Abstract
Cancer cells are able to avoid immune surveillance and exploit the immune system to grow and metastasize. With the development of nano- and micro-particles, there has been a growing number of immunotherapy delivery systems developed to elicit innate and adaptive immune responses to eradicate cancer cells. This can be accomplished by training resident immune cells to recognize and eliminate cells with tumor-associated antigens or by providing external stimuli to enhance tumor cell apoptosis in the immunosuppressive tumor microenvironment (TME). In this review we will focus on nano- and micro-particle (NP and MP) based immunotherapies and vaccines used to elicit a potent and sustained antitumor immune response.
Keywords: Adjuvant, antigen, cytokine, dendritic cells, lymph nodes, microparticle, nanoparticle
1. INTRODUCTION
Nearly 1.7 million new cancer diagnoses and 610,000 cancer-related deaths are projected to occur in 2018 in the US alone [1, 2]. Traditional first line treatments for cancer include surgery, chemotherapy, radiation, or a combination of the three [3]. However, all of these treatments have drawbacks and relapse can reduce the 5-year survival rate by more than 50% in aggressive cancers [1, 4–6]. Tumor resection is most effective in the early stages of cancer, before metastasis has occurred. Cancer is, however, oftentimes detected at late stages when metastasis has already occurred, further limiting treatment options [3, 8]. Furthermore, patients treated with either chemotherapy or radiation also have a higher chance of cancer relapse and tumors may gain resistance to treatment [4]. These drawbacks of traditional treatment options have encouraged discovery of small molecules, peptides, and monoclonal antibodies for immunotherapeutic applications to stimulate the body’s own immune defense system to eradicate cancer. Despite the development of these new immunotherapies, efficacy is limited due to challenges in targeted delivery and controlled release. Nano- and micro-particles (NPs and MPs, respectively) have been widely used as carriers in drug delivery and are being investigated as promising delivery vehicles in immunotherapy. In this review, various NP and MP immunotherapies will be discussed as a means to stimulate the immune system and overcome immunosuppression and tumor evasion.
1.1. Innate and Adaptive Immunity
The innate and adaptive immune systems are major components of our defense against foreign invaders and are responsible for our immediate and long-term protection, respectively [7, 8]. A non-antigen-specific innate immune response occurs through pattern recognition receptors (PRRs) on immune and other cells. PRRs recognize pathogen-associated molecular patterns (PAMPs), which include microbial nucleic acids such as unmethylated CpG, double‐stranded RNA (dsRNA), single‐stranded RNA (ssRNA), 5′‐triphosphate RNA, as well as lipoproteins, surface glycoproteins, and components unique to microbial membranes such as lipopolysaccharide (LPS) or LPS fraction monophosphoryl-lipid A (MPL). In addition, they can recognize danger-associated molecular patterns (DAMPs), such as ATP, CXCL10, high-mobility group box 1 (HMGB1), S100 proteins, heat shock proteins (HSPs), and annexin A1 (ANXA1) [9]. Examples of PRRs recognizing these PAMPs and DAMPs include stimulator of interferon genes (STING) and toll-like receptors (TLRs). PRR activation induces the secretion of inflammatory cytokines, which recruits more immune cells to the site of infection. This initiates immune pathways that can eventually lead to pathogen clearance [10–12]. Natural killer (NK) cells also play a critical role in the innate immune system. These cytotoxic cells are activated by interleukin (IL) cytokines (e.g., IL-12, IL-15, IL-18, IL-2) and are able to secrete perforin and granzymes to induce apoptosis of stressed, infected, or senescent cells [13].
The adaptive response occurs over time and is antigen-specific. An adaptive response leads to T- and B- cell activation, and the generation of immunological memory. Pathogens phagocytosed by antigen presenting cells (APCs) (e.g. macrophages, dendritic cells (DCs)) are processed intracellularly and peptide fragments are presented on major histocompatibility complexes (MHCs) [14]. MHC presentation leads to T-cell priming. T-cells then proliferate and can differentiate into effector T-cells. Effector T-cells can be cytotoxic T-cells (cytotoxic lymphocytes (CTLs), CD8+) or T-helper cells (CD4+). CTLs can migrate through tissues in search of target cells that present the antigenic peptide sequence on their surface. When CTLs recognize these sequences, they induce apoptosis through Fas ligand interaction, or secretion of perforin and granzymes [7, 11, 15]. T-cells can also differentiate into memory T-cells. Memory T-cells that have encountered specific antigens during previous exposure (e.g. infection, vaccination) are able to mount faster and stronger immune responses when exposed to the same antigen again.
CD4+ cells have a more indirect role than CD8+ T cells. Once CD4+ cells are activated, they can differentiate into several subtypes, including Th1 and Th2. IL-12 induces CD4+ cells to differentiate into Th1 cells, which can secrete IL-2 and IFN-γ to activate macrophages and cell-mediated immunity [7]. Th2 differentiation takes place in high IL-4, low IL-12 environments, with other factors favoring a Th2 response including low antigen dose and the presence of co-stimulatory molecules such as OX-40L and Jagged on activating DCs [16]. Th2 cells secrete IL-4, IL-5, IL-10, and IL-13 and stimulate B-cells to proliferate and produce neutralizing antibodies. Specifically, IL-4, which is an autocrine agent, can stimulate class-switching in B-cells to produce IgE antibodies. These cytokines can also inhibit macrophage functions and prevent Th1 differentiation [8, 12, 14].
1.2. Immunosuppressive Tumor Microenvironment
Cancer immunity ideally is initiated by the release of antigens from the tumor bed, which are then endocytosed by APCs. These APCs present tumor antigens to prime and activate T-cells, which can differentiate into effector T-cells and eradicate tumor cells displaying specific antigens [8]. However, cancer cells can evade the immune system and immune cells can be exploited to further tumor progression in a process called immunoediting. Immunoediting consists of elimination, equilibrium, and escape phases [8]. During the elimination phase, antitumor responses from the activation of the innate and adaptive immunity occur, but tumor cells that have escaped the elimination phase can continue to proliferate in the equilibrium phase [1, 5, 11, 15]. The escape phase continues with tumor progression and infiltration.
Progression to the equilibrium and escape phases is strongly associated with a range of immunosuppressive characteristics of the tumor microenvironment (TME). One of these characteristics is the presence of a diverse population of myeloid-derived suppressor cells, induced by the aberrantly high levels of GM-CSF and IL-6 produced by tumor cells [17]. These abnormally-differentiated cells of the myeloid lineage accumulate preferentially in the tumor and suppress the immune response via increased levels of nitric oxide, arginase, and reactive oxygen species [18, 19]. As a direct consequence of altered myelopoiesis, the TME is also characterized by a deficient population of effective APCs, which are critical for inducing an effective T-cell response [20].
Many tumor cells also constitutively express programmed death ligand 1 (PDL1), a ligand for programmed death protein 1 (PD1), expressed on activated T cells. Blockade of PD1 on activated T-cells leads to inhibition of their activity [21]. Administration of PD1 or PDL1-binding antibodies has shown remarkable efficacy in many patients, especially those with a high mutational burden [22]. This efficacy has lead to FDA approval of a number of these antibodies for the treatment of a range of cancers.
Another factor is regulatory T-cells (Tregs), which are found in high quantities in multiple cancers such as skin, breast, lung, and liver. Tregs can mediate self-tolerance to tumor antigens. They achieve this by secreting IL-10 and TGF-β that can suppress the activity of CTL and NK cells [14, 23]. The high presence of Tregs is correlated with reduced survival in patients and poor prognosis caused by tumor growth, angiogenesis, and metastasis [5, 14]. To overcome these immunosuppressive obstacles, immunotherapy is focused on re-educating immune cells in the TME and skewing the immune system towards a Th1 response to elicit a potent anti-cancer response.
1.3. Using Nano- and Micro-particles in Immunotherapy
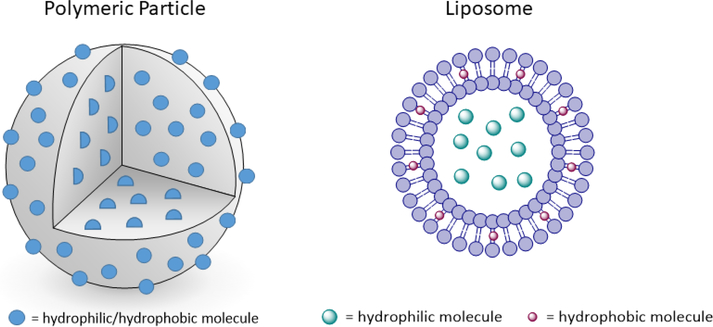
Some of the major challenges facing immunotherapy include off-target toxicity and non-specific immune activation [24, 25]. In order to help address this, NPs and MPs can be used as efficient drug delivery systems for immunotherapies to help modulate the immune system. NP/MPs come in many types, however, this review will investigate only polymeric particles and liposomes. Of the common polymeric particles used, most are made from biodegradable polyesters, polyketals, chitosan, and modified dextrans. Many of the biodegradable polyesters (e.g. poly(lactic-co-glycolic) acid (PLGA)), are FDA-approved drug delivery vehicles [26, 27]. Encapsulation of immunostimulatory agents into polymeric particle carriers can improve delivery and control release of these agents [3, 28]. Due to the hydrophobic nature of the polymer matrix hydrophobic compounds can be loaded at high concentrations into polymeric particles and are distributed throughout the particle’s matrix (Fig. 1). Hydrophilic compounds can be encapsulated as well, however, drug loading efficiency can be drastically decreased if particles are suspended in an aqueous phase during particle manufacture.
