isoprostane pathway of free radical‐mediated lipid peroxidation (Salomon and Miller, 1985). Isolevuglandin (isoLG)–protein adducts were initially found in patients with atherosclerosis and end‐stage kidney disease (Salomon et al., 1997) and since then have been linked to a large number of disease conditions (Salomon and Bi, 2015) including hypertension (Kirabo et al., 2014; Wu et al., 2016; Dixon et al., 2017). In hypertension, isoLG adducts formed in dendritic cells are presented in the context of MHC I to effector CD8+ T‐cells (Trott et al., 2014). To our knowledge, there is at present no evidence of isoLG–protein adducts interacting with HSPs, but it appears likely that their intracellular traffic and delivery to MHC I is accomplished in association with HSPs. The reason for this assertion is that free antigenic peptides cannot be detected in cells unless they are released from chaperones by mild acid treatment (Menoret et al., 1999), and disruption of the binding of peptides with HSP70, HSP60 and HSP90 abolishes the presentation of peptides to MHC I (Binder et al., 2001; Callahan et al., 2008). Therefore, the evidence available indicates that peptides depend on the functional integrity of HSPs to gain access by canonical as well as by cross‐presentation pathways to both MHC I and MHC II molecules in APCs. The role of HSP70 in intracellular antigenic trafficking is complemented by its participation in endocytosis, receptor‐mediated binding and chaperone‐mediated autophagy (Crotzer and Blum, 2008).
Pathogenic relevance of HSP70 in hypertension
While increased HSP70 is a feature of hypertension, the existence of a causal relationship needs to be established. It should be recognized that the participation of HSP in adverse biological events depends on the circumstances and timing of their overproduction. Immediate effects of HSPs in hypertension are protective, suppressing the activation of NF‐κB and ameliorating the BP response to angiotensin II (Chen et al., 2004). However, chronic HSP70 overexpression is likely to be pro‐hypertensive because of its ability to induce autoimmune responses. The administration of exogenous HSP70 also induces different responses depending on the route of administration; for instance, delayed‐type hypersensitivity results from administration of HSP70 in the footpads (Pons et al., 2013), while administration of HSP70 by nasal or i.p. routes induces IL‐10‐mediated anti‐inflammatory responses (Wendling et al., 2000; van Eden et al., 2005; Pons et al., 2013).
Several observations suggest that immune responses activated by HSP70 in fact play a role in the long‐term pathogenesis of hypertension. These observations are summarized in Table 1:
Circulating HSP70, anti‐HSP70 and HSP70 mRNA in essential hypertension. As noted earlier, the European Lacidipine Study on Atherosclerosis found high titres of anti‐HSP70 antibodies in hypertensive individuals independently of age, smoking habits or blood lipids (Pockley et al., 2002). Our own studies in selected patients with essential hypertension (Figure 2) and in the SHR (Rodriguez‐Iturbe and Johnson, 2010) are concordant with these findings (Chavez et al., 2006; Rodriguez‐Iturbe and Johnson, 2010). Not only are the blood levels of HSP70 mRNA increased in essential hypertension but also they are directly correlated with the levels of TNF‐α, IL‐6 and C‐reactive protein (CRP) (Srivastava et al., 2016). These relationships are suggestive of an association between HSP70 overproduction and systemic inflammation.
T‐cell reactivity to HSP70 in human and experimental hypertension. T lymphocytes of rats with salt‐sensitive hypertension induced by transient administration of angiotensin II and l‐NAME develop a proliferative response when cultured with HSP70 derived from M. tuberculosis. More importantly, T lymphocytes of patients with essential hypertension also show reactivity to mycobacterial HSP70 (Parra et al., 2008; Pons et al., 2013) (Figure 3). These studies demonstrate immune recognition of specific epitopes of mycobacterial HSP70 in essential hypertension.
