Abstract
Preeclampsia (PE) is a hypertensive disorder that occurs after 20 weeks of gestation, implicating the placenta as a key offender. PE is associated with an imbalance among B lymphocytes, CD4+ T lymphocytes, NK cells and increased inflammatory cytokines. During early onset PE, trophoblast invasion and placentation are impaired, leading to reduced blood flow to the fetus. In all spectrums of this disorder, a shift towards a pro‐inflammatory state where regulatory cells and cytokines are decreased occurs. Specifically, inflammatory CD4+ T‐cells and inflammatory cytokines are increased while CD4+ T regulatory cells (Tregs) and immunosuppressive cytokines such as IL‐4 and IL‐10 are decreased resulting in B cell activation, production of autoantibodies, endothelial dysfunction and hypertension associated with PE. However, the stimulus for these imbalances is unknown and need to be fully understood so that effective treatments that target the pathogenesis of the disease can be designed. Therefore, this review will focus on the pathways involving CD4+, TH1, TH2, Tregs, TH17s, B cells, and NK cells in the pathophysiology of PE.
Linked Articles
This article is part of a themed section on Immune Targets in Hypertension. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v176.12/issuetoc
Abbreviations
- PE
preeclampsia
- AT1‐AA
agonistic autoantibodies to the angiotensin II AT1 receptor
- TH
T helper
- Tregs
T regulatory cells
- NCRs
natural cytotoxicity receptors
- dNK cells
decidual NK cells
- RUPP
reduced uterine perfusion pressure
- NP
normal pregnant
Introduction
The hypertensive disorders of pregnancy complicate 6 to 11% of all pregnancies (Creasy et al., n.d.; Duley, 1992; Berg et al., 2003; Chang et al., 2003). They are a leading cause of iatrogenic preterm birth and significant contributors to maternal and perinatal morbidity and mortality (Duley, 1992; World Health Organization, 1995). The spectrum of disorders includes preeclampsia (PE)–eclampsia, PE superimposed on chronic hypertension and gestational hypertension (transient hypertension of pregnancy or chronic hypertension identified in the latter half of pregnancy (Mammaro et al., 2009). The clinical complications of these disorders include fetal growth restriction, preterm birth, placental abruption, cardiovascular disease and end‐organ damage (Creasy et al., n.d.). While advances have been made in our understanding of the pathophysiology of the hypertensive disorders of pregnancy, the treatment has not changed in 50 years with delivery remaining the only known definitive cure (Noris et al., 2005). Although the recent Aspirin for Evidence‐Based Preeclampsia Prevention trial demonstrated that daily low‐dose aspirin significantly decreased the incidence of PE in high‐risk pregnancies (Rolnik et al., 2017), aspirin was not proven to improve management or outcomes of women who go on to develop PE or others hypertensive disorders of pregnancy (Poon et al., 2017).
The precipitating cause during pregnancy of new onset hypertension or exacerbation of previously existing hypertension has yet to be fully elucidated. It is postulated that maternal endothelial dysfunction most often incites or exacerbates the clinical signs used for diagnostic purposes. This maternal endothelial dysfunction, manifesting as placental ischaemia, results from improper vascular remodelling due to insufficient trophoblast invasion, which is tightly regulated by immune cells in the decidua during a normal pregnancy (Cornelius, 2018). Placental ischaemia is believed to be the inciting event resulting from a hindrance of proper trophoblast invasion and placentation that leads to reduced blood flow to the fetus and a resulting shift in immune function towards a proinflammatory state characterized by increased pro‐inflammatory immune cells and cytokines and decreased regulatory cells and anti‐inflammatory cytokines (Saito et al., 1999; Lamarca, 2010; Saito et al., 2010; Tam Tam et al., 2011).
Shifts in immune cell populations
The normal cellular milieu of pregnancy involves both pro‐inflammatory and anti‐inflammatory components from the innate and adaptive immune arms (Granger, 2004; Noris et al., 2005; Redman and Sargent, 2005; LaMarca et al., 2007; Gilbert et al., 2008; Toldi et al., 2008; Prins et al., 2009; Lamarca, 2010; Matsubara et al., 2010). On the cellular level, normal pregnancy displays increases in innate immune cells compared to the non‐pregnant state. In the circulation, there is an increase in monocytes and granulocytes, as well as an increase in the activation of monocytes (Faas and de Vos, 2017) in normal pregnancy, compared to non‐pregnant women. Furthermore, an increase in decidual macrophages and NK cells are also observed during normal pregnancy versus non‐pregnancy in order to facilitate trophoblast invasion and uterine spiral artery remodelling (Faas et al., 2014). On a molecular level, the hypertensive disorders of pregnancy are marked by decreased vasodilators, significantly increased inflammatory cytokines, agonistic autoantibodies to the angiotensin II AT1 receptor (AT1‐AA) and chronic immune activation (Conrad and Benyo, 1997; Schlembach, 2003; Granger, 2004; Noris et al., 2005; Redman and Sargent, 2005; LaMarca et al., 2007; Matsubara et al., 2010). Specifically, women who develop PE exhibit elevated circulating and placental levels of TNF‐α and IL‐6 (Conrad and Benyo, 1997; Lam et al., 2005). Anti‐inflammatory cytokines also play a pivotal role in maintaining a normal, successful pregnancy by providing balance to the immune system (Piccinni and Romagnani, 1996; Hambartsoumian, 1998; Chatterjee et al., 2014). During PE, there is evidence of increased AT1‐AA, inflammatory CD4+ T‐cells and inflammatory cytokines such as TNF‐α, IL‐6 and TGF‐β. Previously, our laboratory have demonstrated that normal pregnant (NP) rats receiving either TNF‐α or IL‐6, developed signs of PE such as hypertension, oxidative stress and impaired endothelial function (Murphy et al., 2010; Amaral et al., 2014b). Furthermore, we have reported that AT1‐AA infusion into NP rats mediates the pathophysiology of PE (LaMarca et al., 2012).
