Abstract
Background:
Treatment non-adherence is a leading cause of rehospitalization among patients with chronic obstructive pulmonary disease. Motivational interviewing is a client-centered participatory counseling strategy which enhances motivation for change. The aim of this study was to examine the effects of motivational interviewing on treatment adherence among patients with chronic obstructive pulmonary disease.
Materials and Methods:
This randomized controlled clinical trial was done on 54 hospitalized patients using a two-group repeated measures design. Patients in the intervention group (n=27) received motivational interviewing and lifestyle-related educations, while their counterparts in the comparison group (n=27) solely received lifestyle-related educations. Treatment adherence was measured before, one month, and two months after the intervention.
Results:
At baseline, there was no significant difference between the groups regarding treatment adherence (P>0.05); however, one and two months after the intervention, between-group differences regarding treatment adherence were statistically significant (P<0.05).
Conclusion:
Motivational interviewing promotes treatment adherence among patients with chronic obstructive pulmonary disease.
Keywords: Treatment adherence, Motivational interviewing, Chronic obstructive pulmonary disease
INTRODUCTION
Pulmonary diseases have turned into a leading cause of morbidity and mortality in the world (1). Chronic Obstructive Pulmonary Disease (COPD) is one of the most prevalent pulmonary diseases. Currently, COPD is the sixth leading cause of death and it is estimated that it becomes the third leading cause of death and the fifth most debilitating disease by 2020 (2). Since 2010, COPD-related healthcare costs have been about $2.1 trillion, $1.9 trillion of which have been related to direct costs (such as the costs of healthcare services), while $0.2 trillion have been related to indirect costs (such as loss of employment). It is estimated that indirect COPD-related costs reach $4.8 trillion by 2030 (3). About 51% of COPD-related costs are related to the acute exacerbation of the disease (4). Thus, the best way for cutting such costs is to prevent disease exacerbation or recurrence. Studies showed that most COPD-related rehospitalizations can be prevented through close adherence to treatment strategies such as smoking cessation, medication therapy, clear air breathing, avoidance from going outdoor in polluted days (5), pursed-lip breathing, and lifestyle modifications (6,7).
Poor treatment adherence, particularly among patients with chronic health conditions, is one of the main health-related concerns worldwide. It is a major reason behind the failure of treatments, increased risk for disease recurrence and exacerbation, treatment prolongation, and increased healthcare costs. Moreover, the prognosis of a chronic health condition largely depends on treatment adherence (8).
Treatment adherence among patients with COPD widely varies from 22 to 78% (9). About 200 factors have been identified to contribute to treatment non-adherence (10), the most important of which are the types and the courses of the underlying diseases and their treatments, health-related knowledge, beliefs and faith in treatments, healthcare provider-client relationship, normalization of medication use (11), personality character (12), and the process of medical referrals and visitations (13).
There are different strategies, theories, and models for promoting treatment adherence, each of which focuses on certain psychological aspects of health and behavior. However, for patients with chronic conditions such as COPD, promoting treatment adherence needs strategies which actively involve patients in the process of treatment, motivate them for treatment adherence, and help them internalize healthy behaviors. Compared with other strategies, Motivational Interviewing (MI) embodies more of these attributes (14).
Motivational Interviewing was first introduced by Miller in 1983 as a primary treatment to enhance motivation for subsequent treatments. It is a client-centered participatory counseling strategy which motivates people for change. It comprises empathy and internal conflict externalization and enhances intrinsic motivation through counseling techniques such as asking open-ended questions, reflective listening, summarizing, preparation for change (15). Clinical trials on MI reported its effectiveness in facilitating weight loss among overweight people (16), modifying lifestyle among hypertensive patients (17), improving oral self-care (18), and promoting treatment adherence among patients with transient ischemic attack (19), cystic fibrosis (20), psychosis, pathological gambling, and human immunodeficiency virus infection (21).
To the best of our knowledge, no study has yet investigated the effectiveness of MI in promoting treatment adherence among patients with COPD. Thus, this study was undertaken to examine the effects of MI on treatment adherence in this patient population.
MATERIALS AND METHODS
Study design
This randomized controlled clinical trial was done from January to October 2015 using a two-group repeated-measure design. The trial was registered in the Iranian Registry of Clinical Trials under the registration number of IRCT201604128650N7.