HSP70 prevents and corrects hypertension in animal models. Specific amino‐acid sequences of HSP70 derived from Mycobacterium tuberculosis are reactive with both human and rat T‐cells (Wendling et al., 2000). Intraperitoneal administration of one such peptide induced an IL10‐driven anti‐inflammatory response that suppressed the immune reactivity to HSP70 and prevented the development of experimental salt‐dependent hypertension. Furthermore, the adoptive transfer of T‐cells from rats that had been tolerized to HSP70 was found to improve established salt‐sensitive hypertension (Pons et al., 2013).
Sensitization to HSP70 induces hypertension in normotensive naive rats. Experiments were also done to show that de novo sensitization of T‐cells to HSP70 in the kidney could induce the development of salt‐sensitive hypertension. Specifically, we found that immunization of rats with HSP70 followed by intrarenal induction of the HSP70 gene resulted in renal inflammation and an increase in BP in response to a high‐salt diet (Pons et al., 2013) (Figure 4).
Genetic associations of HSP70 and essential hypertension. A fivefold increased risk of hypertension was found in association with specific haplotypes of HSP70 (haplotypes H5 and H8) in ethnic minorities in China (Li et al., 2009). In addition, studies of the human genome have demonstrated an association between essential hypertension and single nucleotide polymorphisms in the HSP70 family (HSPA1L, HSPA1A and HSPB1) (International Consortium for Blood Pressure Genome‐Wide Association Studies, 2011). To be noted, the expression of inducible HSPA1 and HSPB1 genes located within the MHC has been related to the generation of autoantibodies in systemic autoimmune disorders (Mišunová et al., 2017).
Table 1.
Studies suggesting the association of HSP70 with primary hypertension and high BP
| Finding | Reference |
|---|---|
| HSP70 overexpression in hypertension | |
| Patients with essential hypertension have increased HSP70 levels and a sixfold increment in HSP70 mRNA in peripheral circulation that correlates with markers of systemic inflammation | Srivastava et al. (2016) |
| High BP induces genetic increments of HSP70 in aorta related to the changes in BP | Xu et al. (1995) |
| Lymphocytes of patients with essential hypertension have augmented HSP70 gene expression under heat stress | Kunes et al. (1992) |
| HSP70 is overexpressed in the kidney and aorta in the SHR. Expression of HSP72 predates the development of hypertension | Bravo et al. (2003) and Rodriguez‐Iturbe et al. (2017) |
| Increased immune reactivity to HSP70 in experimental and human essential hypertension | |
| High titres of serum anti‐HSP70 in the SHR and in patients with essential hypertension | Pockley et al. (2002), Rodriguez‐Iturbe and Johnson (2010) and Chavez et al. (2006) |
| T lymphocytes of rats with salt‐sensitive hypertension and of patients with essential hypertension proliferate strongly in response to specific peptide sequences of mycobacterial HSP70 | Parra et al. (2008) and Pons et al. (2013) |
| Intrarenal genetic induction of HSP70 in rats immunized to HSP70 induces renal inflammation and salt‐driven hypertension | Pons et al. (2013) |
| I.p. HSP70 administration induces an IL‐10‐driven T‐cell response that prevents SSHTN. Adoptive transfer of T‐cells from protected rats improves established salt‐sensitive hypertension | Pons et al. (2013) |
| Genetic association of HSP70 and essential hypertension | |
| Increased incidence of hypertension in individuals with specific HSP70 haplotypes in a highly homogeneous ethnic minority | Li et al. (2009) |
| Single nucleotide polymorphisms in the HSP70 family group (HSP1A and HSP1B; SNP: rs805303 and locus; and BAT2–BAT5) associated with essential hypertension | International Consortium for Blood Pressure Genome‐Wide Association Studies (2011) |
Figure 2.