Immunosuppressive cytokines such as IL‐4 and IL‐10, which are present at the fetal–maternal interface and should regulate and control inflammation, are decreased resulting in hypertension and other clinical complications (Conrad and Benyo, 1997; Granger, 2004; LaMarca et al., 2007; Toldi et al., 2008; Prins et al., 2009; Lamarca, 2010; Toldi et al., 2012; Chatterjee et al., 2014; Amaral et al., 2014a; Chatterjee et al., 2015). The main anti‐inflammatory cytokines produced by TH2 cells that are involved in normal pregnancy are IL‐4 and IL‐10 (Nickerson et al., 1994; Strom et al., 1996) and reduction in TH2 cells or IL‐4 contributes, at least in part, to the pathophysiology seen during PE. In agreement with this, clinical studies have demonstrated woman with PE have alterations in the NK cell population. There is also an association between low levels of IL‐4 and elevated levels of NK cells with multiple spontaneous abortions and PE, (Piccinni and Romagnani, 1996; Hambartsoumian, 1998), and supplementation with IL‐4 has improved TH2 cell counts while reducing cytolytic NK cells in pregnant rodent models of PE (Chatterjee et al., 2015; Elfarra et al., 2017).
An exciting area of research involves manipulating such inflammatory mediators responsible for blood pressure fluctuations during pregnancy to better understand the mechanisms involved in PE or miscarriage. We are now just beginning to learn about immune mechanisms and pathways required for normal pregnancies and the alterations seen in those complicated by the spectrum of hypertensive disorders. Although imbalance and improper function of inflammatory mediators is known to exacerbate and incite the hypertensive disorders of pregnancy, the exact role that these cells and cytokines play during this multi‐system disease process is not yet fully understood. Furthermore, improvements in treatment strategies that target this pregnancy disorder need to be considered for better understanding, clinical management and improved outcomes.
CD4+ T‐cell populations
During pregnancy, the abundance of leukocytes is significantly increased in the uterus. Leukocytes make up 30–40% of the decidua cells at the maternal–fetal interface and consist mainly of NK cells, CD14+ myelomonocytic cells and T lymphocytes. CD4+ T‐cell populations can be composed of helper T‐cells including type 1 (TH1), type 2 (TH2) and TH17 cells and immunomodulatory regulatory T‐cells (Treg) (Sykes et al., 2012). The imbalance between the CD4+ T‐cell subsets, T regulatory (Treg) and T helper 17 (TH17) has been positively associated with the pathophysiology of PE (Santner‐Nanan et al., 2009; Toldi et al., 2011; Darmochwal‐Kolarz et al., 2012). There is evidence for a shift towards proinflammatory CD4+ TH1 cells and away from CD4+ Treg and CD4+ TH2, in PE (Raghupathy, 1997; Saito et al., 1999; Veenstra van Nieuwenhoven et al., 2003; Saito et al., 2010). In the uterus, the cytotrophoblast invasion and proliferation can be facilitated by TH2 and Tregs during normal pregnancies (Sargent et al., 2006). However, in pregnancy disorders, there are more effector TH1 and TH17 cells within the circulation and placentas compromising the normal function of TH2 and Tregs.
The importance of Tregs in maintenance of early pregnancy has previously been established (Toldi et al., 2008; Santner‐Nanan et al., 2009; Darmochwal‐Kolarz et al., 2012; Toldi et al., 2012). Clinical studies have reported decreased CD4+ regulatory T‐cells (Tregs) in the decidua and circulation in preeclamptic pregnancies, compared to women with normal pregnancies. These cells mediate tolerance towards the fetus. Failure of the maternal immune tolerance mechanisms could be associated with placental ischaemia and oxidative stress, both of which are known to be involved in the pathophysiology of PE. Our previous findings have demonstrated that reduced uterine perfusion pressure (RUPP) rats have higher total CD4+ T‐cells with a 47% decrease in Tregs compared to NP rats (Wallace et al., 2011). Cornelius et al. published recently that adoptive transfer of Tregs from NP rats into RUPP rats decreases BP and vasoactive factors which contribute to the high BP observed in this rat model of PE (Cornelius et al., 2015a). Furthermore, we have demonstrated that the stimulation of Tregs by either administration of a specific stimulus (superagonistic monoclonal antibody for CD28) or IL‐10 supplementation reduces the signs of PE in response to placental ischaemia (Harmon et al., 2015; Ibrahim et al., 2017). Regulation of the immune response by Tregs is critical to maintenance of maternal health during pregnancy, and the up‐regulation of these cells may help to improve the pathophysiology of PE. The benefits of restoration in number of the Treg population still need to be further investigated.
TH17 cells secrete the proinflammatory cytokine IL‐17 and have been associated with PE. Previous studies have reported that TH17s are increased in preeclamptic women compared to normal pregnancies (Toldi et al., 2011; Darmochwal‐Kolarz et al., 2012). Our laboratory has shown that the abnormally increased TH17 cell population mediates the pathophysiology of PE associated with placental ischaemia, including oxidative stress, intrauterine growth restriction and production of AT1‐AA (Cornelius et al., 2016). In addition to this, we have also demonstrated that infusion of IL‐17 into NP rats lead to an increase in TH17 cells and other signs of PE. Importantly, blockade of IL‐17 function with the soluble receptor, IL‐17 RC, lowered TH17 cell numbers while decreasing hypertension, oxidative stress and AT1‐AA production in response to placental ischaemia (Cornelius et al., 2013). Furthermore, administration of losartan attenuated the hypertension after the adoptive transfer of TH17 cells into NP rats, demonstrating the importance of stimulation of the AT1 receptor in TH17 mediated effects of PE. Importantly, the down‐regulation of this cell population could improve maternal and fetal outcomes.
