Abstract
Background
Physical activity is effective for the prevention and treatment of chronic disease, yet insufficient evidence is available to make comparisons regarding adherence to aerobic physical activity interventions among chronic disease populations, or across different settings.
The purpose of this review is to investigate and provide a quantitative summary of adherence rates to the aerobic physical activity guidelines among people with chronic conditions, as physical activity is an effective form of treatment and prevention of chronic disease.
Methods
Randomized controlled (RCTs) trials where aerobic physical activity was the primary intervention were selected from PsychInfo, PubMed, CINAHL (Cumulative Index to Nursing and Allied Health Literature), Clinical Key, and SCOPUS from 2000 to 2018. Studies were included if the program prescription aligned with the 2008 aerobic physical activity guidelines, were at least 12 weeks in length, and included adult participants living with one of three chronic diseases. The data was extracted by hand and the PRISMA (preferred reporting items for systematic review and meta-analysis) guidelines were used to evaluate risk-of-bias and quality of evidence. Data were pooled using random-effect models. The primary outcome measure was program adherence and the secondary outcome measures were dropout and setting (e.g. home vs. clinic-based). Pooled effect sizes and 95% CiIs (confidence intervals) were calculated using random-effect models.
Results
The literature search identified 1616 potentially eligible studies, of which 30 studies (published between 2000 and 2018, including 3,721 participants) met the inclusion criteria. Three clinical populations were targeted: cancer (n = 14), cardiovascular disease (n = 7), and diabetes (n = 9). Although not statistically significant, adherence rates varied across samples (65, 90, and 80%, respectively) whereas dropout rates were relatively low and consistent across samples (5, 4, and 3%). The average adherence rate, regardless of condition, is 77% (95% CI = 0.68, 0.84) of their prescribed physical activity treatment. The pooled adherence rates for clinic-based and home-based programs did not differ (74% [95% CI, 0.65, 0.82] and 80% [95% CI, 0.65, 0.91], respectively).
Conclusions
The current evidence suggests that people with chronic conditions are capable of sustaining aerobic physical activity for 3+ months, as a form of treatment. Moreover, home-based programs may be just as feasible as supervised, clinic-based physical activity programs.
Keywords: Exercise adherence, Dropout, Chronic disease, Cancer, Cardiovascular disease, Diabetes
Background
Chronic disease is the leading cause of death in America and almost 50% of adults have one or more chronic health conditions [1]. Increasing physical activity has been shown to be an effective form of treatment and prevention of chronic disease [2–4]. The benefits of regular physical activity include, but are not limited to, weight control, strengthening of muscles and bones, increases in balance and general physical functioning, and improvements in mental health [5] and health-related quality of life [6]—all factors negatively affected by chronic disease. Current public health guidelines [7] recommend 150 min of moderate-to-vigorous aerobic exercise per week. However, it has previously been reported that only 35% of women after breast cancer diagnosis [8], 32% of those with cardiovascular disease (CVD) [9], and 46% of people with diabetes met physical activity guidelines [10]. These low adherence rates are not altogether surprising, as individuals with chronic disease have many barriers (i.e. fatigue, pain) to continued physical activity participation relative to those without chronic disease.
Typical treatment for chronic disease involves managing symptoms with medication and accounts for 86% of the total health care expenses in the United States [11]. There is evidence within the medical community that physical activity is comparably effective as an additional treatment of disease [12] and lowering the risk of mortality [13] relative to standard treatment methods (e.g. medications, surgery, chemotherapy and radiation). However, research trials can vary substantially in their methodologies as well as their setting (clinic- vs. home-based). Both settings have unique advantages. Clinic-based programs often provide more detailed and intensive supervision, whereas home-based programs typically provide more autonomy (e.g., more choices regarding training schedule, fewer transportation-related barriers to receive intervention). In a review of physical activity interventions designed for older adults, Conn et al. [14] found a greater effect for clinic-based interventions (d = .26) relative to home-based programs. It is possible that the supervision provided in the clinic-based studies resulted in greater adherence to the program. It cannot be assumed that patients will uniformly adhere to any structured physical activity program, irrespective of their condition. Specifically, do patients’ adherence levels vary across chronic conditions (e.g., cardiovascular vs. metabolic) or type of program (e.g., clinic or home-based)? Answering these questions is essential for practitioners and researchers, as both are interested in understanding how to optimize the delivery of physical activity as medicine as an adjuvant treatment for disease.
