Abstract
Consumption of adenosine triphosphate (ATP) by the heart can change dramatically as the energetic demands increase from a period of rest to strenuous activity. Mitochondrial ATP production is central to this metabolic response since the heart relies largely on oxidative phosphorylation as its source of intracellular ATP. Significant evidence has been acquired indicating that Ca2+ plays a critical role in regulating ATP production by the mitochondria. Here the evidence that the Ca2+ concentration in the mitochondrial matrix ([Ca2+]m) plays a pivotal role in regulating ATP production by the mitochondria is critically reviewed and aspects of this process that are under current active investigation are highlighted. Importantly, current quantitative information on the bidirectional Ca2+ movement across the inner mitochondrial membrane (IMM) is examined in two parts. First, we review how Ca2+ influx into the mitochondrial matrix depends on the mitochondrial Ca2+ channel (i.e., the mitochondrial calcium uniporter or MCU). This discussion includes how the MCU open probability (PO) depends on the cytosolic Ca2+ concentration ([Ca2+]i) and on the mitochondrial membrane potential (ΔΨm). Second, we discuss how steady-state [Ca2+]m is determined by the dynamic balance between this MCU-based Ca2+ influx and mitochondrial Na+/Ca2+ exchanger (NCLX) based Ca2+ efflux. These steady-state [Ca2+]m levels are suggested to regulate the metabolic energy supply due to Ca2+-dependent regulation of mitochondrial enzymes of the tricarboxylic acid cycle (TCA), the proteins of the electron transport chain (ETC), and the F1F0 ATP synthase itself. We conclude by discussing the roles played by [Ca2+]m in influencing mitochondrial responses under pathological conditions.
Introduction
Cardiac mitochondria generate almost all of the cytosolic ATP that is used to meet the massive and variable energy demands of the heart. Heart work is required to maintain blood pressure while pumping oxygenated blood throughout the tissues of the body under diverse highly variable conditions. Contributing to this need are the 10,000 to 20,000 mitochondria within each ventricular myocyte. Their importance is highlighted by their abundance and their dominant presence within the cell; the mitochondria occupy rough 33% of the cellular volume in each ventricular myocyte [1]. This is the largest mitochondrial volume-fraction found in any mammalian cell. The cardiac contraction is activated by a [Ca2+]i transient which triggers crossbridge cycling under load and ATP consumption by the myosin ATPases. Ca2+ is thus an excellent signal for the cell to use to monitor contractile behavior of the heart in part because it can enter the mitochondrial matrix and influence the behavior of key enzymes. Ca2+ readily crosses the outer mitochondrial membrane (OMM) through a large channel that is always permeable to Ca2+. This channel, somewhat erroneously named the “voltage dependent anion channel” (VDAC) [2–4], permits Ca2+ flux into the intermembrane space. Ca2+ entry from the intermembrane space into the mitochondrial matrix is primarily through highly selective, low conductance, Ca2+ channels known as mitochondrial calcium uniporter channels (MCU channels) [5–9]. The Ca2+ that enters the matrix can be pumped back into the intermembrane space by the mitochondrial sodium-calcium exchanger (NCLX) [10,11]. The matrix [Ca2+] (or [Ca2+] m) is thus set by the dynamic balance between MCU Ca2+ entry and NCLX Ca2+ extrusion. The [Ca2+]m is thus the dynamic result of a classic pump-leak balance system. A higher heart rate, for example, would tend to increase [Ca2+] m. In quiescent heart cells the [Ca2+] in the mitochondrial matrix, [Ca2+]m, is estimated to be around 100 nM or similar to the quiescent cytosolic [Ca2+]i [12]. The heart rate in mammals is 1–10 Hz (humans to mice) and the regular [Ca2+] i transients elevate cytosolic [Ca2+]i from about 100 nM to 0.5 to 1.0 μM (cell-wide average at the peak of the [Ca2+] i transient. There is consequently a Ca2+ gradient during the [Ca2+]i transient that favor Ca2+ moving from the cytosol to the mitochondrial matrix and this chemical gradient is augmented by the electrical potential of the inner mitochondrial membrane (ΔΨm= −180 mV) that also favors Ca2+ entry into the mitochondrial matrix [13]. The regular elevation of [Ca2+] i is thought to increase not only the electrochemical gradient favoring Ca2+ entry into the matrix but also increases the open probability (PO) of the MCU. Thus, together they increase Ca2+ entry into the matrix such that [Ca2+]m increases from about 100 nM to 1–3 μM during sustained heart activity [11,12,14]. See Boyman et. al., 2013 [11] for more details regarding [Ca2+] m dynamics during [Ca2+]i transients in heart. The array of [Ca2+]m-sensitive channels, dehydrogenases (DHOs) and proteins within the matrix or in the inner mitochondrial membrane (IMM) enable an activity dependent signal ([Ca2+]m) to regulate the metabolic behavior of the mitochondria. Here we review Ca2+ dependent regulation of the metabolic behavior of the mitochondria in heart. Although there is an emphasis on areas of dispute or controversy and topics with particularly active ongoing research activity, we will also seek to relate our discussion specifically to [Ca2+]m, [Ca2+]i , [ATP], [ADP], inorganic phosphate (Pi), concentrations of reactive oxygen species [ROS], the identity and character of the mitochondrial permeability transition pore (mPTP) and MCU.
Mitochondrial Calcium Dynamics
The mitochondrial membrane potential (ΔΨm).
The proton and electrical gradients are created by the pumping of protons across the IMM into the intermembrane space by Complexes I, III, and IV. These complexes are parts of the electron transport chain (ETC) which consume energy to create the large negative potential (ΔΨm ~ −180 mV) across the IMM which in turn powers ATP synthesis by Complex V. Additionally, this negative membrane potential creates a strong electrochemical driving force for Ca2+ entry into the mitochondrial matrix.
The mitochondrial Ca2+ uniporter (MCU).
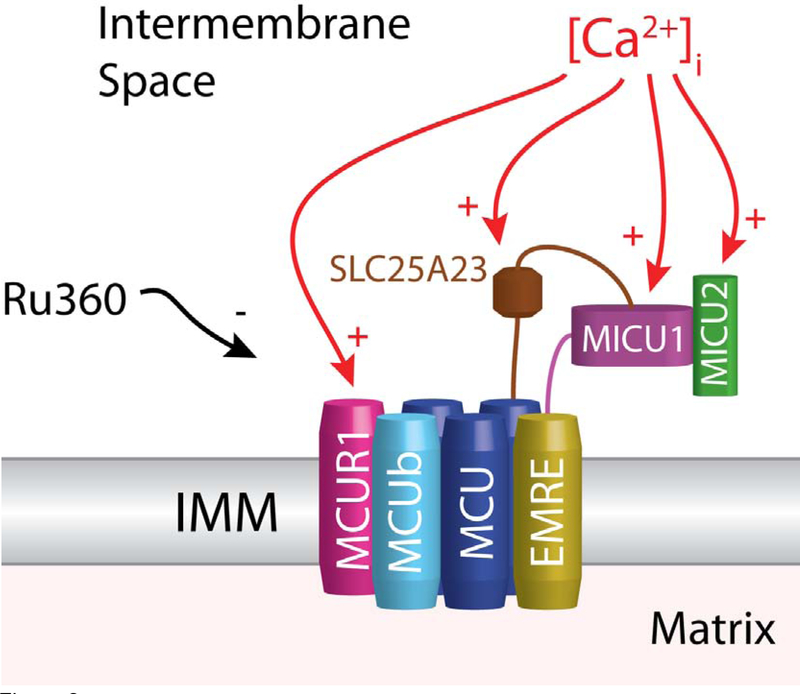
In excitable cells the primary pathway of Ca2+ entry into the mitochondrial matrix is via the MCU. Mitochondria have been known to accumulate Ca2+ ions from surrounding solution since the early 1950s [16]. The uptake of Ca2+ into the mitochondria was first quantified in 1961 using radioactive Ca2+ (Ca45) [17]. This entry of Ca2+ is driven by the highly negative potential of the IMM. However, the mechanism of this uptake remained unknown until in 2004 when the MCU was characterized as a low affinity, highly selective ion channel [5]. The molecular identity of the major channel-forming subunit of the MCU complex (CCDC109A) was first reported in 2011 [6,9]. The pore-forming complex consists of multiple MCU subunits, each with two transmembrane domains [18]. Silencing of the MCU gene abolished Ca2+ entry into the mitochondria. Additionally, recent work has shown that the MCU open probability is critically regulated by other IMM proteins, including MICU1 (Mitochondrial Ca2+ Uptake 1), MICU2 (Mitochondrial Ca2+ Uptake 2), MCUR1 (Mitochondrial Ca2+ Uniporter Regulator 1), SLC25A23, and EMRE (Essential MCU REgulator) (see Fig. 2) [18–31]. A paralog of MCU (MCUb, CCDC109B) also exists but lacks channel activity and typically has lower expression than MCU, however, expression of MCUb is significant in heart, lung, and brain [32]. MICU1 and MICU2 appear to adjust MCU activity by exerting opposing effects in response to changes in [Ca2+]i [22]. MICU1 acts to limit uptake during basal [Ca2+]i levels but activates Ca2+ uptake when [Ca2+]i is elevated while MICU2 appears to predominantly act as a MCU inhibitor. However, many details regarding MICU1’s regulation of MCU PO remain unanswered, especially regarding MICU1 localization. One model suggests that MICU1 localizes to the mitochondrial matrix, while another model suggests MICU1 is localized in the inter-membrane space. It seems that additional work will be required to clarify the exact mechanisms related to MCU regulation. Regardless, this Ca2+-dependent regulation of the IMM Ca2+ entry pathway is important since cardiac mitochondria are routinely bathed in elevated levels of [Ca2+]i during each heartbeat with the ends of the intermyofibrillar mitochondria (IFM) being bathed in up to 10 μM free [Ca2+]i (see Fig. 1C). However, the MCU regulators appear to result in low MCU PO under physiological [Ca2+]i levels [8] and the kinetics of changes in PO remain unknown. The insights and analysis of existing quantitative uptake measurements have allowed the formation of a comprehensive model of mitochondrial Ca2+ uptake,
| (1) |
where PO is sigmoidal function of [Ca2+]i. See [8] for more details.
Figure 2. Ca2+ sensitivity of the MCU complex.
This diagram shows the four known transmembrane components (and regulators) of the MCU complex (MCU,MCUb,EMRE, and MCUR1). Also shown are the known regulators present in the intermembrane space which includes MICU1, MICU2, and SLC25A23. Ca2+ sensitivities are indicated by the red arrows. Black arrow indicates inhibition of MCU by Ru360.
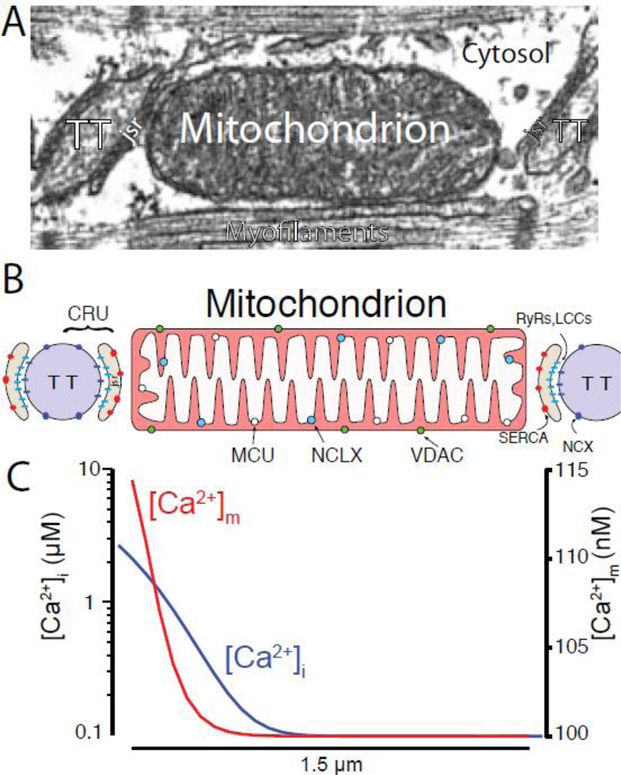
Figure 1. Cardiac Mitochondria and Mitochondrial Ca2+ signaling.