B lymphocytes
An important function of CD4+ T‐cells is to mediate the B lymphocyte memory immune response and specific antibody production towards a single antigen in a process is known as the T‐cell dependent antibody response (Abbus and Lichtman, 2005). Auto‐antibodies are produced during PE, suggesting an important role for B lymphocytes in the pathogenesis of this disease. Liao et al. demonstrated that the percentage of circulating memory B lymphocytes were significantly greater in preeclamptic women than in the NP cohort (Liao et al., 2009). Conventional memory B cells, known as B2 B lymphocytes, undergo antigen processing by recognizing the MHC class II peptide complex with the activated CD4+ T lymphocyte (Abbus and Lichtman, 2005). For B cell maturation and IgG production, several co‐stimulatory signals must occur between the antibody producing B lymphocyte and the CD4+ T helper cell (Abbus and Lichtman, 2005). One of these includes stimulation of the CD20 receptor on the surface of the B cell. This recognition prompts the B cell to enter the circulation and produce antigen specific Ig antibodies. Another necessary co‐stimulatory molecule for B cell maturation is CD40 located on the surface of the B cell which binds with the CD40 ligand on the surface of the T‐cell (Abbus and Lichtman, 2005). B cells then progress through the stages of proliferation, differentiation and internal isotype switching leading to production of specific antigen stimulated antibodies which leads to the formation of short lived plasma cells that secrete antibody and memory B cells residing in the germinal lymph node centres, which will be available for rapid response in future interactions with antigen‐specific T‐cells.
A number of therapeutic agents that inhibit specific interactions between immune molecules on cells have been developed to treat various autoimmune diseases. In a recent study, we utilized a chemotherapeutic agent that has shown efficacy among autoimmune patients by blocking the CD20 (Rutiximab) co‐stimulatory molecule (Cianchini et al., 2007; LaMarca et al., 2011). Rutiximab is used to inhibit B lymphocytes from entering the circulation and secreting antibody, a process known as B cell depletion (Abbus and Lichtman, 2005; Cianchini et al., 2007). We found that with B cell depletion, RUPP rats had lower BP, circulating TNF‐α, autoantibodies and tissue ET‐1 as compared to control RUPP rats (LaMarca et al., 2011). We exposed endothelial cells to serum from B cell depleted RUPP rats and found that ET‐1 secretion was completely attenuated when compared to control RUPP sera. These data supported the hypothesis that B lymphocytes stimulated through T‐cell interaction in response to placental ischaemia in pregnant rats play an important role to increase BP, circulating inflammatory cytokines and ET‐1, possibly via autoantibody production, during pregnancy.
Although this study demonstrated a role for memory B2 B lymphocytes in the pathogenesis of hypertension in response to placental ischaemia, it did not clarify antigenic stimulation or examine the role for the other B cell subtypes in the progression of this disease. B lymphocytes can be characterized as either B1 or B2 cells, each having distinct markers and roles in facilitating immune reactions. B1 lymphocytes can be further divided into B1a or B1b cells (Abbus and Lichtman, 2005; Liao et al., 2009). These cells express IgM in greater quantities than IgG and are the primary source of natural antibodies produced in the absence of antigenic stimulation. These antibodies are polyreactive and cross‐react with multiple antigens such as autoantigens, other immunoglobulins and bacterial polysaccharides (Abbus and Lichtman, 2005; Jensen et al., 2012). B1 B cells have been implicated in the progression of autoimmune diseases and are elevated in lupus erythematosus and rheumatoid arthritis. B1 B cells are present in low numbers in the circulation, lymph nodes and spleen and are predominantly found in the peritoneal and pleural cavities. B1 B lymphocytes are responsible for T‐cell independent antibody production.
We recently demonstrated that importance of B1 lymphocyte communication with activated CD4+ T helper cells in NP rats (Cornelius et al., 2015b). In this study, CD40L on RUPP CD4+ T helper cells was blocked with a neutralizing antibody. Adoptive transfer anti‐CD40L treated RUPP CD4+ T helper cells into NP rats caused a significant decrease in hypertension, placental ROS and importantly AT1‐AA production compared with NP rats who received untreated RUPP CD4+ T helper cells. Importantly, blockade of CD40L on CD4 T‐cells significantly decreased circulating IL‐6. This may be due to decreased CD40 mediated activation of B cells, as CD40‐activated B cells have been shown to secrete IL‐6 (Daudelin et al., 2013). Therefore, the decrease in IL‐6 may also be due to decreased B cell activation by the RUPP CD4 T‐cells which would account for less AT1‐AA. This study demonstrated that interaction between endogenous cells and RUPP CD4+ T helper cells via CD40–CD40L binding is one important mechanism that leads to much of the pathophysiology of PE.
Jensen et al. uncovered an important role for B1 lymphocytes in the progression of PE (Jensen et al., 2012). Preeclamptic placentas stained positive for markers of B1 B lymphocytes (CD19+CD5+). Furthermore, these authors demonstrated that B1 B lymphocytes were stimulated to produce AT1‐AA when co‐cultured with sera from preeclamptic women but not from NP women. This study further illustrated the importance of B cells in the preeclamptic placenta and their stimulation by a soluble factor to produce AT1‐AA and contribute to the progression of this disease. Furthermore, high levels of B1 cells is yet another important characteristic that preeclamptic women share with patients presenting with autoimmune diseases.