Fig. (1).
Schematic of polymeric particles and liposomes. In polymeric particles, the molecules are likely to be spread uniformly throughout. In liposomes, hydrophilic molecules are typically sequestered in the core, whereas hydrophobic molecules are incorporated in the membrane.
Liposomes are another particle delivery vehicle used to encapsulate immunomodulatory reagents. These particles are composed of a phospholipid bilayer and can encapsulate both hydrophobic (within the bilayer) and hydrophilic (encapsulated within the core) compounds (Fig. 1) [8, 26]. Both polymeric particles and liposomes provide temporary protection against drug degradation and can enable cytosolic delivery of immunomodulatory compounds [27, 29].
2. PRINCIPLES AND APPLICATIONS OF NANO- AND MICRO- PARTICLES IN ENHANCING ANTICANCER IMMUNE RESPONSE
2.1. Enhancing Immunogenic Cell Death
Some forms of chemotherapy, radiotherapy, and photodynamic/thermal therapy can induce immunogenic cell death (ICD), a type of cell death with multiple hallmarks. Among these are plasma membrane exposure of the calreticulin (CRT)/ERp57 complex, which increases DC uptake by acting as a ligand for low density lipoprotein receptor-related protein 1 (LRP1) expressed on the DC surface, and secretion of DAMPs including ATP, CXCL10, HMGB1, and ANXA1 [30–32]. Thus, ICD produces both tumor associated antigens and a suite of immunostimulatory DAMPs with the potential to induce an antitumor immune response.
The chemotherapeutics that have been identified as inducers of ICD include doxorubicin, epirubicin, idarubicin, mitoxantrone, bleomycin, bortezomib, cyclophosphamide, oxaliplatin, patupilone, and dinaciclib [33, 34]. While the benefit of formulating some of these compounds into NPs and MPs to diminish systemic toxicity has been established [35], recent studies indicate that micro- or nano-formulation can also augment their ICD effects.
To investigate the role of nanoformulation on the ICD induced by doxorubicin, Rios-Doria et al. examined the effects of the maximum tolerated doses of Doxil (liposomal doxorubicin) or free doxorubicin on a CT26 tumor model in both T-cell-deficient athymic nude mice and immunocompetent BALB/C mice. Free doxorubicin showed no significant antitumor activity in either model, while Doxil exhibited robust antitumor activity in BALB/C mice and significantly less activity in the nude mice, where T cells are mostly absent. This indicates NP doxorubicin’s superior effect was likely immune-mediated [36].
Zhao et al. incorporated oxaliplatin (OXA) into mPEG-PLGA NPs, and incubated Panc-1 pancreatic cancer cells with either the NPs or free OXA. NP OXA resulted in significantly greater ATP release and CRT-positive cells relative to free OXA. Incubation of human primary DCs or mouse DC 2.4 cells with the supernatants from the treated pancreatic cells resulted in significantly increased levels of CD80+ and CD83+ cells with NP OXA versus free OXA, and subsequent co-culture with CD3+ T lymphocytes leads to significantly greater amounts of IFN-γ production in the NP OXA treated cells. In vivo studies of C57BL/6 mice with subcutaneously injected Pan02 tumor cells followed this trend, with significantly greater percentages of IFN-γ+ CTLs in mice treated with NP OXA vs free OXA, leading to a 2.4-fold lower tumor mass in the NP OXA group after 18 days [37].
Photothermal therapy (PTT) achieves local heating and ablation of a tumor by administration of light-absorbing dyes followed by their excitation with near infrared (NIR) light [38]. Photodynamic therapy (PDT) is a similar therapy wherein the administered light-absorbing molecule generates reactive oxygen species instead of heat for tumor ablation [39]. Both therapeutic approaches can also be effective triggers for ICD in tumors, and multiple approaches have been developed to enhance their efficacy using NP/MPs. Chen et al. co-encapsulated indocyanine green (ICG), a photothermal dye, with imiquimod (R837), a TLR-7 agonist, in PLGA NPs. Intratumoral injection of NPs with or without R837 followed by PTT resulted in a greater percentage of mature DCs in the adjuvanted group, while intratumoral injection followed by surgical resection of tumors resulted in lower mature DC percentage for either NP formulation. Combination of PLGA-ICG-R837-based PTT with cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) blockade led to effective inhibition of tumor growth at distant sites, known as the abscopal effect, and subsequent increased survival in multiple tumor models [40].
He et al. leveraged ICD induced by chemotherapy and PDT in tandem by co-encapsulating OXA and the photosensitizer pyropheophorbide-lipid conjugate (pyrolipid) into a core-shell nanoscale-coordination polymeric particle. Combination of PDT and chemotherapy inhibited tumor growth significantly more than PDT alone, and combination with anti-PDL1 therapy induced regression of both PDT-treated primary tumors as well as distant tumors [41].
ICD and the abscopal effect have also been observed during cancer radiotherapy, and immune modulation in combination with radiotherapy unsurprisingly increases this effect [42]. Min et al. have taken an interesting approach to the use of polymeric NPs to modulate the immune response to radiation-induced ICD. They modified batches of PLGA NPs with different functional groups (amine-PEG, N-[1-(2,3-Dioleoyloxy)propyl]-N,N,N-trimethylammonium (DOTAP), maleimide-PEG, and mPEG), and incubated them with lysates from irradiated B16.F10 cells, finding that a range of neoantigen proteins and DAMPs bound to the NPs. They also found that neoantigen binding to NPs led to greater accumulation of APCs, macrophages, and B-cells in the tumor-draining lymph nodes (TDLNs) after intratumoral injection relative to NPs with less binding. Administration of antigen-capturing NPs significantly increased the intratumoral CD8+ T/Treg and CD4+ T/Treg ratios in TDLNs. Intratumorally injected NPs with maleimide or unmodified PLGA surfaces in combination with radiotherapy and anti-PD1 therapy significantly retarded tumor growth and increased survival time relative to radiotherapy and anti-PD1 therapy alone [43].
2.2. Tagging Cancer Cells for Phagocytosis
Two attempts have recently been made to employ polymeric NPs to essentially recapitulate the effects of immunogenic cell death, tagging cancer cells for phagocytosis and subsequent immune processing. Yuan et al. created multivalent bi-specifically conjugated carboxylated polystyrene NPs. One of the targeting ligands was specific for the human epidermal growth factor receptor 2 (HER2) expressed by cancer cells, while the other was CRT. These particles effectively delivered a tag of ICD to cells overexpressing HER2, leading to subsequent phagocytosis by APCs. [44] Ahmed et al. loaded PLGA particles with TLR3 agonist CpG and conjugated their surfaces with streptavidin. They then coated irradiated B16.F10 or RM11 tumor cells with biotin using biotinylated antibody targeting the β1 integrin (anti-mouse/rat CD29). Incubation of the conjugated particles and the biotinylated cells led to cell surface decoration with adjuvant-loaded NPs. Furthermore, subcutaneous vaccination of mice with the RM11 NP-decorated cells conferred significant reduction in the rate of RM11 tumor growth in mice; however, the same strategy was ineffective with the B16.F10 cells and challenge.
2.3. Particle Properties for Lymph Node Drainage and APC Uptake
Several particle properties can affect particle uptake when engineering NPs and MPs for immunotherapy. With regards to particle size, typically particles that are less than 200 nm can be endocytosed by both phagocytic and non-phagocytic cells [45]. In addition, polymeric NPs in this size range made from polystyrene, carboxylated polystyrene, and polypropylene sulphide have been shown to effectively traverse the interstitial space to drain directly to lymphatic vessels and nodes, where they can be taken up by LN-resident APCs such as plasmacytoid DCs [46–49]. Particles greater than 200 nm in diameter can be phagocytosed by naïve peripheral APCs which then migrate to the lymph nodes when activated [26, 47]. Whereas particles that are 1–2 μm are predominately taken up by DCs, 2–3 μm particles are taken up more by macrophages [28, 50]. However, both cell types are capable of engulfing particles nearly as large as the size of the cell-- ~50 μm, with the primary limitation of phagocytosis being contact angle rather than overall volume of the target particle [50].