A) Electron micrograph (EM) of a single cardiac sarcomere showing the location of the transverse tubule (TT), junctional SR compartment (jsr), and intermyofibrillar mitochondrion (IFM). Modified from [15]. B) spatial representation showing the Ca2+ release unit (CRU) located between the transverse-tubule (TT) and junctional SR (JSR) membranes relative to an IFM. C) During a Ca2+ spark, [Ca2+]i briefly bathes the mitochondrion with levels indicated by the blue line and [Ca2+]m rises to levels roughly similar to the red line. Modified from [8]. Note that the left y-axis is log scale.
Our recent investigation indicated that normal physiological mitochondrial Ca2+ uptake is small as is release, and hence unlikely to affect [Ca2+]i signals [12]. Regarding mitochondrial Ca2+ uptake, we have implemented a model to assess how large Jmcu would need to be in order to significantly alter [Ca2+]i signals. We found that to influence the [Ca2+]i transient or local Ca2+ sparks, Jmcu would need to be at least 100 fold higher than its known value. This finding suggest that under normal physiological conditions mitochondria are not significant dynamic buffers of [Ca2+]i in heart and this model-dependent test is supported by direct experimental measurements.
The mitochondrial Na+/Ca2+ exchanger (NCLX).
Na+-dependent Ca2+ efflux from the mitochondria was identified over 40 years ago [33,34]. Surprisingly, Li+ was also able to induce Ca2+ efflux, apparently by serving as a monovalent substitute for Na+. This Li+/Ca2+ exchange capability contrasts with other Na+/Ca2+ exchangers, and therefore led to the “L” (for Li+) in NCLX [10,35]. Located within the IMM, NCLX serves as the primary means of Ca2+ extrusion from the mitochondrial matrix of excitable cells (e.g., heart) while non-excitable cells rely to a certain extent on H+/Ca2+ exchange. Na+ which enters the matrix as part of the NCLX exchange process, and possibly other Na+ leak pathways, is then removed from the mitochondrial matrix to the cytosol by the Na+/H+ exchanger [36,37].
The features of the ion transport mechanism of NCLX remain unknown, and are under active investigation. For example, the exchange stoichiometry (i.e. whether 2, 3 (or more) Na+ ions are exchanged for 1 Ca2+ ion by NCLX) is not known. If 3 Na+ ions are exchange for 1 Ca2+ ion by NCLX then the exchanger would be electrogenic since 1 net electrical charge would be translocated across the IMM when one Ca2+ was transported in the opposite direction. Alternatively, it could be that 2 Na+ ions are exchanged for 1 Ca2+ ion. If so, NCLX would be electroneutral. The electrogenicity of NCLX have profound implication for its sensitivity to changes of ΔΨm. If electrogenic, then NCLX would be modulated by changes in ΔΨm as may happen under conditions of stress or disease and this would intern alter [Ca2+]i which would alter properties of the Ca2+-sensitive proteins. In addition, NCLX probably is sensitive to [Na+]i and pH in the cytosol and in the matrix. Our limited ability to provide calibrated measurements of [Na+] with high temporal resolution makes studies of Na+-dependent modulation of NCLX challenging. Nevertheless, numerous studies have shown that the Na-dependent Ca2+ efflux mediated by NCLX is exquisitely sensitive to [Na+]i changes within the physiological range (i.e., 3–15 mM) [34,38–40].
Under steady-state conditions, the same amounts of Ca2+ which enters the mitochondrial matrix during each [Ca2+]i transient must be extruded by NCLX. Any perturbation of [Na+]i will influence NCLX Ca2+ efflux. In the simplest case this would imply that a rise in [Na+]i would increase NCLX Ca2+ efflux, and will tend to reduce the amounts of Ca2+ that the mitochondria can accumulate, which will also reduce time-averaged [Ca2+]m. Numerous studies have explored this possibility by rising [Na]i to as high as the levels that are expected under pathological conditions (i.e., 15 mM). The effects were pronounced, and included blunted bioenergetic response following sudden transition to strenuous cardiac activity [41,42]. These effects are primarily attributed to alterations of [Ca2+]m dynamics, and are discussed in more details in [8,11].
Using available information, Dash and Beard described a mathematical formulation of NCLX activity during either electroneutral or electrogenic transport [43]. This formulation was extended recently to investigate how NCLX flux (Jnclx) may change during the cardiac [Ca2+ ]i transient, and how Jnclx is expected to change due to [Na+]i perturbations [11]. The effects of [Na+]i on Jnclx are explicit, and further highlight the potential adverse pathological contribution of NCLX to dysregulation of [Ca2+]m.
In addition to learning about the function of NCLX in cardiac mitochondria, recent work has examined NCLX, a ubiquitous mitochondrial protein found in many tissues throughout the body [10]. In HeLa cells NCLX is critical for [Ca2+]m-dependent regulation of NADH production [44]. In astrocytes silencing of NCLX expression affects exocytotic glutamate release, in vitro wound closure, and proliferation [45]. In pancreatic beta cells, silencing the expression of NCLX alter the intracellular signaling pathways that normally activate when the plasma levels of glucose rise [46].
Buffering of [Ca2+]m (βm).
While steady-state [Ca2+]m levels are determined by the balance between influx via MCU (and any other Ca2+ influx pathway) and efflux via NCLX (and any other Ca2+ extrusion mechanisms), transient changes of [Ca2+]m depend also on the “low pass filter” we call Ca2+ buffering within the mitochondrial matrix. This buffering effect is pronounced when the heart changes its rate and/or workload. Ca2+ buffering levels in the mitochondrial matrix will be critical in determining how long it take to reach a new steady-state [Ca2+]m level. Because [Ca2+]m is important in the regulation of mitochondrial metabolism in response to changes in workload (see Mitochondrial Energetics in a Working Heart section below), the effect of buffering is critical to mitochondrial function. Too much buffering would delay the mitochondria’s ability to increase ATP production when demand is great, while too little buffering could produce highly variable and changing [ATP]i. that may adversely affect ATP-dependent processes. Unfortunately, numerous studies into Ca2+ buffering within the mitochondrial matrix have reported vastly different results. Early studies have suggested that matrix buffering is roughly equivalent to the cytosol (i.e., 1% free) [47–49] while more recent studies have reported much larger buffering fractions consisting of differing “classes” of buffers [50–54]. With [Ca2+]m levels regulating so much of mitochondrial function (see below), additional quantitative studies at high temporal and spatial resolution of mitochondrial [Ca2+]m and cytosolic [ATP] are needed to further clarify the dynamics of matrix Ca2+ buffering.
Total mitochondrial Ca2+ content can vary greatly. We, and others, have shown that quiescent cells have a free [Ca2+]m of approximately 100 nM (see [14,55]. Assuming a 100:1 buffering ratio and a 3:1 cytosol to matrix volume ratio, quiescent cells would have approximately 3 μmoles of Ca2+ per liter of cytosol. During pacing [Ca2+]m would likely rise to 15 – 90 μM (per L cytosol) [11,14]. Higher levels of matrix buffering would yield correspondingly higher levels of total [Ca2+]m. It is important to note that mitochondria are certainly capable of taking up significant amounts of Ca2+ under non-physiological conditions. For example, total mitochondrial content from experiments on suspensions of isolated mitochondria suggest that with high [Ca2+]i (e.g. 100 μM) total [Ca2+]m levels can climb to 4–10 millimoles of Ca2+ per liter of cytosol [56]. The release of even a small fraction of this [Ca2+] m would result in significant changes in [Ca2+]i and likely detrimental contracture. However, during normal, quiescent conditions the instantaneous release of all matrix Ca2+ from all the cell’s mitochondria would only raise [Ca2+]i ~ 30 nM. Even during paced activity the complete, instantaneous release of matrix Ca2+ would result in a modest submicromolar increase in [Ca2+]i. Such a release is very unlikely and in our recent paper [55] we observed no influence on [Ca2+]i following the complete loss of ΔΨm. Note, the estimate above assume 40 mg of mitochondrial protein per liter of cell and a cellular to cytosolic volume ratio of 2:1
The Leucine zipper-EF-hand containing transmembrane protein 1 (Letm1).
Letm1 was identified in 1999 as one of the genes contributing to multi-system Wolf-Hirschhorn syndrome [57,58] a multi-system disorder involving loss of variable amounts of the short arm of chromosome 4 including Letm1. Letm1 encodes a mitochondrially targeted, membrane bound protein with 2 EF-hand motifs. Early work showed that Letm1 was critical to mitochondrial shape and function possibly responsible for maintaining K+ homeostasis [59–63]. In 2009, a genome-wide RNAi screen identified Letm1 as an electrogenic (1Ca2+/1H+) calcium-proton antiporter [64] which was sensitive to ruthenium red sensitive. An additional study in 2013 using reconstituted Letm1 in liposomes found Letm1 to be an electroneutral (1Ca2+/2H+) calcium-proton exchanger which was insensitive to ruthenium red [65]. Combined there is now significant evidence that LETM1 is the genetic identity of the mammalian Ca2+/H+ exchanger [64–68]. However, other studies have reinforced the role of Letm1 in K+ and mitochondrial volume homeostasis and suggested Letm1 primarily functions as a electroneutral proton-potassium exchanger (1H+/1K+) [69]. This is supported by work by Demaurex and co-workers which showed that NCLX, and not Letm1, is responsible for extruding Ca2+ from the mitochondria [44]. Some have even suggested that Letm1’s role might change based on conditions [70]. For example, it has been suggested that Letm1 might mediate Ca2+ influx into mitochondria due to the presence of ER, mitochondrial microdomains. However, mechanistic models of such ER/SR/mitochondrial behavior that are constrained by quantitative data do not yet exist. It is important to note, furthermore, that such “privileged” exchange, transport or conductance is rare and experiments to test them are experimentally challenging. Specifically, such hypothesized Letm1 local Ca2+ exchange is thermodynamically very unfavorable and only feasible for Letm1 proteins located on the ends of a mitochondrion closest to the CRU and only when [Ca2+] is very high (i.e., 10 μM) and [Ca2+]m is still low (i.e., 100 nM). Moreover, this estimate assumes a electroneutral exchange of 2H+ for 1 Ca2+. Even this very spatially localized condition would be extremely fleeting as such [Ca2+]i levels only persist for a few milliseconds during a Ca2+ spark and/or [Ca2+]i transient [8]. However, while non-physiological, this behavior could be more relevant during experimental protocols where mitochondria are exposed to large boluses of free [Ca2+]i such as those commonly used to investigate mitochondria Ca2+ fluxes in vitro or [Ca2+]m dynamics in model cells. What is clear is that Letm1 is important for normal mitochondrial function but elucidating the exact details of its function will likely require additional quantitative experiments. Additionally, Letm1 expression has been suggested to be very low in excitable cells [71,72] and therefore likely not relevant to mitochondrial Ca2+ dynamics in heart.