NK ratios
NK cells are granular lymphocytes of the innate immune system that develop in the bone marrow from common lymphoid progenitor cells. NK cells participate in the control of viral or bacterial infection, regulation of haematopoiesis, production of cytokines and cytotoxicity of neoplastic cells. However, a specialized population of NK cells in the decidua also play important roles in the establishment of and maintenance of human pregnancy. The decidual NK (dNK) cells make up approximately 70% of the uterine leukocytes and are the most abundant maternal leukocyte population during the first trimester in human pregnancy (Moffett‐King, 2002; Fukui et al., 2011). The dNK cells have a cytokine‐producing rather than cytotoxic phenotype and have several roles in human pregnancy (Dosiou and Giudice, 2005; Fukui et al., 2011). The dNK cells interact closely with trophoblast cells and secrete cytokines that promote trophoblast growth and mediate trophoblast differentiation, invasion and spiral artery remodelling (Dosiou and Giudice, 2005; LaMarca et al., 2013). The dNK cells also have roles in maintenance of maternal–fetal tolerance throughout pregnancy. The dNK cells recognize HLA antigens on fetal trophoblasts which protect them from cytotoxic targeting. Additionally, the dNK cells have a role to help mediate the increased Treg population in the placenta and inhibit proliferation, survival and activation of fetal‐antigen specific effector T helper cells (Dosiou and Giudice, 2005; Arck and Hecher, 2013; Vacca et al., 2013).
The type 1 shift in the immune profile of women with PE is observed not only in adaptive immune cells but also in innate immune cells, specifically NK cells. The type 1 (NK1) subset of NK cells is characterized by release of IFN‐γ and TNF‐α and potent cytolytic activity upon activation (Peritt et al., 1998; Vacca et al., 2011). Tregs can inhibit IL‐2 mediated polarization of NK1 cells by decreasing availability of IL‐2 (Katsumoto et al., 2004; Kerdiles et al., 2013). The NK2 subset is characterized by their release of the anti‐inflammatory cytokines IL‐5 and IL‐13 and a decrease in their cytolytic activity (Peritt et al., 1998; Vacca et al., 2011). NK2 differentiation is mediated through signalling by the anti‐inflammatory cytokine IL‐4 (Peritt et al., 1998; Vacca et al., 2011). This suggests a role for anti‐inflammatory and regulatory CD4+ T‐cells are able to regulate innate immune cell polarization and activation (Figure 1).
Figure 1.
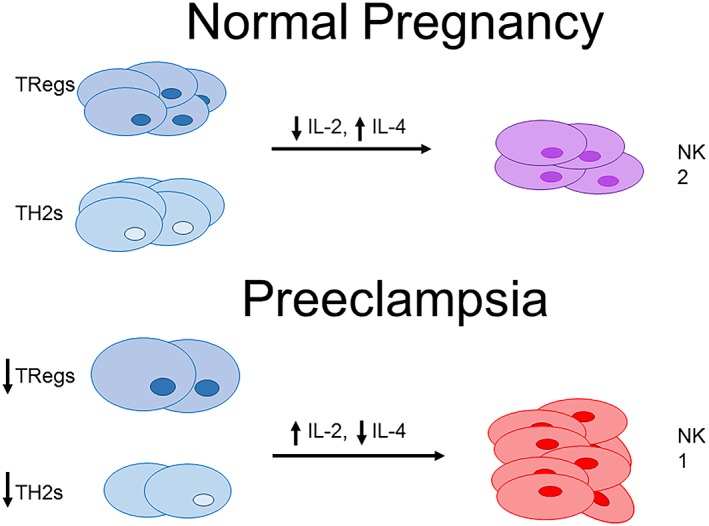
Adaptive CD4+ T‐cell populations may be regulators of NK cell proliferation and polarization. The decreased population of Tregs in PE may increase IL‐2 bioavailability and mediate polarization of NK1 cells. Decreased TH2 populations in PE may lead to decreased levels of NK2 polarizing cytokines, such as IL‐4.
The aberrant activation of NK cells is observed both in the periphery and in the decidua of women with PE (Sargent et al., 2007). A number of studies have demonstrated that changes in the decidual population of NK cells are reflected in the periphery (Park et al., 2010; Fukui et al., 2011). Borzychowski et al. suggested that systemic type 1 and type 2 immunity in normal pregnancy and PE may be mediated by NK cells (Borzychowski et al., 2005). This study demonstrated significant increases in type 1:type 2 ratios in both CD56bright and CD56dim NK cells from peripheral blood mononuclear cells in women with PE, compared with women with normal pregnancies (Borzychowski et al, 2005). The results of these studies were further supported by findings that expression of IFN‐γ was higher in NK cells from women with PE, than in normal pregnancy (Darmochwal‐Kolarz et al., 2002; Germain et al., 2007; Saito et al., 2010).
Natural cytotoxicity receptors (NCRs) are markers unique to NK cells that regulate NK cell cytokine production and cytotoxicity. Expression of the NCR, NKp46, has been examined in peripheral blood in women with PE. Pregnant women who developed PE demonstrated a significantly decreased percentage of NKp46+ cells compared to women without PE (Fukui et al., 2011). Furthermore, the decrease in NKp46 NK cells correlated with an increase in IFN‐γ and TNF‐α positive NK cells, suggesting that the lower expression of NKp46 is associated with the type 1 shift in NK cells in PE. Additional in vitro studies of the activity of NK cells from patients with PE and normal pregnancies investigated the proliferative state and cytotoxic function of NK cells from maternal and cord blood. These studies demonstrated that the proliferative and killing ability of NK cells in PE patients was significantly higher than in normal third trimester pregnant women (Zhang et al., 2004). The increased number of cytotoxic NK cells in this study further supports the increased ratio of type 1 to type 2 NK cells in PE and may identify this cytotoxic population of type 1 NK cells as a therapeutic target in PE.