Study population was comprised of all patients with COPD who lived in Tehran, Iran, and were hospitalized in Masih-Daneshvari Hospital, Tehran, Iran. During the course of the study, 200 patients were hospitalized in the study setting, 140 of them were either ineligible for the study or unwilling to participate. Thus, the remaining 60 patients were randomly allocated to a comparison and an intervention group using the block randomization method. The number of patients in each block was four. Eligibility criteria were a positive diagnosis of COPD by a pulmonologist, an age of less than 65, no comorbid serious health condition (such as stroke or diabetes mellitus), the ability to speak and understand Persian, no history of mental disorder or Alzheimer’s disease, and basic literacy skills.
Sample size calculation
Sample size was calculated using Altman’s nomogram, a power of 0.9, a significance level of 0.05, and the standard deviation values reported by Karimi Moonaghi et al (22). Accordingly, Altman’s monogram revealed that 27 patients were needed for each group.
Measures
Data collection instruments were a demographic questionnaire and the Adherence among Patients with Chronic Disease (APCD) questionnaire. The items of the demographic questionnaire were age, gender, marital and educational status, insurance coverage, satisfaction with personal financial status, cigarette smoking status (pack/year), duration of affliction by COPD, drug history, disease severity base on The Global Initiative for Chronic Obstructive Lung Disease (GOLD)(2), place of residence, and history of hospitalization in the last year. APCD was developed .This questionnaire contains forty items in seven dimensions, namely making effort for treatment (9 items), intention to take treatment (7 items), adaptability (7 items), integrating illness into life (5 items), sticking to treatment (4 items), commitment to treatment (5 items), and indecisiveness for using treatments (3 items). The items are scored using a six-point Likert scale from 0 (stands for “Never”) to 5 (stands for “Completely”), resulting in a total score of 0–200. Higher scores represent closer treatment adherence. The Cronbach’s alpha and the test-retest correlation coefficient of the Persian APCD were 0.921 and 0.875, respectively (23).
Intervention
Initially, patients in both groups completed the study questionnaires. Then, patients in the comparison group attended two training sessions on lifestyle, respiratory chest physiotherapy, and medication use. Both sessions were held in one day and lasted 15–45 minutes depending on participants’ needs and tolerance. Patients in the intervention group were provided with five one-to-one MI sessions. These sessions were held in two consecutive days. The first session was held to introduce MI and prepare patients for it. In the second session, we focused on patients’ feelings in order to help them move from extrinsic toward intrinsic motivation for change. The third session dealt mainly with identifying and resolving patients’ ambivalences and uncertainties. The aim of the fourth session was to create and stimulate an intrinsic desire for change as well as to identify, clarify, and acknowledge participants’ values. Finally, the main focus of the fifth session was on identifying tempting situations and closing the program. After MI sessions, patients were also provided with two sessions on lifestyle, respiratory chest physiotherapy, and medication use. The contents of these two sessions were the same for both groups and had been approved by a pulmonologist. All sessions for patients in both groups were held by the first author. The length of all sessions held for the patients in the intervention group varied from 15 to 45 minutes depending on participants’ needs and tolerance. One and two months after the last session, patients in both groups recompleted APCD questionnaire. During this two-month period, we made several telephone contacts with patients in both groups in order to answer their probable questions. It is noteworthy that the contents of the MI sessions were developed based on the Miller’s recommendations(24) and was approved by an MI specialist.
Data analysis
Data analysis was done using the SPSS software (version 22). The Kolmogorov-Smirnov test was done to compare the study variables with the normal distribution. Moreover, the Chi-square and the independent-sample t tests were used to compare the groups regarding participants’ demographic characteristics and treatment adherence. The repeated-measure analysis of variance was also employed to compare the groups regarding the variations of treatment adherence scores across the three measurement time points.