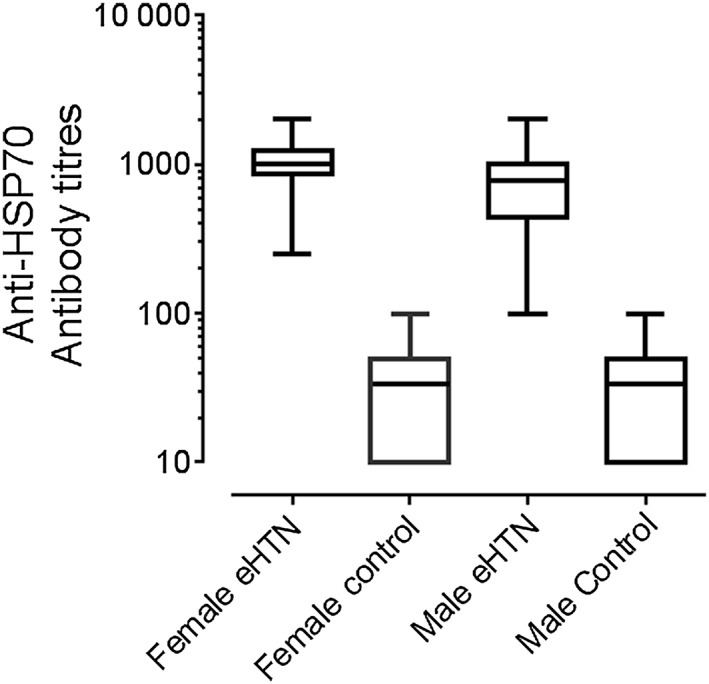
Plasma anti‐HSP70 antibody titres in female (n = 15) and male (n = 15) patients with severe (≥160/110) essential hypertension (eHTN) and normotensive controls of both sexes (male n = 15; female n = 13). Differences between eHBP and controls, P < 0.001 (figure made with data from Chavez et al., 2006).
Figure 3.
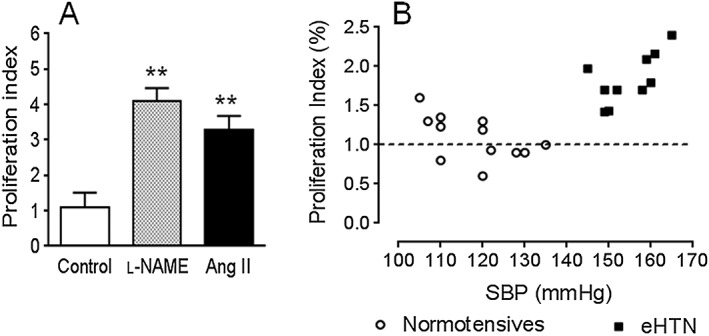
Proliferation of splenic T‐cells from (A) rats with salt‐sensitive hypertension induced by transient inhibition of NOS (l‐NAME) or by transient administration of angiotensin II (Ang II), cultured for 72 h with recombinant rat HSP70. **P < 0.01 versus control. (B) Proliferation index of peripheral blood T‐cells of patients with essential hypertension (eHTN) and normotensive controls, incubated with a specific HSP70 peptide sequence (HSP70 AA 139–153 1A/1B). Difference in mean values of proliferation index in eHTN and normotensive controls, P < 0.01. In the patients with eHTN (B), a significant correlation exists (P < 0.05) between systolic BP (SBP) and proliferation index. Proliferation index = CPM of antigen‐treated cells − background∕CPM of untreated cells − background. Phytoheamagglutinin and ovalbumin used as positive and negative controls respectively (not shown). Figures made using data from Parra et al. (2008) and Pons et al. (2013).
Figure 4.