Targeting the abnormal population of NK cells activated in PE may be a potential therapeutic option to improve treatment or management of PE. Although it is known that IL‐2 and IL‐12 signalling promote differentiation of NK cells to the NK1 subset, it has also been demonstrated that IL‐17 may enhance cytolytic activity of NK cells, suggesting that TH17 cells may play a role in mediating differentiation into the NK1 population subset (Al Omar et al., 2013). IL‐17 induced cytolytic NK cell activity against tumours which suggests a possible therapeutic strategy in cancer treatment (Qian et al., 2017). IL‐17 was also shown to enhance NK cytolytic competence in fungal infection (Bar et al., 2014). Thus, blockade or neutralization of these cytokines may inhibit polarization of NK1 cells in PE. Additionally, expansion of the endogenous Treg population in PE may favour a type 2 shift in NK cells through decreasing IL‐2‐induced NK1 polarization.
Conclusion
The spectrum of hypertensive disorders of pregnancy is associated with inflammation and chronic immune activation. The immune profile of women with PE shifts from a controlled state of mild inflammation with a balance of both proinflammatory and anti‐inflammatory components, to an altered state characterized by increased inflammatory cytokines and effector immune cells with a concomitant decrease in anti‐inflammatory factors and regulatory cells (Figure 2). Preclinical studies suggest that normalization of the immune imbalance in PE may be a potential strategy for the development of therapeutic interventions that could improve maternal and fetal outcomes associated with this maternal syndrome.
Figure 2.
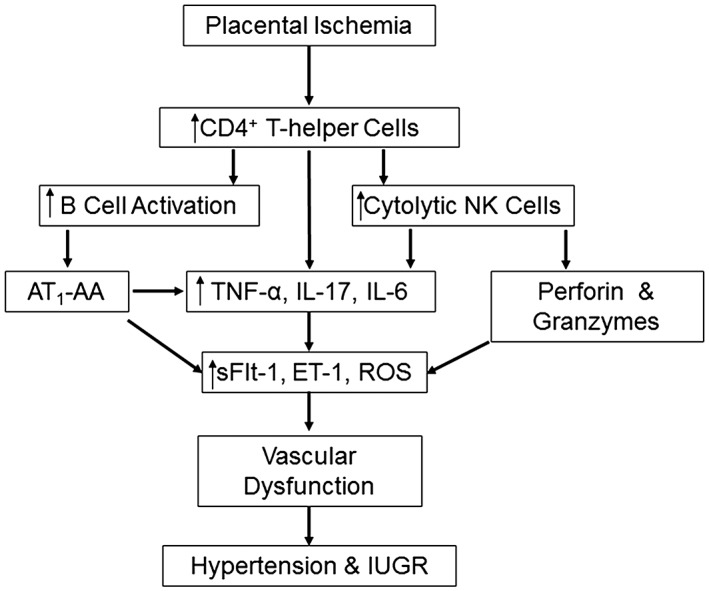
Placental ischaemia leads to an altered population of CD4+ T helper cells that facilitate B cell activation and NK cytolytic polarization leading to increased inflammatory cytokines and effector proteins. This in turn causes maternal system endothelial dysfunction resulting in the development of hypertension and intrauterine growth restriction (IUGR).
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a, 2017b, 2017c).
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgement
This study was supported by NIH grant RO1HD067541 (B.L.) and NIH grant HL130456 (D.C.).
Cornelius D. C., Cottrell J., Amaral L. M., and LaMarca B. (2019) Inflammatory mediators: a causal link to hypertension during preeclampsia, British Journal of Pharmacology, 176, 1914–1921, doi: 10.1111/bph.14466.
References
- Abbus A, Lichtman A (2005). General properties of the immune response, cells and Tissues of the Immune system In: Cellular and Molecular Immunolog. Elsevier Health Sciences: Philadephia, Pennsylvania, pp. 189–215. [Google Scholar]
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Catalytic receptors. Br J Pharmacol 174: S225–S271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD et al (2017c). The Concise Guide To PHARMACOLOGY 2017/18: Other proteins. Br J Pharmacol 174: S1–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Omar S, Flanagan BF, Almehmadi M, Christmas SE (2013). The effects of IL‐17 upon human natural killer cells. Cytokine 62: 123–130. [DOI] [PubMed] [Google Scholar]
- Amaral LM, Kiprono L, Cornelius DC, Shoemaker C, Wallace K, Moseley J et al (2014a). Progesterone supplementation attenuates hypertension and the autoantibody to the angiotensin II type I receptor in response to elevated interleukin‐6 during pregnancy. Am J Obstet Gynecol 211: 158.e1–158.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral LM, Kiprono L, Cornelius DC, Shoemaker C, Wallace K, Moseley J et al (2014b). Progesterone supplementation attenuates hypertension and the autoantibody to the angiotensin II type I receptor in response to elevated interleukin‐6 during pregnancy. Am J Obstet Gynecol 211: 158 e151–e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arck PC, Hecher K (2013). Fetomaternal immune cross‐talk and its consequences for maternal and offspring's health. Nat Med 19: 548–556. [DOI] [PubMed] [Google Scholar]
- Bar E, Whitney PG, Moor K, Reisesousa C, LeibundGut‐Landmann S (2014). IL‐17 regulates systemic fungal immunity by controlling the functional competence of NK cells. Immunity 40: 117–127. [DOI] [PubMed] [Google Scholar]
- Berg CJ, Chang J, Callaghan WM, Whitehead SJ (2003). Pregnancy‐related mortality in the United States, 1991–1997. Obstet Gynecol 101: 289–296. [DOI] [PubMed] [Google Scholar]
- Borzychowski AM, Croy BA, Chan WL, Redman CW, Sargent IL (2005). Changes in systemic type 1 and type 2 immunity in normal pregnancy and pre‐eclampsia may be mediated by natural killer cells. Eur J Immunol 35: 3054–3063. [DOI] [PubMed] [Google Scholar]
- Chang J, Elam‐Evans LD, Berg CJ, Herndon J, Flowers L, Seed KA et al (2003). Pregnancy‐related mortality surveillance – United States, 1991–1999. MMWR Surveill Summ 52: 1–8. [PubMed] [Google Scholar]