RESULTS
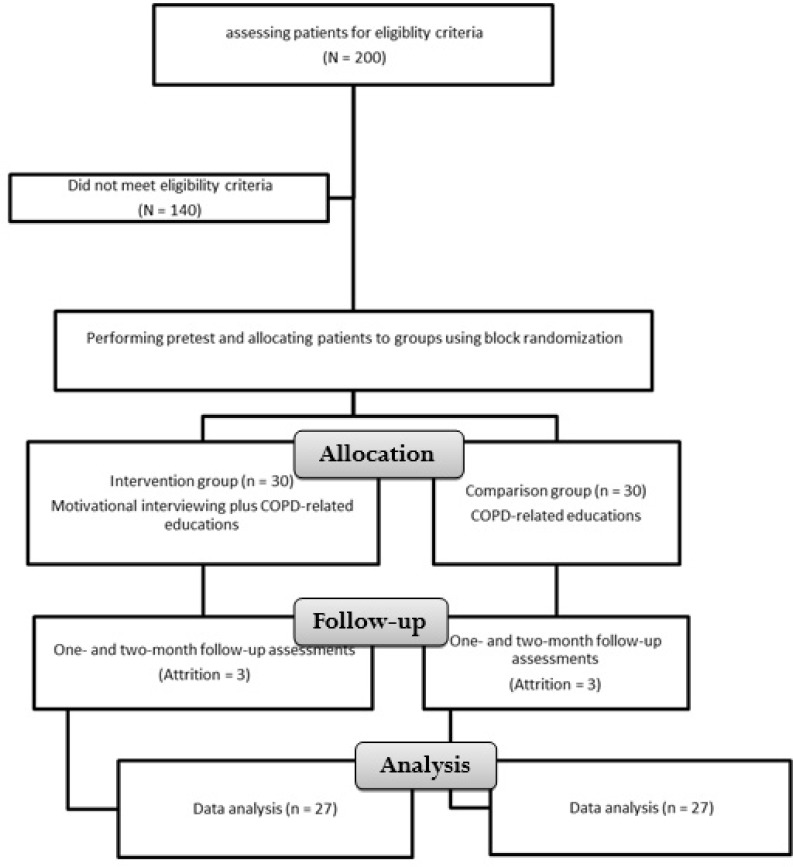
In total, sixty patients were recruited to the study. In the comparison group, two patients were unwilling to stay in the study and one died during the study. Moreover, in the intervention group, three patients were unwilling to stay in the study. All these six patients were excluded and therefore, final data analysis was done on the data retrieved from 27 patients in each group. Figure 1 shows the flow of participants in the trial.
Figure 1.
The flow of participants in the trial
Most patients were male (57.4%) and married (79.62%) and suffered from very severe COPD (51.58%). Moreover, their age mean was about 54 and they were suffering from COPD for about nine years on average. The results of the independent-sample t, the Chi-square, and the Fisher exact tests showed no significant within-group differences regarding participants’ demographic characteristics, clinical characteristics, and drug history (P<0.05; Table 1).
Table 1.
Comparing the groups regarding participants’ characteristics
|
Group Characteristics |
Comparison (Mean ± SD) | Intervention (Mean ± SD) | The results of the Independent-sample t test | |
|---|---|---|---|---|
| Age (year) | 55.04 (7.8) | 53.07 (10.06) | t = 0.76 P =0.44 |
|
| Duration of affliction by COPD (year) | 9.52 (9.4) | 9.07 (10.6) | t = 0.16 P =0.87 |
|
| Number of rehospitalizations | 1.93 (1.1) | 2.37 (1.4) | t =−1.2 P =0.22 |
|
| Cigarette smoking (pack/year) | 15.14 (23) | 10.5 (15.6) | t =0.86 P =0.39 |
|
| N (%) | N (%) | The results of the Chi-square test | ||
| Gender | Male | 17 (63) | 14 (51.9) | χ2= 0.68 P = 0.58 |
| Female | 10 (37) | 13 (48.1) | ||
| Marital status | Single | 5 (18.5) | 6 (21.2) | χ2= 0.114 P < 0.001 |
| Married | 22 (81.5) | 21 (77.8) | ||
| Educational status | Elementary and less | 17 (63) | 9 (33.3) | χ2= 4.98 P = 0.083 |
| Under the diploma | 6 (22.2) | 9 (33.3) | ||
| Diploma and higher | 4 (14.8) | 9 (33.3) | ||
| Disease severity | Mild | 4 (14.8) | 2 (7.4) | χ2= 0.81 P = 0.84 |
| Moderate | 2 (7.4) | 2 (7.4) | ||
| Severe | 8 (29.6) | 8 (29.8) | ||
| Very severe | 13 (48.1) | 15 (55.6) | ||
Table 2 shows the mean scores of treatment adherence and all its dimensions at different measurement time points. The results of the independent-sample t test illustrated no significant difference between the groups regarding baseline scores of treatment adherence (P>0.05).