Mitochondrial Metabolism
Glycolysis alone can not meet the massive energy demands of a beating heart cell. The heart relies on oxidative phosphorylation to generate ATP within the mitochondria primarily from lipid sources. Cytosolic pyruvate is translocated into the mitochondria [73,74] and converted to acetyl-CoA which then enters the TCA cycle. The TCA cycle utilizes dehydrogenases and enzymes to break down acetyl-CoA to oxaloacetate in a process involving 7 intermediates (see Fig. 3) {Krebs:1970vh}. The decarboxylation step in the conversion of isocitrate to α -ketoglutarate by isocitrate dehydrogenase is the rate limiting step for the entire citric acid cycle [75]. For every acetyl-CoA molecule that enters the cycle, three NAD and one FAD molecules are reduced to NADH and FADH2, respectively. These reduced, high energy dinucleotides then provide the energy to pump protons across the IMM through a series of inner membrane clustered interacting proteins known as the electron transport chain (ETC). The detailed workings of the electron transport chain are well documented [76,77]. Briefly, four membrane bound complexes make up the ETC. Complex I removes two electrons (e−) from NADH and transfers them to ubiquinone (Q) and then translocates four protons (H+) across the IMM. Complex II transfers additional e− to Q but pumps no H+. Complex III transfers four H+ to the intermembrane space in a two step process which includes the transfer of two other H+ to Q. Complex IV is the final H+ pump in the ETC and transfers four H+ across the IMM and transfers four e− from cytochrome c (C) to molecular oxygen, creating two molecules of water (H20). This pumping of H+ by the ETC generates the “H+-motive force” or “proton-motive force” utilized by Complex V to phosphorylate adenosine from the lower energy diphosphate form (ADP) to the higher energy triphosphate form, ATP. Each glucose molecule generates two acetyl-CoAs resulting in a net production of 30 ATP molecule from two full cycles of the TCA cycle and sequential oxidative phosphorylation. In a single cardiomyocyte, the large number of IFMs combine to generate approximately 100 – 500 (depending on heart rate) μmoles of ATP per liter of cytosol every second.
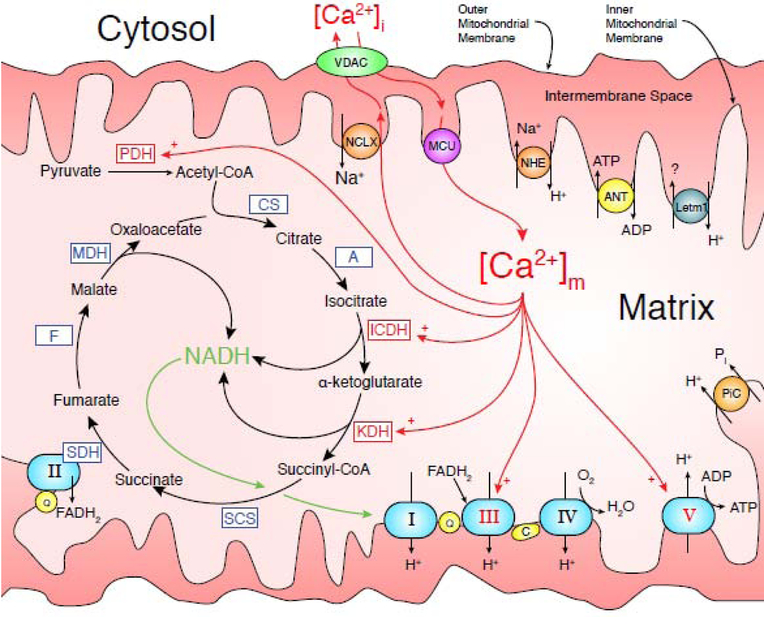
Figure 3. Diagram of Mitochondrial Metabolism Components.
Enzymes with known Ca2+ sensitivities are indicated by red arrow labels with a plus (+) while Ca2+-insensitives ones are indicated by blue labels. The five complexes responsible for oxidative phosphorylation are light blue ovals and labeled with their respective Roman numerals (i.e., I,II,..,V). The TCA cycle is responsible for converting Acetyl-CoA into NADH and is indicated with curved black arrows. Red arrows indicate Ca2+ interactions/pathways. Protein and enzyme abbreviations: pyruvate dehydrogenase (PDH); citrate synthase (CS); aconitase (A); isocitrate dehydrogenase (ICD); a-ketoglutarate dehydrogenase (KDH); succinyl CoA synthetase (SCS); succinate dehydrogenase (SDH); fumarase (F); malate dehydrogenase (MDH); mitochondrial Ca2+ uniporter (MCU); mitochondrial Na+/Ca2+ exchanger (NCLX); Leucine zipper-EF-hand containing transmembrane protein 1 (Letm1); cytochrome C (C); ubiquinone (Q); voltage-dependent anion channel (VDAC); ATP/ADP translocase (ANT); inorganic phosphate carrier (PiC); and the mitochondrial Na+/H+ exchanger (NHE).
Calcium-Dependent Regulation of NADH Production
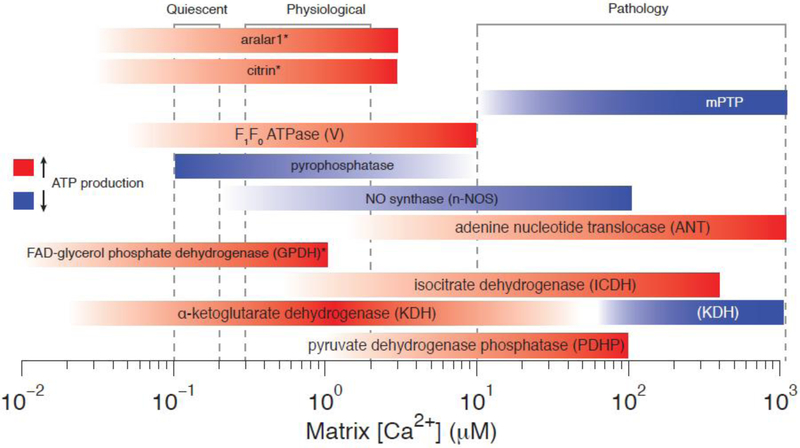
Early understanding of Ca2+-dependent regulation of some of the key enzymes in the TCA cycle comes from an investigation of pyruvate dehydrogenase (PDH) and the role played by phosphorylation [78,79] with the rapid development of an appreciation of how the process to Ca2+ [80]. The apparent KD for this Ca2+ sensitivity was found to be abouot 10 μM [81]. The discovery by Denton et al. of the role played by Ca2+ was soon followed by a more comprehensive screening of the entire TCA cycle for Ca2+ sensitivity [82]. This effort determined that the affinity of α-ketoglutarate dehydrogenase (KDH) for α-ketoglutarate increased in the presence of Ca2+ without a change in Vmax. The apparent KD for KDH Ca2+ sensitivity varies based on based on ADP and ATP levels but ranged from 200 nM to 2 μM [83]. Ca2+ can also indirectly increase KDH activity by 100 fold via Ca2+ binding to ATP, ADP, NADH, and Pi [84]. Finally, both Ca2+ and ADP increase the affinity of isocitrate dehydrogenase (ICDH) for isocitrate without changing its Vmax [85]. The KD for ICDH’s Ca2+ sensitivity varies based on ADP and ATP levels but ranges from 5 μM to 50 μM [83]. Additionally, Ca2+ sensitivities for enzymes and transporters affiliated with the TCA cycle have been observed. For example, membrane bound FAD-glycerol phosphate dehydrogenase (GPDH) activity increases with Ca2+ (KD ~ 100 nM) [86]. Note that GPH likely senses the [Ca2+]i present in the intermembrane space ([Ca2+]ims) and not [Ca2+]m as with PDHP, KDH, and ICDH. Also sensitive to [Ca2+]i in the intermembrane space are the two aspartate-glutamate carriers, Aralar1 and Citrin. Both these carriers have half maximal Ca2+ sensitivity constants of approximately 350 nM. Pyrophosphatase, the enzyme which catalyzes the conversion of one molecule of pyrophosphate to two phosphate ions, and NO synthase (n-NOS), the enzyme responsible for catalyzing the production of nitric oxide (NO) from L-arginine are also sensitive to [Ca2+]m [87,88]. Activation of n-NOS by [Ca2+]m (KD ~5 μM) could result in diminished ATP levels. Additionally, the ADH has been suggested to be regulated by Ca2+ [89,90], however, this effect may be indirect [91]. Figure 4 shows a visual summary of the diverse ways that Ca2+ regulates mitochondrial matrix proteins and enzymes over physiological and pathological ranges of [Ca2+]m. The largest source of acetyl-CoA and substrates for oxidative phosphorylation (e.g., NADH and FADH2) in the heart is via beta-oxidation of fatty-acids within the mitochondria (and peroxisomes) [92]. However, the key enzymatic steps of beta-oxidation are less sensitive to [Ca2+]m than those of glycolysis, the TCA cycle, and oxidative phosphorylation itself [93]. For a more details regarding beta-oxidation in heart we refer the reader to more comprehensive reviews on this topic [92,94].
Figure 4. Calcium sensitivities of Mitochondrial Enzymes/Proteins.
Colored bars indicate ranges of Ca2+ sensitivity. Red bars indicate Ca2+-dependent sensitivities likely to increase ATP production while blue bars indicate likely Ca2+-dependent decreases in ATP production. Asterices (*) indicate entities which sense [Ca2+]ims and not [Ca2+]m (e.g., GPDH, aralar1, and citrin).
Calcium-Dependent Regulation of ETC and F1F0 ATP synthase.
While Ca2+-dependent activation of PDHP, KDH, and ICDH have been well established for many years, more recently investigations have revealed Ca2+ sensitivities of the proteins and complexes associated with the ETC. The electron flow through ubiquinone and Complex III has been shown to be sensitive to Ca2+ [95]. However, this sensitivity appears to be to intermembrane space (ims) Ca2+, [Ca2+]ims. Even more recently work by Balaban et al. in 2013 showed Ca2+ sensitivity for all three proton pumps in the ETC (i.e., Complexes I,III, and IV) [96]. The turnover rate of these three proton pumps increased over 2-fold in response to elevations of Ca2+. One unfortunate consequence of the approach used is that they were unable to determine if the Ca2+ sensitivities were in response to changes in [Ca2+]m or [Ca2+]ims. Furthermore, the effect appears to be due to indirect Ca2+-dependent activation as direct binding studies of Ca2+ to isolated IMM enzymes showed no effect [97,98]. While these results are enticing additional work will be required to elucidate the precise mechanism of Ca2+-dependent activation of the ETC.
In addition to potentially regulating the ETC proton pumps, Ca2+ has been suggested to critically regulate the F1F0 ATP synthase (Complex V). Activation of the F1F0 ATP synthase has been hypothesized as a way to explain the balancing of ATP production during workload that activation of CDHs cannot explain alone. Early work in sonicated cardiomyocytes suggested Ca2+-dependent activation of F1F0 ATP synthase but this effect seemed to be indirect via the release of an inhibitor [99]. More recently, Ca2+ was shown to directly regulate the F1F0 ATP synthase (Complex V) [100]. Using “force-flow relationships”, Balaban and co-workers showed that increases in [Ca2+]m resulted in a two-fold increase ATP production beyond what could be explained by changes in NADH or ΔΨm alone. The KD for this effect was found to be approximately 150 nM. This sensitivity is particularly interesting as we (and others) have shown that quiescent cells have [Ca2+]m levels near the [Ca2+]i (i.e., 100 nM) but during stimulation [Ca2+]m slowly rises [11,12,14]. The activation of F1F0 ATP synthase due to slow rises in [Ca2+]m would allow the mitochondria to increase ATP production as demand increases with greater heart work.