Particle surface charge, commonly quantified by the measurement of a particle’s zeta potential, is also a critical parameter governing the uptake of MPs and NPs. The surface charge of a particle can determine the characteristics of the adsorbed protein corona that forms upon its administration into the body [49, 51], as well as the rate and extent of particle internalization.For example, Yue et al. functionalized the surfaces of carboxylmethyl chitosan NPs (negatively charged due to carboxyl groups) with either chitosan or N-[(2-hydroxy-3- trimethylammonium) propyl] chitosan chloride to create neutral or positively-charged NPs, respectively. They then investigated the rate and extent of uptake, as well as the extent of lysosomal escape into the cytosol, in eight distinct cell lines. They determined that increasing surface charge from positive to negative correlated with increased rate and extent of particle internalization, and that many of the positively-charged particles escaped the endosome/lysosome [52]. This last phenomenon has been attributed to the so-called ‘proton sponge’ effect, wherein the positive charge of the particles causes an influx of negatively-charged Cl− ions into the endosome, subsequently causing osmotic rupture of the endosome [53, 54].
The stiffness or softness of a particle can also impact its uptake into APCs. Stiffer particles have been shown to be subject to increased Fc-receptor-mediated phagocytosis [55], with a recent study of soft and stiff polymeric nanoconstructs, conducted by Palomba et al. indicating that stiff particles are uptaken by bone-marrow-derived monocytes at five times the extent of soft particles [56]. This relationship has been observed across a variety of other delivery platforms [57–59]. It has been proposed that this is due to the reduced membrane deformation and energy expenditure required to phagocytose a rigid particle compared to a soft one [60]. Thus, physical properties like size and shape can play an important role in directing NPs and MPs to their targets to elicit and effective anticancer immune response.
2.4. Tuning Antigen and Adjuvant Presentation
Varying the composition of NPs and MPs allows for control of the processing and subsequent presentation of antigens via either MHC-I or MHC-II, inducing primarily cellular or humoral immunity, respectively. In an early study, Harding et al. varied the membrane composition of liposomes containing a model antigen, egg lysozyme. They found that liposomes sensitized to acid via the inclusion of palmitoyl-homocysteine in their membranes dissociated in the early endosome, while non-acid-sensitive liposomes did not release their cargo until being delivered to lysozymes. Correspondingly, antigen encapsulation in acid-resistant liposomes resulted in more efficient antigen presentation via MHC-II [61].
Exogenous soluble antigens are usually weakly antigenic. A particularly useful property for immunotherapy is particles’ ability to elicit cross-presentation of antigens, whereby particle-associated exogenous antigens enter the cytosol of APCs and are processed for presentation onto MHC-I to stimulate antigen-specific CTL responses, and subsequent killing of antigen-expressing tumor cells [62–64]. Demento et al. demonstrated that prolonging the kinetics of antigen release inside APCs via encapsulation in PLGA NPs led to an enhanced CD8+ T-cell recall response relative to liposomally-encapsulated or alum-adjuvanted antigen [65].
Recently, our group has examined the effects of varied release kinetics of antigens and adjuvants from APC-internalized MPs, leveraging the facile tunable degradation kinetics of an acid sensitive polysaccharide-based polymer, acetalated dextran. We found that delayed release of nucleotide-binding oligomerization domain 2 (NOD2) agonist murabutide via more slowly-degrading particles correspondingly delayed the development of maximal antigen-specific antibody titers, while more quickly-degrading antigen-containing particles induced increased humoral and cellular immune responses at all time points [66].
Stano et al. compared the immune response induced by a self-assembled polymersome made from poly(propylene sulfide) (PPS)-bl-PEG copolymer loaded internally with ovalbumin (OVA) antigen to the immune response to a solid PPS NP with OVA conjugated to its surface. They found that the polymersomes elicited a greater CD4(+) T cell response, while the solid NPs elicited a greater CD8(+) response. Coadministration of both particle types elicited both responses robustly [67].
2.5. Exploiting Inherent Immunogenicity of Micro- and Nano- Particle Materials
Many of the components of NPs and MPs have been engineered or serendipitously discovered to have immune-stimulating properties independent of their cargoes. While this property can potentially be a major hurdle to the use of these materials due to the potential for immune-mediated toxicity, judicious use of these materials offers a promising methodology for inducing an antitumor immune response.
For example, in addition to their aforementioned drainage to lymph nodes due to their small size, the pluronic-stabilized polypropylene sulfide NPs formulated by Reddy et al. also induced substantial complement binding, primarily driven by binding of the C3b protein fragment to exposed hydroxyl groups on the particles’ surfaces. These complement-bound NPs induced significant maturation of DCs in the draining lymph node [48].
Liposomal components have also been shown to have inherent immunomodulatory potential. Chen et al. compared treatment with DOTAP-containing liposomes containing the E7 peptide antigen for HPV positive TC-1 tumors, with a CpG adjuvanted formulation using the same peptide. Non-adjuvanted DOTAP liposomes exhibited similar antitumor efficacy to the CpG-adjuvanted particles, and DOTAP/E7 liposomes resulted in accumulation of CD8+ tumor infiltrating T cells at tumor sites [68]. Further studies of this adjuvant system found it to be enantiospecific to R-DOTAP and dependent on the presence of the quaternary ammonium group on the lipid [69, 70].
Recently, Shetab Boushehri et al. have reported that another commonly-used drug delivery material bearing quaternary ammonium groups, ammonio methacrylate copolymers, also known as Eudragit® RL and RS, exhibit immunostimulatory properties when formulated into NPs. The mechanism of their stimulation of DCs was shown to be partly mediated by TLR4 and nuclear factor κB (NF-κB). They demonstrated significant antitumor effects and extended survival from peritumoral injection of copolymer NPs with a syngeneic colorectal tumor model in mice [71].
Polyanhydrides are another class of common polymeric drug delivery vehicle [72] which have recently been investigated for their potential immune stimulating effects. Tamayo et al. created NPs from poly(methyl vinyl ether-co-maleic anhydride) and found that the NPs stimulated TLRs 2, 4, and 5 and resulted in significant IFN-γ release on incubation with DCs [73]. Examining the immuno-stimulatory effects in mice from particles made with different copolymer ratios of the polyanhydrides 1,8-bis-(p-carboxyphenoxy)-3,6-dioxaoc-tane (CPTEG), 1,6-bis-(p-carboxyphenoxy)-hexane (CPH), and sebacic anhydride (SA), Wafa et al. identified 20:80 CPTEG:CPH particles as having the strongest CD8(+) and CD4(+) T-cell response, corresponding with the longest survival in mice challenged with OVA-expressing thymoma cell line. Interestingly, coadministration of CpG and the poly-anhydride NPs did not enhance OVA immunogenicity relative to administration of OVA-encapsulating NPs alone [74].
These studies illustrate the potential for formulation of NPs and MPs to transform even some of the most common excipients into potent anticancer immunotherapies.
2.6. DC Targeting
Receptors expressed on the surface of DCs, such as Fc receptors (FcRs) and C-type lectin receptors (CLRs), can be targeted by conjugating their respective ligands onto antigen-containing NPs and MPs, increasing the specificity of delivery to DCs and potentially skewing the subsequent processing of the antigen toward a Th1 or Th2 response. Cruz et al. conjugated hD1, an antibody that binds to the CLR DC-specific ICAM3-grabbing nonintegrin (DC-SIGN) to both 200 nm and 2 μm PLGA particles containing a tetanus toxoid antigen. They found that DC-SIGN targeting effectively increased antigen presentation for NPs (10–100-fold increase) but not for MPs. The mannose receptor represents another CLR on DCs that has been used for targeted antigen delivery to DCs to induce tumor immunity [75]. Mannose receptor-mediated internalization of antigen has been shown to lead to an increased cellular immune response to the antigen by increased cross-presentation [76]. Cui et al. mannosylated Ac-DEX NPs and found increased MHC-I presentation of a model antigen relative to control particles that were functionalized with galactose [77]. Using mannose-functionalized PCL-PEG and PLGA-PEG NPs encapsulating an OVA antigen as well as polyinosine-polycytidylic acid (Poly I:C) and CpG, Silva et al. demonstrated increased Th1 response to their antigen relative to non-mannosylated particles, as well as decreased growth rate of B16.F10 tumors in mice [78].
2.7. Delivery of Cytokines
Cytokines can be used to elicit a potent immune response against cancer. They are signaling proteins secreted primarily by immune cells to propagate an immune response. Interferon-α (IFN-α) and IL-2 are two FDA approved cytokine therapies used to treat patients with leukemia and advanced stages of melanoma [1, 79]. Currently, granulocyte macrophage colony-stimulating factor (GM-CSF), IL-7, IL-12, IL-15, IL-18, and IL-21 are also in clinical trials as immunotherapies for cancer. However, one challenge with systemic delivery of cytokines is their rapid clearance, resulting in sub-optimal therapeutic effects. High concentrations and repeated dosing must be used to offset the clearance rate, often resulting in toxic and life-threatening side effects such as systemic inflammation and increased vascular permeability [14, 79]. One way to overcome this is by delivering these cytokines in either lipid or polymeric particles. By packaging the cytokines in a particle, their half-life can be increased because they can be released over time and protected from degradation [14, 80].