Table 2.
Comparing the groups regarding the mean scores of treatment adherence and all its dimensions
| Treatment adherence and its dimensions | Time | Pretest (Mean ± SD) | Posttest 1 (Mean ± SD) | Posttest 2 (Mean ± SD) | Within-group repeated-measure analysis of variance |
|---|---|---|---|---|---|
| Group | |||||
| Making effort for treatment | Comparison | 34.93 (4.5) | 32.3(5.3) | 31.7(6) | F = 11.41 |
| Intervention | 32.7(5.5) | 36.78(4.7) | 36.5(6.8) | P <0.001 | |
| Independent-sample | t =1.59 | t =−3.27 | t =−.2.73 | ||
| t test | P =0.117 | P =0.002 | P =0.008 | ||
| Intention to take treatment | Comparison | 29 (3.6) | 24.8(4.6) | 23.9(4.6) | F = 15.28 |
| Intervention | 26.7(4.8) | 29(5) | 28.5(5.4) | P <0.001 | |
| Independent-sample | t =1.94 | t =−3.15 | t =−3.34 | ||
| t test | P =0.057 | P =0.003 | P =0.002 | ||
| Adaptability | Comparison | 26.7 (4.5) | 22.3(4.8) | 23.5(5.4) | F = 13.54 |
| Intervention | 24.7 (5.2) | 27.3(4.8) | 27.3(5.8) | P <0.001 | |
| Independent-sample | t =1.51 | t =−3.77 | t =−2.43 | ||
| t test | P =0.136 | P =0.000 | P =0.018 | ||
| Integrating illness into life | Comparison | 19.8 (2.8) | 17.3(3.3) | 17.2(3.8) | F = 9.62 |
| Intervention | 17.9(3.7) | 19.8 (3.6) | 19.5(3.7) | P <0.001 | |
| Independent-sample | t =2.17 | t =−2.6 | t =−2.29 | ||
| t test | P =0.035 | P =0.012 | P =0.026 | ||
| Sticking to treatment | Comparison | 15.1 (2.9) | 13.7(2.7) | 13.3(2.8) | F = 5.85 |
| Intervention | 14.2(3.4) | 15.8(4) | 15.8(3.2) | P = 0.004 | |
| Independent-sample | t =1.01 | t =−2.3 | t =−2.93 | ||
| t test | P =0.316 | P =0.025 | P =0.005 | ||
| Commitment to treatment | Comparison | 14.7(3.6) | 15.3(3.1) | 15.9(3.2) | F =4.05 |
| Intervention | 15.3(4.6) | 19.2(3.5) | 18.4(3.9) | P = 0.02 | |
| Independent-sample | t =۰/۵۵ | t =−4.33 | t =−2.55 | ||
| t test | P =0.58 | P =0.000 | P =0.014 | ||
| Indecisiveness for applying treatments | Comparison | 10(3) | 10.3(1.7) | 10.4(2.4) | F = 6.52 |
| Intervention | 10.1(2.5) | 12.1(2.7) | 12.2(2.4) | P <0.001 | |
| Independent-sample | t =−0.19 | t =−2.98 | t =−2.63 | ||
| t test | P =0.84 | P =0.004 | P =0.011 | ||
| Total treatment adherence score | Comparison | 150.48(16.4) | 136.19(19.8) | 136.26(24) | F = 17.46 |
| Intervention | 141.89(24) | 160.26(20.9) | 158.48(27.6) | P <0.001 | |
| Independent-sample | t =1.53 | t =−4.34 | t =−3.15 | ||
| t test | P =0.13 | P =0.000 | P =0.003 |
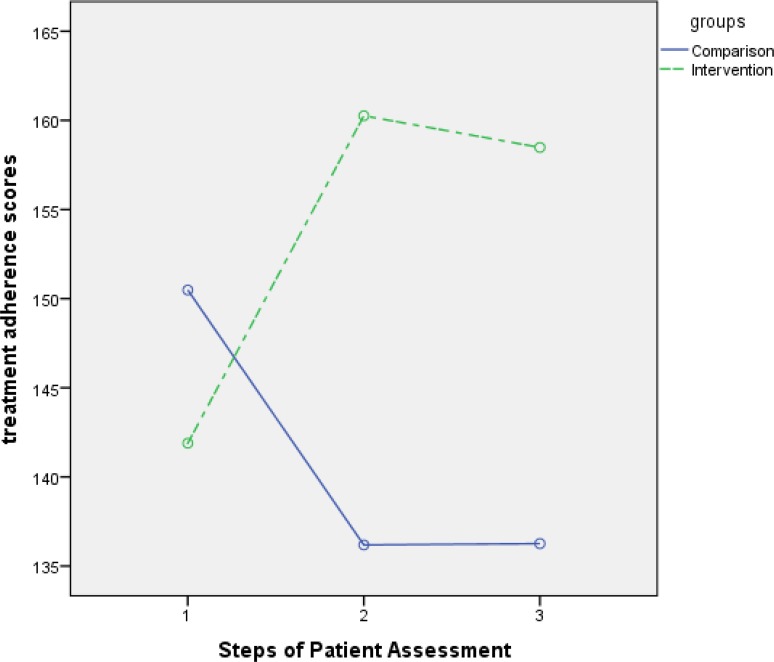
However, at the second and the third time points (i.e. at the first and the second post-tests), between-group differences regarding the scores of treatment adherence and all its dimensions were statistically significant (P<0.05). The results of the repeated-measure analysis of variance also indicated significant between-group differences regarding the variations of the mean scores of treatment adherence and all its dimensions across the three measurement time points (P<0.0001)(Figure 2).
Figure 2.
Variations of treatment adherence scores in both groups over time