The three most commonly studied chronic diseases in the context of physical activity interventions are cancer, CVD, and diabetes. Physical activity has been shown to be an effective treatment for each of these diseases and current evidence suggests exercise has a positive effect on patient quality of life, physical functioning, and fatigue compared to usual care. For example, Gerritsen and Vincent [15] examined the evidence from randomized controlled trials involving cancer patients in a systematic review and meta-analysis and determined that exercise significantly improved self-esteem, physical performance and functioning, fatigue, and social functioning. According to an observational study of 2987 women diagnosed with breast cancer [16], those who participated in regular physical activity (9+ MET (metabolic equivalent task)-hours per week) saw reductions in breast cancer mortality (relative risk: 0.50, 95% CI: 0.34–0.74). Similarly, Anderson et al. [17] reviewed exercise-based cardiac rehabilitation programs and, relative to usual care, exercise improved quality of life, reduced hospital admissions post-treatment (relative risk: 0.82, 95% CI: 0.70 to 0.96), and reduced cardiovascular mortality (relative risk: 0.74, 95% CI: 0.64 to 0.86), independent of study quality, setting, and publication date. Likewise, Umpierre et al. [18] provided substantial evidence that structured exercise training is associated with reduced levels of hemoglobin A1C (HbA1C) (− 0.67%; 95% CI: -0.84% to − 0.49%, p < 0.001), as well as reduced risks for diabetes-related complications in patients with type 2 diabetes. Hu et al. conducted a longitudinal study of 3708 patients with type 2 diabetes [19] and showed a reduction in mortality risk across low, moderate, and high physical activity levels (relative risk: 1.00, 0.59, and 0.49, respectively). Altogether, there is substantial evidence for using exercise as medicine [20] (i.e. treating disease, lowering mortality), but comparisons regarding adherence to aerobic exercise prescriptions across these conditions, and between clinic- and home-based settings, have not been made. Given that exercise can serve as a standalone and complementary medicine, more research is needed examining the relative acceptability of activity prescriptions across populations.
In this systematic review and meta-analysis, we aimed to test the potential differences in adherence and dropout rates among patients involved in aerobic physical activity interventions. We hypothesized that cancer patients would exhibit the lowest adherence rates with the knowledge that very few cancer patients meet the recommended physical activity guidelines (2008 or 2018) for aerobic exercise, and given the long-lasting and debilitating effects of chemotherapy and radiation treatment (e.g., fatigue, cognitive impairment) [21] compared to CVD or diabetes. In addition, we also hypothesized higher adherence associated with clinic-based programs relative to home-based programs because there is arguably more supervision and accountability in such programs.
Methods
Study inclusion criteria
RCTs with an aerobic (only) exercise intervention were included in the review. Specifically, trials must have included an explicit program prescription aligned with the 2008 Physical Activity Guidelines for aerobic exercise (i.e., a minimum of 150 min per week). Also, trials that included adult participants (age 18+), and published results met study inclusion criteria. Physical activity interventions lasting at least 12 weeks were included to align with expected dropout trends previously reported in the literature among people with and without clinical conditions [22, 23]. Studies were included if rates of adherence and dropout were explicitly reported. Author confirmation was required if study data was not reported in sufficient detail. In this study, adherence was defined as meeting the aerobic physical activity recommendations of 150 min/week across the study duration (expressed as a percentage). Dropout was defined as participants who formally withdrew or left the study and did not return (e.g. non-responders who did not officially relinquish their consent to participate). This threshold was determined as one month or longer as a substantial period of time, whereas less than one month could reflect brief illness or vacation. If the paper provided definitions that varied from the ones above, or did not explicitly provide values, the author was contacted and asked to provide the information as requested in order to standardize the data. The intervention frequency, intensity, type, and duration were recorded for each study and each intervention was identified as either home- or clinic-based. Inclusionary criteria allowed for all types of cancer, CVD, and diabetes, as long as patients were currently diagnosed with one of the targeted diseases. Although type-1 diabetes was not exclusionary, all diabetes studies in this review included populations with type-2 diabetes. The type of cancer patients’ treatment (e.g. chemotherapy, radiation, surgery) was not exclusionary.