IL-2 is essential in activating CD8+ T-cells and NK cells to trigger tumor regression [14, 80]. Kedar et al. demonstrated that IL-2 could be encapsulated in liposomes and when injected with irradiated tumor cells intravenously, the therapy was able to reduce tumor growth in a B16.F10 melanoma model [81]. This therapy required lower cumulative doses and few administrations of liposomal IL-2 compared to soluble IL-2.
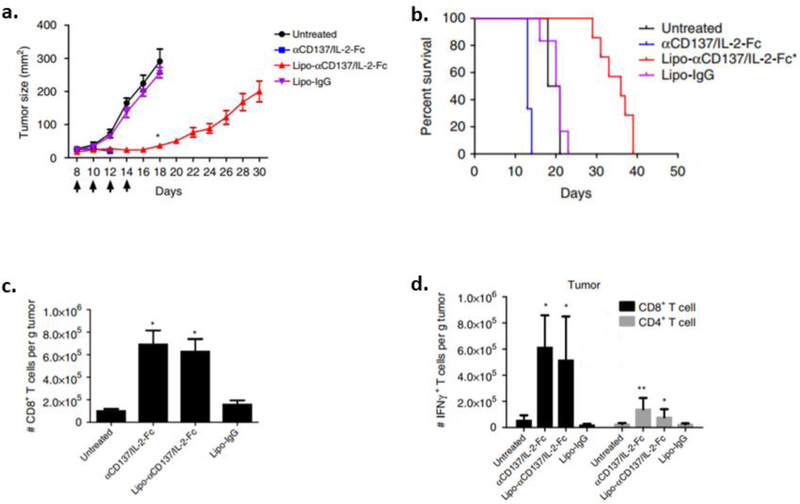
IL-2 therapies in combination with a monoclonal antibody (anti-CD137) have also been explored. CD137, a member of the tumor necrosis factor receptor family, is a costimulatory receptor of CD8+ T-cells and NK cells [82]. The combination of IL-2 and anti-CD137 has been previously shown to elicit potent antitumor immunity along with severe systemic toxicity [83]. In Zhang et al., IL-2 was fused with the Fc fragment of IgG2a [80]. The Fc domain contained a D265A mutation that eliminated Fcγ receptor binding on phagocytes. IL-2-Fc had an increased half-life of more than three-fold compared to soluble IL-2 [83]. However, systemic administration of IL-2-Fc caused severe toxicity and weight loss in C57Bl/6 mice bearing B16.F10 tumors. Due to this, liposomes were generated using 1,2 dioleoyl-sn-glycero-3-phosphocholine (DOPC), cholesterol, 1,2-distearoyl-sn-glycero-3- phosphoethanolamine-N-[amino(polyethylene glycol)] (DSPE-PEG), and DSPE-PEG maleimide. The thiols present on IL-2-Fc and agonistic anti-CD137 were coupled to maleimide groups on the liposomes. By anchoring IL-2-Fc and anti-CD137 onto these liposomes (Lipo-αCD137/IL-2-Fc), systemically administered particles were able to accumulate in the tumor site and prolong survival by inhibiting tumor growth (Fig. 2a). Mice experienced minimal toxicity and weight loss (Fig. 2b) as well as prolonged survival (Fig. 2c) when administered Lipo-αCD137/IL-2-Fc. Additionally, Lipo-αCD137/IL-2-Fc was able to induce CD8+ T-cell infiltration in tumors (Figs. 2d, e, f). After T-cells were restimulated ex vivo, a high population of IFN-γ producing T-cells were found in both the tumor and TDLNs of mice treated with Lipo-αCD137/IL-2-Fc, as well as with the soluble compounds. However, severe toxicity resulting in body weight loss was not observed with the liposomal formulation.
Fig. (2).
Antitumor efficacy using NPs to deliver IL-2. C57BL/6 mice were subcutaneously injected with B16.F10 tumors and treated with either PBS, anti-CD37/IL-2 (αCD137/IL-2-Fc), liposome encapsulated αCD137/IL-2-Fc (Lipo-αCD137/IL-2-Fc), or liposome encapsulated IgG (Lipo-IgG) on days 18, 10, 12, and 14. Shown are a) tumor size, b) survival curve after systemic injections. c) Flow cytometry was used to measure the amount of CD8+ T-cells from the tumor. d) Lymphocytes from the tumor were stimulated ex vivo to determine amount of IFN-γ produced by T-cells [80].
2.8. Delivery of Adjuvants
Oftentimes, adjuvants are required to cue a robust immune response against tumor-associated or -specific antigens [23, 26, 84]. Alum, an FDA-approved adjuvant consisting of particulate aluminum salts, is ubiquitously used in subunit vaccine formulations to increase immunogenicity. Although alum is able to induce a humoral immune response, it does not stimulate a strong Th1 cell-mediated immune response [85]. Since Th1 responses are typically needed to prime CTLs, alternative adjuvants necessary to create an effective cancer vaccine are explored in this section.
2.8.1. STING Agonists
Stimulator of interferon receptor (STING) is a cytoplasmic adapter protein that functions as a DNA sensor [86]. Aduro Biotech and Merck are both currently performing clinical trials using intratumorally injected synthetic STING agonists for cutaneously-accessible tumors. In addition to synthetic agonists, cyclic dinucleotide STING agonists, such as cyclic GMP-AMP (cGAMP), have been shown to induce potent antitumor activity when delivered to the TME. If cytosolic DNA is present, cyclic-GMP-AMP synthase (cGAS) dimerizes and produces cGAMP which can bind and activate STING [86, 87]. When tumor cells undergo apoptosis, DNA is shed, and the STING pathway can be triggered by cGAMP, leading to the activation of DCs and production of type I interferons and other cytokines. These cytokines are crucial to stimulate antitumor responses and to recruit cytotoxic T- cells and NK cells [88, 89]. However, the anionic nature of cGAMP hinders its ability to enter the cytosol and activate STING due to its poor membrane permeability. Other challenges include off-target inflammation or autoimmunity when cGAMP is injected systemically [90]. Currently, strategies are being developed to deliver the agonist more efficiently to the TME using a particle delivery system.
In Koshy et al. 2’3’ cGAMP was encapsulated within cationic liposomes composed of DOTAP, cholesterol, and various concentrations of DSPE-PEG [91]. In vitro studies showed significant uptake of these NPs in bone-marrow derived dendritic cells (BMDCs) compared to soluble fluorescein-conjugated cGAMP after 6 hours. When added to RAW-Blue macrophages, a reporter cell that secretes alkaline phosphatase when the interferon regulatory factor pathway is activated, low concentrations of liposomal cGAMP induced higher levels of enzymatic activity than soluble cGAMP. When used to treat metastatic melanoma tumors by intratumoral injection, liposomal cGAMP with 10 mol% polyethylene glycol (PEG10 cGAMP) and soluble cGAMP led to complete regression in half the mice. Treatment with PEG10 cGAMP liposomes induced tumor regression as well as an adaptive immune response that protected mice when they were re-challenged with B16.F10 tumor cells in the opposite flank. The soluble cGAMP treated mice showed 50% survival, whereas the liposomal cGAMP formulation resulted in 100% survival when rechallenged [91].
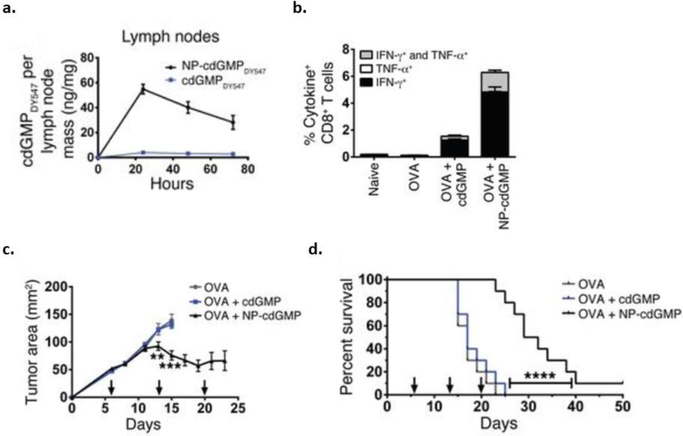
Investigations into using NPs containing STING agonists as cancer vaccines for lymph node targeting are also being explored. In Hanson et al. cyclic di-GMP (cdGMP), a STING agonist, was encapsulated in PEGylated liposomes (NP-cdGMP) [87]. When NP-cdGMP was injected subcutaneously (SC) in BALB/C mice, there was a substantial increase in cdGMP in the draining lymph nodes compared to soluble cdGMP after 24 hours (Fig. 3a) [87]. In order to determine antitumor efficacy, C57BL/6 mice were inoculated with EG.7-OVA (lymphoma cells) and vaccinated with OVA mixed with either soluble cdGMP or NP-cdGMP. Mice treated with OVA+NP-cdGMP had high levels of IFN-γ and TNF-α producing CD8+ T-cells compared to OVA+soluble cdGMP conditions (Fig. 3b). Significant reduction in tumor volume and prolonged survival were also seen in mice administered OVA+NP-cdGMP (Figs. 3c, d) [87].