Mitochondrial Energetics in a Working Heart: Balancing ATP Production and Consumption.
Given the available evidence, [Ca2+]m is thought to be the primary dynamic regulator of ATP production in heart. This conclusion is supported by measurements of ATP by 31P nuclear magnetic resonance (NMR) and by studies that center on [Ca2+]m and its dynamic effects.
Critical insights into the regulation of ATP production in the working heart have been gained by NMR measurements, which not only provide a measure of cellular ATP ([ATP]), but also provide a simultaneous measure of its hydrolysis end products, [ADP] and [Pi]. These investigations report that even after the workload of the heart changes profoundly, [ATP] and its hydrolysis end products remain virtually unchanged [101–103]. Within the temporal resolution of this approach (10–15 seconds) even an abrupt increase in the workload had no measurable effect on these metabolic products [104]. These findings suggest that because ADP and Pi levels remain constant some other signaling mechanism must be responsible for signaling mitochondria to increase ATP production in order to match increased demand [105]. Ca2+ was indicated as the most likely candidate for this mechanism. However, signaling via local subcellular gradients of adenine nucleotides could not be excluded because of the limited spatiotemporal resolution of the NMR measurements, which provide a time-averaged signal from numerous myocardium cells at once. If local subcellular gradients of ATP or ADP do indeed exist, and are changing together with the heart’s workload, this may well be significant. The possible existence of local [ATP] gradients in the cytosol have been supported by several theoretical studies, which suggest that local microdomain of high ATP should form. Such cytosolic compartmentalization is proposed to occur since the IMM and other structural elements in the cell should pose barriers, which will restrict the diffusion of adenine nucleotides between the sites of ATP production and consumption [106–108]. However, due to the availability of selective ATP or ADP indicators the possible existence and role(s) of such gradients have not been tested so far in cardiac cells or tissue. Nevertheless, the recent development of novel new genetically-encoded probes for adenine nucleotides [109–111] may provide exciting new opportunities to test this hypothesis, and perhaps if the temporal resolution of such measurements will permit, the kinetic features of such microdomains may be explored.
In stark contrast to ATP or ADP, a large body of evidence suggests that when the workload of the heart increases, [Ca2+]m increases. The magnitude and dynamics of this increase in [Ca2+]m remain contested however [8,11,12]. Regardless, when the heart workload suddenly increases IFM are exposed to more frequent elevation in [Ca2+]i and possibly increased levels of diastolic [Ca2+]i. This results in an increased driving force for Ca2+ entry into the mitochondrial matrix. Additionally, this effect is further magnified during β-adrenergic stimulation. A sequential increase in [Ca2+]m appears to be critical for stimulating NAD reduction [41,42,47,112] therefore increasing NADH production in the mitochondrial matrix allowing increased ATP production to match demand. The mitochondrial matrix NADH ([NADH]m) is typically measured using its robust signal at 445–455 nm, which comprise the majority of the cell’s (or myocardium) autofluorescence at these wavelengths [113–116]. Utilizing this approach, the critical role of mitochondrial Ca2+ uptake in stimulating the production of NADH has been explicitly demonstrated in 3 different experimental preparations; whole intact hearts [114,117], cardiac muscle strips [112,118–121] and isolated heart cells [41,42]. Despite the general agreement on the role [Ca2+]m, some discrepancies do exist among these studies regarding the mechanisms by which [Ca2+]m dynamically regulates NADH production. Some studies report that [NADH]m is not altered when the workload of the heart increases [114,117], or synonymously, when the stimulation rate of isolated heart cells is increased [41,42]. This evidence suggests that such regulatory actions of [Ca2+]m, and possibly additional factors, are instantaneous. In which case a rise in NADH oxidation by Complex I is matched by an equivalent and near simultaneous rise in the rate of NADH production. Conversely, other studies with heart muscle strips under normal load [112,118–121] suggest that immediately after a rise in the workload, [NADH]m initially declines rapidly (over 5–10 seconds) but later rises slowly (over 30–60 seconds). The decline of [NADH]m is presumed to be due to faster consumption of [NADH]m due to stimulation of oxidative phosphorylation via a yet unknown Ca2+-independent mechanism [119]. These results suggest that [Ca2+]m-dependent stimulation of NADH production by the krebs cycle dehydrogenases occurs slowly, with a time constant of approximately 30 seconds [112]. This would be consistent with the kinetics of [Ca2+]m dynamics, where [Ca2+]m slowly increases with increases in [Ca2+]i, due to mitochondria serving as low-pass filters for changes in [Ca2+]i. Regardless, it seems that additional work will be required to elucidate the exact mechanism by which heart cells rapidly balance ATP production with consumption and whether [Ca2+]m is indeed the primary signaling pathway for this behavior.
Mitochondrial Calcium and its Implications in Cardiac Pathology
Mitochondrial Permeability Transition Pore (mPTP).
Recent publications [122–124] propose a detailed molecular description to a mitochondrial process that was originally described over 60 years ago. It was observed that high levels of [Ca2+]m could cause mitochondrial swelling and dysfunction [125–127]. This process was later attributed to a mitochondrial permeability transition [128], and over the years was broadly accepted as a common process that underlies or contributes to cell death [129]. Early on, this mitochondrial permeability transition was proposed to be caused by a contiguous pore (mPTP) spanning both mitochondrial membranes and consisting of VDAC on the OMM and ANT on the IMM [130]. However this theory was undermined in 2004 when Kokoszka et al. showed that mitochondria from mice lacking ANT still displayed mPTP openings [131]. More recently, several groups have proposed that mPTP is formed by F1F0 ATP synthase [122,124,132,133], specifically the C subunit which has been shown to form large conductance pores when purified and reconstituted in lipid bilayer [123]. The additional features of the in-vitro (ex-mitochondrial) formed pore, such as inhibition by cyclosporin-A and cyclophilin D dependency, also resemble the described features of the putative mPTP. However, questions remain regarding this mPTP candidate [134,135]. Unfortunately, the most compelling evidence would come from genetic testing of F1F0 ATP synthase as mPTP, however, this would be challenging as many mammalian cells (especially cardiomyocytes) rely heavily on F1F0 ATP synthase to generate the ATP necessary for normal cellular function. In heart, mPTP formation has been linked to numerous adverse pathological effects [136,137], but also to critical adaptive compensatory mechanisms [138], and is therefore a compelling area of future research.
Does mPTP function as a mitochondrial Ca2+ release valve at times of stress?
In addition to the adverse effects attributed to mPTP activity (detailed in the next section), some have suggested that mPTP may also protect the mitochondria and cardiomyocytes from metabolic dysfunction [139–142]. Perhaps the most explicit examples arise from the investigations with the cyclophilin D null mice (CypD−/−) [141,142]. CypD is the only protein that remains in consensus as a bonafide molecular component of mPTP, and mitochondria isolated from the heart [142,143] or liver [144–146] of CypD−/− mice are devoid of the general attribute of mPTP. These mitochondria are insensitive to the standard supraphysiological Ca2+ challenge assay, and can uptake substantially larger amounts of Ca2+ before they swell and release their Ca2+ content. CypD−/− is not thought to be an element of the pore forming structure, but rather an interacting regulator that sensitize the opening of mPTP to [Ca2+]m, and also the protein target of cyclosporin A [138]. The initial characterization of the CypD−/− mice revealed that they are born at the expected mendelian frequency, develop normally, and do not display any phenotype when kept under non-stressful conditions [143,144]. However, in a subsequent study with the CypD−/− mice, Elrod and co-workers [142] reported that the repercussions of the lack of CypD only become evident in these animals during strenuous activity or during heart disease. The hemodynamic performances of the CypD−/− mouse heart do not increase following systemic isoproterenol infusion (a β-adrenergic receptor agonist), and once the CypD−/− mice are subjected to a swimming exercise they develop more pronounced cardiac hypertrophy, pulmonary edema, and more than 40 percent die of drowning due to fatigue, compared to 10 percent in the wild type (WT) cohort. Gene array analysis revealed alterations in gene products involved in metabolic pathways, and 13C NMR measurements from the hearts of the CypD−/− mice revealed a metabolic shift from beta-oxidation toward glycolysis. Taken together, these findings suggest primary dysfunction in dynamic regulation of mitochondrial oxidative phosphorylation. Furthermore, after these animals undergo a transaortic constriction (TAC) procedure the pressure overload caused by the constricted aorta develops rapidly into uncompensated heart failure. These failing hearts display a large array of metabolic alterations, and since the WT hearts do not deteriorate to this extent after the TAC procedure it suggest that a critical adaptive compensatory mechanism is absent in the CypD−/− mice. These metabolic dysfunctions were attributed to dysregulation [Ca2+]m, and it was proposed that the desensitized mPTP in the CypD−/− mice is the primary underlying mechanism. This suggests that in normal wild-type animals mPTP acts as a Ca2+ release valve that limits excessive accumulation of matrix Ca2+ [138,142]. Two sets of findings were presented to support this conclusion. First, mitochondria isolated from CypD−/− mice retain unusually large amounts of Ca2+, over 12 mmoles of Ca2+ per liter cytosol (150 nmol Ca2+ per mg of mitochondrial protein) compared to 4 mM in WT. Second, [Ca2+]m was measured in parallel tests with neonatal cardiomyocytes from normal rats and cyclosporine A (CsA) was used as a surrogate means of reducing mPTP openings. When these cultured cells are paced via field stimulation (0.2 Hz), [Ca2+]m rises faster in the cells treated with cyclosporin A and declines to its quiescent levels more slowly when stimulation is halted. These findings are supported by analogous effects of cyclosporin A as reported by other investigators [139,140]. However, quantitative measurements of [Ca2+]m dynamics in the adult cardiomyocytes from the CypD−/− mice have not yet been reported and these tests are more feasible now that new approaches are available for transfection of adult cardiomyocytes with mitochondrially targeted fluorescent probes [14,55,147]. In conclusion, we believe that quantitative measurements of alteration in [Ca2+]m in CypD−/− are required before we can conclude that mPTP serves as a Ca2+ leak pathway under physiological conditions.
Ischemia Reperfusion and [Ca2+]m.
The loss of blood flow to a region of the myocardium due to an arterial occlusion or restriction has adverse effects. In that region the consumed oxygen and metabolites are not replenished, catabolites such as lactate are not cleared, and ischemia develops within several minutes. Therapy usually involves reperfusion of the area with oxygenated blood. However, the sudden reperfusion is also known to cause vast cellular death. In heart, cardiac ischemia has been studied using animal models where coronary arteries are occluded via artificial means. It was demonstrated that the myocardium can endure short periods of ischemia [148], and that subsequently the hemodynamic performances of the heart bounce back to normal [149–152]. But as the duration of the ischemic period is extended, the ATP content of the heart gradually declined due to uncompensated consumption, and irreversible tissue damage due to death of cardiomyocytes occurs. Within the necrotic region where cardiomyocytes had died the EM sections revealed lesions in the contact points between cells, hyper contracted sarcomeres due to rigor, and the mitochondria are swollen and contain amorphous matrix densities, all of which indicate that prior to death these cells where ATP deficient [148]. Since the extent to which ATP had declined correlated with the volumetric size of the necrotic region it was concluded that cell death is due to a gradual ATP depletion [153]. Given these findings it was suggested that the mitochondria do not undergo abrupt functional collapse, but instead that their function is limited due to several factors, such as the availability of metabolites and oxygen, and perhaps are even consuming ATP via the reverse reaction of F1F0 ATP synthase, which would further lower [ATP]. When the cellular availability of free energy potential provided by hydrolysis of ATP declines, the cell cannot maintain electrochemical gradients. This further degrades ATP production, until ultimately the ATP levels are sufficiently low to cause rigor and sarcolemmal raptures [154].