Study exclusion criteria
Studies were excluded for a variety of reasons. The most common reason for exclusion was that exercise was not the primary intervention. For example, a lifestyle intervention study [24] provided counseling with the primary outcome being weight loss. Although physical activity was assessed within the study, it was not a primary outcome of the intervention. Exclusionary criteria did not allow for healthy populations (studies that did not include patients with cancer, CVD, or diabetes), or if the patients did not currently have the disease (e.g., cancer survivors).
Search strategy
The research databases PsychInfo, PubMed, CINAHL, Clinical Key, and SCOPUS were searched limiting the publication range from 2000 to 2018. The keywords were ‘physical activity’ and ‘exercise,’ with ‘adherence,’ ‘compliance,’ or ‘drop out’ for ‘cancer’, ‘cardiovascular disease,’ ‘coronary disease,’ ‘coronary risk,’ or ‘diabetes’. Filters were used to select only RCTs when available. Additional studies were added through a manual search targeting existing meta-analyses and systematic reviews of physical activity interventions for specific clinical populations. Experts within the field were contacted for any published papers the authors may have omitted.
Study selection
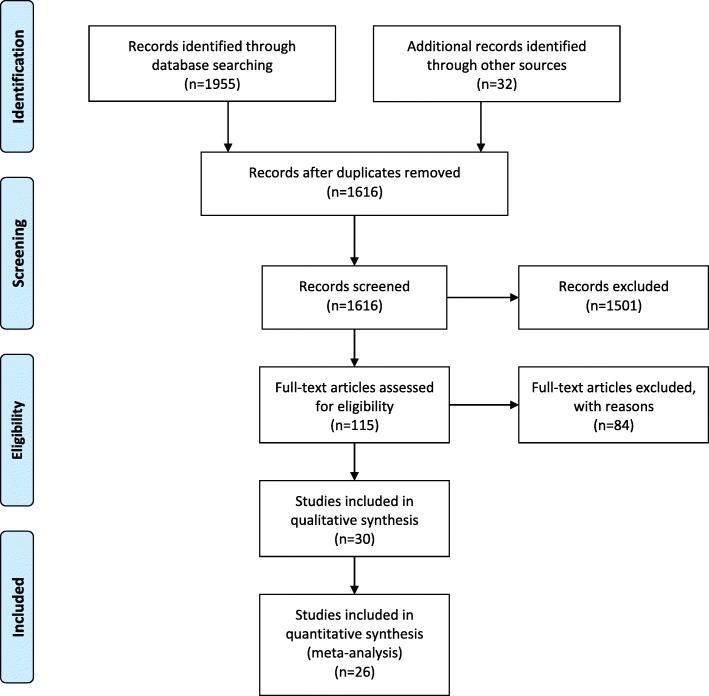
The PRISMA-P (preferred reporting items for systematic review and meta-analysis protocols) guidelines [25] were followed in the reporting of this systematic review protocol (see Figs. 1 and 2). The search was conducted from February 2016 to October 2018. Titles and abstracts containing the key words were searched more thoroughly to ensure the selection criteria was met. Articles needed to be written in English and included adult samples only to be considered for the review. The included and excluded publications were subsequently reviewed by a second author (S. P. M.) until 100% consensus was reached regarding the final sample of studies to be included in the analysis. Disagreements about papers meeting all requirements were discussed amongst the authors until a consensus was reached.
Fig. 1.
PRISMA Flow Diagram
Fig. 2.
PRISMA Checklist
Data extraction
The data for this review and meta-analysis was extracted by hand and stored within a Microsoft Excel spreadsheet. Data were extracted by the first author and checked for alliance with search criteria by the senior author. Data included information about the publication (authors, year, title), participant characteristics (number, age, gender, disease type), intervention characteristics (home- vs. clinic-based, length), and measurement characteristics (adherence definition and rates, dropout definition and rates, confirmation of data by author, intention-to-treat analysis). Authors were contacted if a measure was not explicitly reported within the publication.