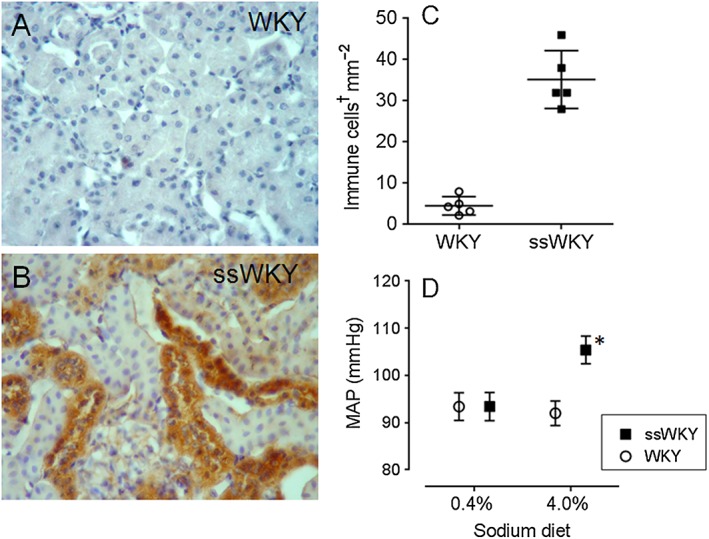
Induction of renal HSP70 expression in rats immunized with HSP70. Normotensive Wistar Kyoto (WKY) rats immunized with injections of HSP70 and adjuvant in foot pads (ssWKY) received HSP70 gene or empty plasmid in both renal veins. Renal sections of rats untreated (A) and 72 h after delivery of HSP70 gene (B), demonstrating HSP70‐positive tubular areas. ssWKY rats developed immune cell infiltration (P < 0.001) in the kidneys after HSP70 gene delivery (C) and responded to a 4% sodium diet with an increase in BP (*P < 0.05) (D). †Immune cells in (C) are the sum of CD5+ and CD68+ (lymnphocytes and macrophages) cells. Figures made using data from Pons et al. (2013).
Concluding remarks
Immune reactivity to and overexpression of HSP70 is a feature of experimental and human essential hypertension. Recent evidence suggests that HSP70 plays a role in the pathogenesis of hypertensive cardiovascular disease. HSP70 may facilitate the development of hypertension by being both a target for autoimmune responses, as well as its ability to enhance the antigenic potential of extracellular or intracellular peptides generated by cellular stress by permitting their traffic to the MHC I and II molecules in APCs (Figure 5). The therapeutic potential of anti‐inflammatory Treg responses elicited by administration of HSP70 and HSP70‐derived peptides to ameliorate established primary hypertension deserves to be investigated.
Figure 5.
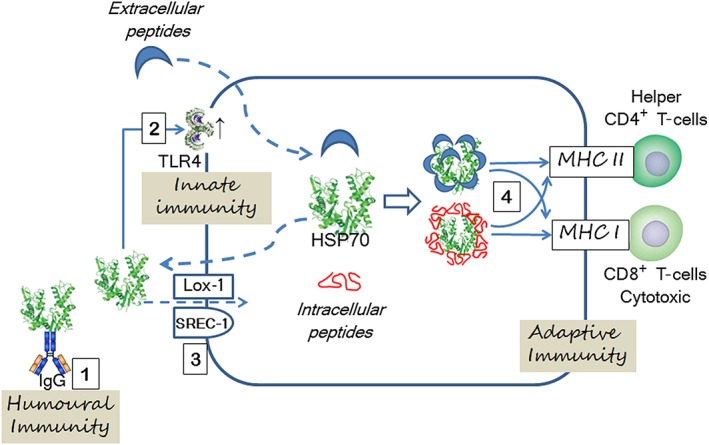
The role of HSP70 in essential hypertension depends on activation of adaptive autoimmune reactivity to itself or to the generation of neoantigens and, possibly, stimulation of innate immunity. Extracellular HSP70 induces the generation of anti‐HSP antibodies (1) and overexpression of TLR2 and TLR4 (2). A direct interaction between HSPs and TLRs has not been demonstrated, and it is possible that stimulation of TLRs by HSPs is indirect. A lectin‐type oxidized LDL receptor (Lox‐1) and scavenger receptor associated with endothelial cells (SREC‐1) mediate HSP70 endocytosis (3). Extracellular and intracellular peptides (isoketal–protein adducts and others) are associated with HSP70 that by repeated folding prevent their aggregation and support their traffic to MHC I and MHC II in APCs by both canonical and cross‐presentation pathways (4) and augment their antigenic potential. Interrupted lines indicate transmembrane displacement.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a,b).