Fig. (3).
Antitumor efficacy using liposomal NPs to deliver STING agonists. a) BALB/c mice were subcutaneously injected with liposomal cyclic di-GMP (NP-cdGMP) or soluble cdGMP. The accumulation of fluorescently tagged cDGMP in lymph nodes was traced by fluorescence spectroscopy. C57BL/6 mice were immunized with OVA, OVA+cdGMP, or OVA+NP-cdGMP on days 0 and 14. b) PBMCs were restimulated ex vivo and analyzed by flow cytometry to determine the population of IFN-γ and TNF-α producing CD8+ T-cells. C57BL/6 mice were subcutaneously injected with EG.7-OVA cells and vaccinated with OVA, OVA+cdGMP, or OVA+NP-cdGMP on days 6, 13, and 20. c) Tumor area and d) survival were measured over time [87].
While encapsulation of STING agonists in liposomal vehicles has demonstrated promising anticancer activity, a major barrier towards widespread use of these formulations is the poor scalability and encapsulation efficiency of the liposome manufacturing process. For example, reported encapsulation efficiencies of cGAMP in liposomes have ranged from 2 to 35% [87, 91–93]. To address these issues, Junkins et al. encapsulated cGAMP into Ac-DEX MPs using coaxial electrospray, a scalable, continuous manufacturing process which permitted a cGAMP encapsulation efficiency of 90%. Upon treating BMDCs with equivalent doses of cGAMP delivered via electrosprayed Ac-DEX MPs, electrosprayed PLGA MPs, Ac-DEX MPs prepared by double emulsion, SoyPC-DOTAP liposomes, or Lipofectamine 3000 transfection agent, they found that the electrosprayed Ac-DEX MPs resulted in significantly greater IFN-β secretion than all other vehicles, and significantly greater IL-6 secretion than all vehicles except emulsion Ac-Dex MPs [90]. These in vitro results demonstrate the potential for improvements in the development of NPs and MPs containing STING agonists for antitumor therapy.
2.8.2. TLR-3 Agonists
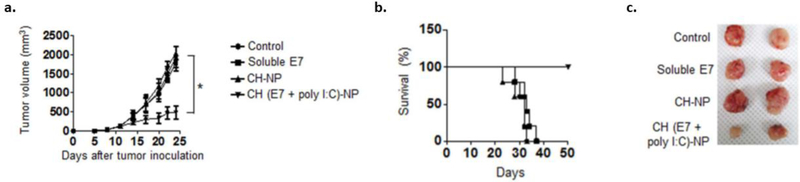
Poly I:C is an analogue of viral double stranded RNA that is recognized by TLR-3, which is localized in the endosomes of APCs [94, 95, 96]. The co-encapsulation of Poly I:C with the E7 peptide in chitosan NPs (CH(E7+poly I:C) was investigated in Han et al. to determine antitumor efficacy [94]. After tumor inoculation with the HPV-E7 expressing TC-1 cells, CH(E7+polyI:C) particles were injected intraperitoneally. Mice vaccinated with CH(E7+poly I:C) particles were able to see significant reduction in tumor volume and 100% survival after 50 days (Figs. 4a–c) [94].
Fig. (4).
Antitumor efficacy using MPs to deliver TLR-3 agonists. TC-1 tumor cells were subcutaneously injected into C57BL/6 mice and treated. Three IP injections of PBS, soluble E7 peptide, chitosan particles (CH-NP) (empty), or chitosan encapsulated E7/Poly I:C particles (CH (E7+polyI:C)-NP) were administered once a week. a) Tumor volume and b) survival were measured over time. c) Tumors were harvested from each treatment group and imaged [94].
2.8.3. TLR-9 Agonists
TLR-9 is expressed in the endosomes of APCs such as macrophages and DCs. Unmethylated CpG is a single-stranded oligodeoxynucleotide (ODN) strand containing cytosine triphosphate deoxynucleotide followed by a guanine triphosphate deoxynucleotide that signals through TLR-9. Upon activation of TLR-9, downstream immunogenic responses are stimulated, such as type I IFN production [97]. CpG is currently employed as an adjuvant in Dynavax’s FDA-approved Hepatitis B vaccine, Heplisav-B [98]. Bourquin et al. used cationized gelatin NPs to complex CpG for delivery to APCs. They noted significantly greater fractions of OVA-specific CD8(+) T cells and IFN-γ(+) CD8(+) T cells one week after s.c. immunization of C57BL/6 mice with OVA and their CpG-gelatin NPs compared to soluble CpG. In addition, they found that similar s.c. immunization with the CpG-gelatin NPs reduced the growth rate of B16-OVA tumors implanted s.c. in C57BL/6 mice, as well as significantly improving their overall survival time. In addition, they found that s.c. administration of soluble CpG induced significant systemic cytokine (IL-12p70, IL-6, and IFN-α) production, while an equivalent dose of CpG-NPs resulted in no detectable systemic cytokines [99].
Another instance of using CpG conjugated to the surface of polymeric NPs can be found in Thomas et al. Here, they conjugated CpG to pluronic block copolymers stabilizing the surfaces of polypropylene sulfide NPs. Ipsilateral administration of the CpG-conjugated NPs to target the TDLN of B16.F10 melanoma, DC maturation within the TDLN and skewed the CD4(+) T cell distribution within the tumor towards a Th1 phenotype. They found that this increased the number of antigen-specific CD8(+) T cells within the tumor, and led to slower tumor growth with NP-conjugated CpG relative to soluble CpG [100].
In Sato et al. CpG was adsorbed onto the surface of polyketal MPs (CpG-MP) [101]. A lung cancer model was developed by administering Lewis lung carcinoma (LLC1) cells via orotracheal intubation in C57BL/6 mice. Mice were administered CpG-MP intratracheally. After 6 hours, CpG-MP had accumulated in the bronchial and alveolar space. In mice treated with CpG-MP, the bronchoalveolar lavage fluid contained higher populations of macrophages and lymphocytes, as well as higher levels of IL-12 compared to free CpG. Intratracheal administration of CpG-MP increased survival by 80%, caused the M1 macrophage population to double, and M2 and Treg populations to decline compared to free CpG [101].
In Xu et al. CpG and the Trp2 peptide were encapsulated in lipid-calcium-phosphate (LCP) NPs [102]. In order to target DCs, these particles were modified with mannose (Mannose-LCP). The calcium-phosphate cores were mixed with DOTAP/cholesterol, DSPE-PEG, and DSPE-PEG-Mannose. Mannose-LCP particles containing 125I-labeled Trp-2 and Texas-Red labeled CpG were subcutaneously injected into C57BL/6 mice. After 18 hours, accumulation of the Mannose-LCP NPs was detected in the lymph nodes and persisted for 4 days. When tested in a B16.F10 model, mice treated with Mannose LCP-p-Trp2/CpG NPs exhibited significant tumor inhibition [102].
Each of these examples of CpG encapsulation either employed conjugation of CpG to the particle surface, or encapsulation via the inclusion of cations in the core to complex the negatively-charged CpG. This reflects a general challenge of CpG administration using polymeric NPs and MPs, namely the very low encapsulation efficiencies observed when encapsulating anionic CpG ODNs into hydrophobic polymer matrices like PLGA. While inclusion of a cationic polymer (e.g. chitosan, protamine, CTAB, or PEI) within the polymeric matrix has been demonstrated to increase the encapsulation efficiency of CpG ODNs [103–107], the toxicity of these cationic materials is likely to limit their therapeutic applicability [108]. To address this issue, Peine et al. used a double emulsion method to encapsulate CpG as well as poly(I:C) into Ac-DEX MPs, achieving an encapsulation efficiency of 36%, versus 3% for PLGA MPs prepared through the same process [109]. Thus Ac-Dex is a promising platform for further development of polymeric NPs or MPs containing CpG ODNs for cancer immunotherapy.
CONCLUSION
Immunotherapeutic strategies using NPs or MPs can enhance antitumor efficacy by eliciting a potent innate and adaptive immune response. In this review, various applications have been covered ranging from delivering cytokines to improving APC uptake and lymph node delivery of TLR and/or STING agonists (Table 1). These therapeutic delivery systems have been applied in stimulating and activating CTLs and NK cells as well as providing immunological memory. The combination of adjuvants and tumor antigen within a particle system proved to increase immunogenicity and reduce tumor progression.
Table 1.