Analysis and synthesis
A meta-analysis was performed to estimate the pooled difference in dropout rates between the intervention and control group and the pooled adherence rate in the intervention group. In addition, separate pooled effect sizes were estimated based on studies stratified by disease type (i.e., cancer, CVD, and diabetes). A random-effect model was estimated given a p-value less than 0.05 from the Cochran’s Q test or an I2 statistics greater or equal to 50%; otherwise, a fixed-effect model was estimated. Meta-regressions were conducted to examine the potential heterogeneities in differential dropout rates between the intervention and control group and adherence rate in the intervention group attributable to different disease type. The independent variables in both meta-regressions were two categorical variables for CVD and type 2 diabetes, with cancer as their common reference group. Additional meta-regressions were conducted to assess dropout/adherence rates in relation to intervention duration (measured by a continuous variable for trial length in weeks), intention to treat (ITT) status (measured by a dichotomous variable for intervention conducted following the ITT principle), age (measured by a continuous variable for mean age of the study sample), and intervention setting (measured by a dichotomous variable for home-based intervention, with clinic-based intervention as the reference group). If studies with multiple intervention groups were included in the review, only the aerobic exercise group was included included in the analysis in comparison to the usual care group.
Publication bias was assessed using the Begg’s test and Egger’s test. All analyses used two-sided tests, and p-values less than 0.05 were considered statistically significant. All statistical analyses were conducted using Stata 15.1 SE version (StataCorp, College Station, TX).
Results
Characteristics of included studies
Following a comprehensive literature search, there were 1616 eligible studies, published between 2000 and 2018. The search and selection of articles are summarized in the study flow diagram (Fig. 1). Study characteristics can be found in Table 1. The median sample size was 81 participants (range 14 to 606). A total of 3,721 participants were included in this review. The 30 studies included examined cancer (n = 14), CVD (n = 7), and diabetes (n = 9). All studies included patients that were currently diagnosed with any type of cancer, CVD, or diabetes. Among the cancer studies, there were four types included in this review: breast, prostate, colorectal, and ovarian. The CVD studies included heterogeneous samples (any CVD-related diagnoses) and homogeneous samples (e.g. coronary disease, heart failure, and hypertension). Although we did not exclude any specific type of diabetes, all studies within this review included patients diagnosed with type 2 diabetes. The mean age was 57.32 (SD = 7.40). All studies included an aerobic exercise program, meeting the 2008 and 2018 Physical Activity Guidelines of a minimum of 150 min of moderate-intensity aerobic activity per week. Half of the studies included were clinic-based (n = 16, 53.3%). The pooled adherence rates for clinic-based and home-based programs were 74% [95% CI, 0.65, 0.82] and 80% [95% CI, 0.65, 0.91], respectively. Across conditions, there was greater variability for the number of clinic-based programs: cancer (n = 7, 50.0%), CVD (n = 2, 28.6%), and diabetes (n = 7, 77.8%). The mean length of the intervention was 20 weeks (range 12 to 52 weeks). Of the 30 studies, 12 studies report the use of intention-to-treat (ITT) method for missing data, 13 studies report not using ITT, and 5 studies did not confirm the type of analysis used.
Table 1.