Perhaps the first direct evidence that ischemic damage is due to an ATP deficiency which can be ameliorated comes from a study by Murry et al., 1986 [155]. It was shown that brief 10 minute periods of ischemic preconditioning reduced the damage that is caused by a subsequent 40 minutes of ischemia. The protective effect of the ischemic-preconditioning is profound, and the necrotic region was reported to be 30% smaller than its size when no preconditioning procedure is carried out. Measurements of ATP content from these hearts indicate that during the ischemia ATP declines slower in the preconditioned hearts, perhaps indicating that the process of matching energy demand with production can become more resilient to ischemia, but a specific mechanism that underlies these effects was not proposed. That same year, 1986, Ibrahim Al-Nassar and Martin Crompton [128] presented evidence that the previously known mitochondrial swelling and collapse caused by high [Ca2+]i is due to reversible permeabilization of the mitochondria. It was found that during prolonged exposure of isolated mitochondria to high [Ca2+]i mitochondrial Ca2+ uptake does not cease, until the accumulated amounts of Ca2+ trigger a mitochondrial permeability transition, and degradation of the ionic gradients across the IMM. The permeabilization itself was attributed to excessive matrix loads of Ca2+, and it was suggested to occur during pathological sustained elevation of [Ca2+]i such that may prevail during ischemia, and was speculated to be more pronounced during the subsequent reperfusion phase [128]. This permeability transition was shown to be fully reversible, and the IMM regains its normal permeability within a few seconds after Ca2+ is removed by chelation [156], which further indicates that these are the actions of a pore rather than non-specific tears in the IMM, and the putative pore was termed mPTP. High levels of ROS reduces the amounts of Ca2+ that are required to trigger mPTP formation [156], and on the other hand, cyclosporin-A, an immunosuppressive agent, exert the opposite effect by desensitizing mPTP formation to Ca2+ [157]. Given the dependencies on Ca2+, Pi, ATP/ADP and ROS it was proposed that mPTP formation may occur during ischemia, and is perhaps more inducible early after the onset of myocardial reperfusion [158]. The possibility that the IMM contains high conductance pore(s) was further supported by subsequent electrophysiological studies conducted by patch-clamping mitoplasts (i.e., IMM vesicles) isolated from rat liver [159,160]. Further applying these electrophysiological approaches revealed that high [Ca2+] of 0.3 mM leads to a formation of a large IMM conductance pathway, having a conductance of 1–2 nS, which exceeds the theoretical upper boundary of a selective ion channels, further indicated a non-selective pore which was termed the mitochondrial megachannel (MMC). The open probability of MMC was shown to decrease by at least 10 fold after exposure to 100 nM of cyclosporin-A [161,162], and this pharmacological and other shared features further suggested that MMC and mPTP are synonymous [163]. Since these reports were presented, additional tools have been developed to investigate changes in the permeability of the IMM in cells [164], and it has been reported that in a large array of different cell types acute stress conditions (e.g., high [Ca2+]i and high [ROS]i) lead to increase in the permeability of the IMM to large molecular weight solutes, and consequently degradation of ΔΨm [165,166].
The involvement of mPTP has been implicated by many studies as a central mechanism that underlie the adverse effects of cardiac ischemia. Cyclosporin-A reduces the extent of the cardiac ischemia-reperfusion injury [167], and so does knocking-out cyclophilin D [143], the known mitochondrial protein target of Cyclosporin-A. Additional evidence suggests that detrimental mPTP opening do not occur during 30–40 minutes of ischemia, but rather immediately after the onset of tissue reperfusion [168]. These observations suggest complex regulated behavior, and were attributed in part to inhibition of mPTP due to the cytosolic acidification that prevails during the ischemic phase. Conversely, as soon as the myocardium is reperfused, the cytosolic pH rebounds, and the less acidified environment is suggested to promote mPTP formation[137]. These findings further highlight the need to understand the molecular nature of the mPTP and how it is activated and inhibited. Furthermore, the roles played by mPTP in the cellular response to ischemia, reperfusion, and cardiac stress should be identified and characterized. Moreover, the recent investigation with the MCU null mouse (MCU−/−) [169] highlights the need to further investigate the role of high [Ca2+]m in IR injury and how it relates to our understanding of mPTP. Evidently, the MCU−/− mouse is as vulnerable to IR injury as its WT littermates [169], which challenges the existing view that reduction of IMCU should reduce the extent of IR injury [170]. But even more perplexing is why CsA treatment becomes ineffective (i.e., fail to protect from IR injury) in MCU−/− mouse hearts. Does mPTP underlie cell death in the MCU−/− mouse? Are the features of mPTP in this genetic model altered? Pan X et al., 2013 did not test the [Ca2+]m sensitivity of mPTP, presumably because mitochondria isolated from the hearts of the MCU−/− mouse do not take up sufficient amounts of Ca2+ and the standard Ca2+ challenging test could not be applied to test how much [Ca2+]i is required to induce mitochondrial swelling. In addition to these uncertainties regarding the [Ca2+]m sensitivity of mPTP, there are even greater unknowns regarding the joint actions of [Ca2+]m and ROS in regulating mPTP. For example, does the recent exciting work from Cheng’s group [171–175] on mitochondrial superoxide flashes depend on mPTP in some way? A recent study showed that the frequency of flashes in cardiac cells depends on stimulating normal [Ca2+]i transients, on various metabolic substrates, and hence the mitochondrial flashes are suggested to be a normal physiological occurrence in heart [176]. This suggests that mPTP is reversible in the case of the mitochondrial flashes. If so, what activates mPTP and what controls its reversal? Current uncertainties regarding the mechanisms that underlie mPTP activation during both normal and pathological conditions are exciting albeit challenging questions. One of the few common threads in the work on mPTP and on mitochondrial metabolic regulation is the central role that appears to be played by [Ca2+]m. Nevertheless, the questions abound.
Summary
In the heart, Ca2+ and mitochondria are intimately connected as cardiac mitochondria are very abundant and often in very close proximity to cardiac CRUs. That Ca2+ enters the mitochondrial matrix is beyond dispute. Nevertheless the amount that enters and the speed with which it traverses the IMM are in dispute. There is consensus that [Ca2+]m is the ideal candidate for providing the feed-forward signal responsible for altering ATP production to meet changes in cardiac energy demand. [Ca2+]m is, moreover, implicated in regulating, either directly or indirectly, nearly every major component involved in mitochondrial ATP production, including key enzymes in the TCA cycle, proteins of the ETC, the F1F0 ATP synthase itself as well as many important transporters and carriers. While under physiological conditions the Ca2+ fluxes across the IMM which determine the steady-state levels of [Ca2+]m appear to be modest in magnitude they are still capable of critically altering mitochondrial function. During pathology, [Ca2+]m also appears to play a critical role in the balance between cell life and death. In fact, some of these [Ca2+]m sensitive processes appear to be substantial targets for the development of future therapeutics. Developing and implementing new techniques and tools to investigate these pathways in a quantitative manner will provide critical information to the field of mitochondrial and cardiac biology.
Highlights.
How mitochondrial calcium influences metabolism in heart is reviewed.
How mitochondrial calcium may influence ischemia-reperfusion injury is discussed.
What the mitochondrial permeability transition pore (mPTP) may do under physiological and pathophysiological conditions is presented.
Acknowledgements
The authors declare no conflicts of interest. This work was partially supported by the USA-Israeli Binational Research Grant # 2009–334 (to WJL). Also by grants to WJL from the Leducq Foundation (European-North American Atrial Fibrillation Research Alliance, ENAFRA), and by the NHLBI R01 HL106059 (to WJL), R01 HL105239–01 (to WJL), and P01 HL67849 (to WJL) 1U01HL116321 (to WJL), and NIH Grant F32 HL108604 (to GSBW). In addition, the research leading to these results has received funding from the European Community’s Seventh Framework Programme FP7/2007–2013 under grant agreement No. HEALTH-F2-2009-241526, EUTrigTreat (to WJL). Additionally, the authors would like to acknowledge Andrew P. Wescott for his assistance in the creation of Fig. 1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. Springer; 2001. [Google Scholar]
- [2].Shoshan-Barmatz V, Ben-Hail D. VDAC, a multi-functional mitochondrial protein as a pharmacological target. Mitochondrion 2012;12:24–34. [DOI] [PubMed] [Google Scholar]
- [3].Komarov AG, Deng D, Craigen WJ, Colombini M. New insights into the mechanism of permeation through large channels. Biophys J 2005;89:3950–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tan W, Colombini M. VDAC closure increases calcium ion flux. Biochim Biophys Acta 2007;1768:2510–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature 2004;427:360–4. [DOI] [PubMed] [Google Scholar]
- [6].De Stefani D, Raffaello A, Teardo E, Szabò I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 2011;476:336–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fieni F, Lee SB, Jan YN, Kirichok Y. Activity of the mitochondrial calcium uniporter varies greatly between tissues. Nat Commun 2012;3:1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Williams GSB, Boyman L, Chikando AC, Khairallah RJ, Lederer WJ. Mitochondrial calcium uptake. Proc Natl Acad Sci USA 2013;110:10479–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 2011;476:341–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Palty R, Silverman WF, Hershfinkel M, Caporale T, Sensi SL, Parnis J, et al. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc Natl Acad Sci USA 2010;107:436–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Boyman L, Williams GSB, Khananshvili D, Sekler I, Lederer WJ. NCLX: the mitochondrial sodium calcium exchanger. J Mol Cell Cardiol 2013;59:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Boyman L, Chikando A, Williams GSB, Lederer JW. Calcium Movement in Cardiac Mitochondria. HttpdxDoiorgJBpj 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lehninger AL, Reynafarje B, Vercesi A, Tew WP. Transport and accumulation of calcium in mitochondria. Ann N Y Acad Sci 1978;307:160–76. [DOI] [PubMed] [Google Scholar]
- [14].Lu X, Ginsburg KS, Kettlewell S, Bossuyt J, Smith GL, Bers DM. Measuring local gradients of intramitochondrial [Ca(2+)] in cardiac myocytes during sarcoplasmic reticulum Ca(2+) release. Circ Res 2013;112:424–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Brochet DXP, Yang D, Di Maio A, Lederer WJ, Franzini-Armstrong C, Cheng H. Ca2+ blinks: rapid nanoscopic store calcium signaling. Proc Natl Acad Sci USA 2005;102:3099–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].SLATER EC, CLELAND KW. The calcium content of isolated heart-muscle sarcosomes. Biochem J 1953;54:xxii. [PubMed] [Google Scholar]