Nano- and micro-particle immunotherapeutic drug delivery systems for the treatment of cancer.
| Particle Formulation | Particle Diameter (nm) | Immunomodulatory Reagent (Encapsulation Efficiency) | Cancer Model | References |
|---|---|---|---|---|
| Liposome (Doxil) | ~100 | Doxorubicin (98–100%) [113] | CT26, MCA205 | (Rios-Doria et al. 2015) |
| Polymeric (PEG-PLGA) | ~100 | Oxaliplatin (20–30%) | PANC-1 | (Zhao et al. 2016) |
| Polymeric (PLGA) | ~100 | ICG, R837 (R837 80–90%, ICG n/a) | 4T1, C26T | (Chen et al. 2016) |
| Polymeric (Nanoscale coordination polymer) | ~50 | Oxaliplatin, pyropheophorbide conjugate (n/a) | MC38 and CT26 | (He et al. 2016) |
| Polymeric (Surface-modified PLGA) | 50–100 | Amine-PEG, DOTAP, maleimide-PEG, mPEG (n/a) | B16.F10 | (Min et al. 2017) |
| Polymeric (Carboxyl-terminated PS) | ~45 | Anti-HER2 antibody, CRT (~1%) | HER2high E0771/E2, HER2neg E0771 | (Yuan et al. 2017) |
| Polymeric (PLGA) | 500–700 | CpG (2–11.5%) | B16.F10, RM11 | (Ahmed et al. 2017) |
| Liposome | ~100 | DOTAP (n/a) | TC-1, BL6 | (Chen et al. 2008) |
| Polymeric (Eudragit® RL and RS) | ~130 and ~430 | Eudragit® RL and RS (n/a) | C26 | (Shetab Boushehri et al. 2018) |
| Polymeric (Varied polyanhydride copolymers) | ~1000 | CPTEG:CPH, CPH:SA, CpG (OVA: 25–47%) | E.G7-OVA | (Wafa et al. 2017) |
| Polymeric (PCL-PEG, PLGA-PEG) | ~150 to ~200 | (Poly I:C), CpG (60–84% antigen, 47–78% adjuvant) | B16.F10 | (Silva et al. 2015) |
| Liposome | 750–1500 and 60 | IL-2 (83–99%) | B16.F10 | (Kedar et al. 2000) |
| Liposome | ~100 | IL-2-Fc, αCD137 (n/a) | B16.F10 | (Zhang et al. 2018) |
| Liposome | ~160 | cGAMP (~20%) | B16.F10 | (Koshy et al. 2017) |
| Liposome | 150 | cdGMP (n/a) | E.G7-OVA | (Hanson et al. 2015) |
| Polymeric (chitosan) | ~250 | poly I:C (OVA: 50%, poly I:C: 70%) | TC-1 | (Han et al. 2016) |
| Polymeric (Cationized gelatin) | ~250 | CpG (n/a) | B16-OVA | (Bourquin et al. 2008) |
| Polymeric (Pluronic-stabilized PPS) | 30 | CpG (n/a) | B16.F10 | (Thomas et al. 2014) |
| Polymeric (Polyketal) | 1000–5000 | CpG (n/a) | LLC1 | (Sato et al. 2015) |
| Lipid-calcium phosphate | 30 | CpG (Trp-2: ~60%, CpG: ~40%) | B16.F10 | (Xu et al. 2013) |
All of these applications have potential; however, they require further review and optimization in order to understand the dynamic interactions between particles and the immune system. While significant dose sparing can be achieved by the targeted delivery of immunomodulatory compounds by NPs and MPs, there is also the potential for unpredictable side effects due to the accumulation of these particulate systems in other undesired areas, analogous to the ‘hand-foot syndrome’ observed in cancer patients treated with Doxil [110], as more of these therapies reach clinical trials. In addition, manufacturing stable formulations in a cost-effective and scalable fashion remains a challenge for most or all NP and MP delivery systems, as again exemplified by the recurrent critical supply shortages of Doxil due to manufacturing challenges [111–113].
However, particulate immunotherapy delivery systems have a promising future as we further refine our knowledge of the interactions of particles with the immune system and our ability to scalably manufacture these particle systems. As demonstrated by several of the studies mentioned herein, combination therapies (cancer vaccine/adjuvants, checkpoint inhibitors, chemotherapeutics, radiation, and/or cytokine delivery) have immense potential as well. The combination of these various therapeutics could result in synergy and improved efficacy. It is an exciting time to be in a field in which the lessons learned from cancer nanotherapeutics and immunotherapy can be combined to further our progress in the fight against cancer.
Footnotes
CONFLICT OF INTEREST
Drs. Ainslie and Bachelder serve on the advisory board for IMMvention Therapeutix, Inc. Although a financial conflict of interest was identified for management based on the overall scope of the project and its potential benefit to IMMvention Therapeutix, Inc., the research findings reported in this publication may not necessarily be related to the interests of IMMvention Therapeutix, Inc. The terms of this arrangement have been reviewed by the University of North Carolina at Chapel Hill in accordance with its policy on objectivity in research.
CONSENT FOR PUBLICATION
Not applicable.
REFERENCES
- [1].Maciejko L, Smalley M, Goldman A, Cancer Immunotherapy and Personalized Medicine: Emerging Technologies and Biomarker-Based Approaches. J. Mol. Biomark. Diagn 8 (2017), doi: 10.4172/2155-9929.1000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Siegel RL, Miller KD, Jemal A, Cancer Statistics, 2017. CA Cancer J. Clin 67, 7–30 (2017). [DOI] [PubMed] [Google Scholar]
- [3].Velpurisiva P, Gad A, Piel B, Jadia R, Rai P, Nanoparticle design strategies for effective cancer immunotherapy. J Biomed (Syd). 2, 64–77 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Goldman A et al. , Rationally Designed 2-in-1 Nanoparticles Can Overcome Adaptive Resistance in Cancer. ACS Nano. 10, 5823–5834 (2016). [DOI] [PubMed] [Google Scholar]
- [5].Shao K et al. , Nanoparticle-based immunotherapy for cancer. ACS Nano. 9, 16–30 (2015). [DOI] [PubMed] [Google Scholar]
- [6].Restifo NP, Smyth MJ, Snyder A, Acquired resistance to immunotherapy and future challenges. Nat. Rev. Cancer 16, 121–126 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fan Y, Moon JJ, Nanoparticle drug delivery systems designed to improve cancer vaccines and immunotherapy. Vaccines (Basel). 3, 662–685 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Conniot J et al. , Cancer immunotherapy: nanodelivery approaches for immune cell targeting and tracking. Front. Chem 2, 105 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT, PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol. Rev 249, 158–175 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Li K, Qu S, Chen X, Wu Q, Shi M, Promising Targets for Cancer Immunotherapy: TLRs, RLRs, and STING-Mediated Innate Immune Pathways. Int. J. Mol. Sci 18 (2017), doi: 10.3390/ijms18020404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zang X et al. , Nanoparticles for tumor immunotherapy. Eur. J. Pharm. Biopharm 115, 243–256 (2017). [DOI] [PubMed] [Google Scholar]
- [12].Mantegazza AR, Magalhaes JG, Amigorena S, Marks MS, Presentation of phagocytosed antigens by MHC class I and II. Traffic. 14, 135–152 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chan CJ, Smyth MJ, Martinet L, Molecular mechanisms of natural killer cell activation in response to cellular stress. Cell Death Differ. 21, 5–14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kapadia CH, Perry JL, Tian S, Luft JC, DeSimone JM, Nanoparticulate immunotherapy for cancer. J. Control. Release 219, 167–180 (2015). [DOI] [PubMed] [Google Scholar]
- [15].Frankel T, Lanfranca MP, Zou W, The role of tumor microenvironment in cancer immunotherapy. Adv. Exp. Med. Biol 1036, 51–64 (2017). [DOI] [PubMed] [Google Scholar]
- [16].Vroman H, van den Blink B, Kool M, Mode of dendritic cell activation: the decisive hand in Th2/Th17 cell differentiation. Implications in asthma severity? Immunobiology. 220, 254–261 (2015). [DOI] [PubMed] [Google Scholar]
- [17].Marigo I et al. , Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 32, 790–802 (2010). [DOI] [PubMed] [Google Scholar]
- [18].Marvel D, Gabrilovich DI, Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. The Journal of Clinical Investigation (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ugel S, De Sanctis F, Mandruzzato S, Bronte V, Tumor-induced myeloid deviation: when myeloid-derived suppressor cells meet tumor-associated macrophages. The Journal of Clinical Investigation (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Broz ML et al. , Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell. 26, 638–652 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hoos A, Development of immuno-oncology drugs - from CTLA4 to PD1 to the next generations. Nat. Rev. Drug Discov 15, 235–247 (2016). [DOI] [PubMed] [Google Scholar]
- [22].Rizvi NA et al. , Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 348, 124–128 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chesson CB, Zloza A, Nanoparticles: augmenting tumor antigen presentation for vaccine and immunotherapy treatments of cancer. Nanomedicine (Lond). 12, 2693–2706 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Carson WE et al. , Coadministration of interleukin-18 and interleukin-12 induces a fatal inflammatory response in mice: critical role of natural killer cell interferon-gamma production and STAT-mediated signal transduction. Blood. 96, 1465–1473 (2000). [PubMed] [Google Scholar]