Study Characteristics
| First author, year (superscript = references) | Sample size | Duration | Chronic disease | Type | Age in years (mean) | Location |
|---|---|---|---|---|---|---|
| Gokal et al., 2016 [35] | 50 | 12 weeks | cancer | breast | 52.00 | home-based |
| Huang et al., 2015 [36] | 159 | 12 weeks | cancer | breast | 48.27 | home-based |
| Cadmus et al., 2009 [37] | 50 | 24 weeks | cancer | breast | 55.80 | home-based |
| Courneya et al., 2009 [38] | 122 | 12 weeks | cancer | lymphoma | 53.20 | clinic-based |
| Segal et al., 2008 [39] | 81 | 24 weeks | cancer | prostate | 65.75 | clinic-based |
| Al-Majid et al., 2015 [40] | 14 | 12 weeks | cancer | breast | 50.30 | clinic-based |
| Courneya et al., 2008 [41] | 242 | 17 weeks | cancer | breast | 50.00 | clinic-based |
| Dodd et al., 2010 [42] | 119 | 12 weeks | cancer | breast, colorectal, or ovarian | 50.50 | home-based |
| Duijts et al., 2012 [43] | 422 | 12 weeks | cancer | breast | 48.20 | home-based |
| Giallauria et al., 2015 [44] | 94 | 52 weeks | cancer | breast | 53.50 | clinic-based |
| Nikander et al., 2007 [45] | 28 | 12 weeks | cancer | breast | 53.00 | clinic-based |
| Pickett et al., 2002 [46] | 52 | 12 weeks | cancer | breast | 52.00 | home-based |
| Shang et al., 2012 [47] | 126 | 20 weeks | cancer | breast, colorectal, or prostate | 60.20 | clinic-based |
| Courneya et al., 2003 [48] | 102 | 16 weeks | cancer | colorectal | 60.00 | home-based |
| Lian et al., 2014 [49] | 330 | 12 weeks | CVD | coronary artery disease | 62.30 | home-based |
| Li et al., 2015 [50] | 77 | 12 weeks | CVD | CVD | 80.68 | home-based |
| Salvetti et al., 2008 [51] | 39 | 12 weeks | CVD | coronary disease | 53.00 | home-based |
| Gary et al., 2011 [52] | 24 | 12 weeks | CVD | heart failure | 60.00 | home-based |
| Guimaraes et al., 2014 [53] | 32 | 12 weeks | CVD | hyper-tension | 53.70 | clinic-based |
| Houle et al., 2011 [54] | 65 | 52 weeks | CVD | acute coronary syndrome | 59.00 | home-based |
| Kitzman et al., 2010 [55] | 53 | 16 weeks | CVD | heart failure | 70.00 | clinic-based |
| Lee et al., 2015 [56] | 80 | 12 weeks | diabetes | type 2 | 56.08 | home-based |
| Church et al., 2010 [57] | 113 | 36 weeks | diabetes | type 2 | 55.80 | clinic-based |
| Dela et al., 2004 [58] | 24 | 12 weeks | diabetes | type 2 | 51.50 | home-based |
| Balducci et al., 2014 [59] | 127 | 48 weeks | diabetes | type 2 | 60.00 | clinic-based |
| Negri et al., 2010 [60] | 60 | 16 weeks | diabetes | type 2 | 65.70 | clinic-based |
| Nicolucci et al., 2012 [61] | 606 | 49 weeks | diabetes | type 2 | 60.00 | clinic-based |
| Sigal et al., 2007 [62] | 251 | 22 weeks | diabetes | type 2 | 53.50 | clinic-based |
| Nam et al., 2012 [63] | 140 | 24 weeks | diabetes | type 2 | 56.39 | clinic-based |
| Tessier et al., 2000 [64] | 39 | 16 weeks | diabetes | type 2 | 69.40 | clinic-based |
Dropout and adherence rates of the interventions
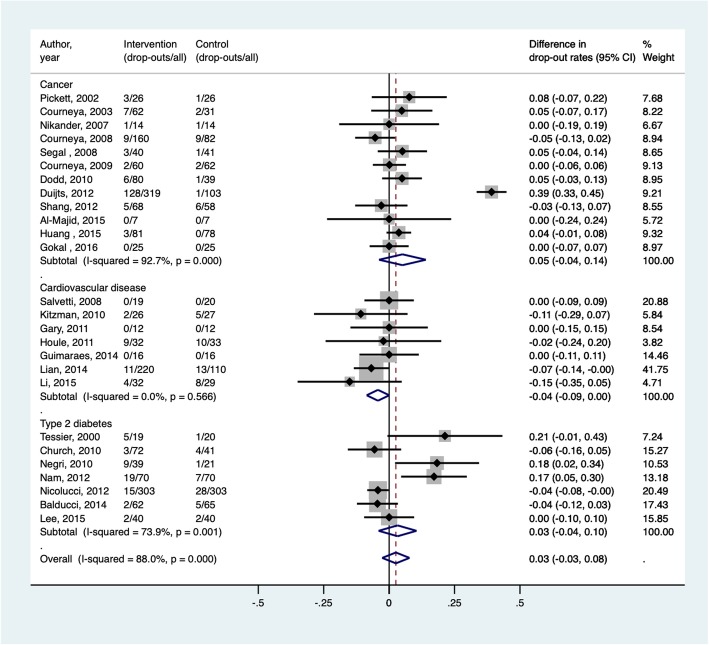