DISCUSSION
The aim of this study was to examine the effects of MI on treatment adherence among patients with COPD. Findings showed that after the intervention, treatment adherence mean score in the MI group was significantly greater than the comparison group.
At baseline, treatment adherence in both groups was poor and there was no significant difference between the groups regarding treatment adherence mean score. Other studies also reported poor treatment adherence among patients with chronic diseases (25–27). Poor treatment adherence is associated with many different negative outcomes both for patients and healthcare systems, the most common of which include, but not limited to, disease exacerbation, short life expectancy, low quality of life, family problems, hospital over-crowdedness, healthcare providers’ heavy workload and exhaustion, and increased healthcare costs (8).
Study findings also revealed that despite routine counseling and educational services, treatment adherence in the comparison group had a downward trend while in the MI group, the trend was upward. Other studies also reported the effectiveness of MI in promoting treatment adherence among patients with psychiatric disorders (28), pneumonia (29), and hypertension (30). Similarly, Zidarn and Kolenko found that MI had significant positive effects on smoking cessation (31). Di Marco et al. also reported that MI can potentially improve the efficacy of guided self-help weight loss treatments (32). A systematic review and meta-analysis on seventeen clinical trials also reported the effectiveness of MI in promoting treatment adherence (14). The results of another meta-analysis of controlled clinical trials illustrated that when used as a primary treatment, MI produces more significant and more stable effects than when used as an independent treatment. In other words, the stability of MI effects on patients with chronic diseases largely depends on its association with other treatment strategies and strong support. Similarly, Arkowitz et al. reported that successful long-term behavioral modification necessitates the combination of MI with other treatments (33). Hence, it can be used as a primary treatment to enhance patient motivation for adhering to other treatments (34).
Despite the abundance of studies which reported the effectiveness of MI, some studies showed its ineffectiveness. For instance, Stenman et al. found that MI was ineffective in promoting treatment adherence and reducing gingival bleeding and plaque among patients with periodontal infection. These conflicting findings may be due to the fact that their MI intervention was implemented only in a single 44-minute session (35).
CONCLUSION
MI can promote treatment adherence among patients with COPD. Of course, strong supportive, counseling, and educational interventions are needed to improve the effectiveness of MI. Future studies are recommended to assess the long-term effects of MI on treatment adherence, rehospitalization, and quality of life.