- [25].Phan GQ et al. , Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 100, 8372–8377 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bookstaver ML, Tsai SJ, Bromberg JS, Jewell CM, Improving vaccine and immunotherapy design using biomaterials. Trends Immunol. 39, 135–150 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gregory AE, Titball R, Williamson D, Vaccine delivery using nanoparticles. Front. Cell. Infect. Microbiol 3, 13 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chen N, Peine K, Bachelder E, Ainslie K, Micro- and Nano-particulate Strategies for Antigen Specific Immune Tolerance to Treat Autoimmune Diseases. Pharm. Nanotechnol 3, 85–100 (2015). [Google Scholar]
- [29].Jiang H, Wang Q, Sun X, Lymph node targeting strategies to improve vaccination efficacy. J. Control. Release 267, 47–56 (2017). [DOI] [PubMed] [Google Scholar]
- [30].Obeid M et al. , Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med 13, 54–61 (2007). [DOI] [PubMed] [Google Scholar]
- [31].Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G, Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol 17, 97–111 (2017). [DOI] [PubMed] [Google Scholar]
- [32].Kono H, Rock KL, How dying cells alert the immune system to danger. Nat. Rev. Immunol 8, 279–289 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pol J et al. , Trial Watch: Immunogenic cell death inducers for anticancer chemotherapy. Oncoimmunology. 4, e1008866 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hossain Dewan Md Sakib et al. , Dinaciclib induces immunogenic cell death and enhances anti-PD1-mediated tumor suppression. The Journal of Clinical Investigation (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].O’Brien MER, Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYXTM/Doxil”) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann. Oncol 15, 440–449 (2004). [DOI] [PubMed] [Google Scholar]
- [36].Rios-Doria J et al. , Doxil synergizes with cancer immunotherapies to enhance antitumor responses in syngeneic mouse models. Neoplasia. 17, 661–670 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhao X et al. , Inducing enhanced immunogenic cell death with nanocarrier-based drug delivery systems for pancreatic cancer therapy. Biomaterials. 102, 187–197 (2016). [DOI] [PubMed] [Google Scholar]
- [38].Cheng L, Wang C, Feng L, Yang K, Liu Z, Functional nanomaterials for phototherapies of cancer. Chem. Rev. 114, 10869–10939 (2014). [DOI] [PubMed] [Google Scholar]
- [39].Dolmans DEJGJ, Fukumura D, Jain RK, Photodynamic therapy for cancer. Nat. Rev. Cancer 3, 380–387 (2003). [DOI] [PubMed] [Google Scholar]
- [40].Chen Q et al. , Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat. Commun 7, 13193 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].He C et al. , Core-shell nanoscale coordination polymers combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. Nat. Commun 7, 12499 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Formenti SC, Demaria S, Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst 105, 256–265 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Min Y et al. , Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat. Nanotechnol 12, 877–882 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Yuan H et al. , Multivalent bi-specific nanobioconjugate engager for targeted cancer immunotherapy. Nat. Nanotechnol 12, 763–769 (2017). [DOI] [PubMed] [Google Scholar]
- [45].Oh N, Park J-H, Endocytosis and exocytosis of nanoparticles in mammalian cells. Int. J. Nanomedicine 9 Suppl 1, 51–63 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Fifis T et al. , Size-dependent immunogenicity: therapeutic and protective properties of nano-vaccines against tumors. J. Immunol 173, 3148–3154 (2004). [DOI] [PubMed] [Google Scholar]
- [47].Manolova V et al. , Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol 38, 1404–1413 (2008). [DOI] [PubMed] [Google Scholar]
- [48].Reddy ST et al. , Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat. Biotechnol 25, 1159–1164 (2007). [DOI] [PubMed] [Google Scholar]
- [49].Foged C, Brodin B, Frokjaer S, Sundblad A, Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int. J. Pharm 298, 315–322 (2005). [DOI] [PubMed] [Google Scholar]
- [50].Champion JA, Walker A, Mitragotri S, Role of particle size in phagocytosis of polymeric microspheres. Pharm. Res 25, 1815–1821 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Patil S, Sandberg A, Heckert E, Self W, Seal S, Protein adsorption and cellular uptake of cerium oxide nanoparticles as a function of zeta potential. Biomaterials. 28, 4600–4607 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Yue Z-G et al. , Surface charge affects cellular uptake and intracellular trafficking of chitosan-based nanoparticles. Biomacromolecules. 12, 2440–2446 (2011). [DOI] [PubMed] [Google Scholar]
- [53].Yang S, May S, Release of cationic polymer-DNA complexes from the endosome: A theoretical investigation of the proton sponge hypothesis. J. Chem. Phys 129, 185105 (2008). [DOI] [PubMed] [Google Scholar]
- [54].Akinc A, Thomas M, Klibanov AM, Langer R, Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. J. Gene Med 7, 657–663 (2005). [DOI] [PubMed] [Google Scholar]
- [55].Beningo KA, Wang Y, Fc-receptor-mediated phagocytosis is regulated by mechanical properties of the target. J. Cell Sci 115, 849–856 (2002). [DOI] [PubMed] [Google Scholar]
- [56].Palomba R et al. , Modulating phagocytic cell sequestration by tailoring nanoconstruct softness. ACS Nano. 12, 1433–1444 (2018). [DOI] [PubMed] [Google Scholar]
- [57].Alexander JF et al. , Cubical Shape Enhances the Interaction of Layer-by-Layer Polymeric Particles with Breast Cancer Cells. Adv. Healthc. Mater 4, 2657–2666 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Key J et al. , Soft discoidal polymeric nanoconstructs resist macrophage uptake and enhance vascular targeting in tumors. ACS Nano. 9, 11628–11641 (2015). [DOI] [PubMed] [Google Scholar]
- [59].Cui J et al. , Mechanically tunable, self-adjuvanting nanoengineered polypeptide particles. Adv Mater Weinheim. 25, 3468–3472 (2013). [DOI] [PubMed] [Google Scholar]
- [60].Benne N, van Duijn J, Kuiper J, Jiskoot W, Slütter B, Orchestrating immune responses: How size, shape and rigidity affect the immunogenicity of particulate vaccines. J. Control. Release 234, 124–134 (2016). [DOI] [PubMed] [Google Scholar]
- [61].Harding CV, Collins DS, Slot JW, Geuze HJ, Unanue ER, Liposome-encapsulated antigens are processed in lysosomes, recycled, and presented to T cells. Cell. 64, 393–401 (1991). [DOI] [PubMed] [Google Scholar]
- [62].Falo LD, Kovacsovics-Bankowski M, Thompson K, Rock KL, Targeting antigen into the phagocytic pathway in vivo induces protective tumour immunity. Nat. Med 1, 649–653 (1995). [DOI] [PubMed] [Google Scholar]
- [63].Kovacsovics-Bankowski M, Clark K, Benacerraf B, Rock KL, Efficient major histocompatibility complex class I presentation of exogenous antigen upon phagocytosis by macrophages. Proc Natl Acad Sci USA. 90, 4942–4946 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Graham DB et al. , An ITAM-signaling pathway controls cross-presentation of particulate but not soluble antigens in dendritic cells. J. Exp. Med 204, 2889–2897 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Demento SL et al. , Role of sustained antigen release from nanoparticle vaccines in shaping the T cell memory phenotype. Biomaterials. 33, 4957–4964 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Chen N et al. , Tunable degradation of acetalated dextran microparticles enables controlled vaccine adjuvant and antigen delivery to modulate adaptive immune responses. J. Control. Release 273, 147–159 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Stano A, Scott EA, Dane KY, Swartz MA, Hubbell JA, Tunable T cell immunity towards a protein antigen using polymersomes vs. solid-core nanoparticles. Biomaterials. 34, 4339–4346 (2013). [DOI] [PubMed] [Google Scholar]
- [68].Chen W, Yan W, Huang L, A simple but effective cancer vaccine consisting of an antigen and a cationic lipid. Cancer Immunol. Immunother 57, 517–530 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Vasievich EA, Chen W, Huang L, Enantiospecific adjuvant activity of cationic lipid DOTAP in cancer vaccine. Cancer Immunol. Immunother 60, 629–638 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Vangasseri DP et al. , Immunostimulation of dendritic cells by cationic liposomes. Mol. Membr. Biol 23, 385–395 (2006). [DOI] [PubMed] [Google Scholar]
- [71].Shetab Boushehri MA, Stein V, Lamprecht A, Cargo-free particles of ammonio methacrylate copolymers: From pharmaceutical inactive ingredients to effective anticancer immunotherapeutics. Biomaterials. 166, 1–12 (2018). [DOI] [PubMed] [Google Scholar]