Overall, the un-weighted average adherence rate was 77% and the dropout rate was 7.0%. No significant differences were found between the three chronic diseases. The pattern of data merely suggest that cancer patients had greater variability in adherence and dropout (adherence: min. = 30.1%, max. = 92.9%; dropout: min. = 0%, max. = 40.1%), compared to CVD (adherence: min. = 80.9%, max. = 100%; dropout: min. = 0%, max. = 28.1%) and diabetes (adherence: min. = 48.6%, max. = 100%; dropout: min. = 0%, max. = 27.1%). We also did not see differences in adherence rates between strictly clinic-based (74% [95% CI, 0.65, 0.82]) and home-based (80% [95% CI, 0.65, 0.91]) aerobic exercise programs. Fig. 3 reports the meta-analysis estimates on the pooled difference in dropout rates between the intervention and control group for all studies and studies stratified by disease type (i.e., cancer, CVD, and diabetes). The estimated pooled difference in dropout rates based on all studies who reported dropout rates for both the intervention and control groups (n = 26) was 0.03 (95% confidence interval [CI] = − 0.03, 0.08), denoting a lack of difference in dropout rates between intervention and control groups. Subgroup meta-analysis revealed no statistical significance as well. The estimated pooled differences in dropout rates based on studies with cancer (n = 12), CVD (n = 7), and type 2 diabetes (n = 7) patients were 0.05 (95% CI [− 0.04, 0.14]; I2 = 92.7%), 0.04 (95% CI [− 0.09, 0.00]; I2 = 0.0%), and 0.03 (95% CI [− 0.04, 0.10], I2 = 73.9%). The I2 did show substantial heterogeneity for dropout among the sub-diseases. Meta-regression revealed no difference in the differential dropout rates between intervention and control groups across studies stratified by disease type, intervention duration, ITT status, age, or intervention setting. The p-values for the Begg’s test and Egger’s test were 0.13 and 0.85, respectively, denoting a lack of publication bias.
Fig. 3.
Forest plot of meta-analysis estimates on the pooled difference in dropout rates between the intervention and control group for all studies and studies stratified by disease type
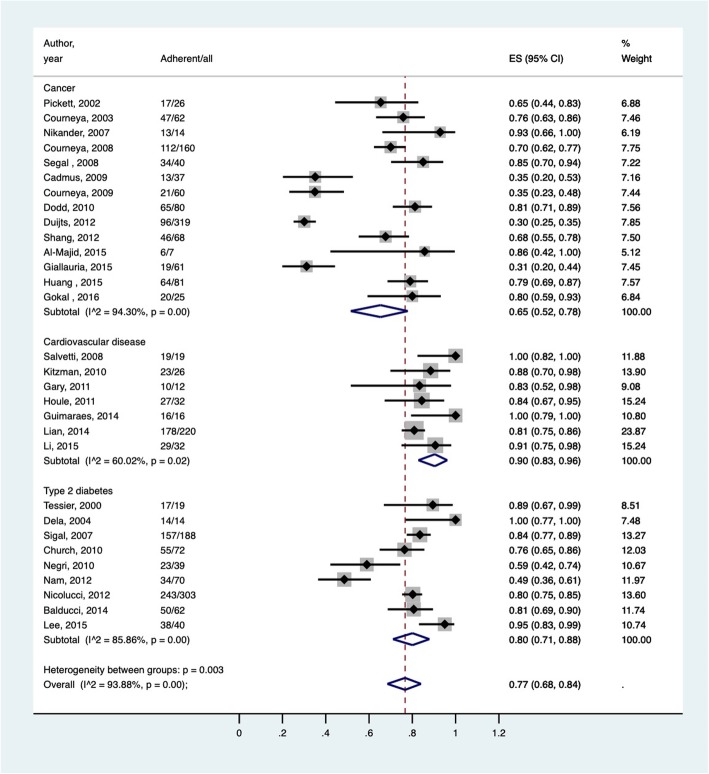
Figure 4 reports the meta-analysis estimates on the pooled adherence rate in the intervention group for all studies and studies stratified by disease type. The estimated pooled adherence rate based on all studies (n = 30) was 0.77 (95% CI = 0.68, 0.84) in the intervention group. The estimated pooled adherence rates for patients in the intervention groups of the cancer (n = 14), CVD (n = 7), and type 2 diabetes (n = 9) studies were 0.65 (95% CI [0.52, 0.78]; I2 = 94.30%), 0.90 (95% CI [0.83, 0.96]; I2 = 60.02%), and 0.80 (95% CI [0.71, 0.88]; I2 = 85.86%), respectively. The I2 showed substantial heterogeneity among adherence rates for the three targeted diseases. Meta-regression found no difference in the pooled adherence rate in the intervention group across studies stratified by disease type, intervention duration, ITT status, age, or intervention setting. The p-values for the Begg’s test and Egger’s test were 0.62 and 0.13, respectively, denoting a lack of publication bias.