- [17].VASINGTON FD, MURPHY JV. Ca ion uptake by rat kidney mitochondria and its dependence on respiration and phosphorylation. J Biol Chem 1962;237:2670–7. [PubMed] [Google Scholar]
- [18].Kamer KJ, Sancak Y, Mootha VK. The uniporter: from newly identified parts to function. Biochem Biophys Res Commun 2014;449:370–2. [DOI] [PubMed] [Google Scholar]
- [19].Perocchi F, Gohil VM, Girgis HS, Bao XR, McCombs JE, Palmer AE, et al. MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake. Nature 2010;467:291–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Plovanich M, Bogorad RL, Sancak Y, Kamer KJ, Strittmatter L, Li AA, et al. MICU2, a Paralog of MICU1, Resides within the Mitochondrial Uniporter Complex to Regulate Calcium Handling. PLoS ONE 2013;8:e55785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kamer KJ, Mootha VK. MICU1 and MICU2 play nonredundant roles in the regulation of the mitochondrial calcium uniporter. EMBO Rep 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Patron M, Checchetto V, Raffaello A, Teardo E, Vecellio Reane D, Mantoan M, et al. MICU1 and MICU2 Finely Tune the Mitochondrial Ca(2+) Uniporter by Exerting Opposite Effects on MCU Activity. Mol Cell 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hoffman NE, Chandramoorthy HC, Shanmughapriya S, Zhang XQ, Vallem S, Doonan PJ, et al. SLC25A23 augments mitochondrial Ca2+ uptake, interacts with MCU, and induces oxidative stress-mediated cell death. Mol Biol Cell 2014;25:936–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sancak Y, Markhard AL, Kitami T, Kovács-Bogdán E, Kamer KJ, Udeshi ND, et al. EMRE is an Essential Component of the Mitochondrial Calcium Uniporter Complex. Science 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mallilankaraman K, Cárdenas C, Doonan PJ, Chandramoorthy HC, Irrinki KM, Golenár T, et al. MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism. Nat Cell Biol 2012;14:1336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kovács-Bogdán E, Sancak Y, Kamer KJ, Plovanich M, Jambhekar A, Huber RJ, et al. Reconstitution of the mitochondrial calcium uniporter in yeast. Proc Natl Acad Sci USA 2014:201400514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Logan CV, Szabadkai G, Sharpe JA, Parry DA, Torelli S, Childs A-M, et al. Loss-of-function mutations in MICU1 cause a brain and muscle disorder linked to primary alterations in mitochondrial calcium signaling. Nat Genet 2014;46:188–93. [DOI] [PubMed] [Google Scholar]
- [28].Hoffman NE, Chandramoorthy HC, Shamugapriya S, Zhang X, Rajan S, Mallilankaraman K, et al. MICU1 motifs define mitochondrial calcium uniporter binding and activity. Cell Rep 2013;5:1576–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kevin Foskett J, Madesh M. Regulation of the mitochondrial Ca(2+) uniporter by MICU1 and MICU2. Biochem Biophys Res Commun 2014;449:377–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Csordás G, Golenár T, Seifert EL, Kamer KJ, Sancak Y, Perocchi F, et al. MICU1 controls both the threshold and cooperative activation of the mitochondrial Ca2+ uniporter. Cell Metab 2013;17:976–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mallilankaraman K, Doonan P, Cárdenas C, Chandramoorthy HC, Müller M, Miller R, et al. MICU1 Is an Essential Gatekeeper for MCU-Mediated Mitochondrial Ca(2+) Uptake that Regulates Cell Survival. Cell 2012;151:630–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Raffaello A, De Stefani D, Sabbadin D, Teardo E, Merli G, Picard A, et al. The mitochondrial calcium uniporter is a multimer that can include a dominant-negative pore-forming subunit. Embo J 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Carafoli E The release of calcium from heart mitochondria by sodium. J Mol Cell Cardiol 1974;6:361–71. [DOI] [PubMed] [Google Scholar]
- [34].Crompton M, KUNZI M, Carafoli E. The Calcium-Induced and Sodium-Induced Effluxes of Calcium from Heart Mitochondria. Evidence for a Sodium-Calcium Carrier. Eur J Biochem 1977;79:549–58. [DOI] [PubMed] [Google Scholar]
- [35].Palty R, Ohana E, Hershfinkel M, Volokita M, Elgazar V, Beharier O, et al. Lithium-calcium exchange is mediated by a distinct potassium-independent sodium-calcium exchanger. J Biol Chem 2004;279:25234–40. [DOI] [PubMed] [Google Scholar]
- [36].Mitchell P, Moyle J. Chemiosmotic hypothesis of oxidative phosphorylation. Nature 1967;213:137–9. [DOI] [PubMed] [Google Scholar]
- [37].Krulwich TA. Na+/H+ antiporters. Biochim Biophys Acta 1983;726:245–64. [DOI] [PubMed] [Google Scholar]
- [38].Crompton M, Moser R, Lüdi H, Carafoli E. The interrelations between the transport of sodium and calcium in mitochondria of various mammalian tissues. Eur J Biochem 1978;82:25–31. [DOI] [PubMed] [Google Scholar]
- [39].Wolkowicz PE, Michael LH, Lewis RM, McMillin-Wood J. Sodium-calcium exchange in dog heart mitochondria: effects of ischemia and verapamil. Am J Physiol 1983;244:H644–51. [DOI] [PubMed] [Google Scholar]
- [40].Wei A-CA, Liu TT, Cortassa SS, Winslow RLR, O’Rourke BB. Mitochondrial Ca2+ influx and efflux rates in guinea pig cardiac mitochondria: low and high affinity effects of cyclosporine A. Biochim Biophys Acta 2011;1813:1373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Maack C, Cortassa S, Aon MA, Ganesan AN, Liu T, O’Rourke B. Elevated cytosolic Na+ decreases mitochondrial Ca2+ uptake during excitation-contraction coupling and impairs energetic adaptation in cardiac myocytes. Circ Res 2006;99:172–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Liu T, O’Rourke B. Enhancing mitochondrial Ca2+ uptake in myocytes from failing hearts restores energy supply and demand matching. Circulation Research 2008;103:279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Dash RK, Beard DA. Analysis of cardiac mitochondrial Na+-Ca2+ exchanger kinetics with a biophysical model of mitochondrial Ca2+ handling suggests a 3:1 stoichiometry. J Physiol (Lond) 2008;586:3267–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].De Marchi U, Santo-Domingo J, Castelbou C, Sekler I, Wiederkehr A, Demaurex N. NCLX, but not LETM1, mediates mitochondrial Ca2+ extrusion thereby limiting Ca2+-induced NAD(P)H production and modulating matrix redox state. 2014:jbc.M113.540898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Parnis J, Montana V, Delgado-Martinez I, Matyash V, Parpura V, Kettenmann H, et al. Mitochondrial exchanger NCLX plays a major role in the intracellular Ca2+ signaling, gliotransmission, and proliferation of astrocytes. J Neurosci 2013;33:7206–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Nita II, Hershfinkel M, Kantor C, Rutter GA, Lewis EC, Sekler I. Pancreatic β-cell Na+ channels control global Ca2+ signaling and oxidative metabolism by inducing Na+ and Ca2+ responses that are propagated into mitochondria. Faseb J 2014;28:3301–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev 1990;70:391–425. [DOI] [PubMed] [Google Scholar]
- [48].Hansford RG, Castro F. Intramitochondrial and extramitochondrial free calcium ion concentrations of suspensions of heart mitochondria with very low, plausibly physiological, contents of total calcium. J Bioenerg Biomembr 1982;14:361–76. [DOI] [PubMed] [Google Scholar]
- [49].Coll KE, Joseph SK, Corkey BE, Williamson JR. Determination of the matrix free Ca2+ concentration and kinetics of Ca2+ efflux in liver and heart mitochondria. J Biol Chem 1982;257:8696–704. [PubMed] [Google Scholar]
- [50].Bazil JN, Blomeyer CA, Pradhan RK, Camara AKS, Dash RK. Modeling the calcium sequestration system in isolated guinea pig cardiac mitochondria. J Bioenerg Biomembr 2013;45:177–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Tewari SG, Camara AK, Stowe DF, Dash RK. Computational Analysis of Ca2+ Dynamics in Isolated Cardiac Mitochondria Predicts Two Distinct Modes of Ca2+ Uptake. J Physiol (Lond) 2014:jphysiol.2013.268847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Blomeyer CA, Bazil JN, Stowe DF, Pradhan RK, Dash RK, Camara AKS. Dynamic buffering of mitochondrial Ca2+ during Ca2+ uptake and Na+-induced Ca2+ release. J Bioenerg Biomembr 2013;45:189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wei A-C, Liu T, Winslow RL, O’Rourke B. Dynamics of matrix-free Ca2+ in cardiac mitochondria: two components of Ca2+ uptake and role of phosphate buffering. J Gen Physiol 2012;139:465–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Boelens AD, Pradhan RK, Blomeyer CA, Camara AKS, Dash RK, Stowe DF. Extra-matrix Mg2+ limits Ca2+ uptake and modulates Ca2+ uptake-independent respiration and redox state in cardiac isolated mitochondria. J Bioenerg Biomembr 2013;45:203–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Boyman L, Chikando AC, Williams GSB, Khairallah RJ, Kettlewell S, Ward CW, et al. Calcium movement in cardiac mitochondria. Biophys J 2014;107:1289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Carafoli E, Lehninger AL. A survey of the interaction of calcium ions with mitochondria from different tissues and species. Biochem J 1971;122:681–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Endele S, Fuhry M, Pak SJ, Zabel BU, Winterpacht A. LETM1, a novel gene encoding a putative EF-hand Ca(2+)-binding protein, flanks the Wolf-Hirschhorn syndrome (WHS) critical region and is deleted in most WHS patients. Genomics 1999;60:218–25. [DOI] [PubMed] [Google Scholar]
- [58].Shanske AL, Yachelevich N, Ala-Kokko L, Leonard J, Levy B. Wolf-Hirschhorn syndrome and ectrodactyly: New findings and a review of the literature. Am J Med Genet A 2010;152A:203–8. [DOI] [PubMed] [Google Scholar]
- [59].Nowikovsky K, Froschauer EM, Zsurka G, Samaj J, Reipert S, Kolisek M, et al. The LETM1/YOL027 gene family encodes a factor of the mitochondrial K+ homeostasis with a potential role in the Wolf-Hirschhorn syndrome. J Biol Chem 2004;279:30307–15. [DOI] [PubMed] [Google Scholar]
- [60].Hasegawa A, van der Bliek AM. Inverse correlation between expression of the Wolfs Hirschhorn candidate gene Letm1 and mitochondrial volume in C. elegans and in mammalian cells. Hum Mol Genet 2007;16:2061–71. [DOI] [PubMed] [Google Scholar]
- [61].Dimmer KS, Navoni F, Casarin A, Trevisson E, Endele S, Winterpacht A, et al. LETM1, deleted in Wolf-Hirschhorn syndrome is required for normal mitochondrial morphology and cellular viability. Hum Mol Genet 2008;17:201–14. [DOI] [PubMed] [Google Scholar]
- [62].McQuibban AG, Joza N, Megighian A, Scorzeto M, Zanini D, Reipert S, et al. A Drosophila mutant of LETM1, a candidate gene for seizures in Wolf-Hirschhorn syndrome. Hum Mol Genet 2010;19:987–1000. [DOI] [PubMed] [Google Scholar]