- [72].Kumar N, Langer RS, Domb AJ, Polyanhydrides: an overview. Adv. Drug Deliv. Rev 54, 889–910 (2002). [DOI] [PubMed] [Google Scholar]
- [73].Tamayo I et al. , Poly(anhydride) nanoparticles act as active Th1 adjuvants through Toll-like receptor exploitation. Clin. Vaccine Immunol 17, 1356–1362 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Wafa EI, Geary SM, Goodman JT, Narasimhan B, Salem AK, The effect of polyanhydride chemistry in particle-based cancer vaccines on the magnitude of the anti-tumor immune response. Acta Biomater. 50, 417–427 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].He L-Z et al. , Antigenic targeting of the human mannose receptor induces tumor immunity. J. Immunol 178, 6259–6267 (2007). [DOI] [PubMed] [Google Scholar]
- [76].Burgdorf S, Kautz A, Böhnert V, Knolle PA, Kurts C, Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science. 316, 612–616 (2007). [DOI] [PubMed] [Google Scholar]
- [77].Cui L, Cohen JA, Broaders KE, Beaudette TT, Fréchet JMJ, Mannosylated dextran nanoparticles: a pH-sensitive system engineered for immunomodulation through mannose targeting. Bioconjug. Chem 22, 949–957 (2011). [DOI] [PubMed] [Google Scholar]
- [78].Silva JM et al. , In vivo delivery of peptides and Toll-like receptor ligands by mannose-functionalized polymeric nanoparticles induces prophylactic and therapeutic anti-tumor immune responses in a melanoma model. J. Control. Release 198, 91–103 (2015). [DOI] [PubMed] [Google Scholar]
- [79].Lee S, Margolin K, Cytokines in cancer immunotherapy. Cancers (Basel). 3, 3856–3893 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Zhang Y, Li N, Suh H, Irvine DJ, Nanoparticle anchoring targets immune agonists to tumors enabling anti-cancer immunity without systemic toxicity. Nat. Commun 9, 6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Kedar E et al. , Delivery of cytokines by liposomes: hematopoietic and immunomodulatory activity of interleukin-2 encapsulated in conventional liposomes and in long-circulating liposomes. J. Immunother 23, 131–145 (2000). [DOI] [PubMed] [Google Scholar]
- [82].Yonezawa A, Dutt S, Chester C, Kim J, Kohrt HE, Boosting Cancer Immunotherapy with Anti-CD137 Antibody Therapy. Clin. Cancer Res 21, 3113–3120 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Zhu EF et al. , Synergistic innate and adaptive immune response to combination immunotherapy with anti-tumor antigen antibodies and extended serum half-life IL-2. Cancer Cell. 27, 489–501 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Wilson DR et al. , Biodegradable STING agonist nanoparticles for enhanced cancer immunotherapy. Nanomedicine. 14, 237–246 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Andorko JI, Jewell CM, Designing biomaterials with immunomodulatory properties for tissue engineering and regenerative medicine. Bioeng. Transl. Med 2, 139–155 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Cai X, Chiu Y-H, Chen ZJ, The cGAS-cGAMP-STING pathway of cytosolic DNA sensing and signaling. Mol. Cell 54, 289–296 (2014). [DOI] [PubMed] [Google Scholar]
- [87].Hanson MC et al. , Nanoparticulate STING agonists are potent lymph node-targeted vaccine adjuvants. The Journal of Clinical Investigation (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Lemos H, Huang L, McGaha T, Mellor AL, STING, nanoparticles, autoimmune disease and cancer: a novel paradigm for immunotherapy? Expert Rev. Clin. Immunol 11, 155–165 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Takashima K et al. , STING in tumor and host cells cooperatively work for NK cell-mediated tumor growth retardation. Biochem. Biophys. Res. Commun 478, 1764–1771 (2016). [DOI] [PubMed] [Google Scholar]
- [90].Junkins RD et al. , A robust microparticle platform for a STING-targeted adjuvant that enhances both humoral and cellular immunity during vaccination. J. Control. Release 270, 1–13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Koshy ST, Cheung AS, Gu L, Graveline AR, Mooney DJ, Liposomal delivery enhances immune activation by STING agonists for cancer immunotherapy. Adv. Biosys. 1, 1600013 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Miyabe H et al. , A new adjuvant delivery system “cyclic di-GMP/YSK05 liposome” for cancer immunotherapy. J. Control. Release 184, 20–27 (2014). [DOI] [PubMed] [Google Scholar]
- [93].Nakamura T et al. , Liposomes loaded with a STING pathway ligand, cyclic di-GMP, enhance cancer immunotherapy against metastatic melanoma. J. Control. Release 216, 149–157 (2015). [DOI] [PubMed] [Google Scholar]
- [94].Han HD et al. , In vivo stepwise immunomodulation using chitosan nanoparticles as a platform nanotechnology for cancer immunotherapy. Sci. Rep 6, 38348 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Ammi R et al. , Poly(I:C) as cancer vaccine adjuvant: knocking on the door of medical breakthroughs. Pharmacol. Ther 146, 120–131 (2015). [DOI] [PubMed] [Google Scholar]
- [96].Bianchi F, Pretto S, Tagliabue E, Balsari A, Sfondrini L, Exploiting poly(I:C) to induce cancer cell apoptosis. Cancer Biol. Ther 18, 747–756 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Krieg AM, Toll-like receptor 9 (TLR9) agonists in the treatment of cancer. Oncogene. 27, 161–167 (2008). [DOI] [PubMed] [Google Scholar]
- [98].Cooper C, Mackie D, Hepatitis B surface antigen-1018 ISS adjuvant-containing vaccine: a review of HEPLISAV™ safety and efficacy. Expert Rev. Vaccines 10, 417–427 (2011). [DOI] [PubMed] [Google Scholar]
- [99].Bourquin C et al. , Targeting CpG oligonucleotides to the lymph node by nanoparticles elicits efficient antitumoral immunity. J. Immunol 181, 2990–2998 (2008). [DOI] [PubMed] [Google Scholar]
- [100].Thomas SN, Vokali E, Lund AW, Hubbell JA, Swartz MA, Targeting the tumor-draining lymph node with adjuvanted nanoparticles reshapes the anti-tumor immune response. Biomaterials. 35, 814–824 (2014). [DOI] [PubMed] [Google Scholar]
- [101].Sato T, Shimosato T, Ueda A, Ishigatsubo Y, Klinman DM, Intrapulmonary delivery of cpg microparticles eliminates lung tumors. Mol. Cancer Ther 14, 2198–2205 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Xu Z et al. , Multifunctional nanoparticles co-delivering Trp2 peptide and CpG adjuvant induce potent cytotoxic T-lymphocyte response against melanoma and its lung metastasis. J. Control. Release 172, 259–265 (2013). [DOI] [PubMed] [Google Scholar]
- [103].San Román B et al. , Co-encapsulation of an antigen and CpG oligonucleotides into PLGA microparticles by TROMS technology. Eur. J. Pharm. Biopharm 70, 98–108 (2008). [DOI] [PubMed] [Google Scholar]
- [104].Fischer S et al. , Concomitant delivery of a CTL-restricted peptide antigen and CpG ODN by PLGA microparticles induces cellular immune response. J. Drug Target 17, 652–661 (2009). [DOI] [PubMed] [Google Scholar]
- [105].Martínez Gómez JM et al. , A protective allergy vaccine based on CpG- and protamine-containing PLGA microparticles. Pharm. Res 24, 1927–1935 (2007). [DOI] [PubMed] [Google Scholar]
- [106].Singh M et al. , Cationic Microparticles Are an Effective Delivery System for Immune Stimulatory CpG DNA. Pharmaceutical Research. [DOI] [PubMed] [Google Scholar]
- [107].Shakweh M, Fattal E, Design and Characterisation of Poly(lactide-co-glycolide) Small Particulate Systems for the Delivery of Immunostimulant CpG Oligonucleotide. J. Nanosci. Nanotechnol 6, 2811–2820 (2006). [DOI] [PubMed] [Google Scholar]
- [108].Ballarín-González B, Howard KA, Polycation-based nanoparticle delivery of RNAi therapeutics: adverse effects and solutions. Adv. Drug Deliv. Rev 64, 1717–1729 (2012). [DOI] [PubMed] [Google Scholar]
- [109].Peine KJ et al. , Efficient delivery of the toll-like receptor agonists polyinosinic:polycytidylic acid and CpG to macrophages by acetalated dextran microparticles. Mol. Pharm 10, 2849–2857 (2013). [DOI] [PubMed] [Google Scholar]
- [110].Lorusso D et al. , Pegylated liposomal doxorubicin-related palmar-plantar erythrodysesthesia (“hand-foot” syndrome). Ann. Oncol 18, 1159–1164 (2007). [DOI] [PubMed] [Google Scholar]
- [111].Ragelle H, Danhier F, Préat V, Langer R, Anderson DG, Nanoparticle-based drug delivery systems: a commercial and regulatory outlook as the field matures. Expert Opin. Drug Deliv 14, 851–864 (2017). [DOI] [PubMed] [Google Scholar]
- [112].Barenholz Y, Doxil®--the first FDA-approved nano-drug: lessons learned. J. Control. Release 160, 117–134 (2012). [DOI] [PubMed] [Google Scholar]
- [113].Zucker D, Marcus D, Barenholz Y, Goldblum A, Liposome drugs’ loading efficiency: a working model based on loading conditions and drug’s physicochemical properties. J. Control. Release 139, 73–80 (2009). [DOI] [PubMed] [Google Scholar]