Fig. 4.
Forest plot of meta-analysis estimates on the pooled adherence rate (proportion) in the intervention group for all studies and studies stratified by disease type
Discussion
Individuals living with chronic disease must cope with a plethora of unique barriers to exercise (e.g., disease-specific symptoms, comorbidities, fatigue) that are irrelevant for healthy populations. The purpose of this systematic review and meta-analysis was to examine the potential differences in adherence and dropout rates of aerobic physical activity interventions among people with chronic disease. We hypothesized that cancer would have the lowest adherence rates, based upon the low percentage of those with cancer who follow aerobic physical activity recommendations and given the known long-term effects of cancer treatment [21]. Contrary to our hypothesis, no statistically significant differences were found between the three chronic diseases. In addition, we did not see differences in adherence rates between strictly clinic-based (74% [95% CI, 0.65, 0.82]) and home-based (80% [95% CI, 0.65, 0.91]) aerobic exercise programs. These findings suggest that home-based programs may be just as feasible and perhaps, equally engaging, as programs designed with more professional supervision in rehabilitation clinics and research settings.
Overall, adherence to the exercise prescription (e.g. meeting aerobic physical activity guidelines) was 77%. It is important to note that nearly half (43.3%) of adherence data reported herein was not based on ITT (calculations only used data from those who completed studies). Failure to adopt ITT methods can inflate primary outcomes in RCTs [26], although ITT status did not appear to contribute to substantive differences in adherence across chronic conditions. The results suggest adherence to the physical activity guidelines is highly feasible among chronic disease populations, in both clinic and home-based physical activity interventions.
Given the substantial heterogeneity in adherence rates across studies within each targeted sub-population, it seems worthwhile to consider new approaches for increasing continued exercise engagement among individuals who may have particular difficulty (falling well short of the average). For example, a precision behavioral medicine approach could begin to identify “red flags,” (scores indicating below-average functioning) to facilitate decisions about the appropriate type or timing of interventions. Such an approach can be used to inform supplemental strategies focusing on the interaction among disease symptomology with physical functioning (e.g., mobility limitations) and psychological functioning (e.g., self-efficacy beliefs), all of which can change, and contributes to variability in adherence. In support of this, Pedersen and Saltin (2006) found that in general, adherence to physical activity prescriptions among patients with chronic disease are more likely to occur when they are individualized to the patient, initially supervised, and include both aerobic and strength components [27]. Interestingly, Wong, McAuley, and Trinh (2018) reviewed physical activity program preferences among cancer survivors and found a wide variation of preferences, suggesting that tailored programs may optimize program adherence [28].
Although the studies herein provide greater insight into aerobic physical activity adherence and dropout rates among chronic disease populations, insufficient information is available to explain our null findings. Many studies have targeted exercise determinants, yet evidence has not pointed to a consistent set of factors associated with adherence to physical activity guidelines or exercise programs. For example, using a broad framework incorporating many social and ecological factors, Kampshoff et al. [29] found that only one’s past exercise experience was associated with adherence among cancer survivors. Few systematic reviews exist, focused on determinants of exercise adherence for people with CVD and diabetes. Daly et al. [30] identified demographic factors as well as perceived benefits and leisure-time physical activity to be associated with non-adherence among cardiac rehabilitation patients. However, the authors pointed out that there were many methodological limitations of the studies in their sample (particularly a lack of randomized controlled designs), making it unclear as to which factors are associated with adherence levels among people with CVD. Allen [31] conducted a review examining exercise adherence in populations with diabetes and found self-efficacy measures to be predictive of exercise initiation and maintenance. However, with most studies included in the review, exercise was not the primary outcome. Rather the primary interventions were self-care regimens that included an exercise monitoring component. The limited research in this area and the variability among studies that do exist make it difficult to assess the most robust determinants of physical activity adherence among chronic disease populations.