- Chatterjee P, Chiasson VL, Bounds KR, Mitchell BM (2014). Regulation of the anti‐inflammatory cytokines interleukin‐4 and interleukin‐10 during pregnancy. Front Immunol 5: 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee P, Chiasson VL, Seerangan G, Tobin RP, Kopriva SE, Newell‐Rogers MK et al (2015). Cotreatment with interleukin 4 and interleukin 10 modulates immune cells and prevents hypertension in pregnant mice. Am J Hypertens 28: 135–142. [DOI] [PubMed] [Google Scholar]
- Cianchini G, Corona R, Frezzolini A, Ruffelli M, Didona B, Puddu P (2007). Treatment of severe pemphigus with rituximab: report of 12 cases and a review of the literature. Arch Dermatol 143: 1033–1038. [DOI] [PubMed] [Google Scholar]
- Conrad KP, Benyo DF (1997). Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol 37: 240–249. [DOI] [PubMed] [Google Scholar]
- Cornelius DC (2018). Preeclampsia: from inflammation to immunoregulation. Clin Med Insights Blood Disord 11 1179545X17752325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius DC, Amaral LM, Harmon A, Wallace K, Thomas AJ, Campbell N et al (2015a). An increased population of regulatory T cells improves the pathophysiology of placental ischemia in a rat model of preeclampsia. Am J Physiol Regul Integr Comp Physiol 309: R884–R891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius DC, Amaral LM, Wallace K, Campbell N, Thomas AJ, Scott J et al (2016). Reduced uterine perfusion pressure T‐helper 17 cells cause pathophysiology associated with preeclampsia during pregnancy. Am J Physiol Regul Integr Comp Physiol 311: R1192–R1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius DC, Castillo J, Porter J, Amaral LM, Campbell N, Paige A et al (2015b). Blockade of CD40 ligand for intercellular communication reduces hypertension, placental oxidative stress, and AT1‐AA in response to adoptive transfer of CD4+ T lymphocytes from RUPP rats. Am J Physiol Regul Integr Comp Physiol 309: R1243–R1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius DC, Hogg JP, Scott J, Wallace K, Herse F, Moseley J et al (2013). Administration of interleukin‐17 soluble receptor C suppresses TH17 cells, oxidative stress, and hypertension in response to placental ischemia during pregnancy. Hypertension 62: 1068–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creasy RK, Resnik R, Greene MF, Iams JD, & Lockwood CJ Creasy and Resnik's maternal‐fetal medicine: principles and practice. Available from http://www.clinicalkey.com/dura/browse/bookChapter/3‐s2.0‐C20110040644 (accessed 5/1/2018).
- Darmochwal‐Kolarz D, Kludka‐Sternik M, Tabarkiewicz J, Kolarz B, Rolinski J, Leszczynska‐Gorzelak B et al (2012). The predominance of Th17 lymphocytes and decreased number and function of Treg cells in preeclampsia. J Reprod Immunol 93: 75–81. [DOI] [PubMed] [Google Scholar]
- Darmochwal‐Kolarz D, Rolinski J, Leszczynska‐Goarzelak B, Oleszczuk J (2002). The expressions of intracellular cytokines in the lymphocytes of preeclamptic patients. Am J Reprod Immunol 48: 381–386. [DOI] [PubMed] [Google Scholar]
- Daudelin JF, Mathieu M, Boulet S, Labrecque N (2013). IL‐6 production by dendritic cells is dispensable for CD8+ memory T‐cell generation. Biomed Res Int 2013: 126189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosiou C, Giudice LC (2005). Natural killer cells in pregnancy and recurrent pregnancy loss: endocrine and immunologic perspectives. Endocr Rev 26: 44–62. [DOI] [PubMed] [Google Scholar]
- Duley L (1992). Maternal mortality associated with hypertensive disorders of pregnancy in Africa, Asia, Latin America and the Caribbean. Br J Obstet Gynaecol 99: 547–553. [DOI] [PubMed] [Google Scholar]
- Elfarra J, Amaral LM, McCalmon M, Scott JD, Cunningham MW Jr, Gnam A et al (2017). Natural killer cells mediate pathophysiology in response to reduced uterine perfusion pressure. Clin Sci 131: 2753–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faas MM, de Vos P (2017). Maternal monocytes in pregnancy and preeclampsia in humans and in rats. J Reprod Immunol 119: 91–97. [DOI] [PubMed] [Google Scholar]
- Faas MM, Spaans F, De Vos P (2014). Monocytes and macrophages in pregnancy and pre‐eclampsia. Front Immunol 5: 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui A, Funamizu A, Yokota M, Yamada K, Nakamua R, Fukuhara R et al (2011). Uterine and circulating natural killer cells and their roles in women with recurrent pregnancy loss, implantation failure and preeclampsia. J Reprod Immunol 90: 105–110. [DOI] [PubMed] [Google Scholar]
- Germain SJ, Sacks GP, Sooranna SR, Sargent IL, Redman CW (2007). Systemic inflammatory priming in normal pregnancy and preeclampsia: the role of circulating syncytiotrophoblast microparticles. J Immunol 178: 5949–5956. [DOI] [PubMed] [Google Scholar]
- Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP (2008). Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol 294: H541–H550. [DOI] [PubMed] [Google Scholar]