- [63].Hashimi H, McDonald L, Stríbrná E, Lukeš J. Trypanosome Letm1 protein is essential for mitochondrial potassium homeostasis. Journal of Biological Chemistry 2013;288:26914–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Jiang D, Zhao L, Clapham DE. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science 2009;326:144–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Tsai M-F, Jiang D, Zhao L, Clapham D, Miller C. Functional reconstitution of the mitochondrial Ca2+/H+ antiporter Letm1. J Gen Physiol 2014;143:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Doonan PJ, Chandramoorthy HC, Hoffman NE, Zhang X, Cárdenas C, Shanmughapriya S, et al. LETM1-dependent mitochondrial Ca2+ flux modulates cellular bioenergetics and proliferation. Faseb J 2014:fj.14–256453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Jiang D, Zhao L, Clish CB, Clapham DE. Letm1, the mitochondrial Ca2+/H+ antiporter, is essential for normal glucose metabolism and alters brain function in Wolf-Hirschhorn syndrome. Proc Natl Acad Sci USA 2013;110:E2249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Waldeck-Weiermair M, Jean-Quartier C, Rost R, Khan MJ, Vishnu N, Bondarenko AI, et al. Leucine zipper EF hand-containing transmembrane protein 1 (Letm1) and uncoupling proteins 2 and 3 (UCP2/3) contribute to two distinct mitochondrial Ca2+ uptake pathways. Journal of Biological Chemistry 2011;286:28444–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Nowikovsky K, Bernardi P. LETM1 in mitochondrial cation transport. Frontiers in Physiology 2014;5:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].O-Uchi J, Pan S, Sheu S-S. Perspectives on: SGP symposium on mitochondrial physiology and medicine: molecular identities of mitochondrial Ca2+ influx mechanism: updated passwords for accessing mitochondrial Ca2+-linked health and disease. J Gen Physiol 2012;139:435–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Pizzo P, Drago I, Filadi R, Pozzan T. Mitochondrial Ca(2+) homeostasis: mechanism, role, and tissue specificities. Pflugers Arch 2012;464:3–17. [DOI] [PubMed] [Google Scholar]
- [72].Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev 2006;86:369–408. [DOI] [PubMed] [Google Scholar]
- [73].Papa S, Paradies G. On the mechanism of translocation of pyruvate and other monocarboxylic acids in rat-liver mitochondria. Eur J Biochem 1974;49:265–74. [DOI] [PubMed] [Google Scholar]
- [74].Bricker DK, Taylor EB, Schell JC, Orsak T, Boutron A, Chen Y-C, et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science 2012;337:96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Zhao WN, McAlister-Henn L. Assembly and function of a cytosolic form of NADH-specific isocitrate dehydrogenase in yeast. J Biol Chem 1996;271:10347–52. [PubMed] [Google Scholar]
- [76].Lehninger AL. Energy coupling in electron transport. Fed Proc 1967;26:1333–4. [PubMed] [Google Scholar]
- [77].Reynafarje B, Brand MD, Lehninger AL. Evaluation of the H+/site ratio of mitochondrial electron transport from rate measurements. J Biol Chem 1976;251:7442–51. [PubMed] [Google Scholar]
- [78].Linn TC, Pettit FH, Reed LJ. Alpha-keto acid dehydrogenase complexes. X. Regulation of the activity of the pyruvate dehydrogenase complex from beef kidney mitochondria by phosphorylation and dephosphorylation. Proc Natl Acad Sci USA 1969;62:234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Linn TC, Pettit FH, Hucho F, Reed LJ. Alpha-keto acid dehydrogenase complexes. XI. Comparative studies of regulatory properties of the pyruvate dehydrogenase complexes from kidney, heart, and liver mitochondria. Proc Natl Acad Sci USA 1969;64:227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Denton RM, Randle PJ, Martin BR. Stimulation by calcium ions of pyruvate dehydrogenase phosphate phosphatase. Biochem J 1972;128:161–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Turkan A, Hiromasa Y, Roche TE. Formation of a complex of the catalytic subunit of pyruvate dehydrogenase phosphatase isoform 1 (PDP1c) and the L2 domain forms a Ca2+ binding site and captures PDP1c as a monomer. Biochemistry 2004;43:15073–85. [DOI] [PubMed] [Google Scholar]
- [82].McCormack JG, Denton RM. The effects of calcium ions and adenine nucleotides on the activity of pig heart 2-oxoglutarate dehydrogenase complex. Biochem J 1979;180:533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Rutter GA, Denton RM. Regulation of NAD+-linked isocitrate dehydrogenase and 2-oxoglutarate dehydrogenase by Ca2+ ions within toluene-permeabilized rat heart mitochondria. Interactions with regulation by adenine nucleotides and NADH/NAD+ ratios. Biochem J 1988;252:181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Lawlis VB, Roche TE. Inhibition of bovine kidney alpha-ketoglutarate dehydrogenase complex by reduced nicotinamide adenine dinucleotide in the presence or absence of calcium ion and effect of adenosine 5’-diphosphate on reduced nicotinamide adenine dinucleotide inhibition. Biochemistry 1981;20:2519–24. [DOI] [PubMed] [Google Scholar]
- [85].Denton RM, Richards DA, Chin JG. Calcium ions and the regulation of NAD+-linked isocitrate dehydrogenase from the mitochondria of rat heart and other tissues. Biochem J 1978;176:899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Hansford RG. Dehydrogenase activation by Ca2+ in cells and tissues. J Bioenerg Biomembr 1991;23:823–54. [DOI] [PubMed] [Google Scholar]
- [87].Ghafourifar P, Richter C. Nitric oxide synthase activity in mitochondria. FEBS Lett 1997;418:291–6. [DOI] [PubMed] [Google Scholar]
- [88].Davidson AM, Halestrap AP. Inhibition of mitochondrial-matrix inorganic pyrophosphatase by physiological [Ca2+], and its role in the hormonal regulation of mitochondrial matrix volume. Biochem J 1989;258:817–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Spencer T, Bygrave FL. Modification by calcium ions of adenine nucleotide translocation in rat liver mitochondria. Biochem J 1972;129:355–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Moreno-Sánchez R Contribution of the translocator of adenine nucleotides and the ATP synthase to the control of oxidative phosphorylation and arsenylation in liver mitochondria. J Biol Chem 1985;260:12554–60. [PubMed] [Google Scholar]
- [91].Mildaziene V, Baniene R, Nauciene Z, Bakker BM, Brown GC, Westerhoff HV, et al. Calcium indirectly increases the control exerted by the adenine nucleotide translocator over 2-oxoglutarate oxidation in rat heart mitochondria. Arch Biochem Biophys 1995;324:130–4. [DOI] [PubMed] [Google Scholar]
- [92].Lopaschuk GD, Ussher JR, Folmes CDL, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev 2010;90:207–58. [DOI] [PubMed] [Google Scholar]
- [93].Schönekess BO, Brindley PG, Lopaschuk GD. Calcium regulation of glycolysis, glucose oxidation, and fatty acid oxidation in the aerobic and ischemic heart. Can J Physiol Pharmacol 1995;73:1632–40. [DOI] [PubMed] [Google Scholar]
- [94].Grynberg A, Demaison L. Fatty acid oxidation in the heart. J Cardiovasc Pharmacol 1996;28 Suppl 1:S11–7. [DOI] [PubMed] [Google Scholar]
- [95].Murphy AN, Kelleher JK, Fiskum G. Submicromolar Ca2+ regulates phosphorylating respiration by normal rat liver and AS-30D hepatoma mitochondria by different mechanisms. J Biol Chem 1990;265:10527–34. [PubMed] [Google Scholar]
- [96].Glancy B, Willis WT, Chess DJ, Balaban RS. Effect of calcium on the oxidative phosphorylation cascade in skeletal muscle mitochondria. Biochemistry 2013;52:2793–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Bender E, Kadenbach B. The allosteric ATP-inhibition of cytochrome c oxidase activity is reversibly switched on by cAMP-dependent phosphorylation. FEBS Lett 2000;466:130–4. [DOI] [PubMed] [Google Scholar]
- [98].Panov AV, Scaduto RC. Influence of calcium on NADH and succinate oxidation by rat heart submitochondrial particles. Arch Biochem Biophys 1995;316:815–20. [DOI] [PubMed] [Google Scholar]
- [99].Harris DA, Das AM. Control of mitochondrial ATP synthesis in the heart. Biochem J 1991;280 ( Pt 3):561–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Territo PR, Mootha VK, French SA, Balaban RS. Ca(2+) activation of heart mitochondrial oxidative phosphorylation: role of the F(0)/F(1)-ATPase. Am J Physiol, Cell Physiol 2000;278:C423–35. [DOI] [PubMed] [Google Scholar]
- [101].Balaban RS, Kantor HL, Katz LA, Briggs RW. Relation between work and phosphate metabolite in the in vivo paced mammalian heart. Science 1986;232:1121–3. [DOI] [PubMed] [Google Scholar]
- [102].Katz LA, Swain JA, Portman MA, Balaban RS. Relation between phosphate metabolites and oxygen consumption of heart in vivo. Am J Physiol 1989;256:H265–74. [DOI] [PubMed] [Google Scholar]
- [103].Neely JR, Denton RM, England PJ, Randle PJ. The effects of increased heart work on the tricarboxylate cycle and its interactions with glycolysis in the perfused rat heart. Biochem J 1972;128:147–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Heineman FW, Balaban RS. Phosphorus-31 nuclear magnetic resonance analysis of transient changes of canine myocardial metabolism in vivo. J Clin Invest 1990;85:843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Glancy B, Balaban RS. Role of mitochondrial Ca2+ in the regulation of cellular energetics. Biochemistry 2012;51:2959–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Aliev MK, Saks VA. Compartmentalized energy transfer in cardiomyocytes: use of mathematical modeling for analysis of in vivo regulation of respiration. Biophys J 1997;73:428–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Saks V, Beraud N, Wallimann T. Metabolic compartmentation - a system level property of muscle cells: real problems of diffusion in living cells. Int J Mol Sci 2008;9:751–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Saks V, Dzeja P, Schlattner U, Vendelin M, Terzic A, Wallimann T. Cardiac system bioenergetics: metabolic basis of the Frank-Starling law. J Physiol (Lond) 2006;571:253–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Tantama M, Martínez-François JR, Mongeon R, Yellen G. Imaging energy status in live cells with a fluorescent biosensor of the intracellular ATP-to-ADP ratio. Nat Commun 2013;4:2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Kioka H, Kato H, Fujikawa M, Tsukamoto O, Suzuki T, Imamura H, et al. Evaluation of intramitochondrial ATP levels identifies G0/G1 switch gene 2 as a positive regulator of oxidative phosphorylation. Proc Natl Acad Sci USA 2014;111:273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Tsuyama T, Kishikawa J-I, Han Y-W, Harada Y, Tsubouchi A, Noji H, et al. In vivo fluorescent adenosine 5’-triphosphate (ATP) imaging of Drosophila melanogaster and Caenorhabditis elegans by using a genetically encoded fluorescent ATP biosensor optimized for low temperatures. Anal Chem 2013;85:7889–96. [DOI] [PubMed] [Google Scholar]
- [112].Brandes R, Bers DM. Simultaneous measurements of mitochondrial NADH and Ca(2+) during increased work in intact rat heart trabeculae. Biophys J 2002;83:587–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Eng J, Lynch RM, Balaban RS. Nicotinamide adenine dinucleotide fluorescence spectroscopy and imaging of isolated cardiac myocytes. Biophys J 1989;55:621–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Nuutinen EM. Subcellular origin of the surface fluorescence of reduced nicotinamide nucleotides in the isolated perfused rat heart. Basic Res Cardiol 1984;79:49–58. [DOI] [PubMed] [Google Scholar]
- [115].Blinova K, Carroll S, Bose S, Smirnov AV, Harvey JJ, Knutson JR, et al. Distribution of mitochondrial NADH fluorescence lifetimes: steady-state kinetics of matrix NADH interactions. Biochemistry 2005;44:2585–94. [DOI] [PubMed] [Google Scholar]
- [116].Blinova K, Levine RL, Boja ES, Griffiths GL, Shi Z-D, Ruddy B, et al. Mitochondrial NADH fluorescence is enhanced by complex I binding. Biochemistry 2008;47:9636–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Heineman FW, Balaban RS. Effects of afterload and heart rate on NAD(P)H redox state in the isolated rabbit heart. Am J Physiol 1993;264:H433–40. [DOI] [PubMed] [Google Scholar]