Conflict of interest
B.R.‐I. has no conflicts of interest. R.J.J. is on the Scientific Board of XORT Therapeutics and has patent and patent applications related to lowering uric acid or blocking fructose metabolism in the treatment of hypertension and metabolic disorders. Both R.J.J. and M.A.L. are members of Colorado Research Partners, LLC, that is developing inhibitors of fructose metabolism.
Acknowledgements
The work in the authors' laboratories is funded by grants from the FONACYT, Venezuela (FC‐2005000283, to B.R.‐I.) and Asociación de Amigos del Riñón (to B.R.‐I.) and by the National Institutes of Health National Heart Lung and Blood Institute, USA (HL‐68607 to R.J.J.).
Rodriguez‐Iturbe B., Lanaspa M. A., and Johnson R. J. (2019) The role of autoimmune reactivity induced by heat shock protein 70 in the pathogenesis of essential hypertension, British Journal of Pharmacology, 176, 1829–1838, doi: 10.1111/bph.14334.
References
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: Catalytic receptors. Br J Pharmacol 174: S225–S271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Overview. Br J Pharmacol 174: S1–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE et al (2002). Novel signal transduction pathway utilized by extracellular HSP70. Role of toll‐like receptor (TLR) 2 and TLR4. J Biol Chem 277: 15028–15034. [DOI] [PubMed] [Google Scholar]
- Auger I, Escola JM, Gorvel JP, Roudier J (1996). HLA‐DR4 and HLA‐DR10 motifs that carry susceptibility to rheumatoid arthritis bind 70‐kD heat shock proteins. Nature Med 2: 306–310. [DOI] [PubMed] [Google Scholar]
- Bausinger H, Lipsker D, Ziylan U, Manie S, Briand J‐P, Cazenave J‐P et al (2002). Endotoxin‐free heat‐shock protein 70 fails to induce APC activation. Eur J Immunol 32: 3708–3713. [DOI] [PubMed] [Google Scholar]
- Binder RJ (2014). Functions of heat shock proteins in pathways of the innate and adaptive immune system. J Immunol 193: 5765–5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder RJ, Blachere NE, Srivastava PK (2001). Heat shock protein chaperoned peptides but not free peptides introduced into the cytosol are presented efficiently by major histocompatibility complex I molecules. J Biol Chem 276: 17163–17171. [DOI] [PubMed] [Google Scholar]
- Bravo J, Quiroz Y, Pons H, Parra G, Herrera‐Acosta J, Johnson RJ et al (2003). Vimentin and heat shock protein expression are induced in the kidney by angiotensin and by nitric oxide inhibition. Kidney Int Suppl 86: S46–S51. [DOI] [PubMed] [Google Scholar]
- Calderwood SK (2007). Heat shock proteins in extracellular signaling. Methods 43: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood SK, Gong J, Murshid A (2016). Extracellular HSPs: the complicated roles of extracellular HSPs in immunity. Front Immunol 7: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood SK, Murshid A, Gong J (2012). Heat shock proteins: conditional mediators of inflammation in tumor immunity. Front Immunol 3: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan MK, Garg M, Srivastava PK (2008). Heat‐shock protein 90 associates with N‐terminal extended peptides and is required for direct and indirect antigen presentation. Proc Natl Acad Sci U S A 105: 1662–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez M, Romero F, Críani L, Barrios Y, Hidalgo‐Useche P, Johnson RJ et al (2006). Patients with essential hypertension have serum antibodies against the inducible heat shock protein 70 (HSP 70) (abstract TH‐FC154). J Am Soc Nephrol 17: 34A. [Google Scholar]
- Chen Y, Ross BM, Currie WR (2004). Heat shock treatment protects against angiotensin II‐induced hypertension and inflammation in aorta. Cell Stress Chaperones 9: 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen IR (2013). Autoantibody repertoires, natural biomarkers, and system controllers. Trends Immunol 34: 620–625. [DOI] [PubMed] [Google Scholar]