- Granger JP (2004). Inflammatory cytokines, vascular function, and hypertension. Am J Physiol Regul Integr Comp Physiol 286: R989–R990. [DOI] [PubMed] [Google Scholar]
- Hambartsoumian E (1998). Endometrial leukemia inhibitory factor (LIF) as a possible cause of unexplained infertility and multiple failures of implantation. Am J Reprod Immunol 39: 137–143. [DOI] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS guide to pharmacology in 2018: updates and expansion to encompass the new guide to immunopharmacology. Nucl Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon A, Cornelius D, Amaral L, Paige A, Herse F, Ibrahim T et al (2015). IL‐10 supplementation increases Tregs and decreases hypertension in the RUPP rat model of preeclampsia. Hypertens Pregnancy 34: 291–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim T, Przybyl L, Harmon AC, Amaral LM, Faulkner JL, Cornelius DC et al (2017). Proliferation of endogenous regulatory T cells improve the pathophysiology associated with placental ischaemia of pregnancy. Am J Reprod Immunol 78: e12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen F, Wallukat G, Herse F, Budner O, El‐Mousleh T, Costa SD et al (2012). CD19+CD5+ cells as indicators of preeclampsia. Hypertension 59: 861–868. [DOI] [PubMed] [Google Scholar]
- Katsumoto T, Kimura M, Yamashita M, Hosokawa H, Hashimoto K, Hasegawa A et al (2004). STAT6‐dependent differentiation and production of IL‐5 and IL‐13 in murine NK2 cells. J Immunol 173: 4967–4975. [DOI] [PubMed] [Google Scholar]
- Kerdiles Y, Ugolini S, Vivier E (2013). T cell regulation of natural killer cells. J Exp Med 210: 1065–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam C, Lim KH, Karumanchi SA (2005). Circulating angiogenic factors in the pathogenesis and prediction of preeclampsia. Hypertension 46: 1077–1085. [DOI] [PubMed] [Google Scholar]
- Lamarca B (2010). The role of immune activation in contributing to vascular dysfunction and the pathophysiology of hypertension during preeclampsia. Minerva Ginecol 62: 105–120. [PMC free article] [PubMed] [Google Scholar]
- LaMarca B, Cornelius D, Wallace K (2013). Elucidating immune mechanisms causing hypertension during pregnancy. Phys Ther 28: 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMarca B, Parrish MR, Wallace K (2012). Agonistic autoantibodies to the angiotensin II type I receptor cause pathophysiologic characteristics of preeclampsia. Gend Med 9: 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMarca B, Wallace K, Herse F, Wallukat G, Martin JN Jr, Weimer A et al (2011). Hypertension in response to placental ischemia during pregnancy: role of B lymphocytes. Hypertension 57: 865–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMarca BD, Ryan MJ, Gilbert JS, Murphy SR, Granger JP (2007). Inflammatory cytokines in the pathophysiology of hypertension during preeclampsia. Curr Hypertens Rep 9: 480–485. [DOI] [PubMed] [Google Scholar]
- Liao AH, Liu LP, Ding WP, Zhang L (2009). Functional changes of human peripheral B‐lymphocytes in pre‐eclampsia. Am J Reprod Immunol 61: 313–321. [DOI] [PubMed] [Google Scholar]
- Mammaro A, Carrara S, Cavaliere A, Ermito S, Dinatale A, Pappalardo EM et al (2009). Hypertensive disorders of pregnancy. J Prenat Med 3: 1–5. [PMC free article] [PubMed] [Google Scholar]
- Matsubara K, Matsubara Y, Hyodo S, Katayama T, Ito M (2010). Role of nitric oxide and reactive oxygen species in the pathogenesis of preeclampsia. J Obstet Gynaecol Res 36: 239–247. [DOI] [PubMed] [Google Scholar]
- Moffett‐King A (2002). Natural killer cells and pregnancy. Nat Rev Immunol 2: 656–663. [DOI] [PubMed] [Google Scholar]
- Murphy SR, LaMarca BB, Cockrell K, Granger JP (2010). Role of endothelin in mediating soluble fms‐like tyrosine kinase 1‐induced hypertension in pregnant rats. Hypertension 55: 394–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson P, Steurer W, Steiger J, Zheng X, Steele AW, Strom TB (1994). Cytokines and the Th1/Th2 paradigm in transplantation. Curr Opin Immunol 6: 757–764. [DOI] [PubMed] [Google Scholar]
- Noris M, Perico N, Remuzzi G (2005). Mechanisms of disease: pre‐eclampsia. Nat Clin Pract Nephrol 1: 98–114 quiz 120. [DOI] [PubMed] [Google Scholar]
- Park DW, Lee HJ, Park CW, Hong SR, Kwak‐Kim J, Yang KM (2010). Peripheral blood NK cells reflect changes in decidual NK cells in women with recurrent miscarriages. Am J Reprod Immunol 63: 173–180. [DOI] [PubMed] [Google Scholar]
- Peritt D, Robertson S, Gri G, Showe L, Aste‐Amezaga M, Trinchieri G (1998). Cutting edge: differentiation of human NK cells into NK1 and NK2 subsets. J Immunol 161: 5821–5824. [PubMed] [Google Scholar]
- Piccinni MP, Romagnani S (1996). Regulation of fetal allograft survival by a hormone‐controlled Th1‐ and Th2‐type cytokines. Immunol Res 15: 141–150. [DOI] [PubMed] [Google Scholar]