- [118].Brandes R, Bers DM. Increased work in cardiac trabeculae causes decreased mitochondrial NADH fluorescence followed by slow recovery. Biophys J 1996;71:1024–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Brandes R, Bers DM. Intracellular Ca2+ increases the mitochondrial NADH concentration during elevated work in intact cardiac muscle. Circ Res 1997;80:82–7. [DOI] [PubMed] [Google Scholar]
- [120].Brandes R, Maier LS, Bers DM. Regulation of mitochondrial [NADH] by cytosolic [Ca2+] and work in trabeculae from hypertrophic and normal rat hearts. Circ Res 1998;82:1189–98. [DOI] [PubMed] [Google Scholar]
- [121].Brandes R, Bers DM. Analysis of the mechanisms of mitochondrial NADH regulation in cardiac trabeculae. Biophys J 1999;77:1666–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Giorgio V, Stockum von S, Antoniel M, Fabbro A, Fogolari F, Forte M, et al. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci USA 2013;110:5887–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Alavian KN, Beutner G, Lazrove E, Sacchetti S, Park H-A, Licznerski P, et al. An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc Natl Acad Sci USA 2014:201401591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Bonora M, Bononi A, De Marchi E, Giorgi C, Lebiedzinska M, Marchi S, et al. Role of the c subunit of the FO ATP synthase in mitochondrial permeability transition. Cell Cycle 2013;12:674–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].RAAFLAUB J [Swelling of isolated mitochondria of the liver and their susceptibility to physicochemical influences]. Helv Physiol Pharmacol Acta 1953;11:142–56. [PubMed] [Google Scholar]
- [126].HUNTER FE, FORD L. Inactivation of oxidative and phosphorylative systems in mitochondria by preincubation with phosphate and other ions. J Biol Chem 1955;216:357–69. [PubMed] [Google Scholar]
- [127].Nicholls D, Akerman K. Mitochondrial calcium transport. Biochim Biophys Acta 1982;683:57–88. [DOI] [PubMed] [Google Scholar]
- [128].Al-Nasser I, Crompton M. The reversible Ca2+-induced permeabilization of rat liver mitochondria. Biochem J 1986;239:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Lemasters JJ, Nieminen AL, Qian T, Trost LC, Elmore SP, Nishimura Y, et al. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta 1998;1366:177–96. [DOI] [PubMed] [Google Scholar]
- [130].Baines CP. The molecular composition of the mitochondrial permeability transition pore. J Mol Cell Cardiol 2009;46:850–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, et al. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature 2004;427:461–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Carraro M, Giorgio V, Sileikyte J, Sartori G, Forte M, Lippe G, et al. Channel Formation by Yeast F-ATP Synthase and the Role of Dimerization in the Mitochondrial Permeability Transition. Journal of Biological Chemistry 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Chinopoulos C, Adam-Vizi V. Modulation of the mitochondrial permeability transition by cyclophilin D: moving closer to F(0)-F(1) ATP synthase? Mitochondrion 2012;12:41–5. [DOI] [PubMed] [Google Scholar]
- [134].Karch J, Molkentin JD. Identifying the components of the elusive mitochondrial permeability transition pore. Proc Natl Acad Sci USA 2014;111:10396–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Halestrap AP. The C Ring of the F1Fo ATP Synthase Forms the Mitochondrial Permeability Transition Pore: A Critical Appraisal. Molecular and Cellular Oncology 2014;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Murphy E, Steenbergen C. What makes the mitochondria a killer? Can we condition them to be less destructive? Biochim Biophys Acta 2011;1813:1302–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Halestrap AP. A pore way to die: the role of mitochondria in reperfusion injury and cardioprotection. Biochem Soc Trans 2010;38:841–60. [DOI] [PubMed] [Google Scholar]
- [138].Elrod JW, Molkentin JD. Physiologic functions of cyclophilin D and the mitochondrial permeability transition pore. Circ J 2013;77:1111–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Altschuld RA, Hohl CM, Castillo LC, Garleb AA, Starling RC, Brierley GP. Cyclosporin inhibits mitochondrial calcium efflux in isolated adult rat ventricular cardiomyocytes. Am J Physiol 1992;262:H1699–704. [DOI] [PubMed] [Google Scholar]
- [140].Korge P, Yang L, Yang J-H, Wang Y, Qu Z, Weiss JN. Protective role of transient pore openings in calcium handling by cardiac mitochondria. Journal of Biological Chemistry 2011;286:34851–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Barsukova A, Komarov A, Hajnóczky G, Bernardi P, Bourdette D, Forte M. Activation of the mitochondrial permeability transition pore modulates Ca2+ responses to physiological stimuli in adult neurons. Eur J Neurosci 2011;33:831–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Elrod JW, Wong R, Mishra S, Vagnozzi RJ, Sakthievel B, Goonasekera SA, et al. Cyclophilin D controls mitochondrial pore-dependent Ca(2+) exchange, metabolic flexibility, and propensity for heart failure in mice. J Clin Invest 2010;120:3680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 2005;434:658–62. [DOI] [PubMed] [Google Scholar]
- [144].Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 2005;434:652–8. [DOI] [PubMed] [Google Scholar]
- [145].Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of Cyclophilin D. J Biol Chem 2005;280:18558–61. [DOI] [PubMed] [Google Scholar]
- [146].Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, et al. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci USA 2005;102:12005–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Kettlewell S, Cabrero P, Nicklin SA, Dow JAT, Davies S, Smith GL. Changes of intramitochondrial Ca2+ in adult ventricular cardiomyocytes examined using a novel fluorescent Ca2+ indicator targeted to mitochondria. J Mol Cell Cardiol 2009;46:891–901. [DOI] [PubMed] [Google Scholar]
- [148].Jennings RB, Hawkins HK, Lowe JE, Hill ML, Klotman S, Reimer KA. Relation between high energy phosphate and lethal injury in myocardial ischemia in the dog. Am J Pathol 1978;92:187–214. [PMC free article] [PubMed] [Google Scholar]
- [149].Jennings RB, Reimer KA. The cell biology of acute myocardial ischemia. Annu Rev Med 1991;42:225–46. [DOI] [PubMed] [Google Scholar]
- [150].Appleyard RF, Cohn LH. Myocardial stunning and reperfusion injury in cardiac surgery. J Card Surg 1993;8:316–24. [DOI] [PubMed] [Google Scholar]
- [151].Bolli R, Marbán E. Molecular and cellular mechanisms of myocardial stunning. Physiol Rev 1999;79:609–34. [DOI] [PubMed] [Google Scholar]
- [152].Skyschally A, Schulz R, Heusch G. Pathophysiology of myocardial infarction: protection by ischemic pre- and postconditioning. Herz 2008;33:88–100. [DOI] [PubMed] [Google Scholar]
- [153].Reimer KA, Jennings RB. Energy metabolism in the reversible and irreversible phases of severe myocardial ischemia. Acta Med Scand Suppl 1981;651:19–27 [DOI] [PubMed] [Google Scholar]
- [154].Jennings RB, Reimer KA, Jones RN, Peyton RB. High energy phosphates, anaerobic glycolysis and irreversibility in ischemia. Adv Exp Med Biol 1983;161:403–19. [DOI] [PubMed] [Google Scholar]
- [155].Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 1986;74:1124–36. [DOI] [PubMed] [Google Scholar]
- [156].Crompton M, Costi A, Hayat L. Evidence for the presence of a reversible Ca2+-dependent pore activated by oxidative stress in heart mitochondria. Biochem J 1987;245:915–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Crompton M, Ellinger H, Costi A. Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem J 1988;255:357–60. [PMC free article] [PubMed] [Google Scholar]
- [158].Crompton M, Costi A. Kinetic evidence for a heart mitochondrial pore activated by Ca2+, inorganic phosphate and oxidative stress. A potential mechanism for mitochondrial dysfunction during cellular Ca2+ overload. Eur J Biochem 1988;178:489–501. [DOI] [PubMed] [Google Scholar]
- [159].Kinnally KW, Campo ML, Tedeschi H. Mitochondrial channel activity studied by patch-clamping mitoplasts. J Bioenerg Biomembr 1989;21:497–506. [DOI] [PubMed] [Google Scholar]
- [160].Petronilli V, Szabo I, Zoratti M. The inner mitochondrial membrane contains ion-conducting channels similar to those found in bacteria. FEBS Lett 1989;259:137–43. [DOI] [PubMed] [Google Scholar]
- [161].Szabo I, Zoratti M. The giant channel of the inner mitochondrial membrane is inhibited by cyclosporin A. J Biol Chem 1991;266:3376–9. [PubMed] [Google Scholar]
- [162].Bernardi P, Vassanelli S, Veronese P, Colonna R, Szabo I, Zoratti M. Modulation of the mitochondrial permeability transition pore. Effect of protons and divalent cations. J Biol Chem 1992;267:2934–9. [PubMed] [Google Scholar]
- [163].Szabo I, Zoratti M. The mitochondrial megachannel is the permeability transition pore. J Bioenerg Biomembr 1992;24:111–7. [DOI] [PubMed] [Google Scholar]
- [164].Miotto G, Canton M, Brini M, Colonna R. Transient and long-lasting openings of the mitochondrial permeability transition pore can be monitored directly in intact cells by changes in mitochondrial calcein fluorescence. Biophys J 1999;76:725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Rasola A, Bernardi P. Mitochondrial permeability transition in Ca(2+)-dependent apoptosis and necrosis. Cell Calcium 2011;50:222–33. [DOI] [PubMed] [Google Scholar]
- [166].Duchen MR. Mitochondria, calcium-dependent neuronal death and neurodegenerative disease. Pflugers Arch 2012;464:111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Griffiths EJ, Halestrap AP. Protection by Cyclosporin A of ischemia/reperfusion-induced damage in isolated rat hearts. J Mol Cell Cardiol 1993;25:1461–9. [DOI] [PubMed] [Google Scholar]
- [168].Griffiths EJ, Halestrap AP. Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem J 1995;307 ( Pt 1):93–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Pan X, Liu J, Nguyen T, Liu C, Sun J, Teng Y, et al. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat Cell Biol 2013;15:1464–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Joiner M-LA, Koval OM, Li J, He BJ, Allamargot C, Gao Z, et al. CaMKII determines mitochondrial stress responses in heart. Nature 2012;491:269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Wang W, Fang H, Groom L, Cheng A, Zhang W, Liu J, et al. Superoxide flashes in single mitochondria. Cell 2008;134:279–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Sheu S, Wang W, Cheng H, Dirksen R. Superoxide flashes: illuminating new insights into cardiac ischemia/reperfusion injury. Future Cardiol 2008;4:551–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Zhang X, Huang Z, Hou T, Xu J, Wang Y, Shang W, et al. Superoxide constitutes a major signal of mitochondrial superoxide flash. Life Sci 2013;93:178–86. [DOI] [PubMed] [Google Scholar]
- [174].Hou Y, Ouyang X, Wan R, Cheng H, Mattson MP, Cheng A. Mitochondrial superoxide production negatively regulates neural progenitor proliferation and cerebral cortical development. Stem Cells 2012;30:2535–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Zhang W, Li K, Zhu X, Wu D, Shang W, Yuan X, et al. Subsarcolemmal Mitochondrial Flashes Induced by Hypochlorite Stimulation in Cardiac Myocytes. Free Radic Res 2014:1–30. [DOI] [PubMed] [Google Scholar]
- [176].Gong G, Liu X, Wang W. Regulation of metabolism in individual mitochondria during excitation-contraction coupling. J Mol Cell Cardiol 2014;76C:235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]