Acknowledgement
The authors need to show their sincere gratitude to the participants of the study and the staff of Masih-Daneshvari Hospital.
Footnotes
Conflict of interest
There is no conflict of interest to declared.
REFERENCES
- 1.Ries AL, Kaplan RM, Myers R, Prewitt LM. Maintenance after pulmonary rehabilitation in chronic lung disease: a randomized trial. Am J Respir Crit Care Med 2003;167(6): 880–8. [DOI] [PubMed] [Google Scholar]
- 2.Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187(4):347–65. [DOI] [PubMed] [Google Scholar]
- 3.Bloom DE, Cafiero E, Jané-Llopis E, Abrahams-Gessel S, Bloom LR, et al. The global economic burden of noncommunicable diseases. Program on the Global Demography of Aging; 2012. January. [Google Scholar]
- 4.Saleh A, López-Campos JL, Hartl S, Pozo-Rodríguez F, Roberts CM, European COPD Audit team The Effect of Incidental Consolidation on Management and Outcomes in COPD Exacerbations: Data from the European COPD Audit. PLoS One 2015;10(7): e0134004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kennedy SM, Chambers R, Du W, Dimich-Ward H. Environmental and occupational exposures: do they affect chronic obstructive pulmonary disease differently in women and men? Proc Am Thorac Soc 2007;4(8):692–4. [DOI] [PubMed] [Google Scholar]
- 6.Roberts MH, Mapel DW, Von Worley A, Beene J. Clinical factors, including All Patient Refined Diagnosis Related Group severity, as predictors of early rehospitalization after COPD exacerbation. Drugs Context 2015. 4. pii: 212278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maddocks M, Kon SS, Singh SJ, Man WD. Rehabilitation following hospitalization in patients with COPD: can it reduce readmissions? Respirology 2015;20(3):395–404. [DOI] [PubMed] [Google Scholar]
- 8.Turan O, Emre JC, Deniz S, Baysak A, Turan PA, Mirici A. Adherence to Current COPD Guidelines in Turkey. Expert Opin Pharmacother 2016;17(2):153–8. [DOI] [PubMed] [Google Scholar]
- 9.Sabaté E. Adherence to long-term therapies: evidence for action. World Health Organization; 2003. [PubMed] [Google Scholar]
- 10.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care 1986;24(1):67–74. [DOI] [PubMed] [Google Scholar]
- 11.George J, Kong DC, Thoman R, Stewart K. Factors associated with medication nonadherence in patients with COPD. Chest 2005;128(5):3198–204. [DOI] [PubMed] [Google Scholar]
- 12.Becker MH, Haefner DP, Kasl SV, Kirscht JP, Maiman LA, Rosenstock IM. Selected psychosocial models and correlates of individual health-related behaviors. Med Care 1977;15(5 SUPPL):27–46. [DOI] [PubMed] [Google Scholar]
- 13.Finnerty FA, Jr, Shaw LW, Himmelsbach CK. Hypertension in the inner city. II. Detection and follow -up. Circulation 1973;47(1):76–8. [DOI] [PubMed] [Google Scholar]
- 14.Palacio A, Garay D, Langer B, Taylor J, Wood BA, Tamariz L. Motivational Interviewing Improves Medication Adherence: a Systematic Review and Meta-analysis. J Gen Intern Med 2016;31(8):929–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller WR. Motivational interviewing with problem drinkers. Behavioural and Cognitive Psychotherapy 1983;11(2):147–72. [Google Scholar]
- 16.Navidian A, Abedi MR, Baghban I, Fatehizade MS, Poursharifi H. Effect of motivational interviewing on the weight self-efficacy lifestyle in men suffering from overweight and obesity. International Journal of Behavioral Sciences 2010;4(2):149–54. [Google Scholar]
- 17.Navidian A, Abedi MR, Baghban I, Fatehizadeh MA, Poorsharifi H. The effects of motivational interviewing on lifestyle modifications of clients suffering from hypertension. Razi Journal of Medical Sciences 2010;17(71):79–94. [Google Scholar]
- 18.Mohammadi Zeidi I, Yekaninejad MS, Akaberi A, Pakpour A. The effectiveness of Motivational interviewing (MI) of oral self care behaviors among high school students in Qazvin. Journal of North Khorasan University of Medical Sciences 2013; 5(1):127–37. persian. [Google Scholar]