- Crotzer VL, Blum JS (2008). Cytosol to lysosome transport of intracellular antigens during immune surveillance. Traffic 9: 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deffit SN, Blum JS (2015). A central role for HSC70 in regulating antigen trafficking and MHC class II presentation. Mol Immunol 68: 85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra R, Larson MG, Benjamin EJ, Lipinska I, Gona P, Corey D et al (2006). Cross‐sectional correlates of serum heat shock protein 70 in the community. Am J Hypertens 19: 227–231. [DOI] [PubMed] [Google Scholar]
- Dixon KB, Davies SS, Kirabo A (2017). Dendritic cells and isolevuglandins in immunity, inflammation and hypertension. Am J Physiol Heart Circ Physiol 312: H368–H374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Tsan M‐F (2003). Endotoxin contamination in recombinant human heat shock protein 70 (Hsp70) preparation is responsible for the induction of tumor necrosis factor release by murine macrophages. J Biol Chem 278: 174–179. [DOI] [PubMed] [Google Scholar]
- Gomez‐Pastor R, Burchfiel ET, Thiele DJ (2017). Regulation of heat shock transcription factors and their roles in physiology and disease. Nature Rev Mol Cell Biol 19: 4–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, Zhu B, Murshid A, Adachi H, Song B, Lee A et al (2009). T cell activation by heat shock protein 70 vaccine requires TLR signaling and scavenger receptor expressed by endothelial cells. J Immunol 183: 3092–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrick JP, Hartl FU (1993). Molecular chaperone functions of heat‐shock proteins. Annu Rev Biochem 62: 349–384. [DOI] [PubMed] [Google Scholar]
- Ichiyanagi T, Imai T, Kajiwara C, Mizukami S, Nakai A, Nakayama T et al (2010). Essential role of endogenous heat shock protein 90 of dendritic cells in antigen cross‐presentation. J Immunol 185: 2693–2700. [DOI] [PubMed] [Google Scholar]
- Imai T, Kato Y, Kajiwara C, Mizukami S, Ishige I, Ichiyanagi T et al (2011). Heat shock protein 90 (Hsp90) contributes to cytosolic translocation of extracellular antigen for cross‐presentation by dendritic cells. Proc Natl Acad Sci U S A 108: 16363–16368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Consortium for Blood Pressure Genome‐Wide Association Studies (2011). Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 478: 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janson AA, Klatser PR, van der Zee R, Cornelisse YE, de Vries RR, Thole JE et al (1991). A systematic molecular analysis of the T cell‐stimulating antigens from Mycobacterium leprae with T cell clones of leprosy patients. Identification of a novel M. leprae HSP 70 fragment by M. leprae‐specific T cells. J Immunol 147: 3530–3537. [PubMed] [Google Scholar]
- Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J et al (2014). DC isoketal‐modified proteins activate T cells and promote hypertension. J Clin Invest 124: 4642–4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kregel KC (2002). Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol 92: 2177–2186. [DOI] [PubMed] [Google Scholar]
- Kunes J, Poirier M, Tremblay J, Hamet P (1992). Expression of hsp70 gene in lymphocytes from normotensive and hypertensive humans. Acta Physiol Scand 146: 307–311. [DOI] [PubMed] [Google Scholar]
- Lancaster GI, Febbraio MA (2005). Exosome‐dependent trafficking of HSP70: a novel secretory pathway for cellular stress proteins. J Biol Chem 280: 23349–23355. [DOI] [PubMed] [Google Scholar]