- Poon LC, Wright D, Rolnik DL, Syngelaki A, Delgado JL, Tsokaki T et al (2017). Aspirin for Evidence‐Based Preeclampsia Prevention trial: effect of aspirin in prevention of preterm preeclampsia in subgroups of women according to their characteristics and medical and obstetrical history. Am J Obstet Gynecol 217: 585 e581–585 e585. [DOI] [PubMed] [Google Scholar]
- Prins JR, Boelens HM, Heimweg J, Van der Heide S, Dubois AE, Van Oosterhout AJ et al (2009). Preeclampsia is associated with lower percentages of regulatory T cells in maternal blood. Hypertens Pregnancy 28: 300–311. [DOI] [PubMed] [Google Scholar]
- Qian X, Chen H, Wu X, Hu L, Huang Q, Jin Y (2017). Interleukin‐17 acts as double‐edged sword in anti‐tumor immunity and tumorigenesis. Cytokine 89: 34–44. [DOI] [PubMed] [Google Scholar]
- Raghupathy R (1997). Th1‐type immunity is incompatible with successful pregnancy. Immunol Today 18: 478–482. [DOI] [PubMed] [Google Scholar]
- Redman CW, Sargent IL (2005). Latest advances in understanding preeclampsia. Science 308: 1592–1594. [DOI] [PubMed] [Google Scholar]
- Rolnik DL, Wright D, Poon LC, O'Gorman N, Syngelaki A, de Paco Matallana C et al (2017). Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med 377: 613–622. [DOI] [PubMed] [Google Scholar]
- Saito S, Nakashima A, Shima T, Ito M (2010). Th1/Th2/Th17 and regulatory T‐cell paradigm in pregnancy. Am J Reprod Immunol 63: 601–610. [DOI] [PubMed] [Google Scholar]
- Saito S, Sakai M, Sasaki Y, Tanebe K, Tsuda H, Michimata T (1999). Quantitative analysis of peripheral blood Th0, Th1, Th2 and the Th1:Th2 cell ratio during normal human pregnancy and preeclampsia. Clin Exp Immunol 117: 550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santner‐Nanan B, Peek MJ, Khanam R, Richarts L, Zhu E, Fazekas de St Groth B et al (2009). Systemic increase in the ratio between Foxp3+ and IL‐17‐producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J Immunol 183: 7023–7030. [DOI] [PubMed] [Google Scholar]
- Sargent IL, Borzychowski AM, Redman CW (2006). NK cells and human pregnancy – an inflammatory view. Trends Immunol 27: 399–404. [DOI] [PubMed] [Google Scholar]
- Sargent IL, Borzychowski AM, Redman CW (2007). NK cells and pre‐eclampsia. J Reprod Immunol 76: 40–44. [DOI] [PubMed] [Google Scholar]
- Schlembach D (2003). Pre‐eclampsia – still a disease of theories. Fukushima J Med Sci 49: 69–115. [PubMed] [Google Scholar]
- Strom TB, Roy‐Chaudhury P, Manfro R, Zheng XX, Nickerson PW, Wood K et al (1996). The Th1/Th2 paradigm and the allograft response. Curr Opin Immunol 8: 688–693. [DOI] [PubMed] [Google Scholar]
- Sykes L, MacIntyre DA, Yap XJ, Teoh TG, Bennett PR (2012). The Th1:th2 dichotomy of pregnancy and preterm labour. Mediators Inflamm 2012: 967629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam Tam KB, George E, Cockrell K, Arany M, Speed J, Martin JN Jr et al (2011). Endothelin type A receptor antagonist attenuates placental ischemia‐induced hypertension and uterine vascular resistance. Am J Obstet Gynecol 204: 330.e331–330.e334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toldi G, Rigo J Jr, Stenczer B, Vasarhelyi B, Molvarec A (2011). Increased prevalence of IL‐17‐producing peripheral blood lymphocytes in pre‐eclampsia. Am J Reprod Immunol 66: 223–229. [DOI] [PubMed] [Google Scholar]
- Toldi G, Saito S, Shima T, Halmos A, Veresh Z, Vasarhelyi B et al (2012). The frequency of peripheral blood CD4+ CD25high FoxP3+ and CD4+ CD25‐ FoxP3+ regulatory T cells in normal pregnancy and pre‐eclampsia. Am J Reprod Immunol 68: 175–180. [DOI] [PubMed] [Google Scholar]
- Toldi G, Svec P, Vasarhelyi B, Meszaros G, Rigo J, Tulassay T et al (2008). Decreased number of FoxP3+ regulatory T cells in preeclampsia. Acta Obstet Gynecol Scand 87: 1229–1233. [DOI] [PubMed] [Google Scholar]
- Vacca P, Mingari MC, Moretta L (2013). Natural killer cells in human pregnancy. J Reprod Immunol 97: 14–19. [DOI] [PubMed] [Google Scholar]
- Vacca P, Moretta L, Moretta A, Mingari MC (2011). Origin, phenotype and function of human natural killer cells in pregnancy. Trends Immunol 32: 517–523. [DOI] [PubMed] [Google Scholar]
- Veenstra van Nieuwenhoven AL, Heineman MJ, Faas MM (2003). The immunology of successful pregnancy. Hum Reprod Update 9: 347–357. [DOI] [PubMed] [Google Scholar]
- Wallace K, Richards S, Dhillon P, Weimer A, Edholm ES, Bengten E et al (2011). CD4+ T‐helper cells stimulated in response to placental ischemia mediate hypertension during pregnancy. Hypertension 57: 949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (1995). The world health report: report of the Director‐General World Health Organization: Geneva, p volumes.
- Zhang Z, Gong F, Jia L, Chang C, Hou L, Yang R et al (2004). Studies on activity of NK cells in preeclampsia patients. J Huazhong Univ Sci Technolog Med Sci 24: 473–475. [DOI] [PubMed] [Google Scholar]