- 19.Hedegaard U, Kjeldsen LJ, Pottegård A, Bak S, Hallas J. Multifaceted intervention including motivational interviewing to support medication adherence after stroke/transient ischemic attack: a randomized trial. Cerebrovasc Dis Extra 2014;4(3):221–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duff AJ, Latchford GJ. Motivational interviewing for adherence problems in cystic fibrosis; evaluation of training healthcare professionals. J Clin Med Res 2013;5(6):475–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rollnick S, Miller WR, Butler CC, Aloia MS. Motivational interviewing in health care: helping patients change behavior. COPD: Journal of Chronic Obstructive Pulmonary Disease 2008;5(3):203. [Google Scholar]
- 22.Karimi Moonaghi H, Hasanzadeh F, Shamsoddini S, Emamimoghadam Z, Ebrahimzadeh S. A comparison of face to face and video-based education on attitude related to diet and fluids: Adherence in hemodialysis patients. Iran J Nurs Midwifery Res 2012;17(5):360–4. [PMC free article] [PubMed] [Google Scholar]
- 23.Fatemi NS, Rafii F, Hajizadeh E, Modanloo M. Psychometric properties of the adherence questionnaire in patients with chronic disease: a mix method study. Koomesh 2018; 20(2): 192–202. [Google Scholar]
- 24.Miller WR, Rollnick S. Motivational interviewing: Preparing people for change New York: Guilford; 2002. p. 218. [Google Scholar]
- 25.Napolitano F, Napolitano P, Angelillo IF, Collaborative Working Group Medication adherence among patients with chronic conditions in Italy. Eur J Public Health 2016;26(1):48–52. [DOI] [PubMed] [Google Scholar]
- 26.Behnood-Rod A, Rabbanifar O, Pourzargar P, Rai A, Saadat Z, Saadat H, et al. Adherence to Antihypertensive Medications in Iranian Patients. Int J Hypertens 2016;2016:1508752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dabaghian FH, Rassouli M, Sadighi J, Ghods R. Adherence to prescribed medications of Iranian traditional medicine in a group of patients with chronic disease. J Res Pharm Prac 2016;5(1):52–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamrin V, Iennaco JD. Evaluation of Motivational Interviewing to Improve Psychotropic Medication Adherence in Adolescents. J Child Adolesc Psychopharmacol 2017;27(2):148–159. [DOI] [PubMed] [Google Scholar]
- 29.Eyler R, Shvets K, Blakely ML. Motivational Interviewing to Increase Postdischarge Antibiotic Adherence in Older Adults with Pneumonia. Consult Pharm 2016;31(1):38–43. [DOI] [PubMed] [Google Scholar]
- 30.Honarvar M, Ariaie M, Bordi R, Kamran A. Effect of Motivational Interviewing on Adherence to Treatment in Patients with Hypertension. The Horizon of Medical Sciences 2015;21(3):213–20. [Google Scholar]
- 31.Zidarn M, Kolenko A. Effectiveness of motivational interview for smoking cessation in hospital setting. European Respiratory Journal 2016. 48: PA4310 [Google Scholar]
- 32.DiMarco ID, Klein DA, Clark VL, Wilson GT. The use of motivational interviewing techniques to enhance the efficacy of guided self-help behavioral weight loss treatment. Eat Behav 2009;10(2):134–6. [DOI] [PubMed] [Google Scholar]
- 33.Arkowitz H, Miller WR, Rollnick S. Motivational interviewing in the treatment of psychological problems. Guilford Publications; 2015. [Google Scholar]
- 34.Burke BL, Arkowitz H, Menchola M. The efficacy of motivational interviewing: a meta-analysis of controlled clinical trials. J Consult Clin Psychol 2003;71(5):843–61. [DOI] [PubMed] [Google Scholar]
- 35.Stenman J, Lundgren J, Wennström JL, Ericsson JS, Abrahamsson KH. A single session of motivational interviewing as an additive means to improve adherence in periodontal infection control: a randomized controlled trial. J Clin Periodontol 2012;39(10):947–54. [DOI] [PubMed] [Google Scholar]