- Li JX, Tang BP, Sun HP, Feng M, Cheng ZH, Niu WQ (2009). Interacting contribution of the five polymorphisms in three genes of Hsp70 family to essential hypertension in Uygur ethnicity. Cell Stress Chaperones 14: 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair‐Rohani H et al (2010). A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2224–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mambula SS, Calderwood SK (2006). Heat shock protein 70 is secreted from tumor cells by a nonclassical pathway involving lysosomal endosomes. J Immunol 177: 7849–7857. [DOI] [PubMed] [Google Scholar]
- Marincek B‐C, Srokowski C, Schild H, Hamerling G, Momburg F (2008). Heat shock protein–antigen fusions lose their enhanced immuno‐stimulatory capacity after endotoxin depletion. Mol Immunol 46: 181–191. [DOI] [PubMed] [Google Scholar]
- Menoret A, Chandawarkar RY, Srivastava PK (2000). Natural autoantibodies against heat‐shock proteins hsp70 and gp96: implications for immunotherapy using heat‐shock proteins. Immunology 101: 364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menoret A, Peng P, Srivastava PK (1999). Association of peptides with heat shock protein gp96 occurs in vivo and not after cell lysis. Biochem Biophys Res Commun 262: 813–818. [DOI] [PubMed] [Google Scholar]
- Mišunová M, Svitálková T, Pleštilová L, Kryštufková O, Tegzová D, Svobodová R et al (2017). Molecular markers of systemic autoimmune disorders: the expression of MHC‐located HSP70 genes is significantly associated with autoimmunity development. Clin Exp Rheumatol 35: 33–42. [PubMed] [Google Scholar]
- Murshid A, Borges TJ, Lang BJ, Calderwood SK (2016). The scavenger receptor SREC‐1 cooperates with toll‐like receptors to trigger inflammatory innate immune responses. Front Immunol 7: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murshid A, Gong J, Calderwood SK (2012). The role of heat shock proteins in antigen cross presentation. Front Immunol 3: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel W, Seedorf M (2008). Unconventional mechanisms of protein transport to the cell surface of eukaryotic cells. Annu Rev Cell Dev Biol 24: 287–308. [DOI] [PubMed] [Google Scholar]
- Parra G, Quiroz Y, Salazar J, Bravo Y, Pons H, Chavez M et al (2008). Experimental induction of salt‐sensitive hypertension is associated with lymphocyte proliferative response to HSP70. Kidney Int 74 (Suppl 111): S55–S59. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Shepherd J, Corton JM (1998). Detection of heat shock protein 70 (Hsp70) and anti‐Hsp70 antibodies in the serum of normal individuals. Immunol Invest 27: 367–377. [DOI] [PubMed] [Google Scholar]
- Pockley AG, De Faire U, Kiessling R, Lemne C, Thulin T, Frostegård J (2002). Circulating heat shock protein and heat shock protein antibody levels in established hypertension. J Hypertens 20: 1815–1820. [DOI] [PubMed] [Google Scholar]
- Pons H, Ferrebuz A, Quiroz Y, Romero‐Vasquez F, Parra G, Johnson RJ et al (2013). Immune reactivity to heat shock protein 70 expressed in the kidney is cause of salt sensitive hypertension. Am J Physiol Renal Physiol 304: F289–F299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qui B, Jia Y, Liu Y, Wang H, Ren G, Wang H (2015). The detection and role of heat shock protein 70 in various nondisease conditions and disease conditions: a literature review. Cell Stress Chaperones 20: 885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritossa F (1962). A new puffing pattern induced by temperature and DNP in Drosophila . Experientia 18: 571–573. [Google Scholar]
- Rodriguez‐Iturbe B, Johnson RJ (2010). The role of renal microvascular disease and interstitial inflammation in salt‐sensitive hypertension. Hypertens Res 33: 975–980. [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Iturbe B, Pons HA, Johnson RJ (2017). The role of the immune system in hypertension. Physiol Rev 97: 1127–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez‐Iturbe B, Vaziri ND, Herrera‐Acosta J, Johnson RJ (2004). Oxidative stress, renal infiltration of immune cells and salt‐sensitive hypertension: all for one and one for all. Am J Physiol Renal Physiol 286: F606–F616. [DOI] [PubMed] [Google Scholar]
- Salomon RG, Bi W (2015). Isolevuglandin adducts in disease. Antioxid Redox Signal 22: 1703–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon RG, Miller DB (1985). Levuglandins: isolation, characterization, and total synthesis of new secoprostanoid products from prostaglandin endoperoxides. Adv Prostaglandin Thromboxane Leukotriene Res 15: 323–326. [PubMed] [Google Scholar]
- Salomon RG, Subbanagounder G, O'Neil J, Kaur K, Smith MA, Hoff HF et al (1997). Levuglandin E2–protein adducts in human plasma and vasculature. Chem Res Toxicol 10: 536–545. [DOI] [PubMed] [Google Scholar]
- Smith CM, Wilson NS, Waithman J, Villadangos JA, Carbone FR, Heath WR et al (2004). Cognate CD4+ T cell licensing of dendritic cells in CD8+ T cell immunity. Nature Immunol 5: 1143–1148. [DOI] [PubMed] [Google Scholar]
- Srivastava K, Narang R, Bhatia J, Saluja D (2016). Expression of heat shock protein 70 gene and its correlation with inflammatory markers in essential hypertension. PLoS One 11: e0151060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricher F, Macri C, Ruff M, Muller S (2013). HSPA8/HSC70 chaperone protein: structure, function, and chemical targeting. Autophagy 9: 1937–1954. [DOI] [PubMed] [Google Scholar]
- Trott DW, Thabet SR, Kirabo A, Saleh MA, Itani H, Norlander AE et al (2014). Oligoclonal CD8+ T cells play a critical role in the development of hypertension. Hypertension 64: 1108–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eden W, Holoshitz J, Nevo Z, Frenkel A, Klajman A, Cohen IR (1985). Arthritis induced by a T‐lymphocyte clone that responds to Mycobacterium tuberculosis and to cartilage proteoglycans. Proc Nat Acad Sci 82: 5117–5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eden W, Spiering R, Broere F, van der Zee RA (2012). A case of mistaken identity: Hsps are no DAMPs but DAMPERs. Cell Stress Chaperones 17: 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eden W, Thole JE, van der Zee R, Noordzij A, van Embden JD, Hensen EJ et al (1988). Cloning of mycobacterial epitope recognized by T lymphocytes in adjuvant arthritis. Nature 331: 171–173. [DOI] [PubMed] [Google Scholar]
- van Eden W, van der Zee R, Prakken B (2005). Heat‐shock proteins induce T‐cell regulation of chronic inflammation. Nat Rev Immunol 5: 318–330. [DOI] [PubMed] [Google Scholar]
- Wendling U, Paul L, van der Zee R, Prakken B, Singh M, van Eden W (2000). A conserved mycobacterial heat shock protein (hsp) 70 sequence prevents adjuvant arthritis upon nasal administration and induces IL‐10‐producing T cells that cross‐react with the mammalian self‐hsp70 homologue. J Immunol 164: 2711–2717. [DOI] [PubMed] [Google Scholar]
- Wu J, Saleh MA, Kirabo A, Itani HA, Montaniel KR, Xiao L et al (2016). Immune activation caused by vascular oxidation promotes fibrosis and hypertension. J Clin Invest 126: 50–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Li D‐G, Holbrook NJ, Udelsman R (1995). Acute hypertension induces heat‐shock protein 70 gene expression in rat aorta. Circulation 92: 1223–1229. [DOI] [PubMed] [Google Scholar]
- Zhou D, Li P, Lin Y, Lott JM, Hislop AD, Canaday DH et al (2005). Lamp‐2a facilitates MHC class II presentation of cytoplasmic antigens. Immunity 22: 571–581. [DOI] [PubMed] [Google Scholar]