Although it is unclear which factors reliably contribute to exercise adherence, specifically among people living with chronic disease, some factors seem to be unrelated (exercise modality, location). For example, Yang et al. [32] compared the effectiveness of aerobic and resistance exercise in populations with diabetes and found no evidence that either modality resulted in more favorable health outcomes. Yang et al. concluded that instead of focusing on the most preferred type of exercise, there should be a greater emphasis on getting chronic disease populations to remain physically active. In two other reviews of cardiac rehabilitation patients’ program adherence, Anderson et al. [33] and Dalal et al. [34] found that home- and center-based programs were equally effective in improving health-related quality of life. Anderson et al. also found programs that included self-regulatory factors (i.e. self-monitoring, action planning) resulted in the greatest levels of program adherence. Together, our findings coupled with prior research underscore the problem of exercise adherence. Perhaps a more patient-centered perspective and targeting self-regulatory deficiencies and strengths may benefit people with chronic conditions more so than generic exercise interventions designed to overcome common barriers.
There are several limitations that should be considered when interpreting the results of this study and should be addressed in future research. First, the exercise prescriptions varied in duration, intensity and complexity. In addition, this study only targeted trials lasting at least three months (again, for consistency purposes, to align with established data regarding exercise program dropout). This resulted in only 30 studies being used for the review and limited our power to test potential moderating characteristics of sample attributes and trial design.
Conclusion
The findings of this review suggest that among people with different chronic conditions participating in aerobic physical activity interventions, there is consistency in the extent to which they are non-adherent. The overall rate of adherence was 77% to the physical activity programs, and the dropout rate was 7%, suggesting that people with chronic conditions are capable of sustaining physical activity for 3+ months, under different degrees of supervision, at levels sufficient for health benefits. Future researchers and healthcare providers should continue to develop adherence-promotion strategies that account for the shared barriers across chronic disease populations, as well as the known variability within these sub-populations.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CI
confidence interval
- CINAHL
Cumulative Index to Nursing and Allied Health Literature
- CVD
cardiovascular disease
- ITT
intention-to-treat
- MET
metabolic equivalent task
- PRISMA
preferred reporting items for systematic review and meta-analysis
- PRISMA-P
preferred reporting items for systematic review and meta-analysis protocols
- RCT
randomized controlled trial
Authors’ contributions
TB carried out the data collection, study design, data analysis and interpretation, and manuscript drafting. MJ carried out the data analysis and interpretation. RA carried out the data analysis and interpretation. LT carried out the data interpretation and manuscript reviewing. MM carried out the data interpretations and manuscript reviewing. SPM carried out the study conceptualization, study design, data interpretation, and manuscript drafting. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ward BW, Schiller JS, Goodman RA. Peer reviewed: multiple chronic conditions among U.S. adults: a 2012 update. Prev Chronic Dis. 2014;11. [DOI] [PMC free article] [PubMed]
- 2.Stevinson C, Lawlor DA, Fox KR. Exercise interventions for cancer patients: systematic review of controlled trials. Cancer Causes Control. 2004;15(10):1035–1056. doi: 10.1007/s10552-004-1325-4. [DOI] [PubMed] [Google Scholar]
- 3.Taylor RS, Brown A, Ebrahim S, et al. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. Am J Med. 2004;116(10):682–692. doi: 10.1016/j.amjmed.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Zanuso S, Jimenez A, Pugliese G, Corigliano G, Balducci S. Exercise for the management of type 2 diabetes: a review of the evidence. Acta Diabetol. 2010;47(1):15–22. doi: 10.1007/s00592-009-0126-3. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC). Physical activity and health. https://www.cdc.gov/physicalactivity/basics/pa-health/index.htm#ReducedCancer. Accessed December 14, 2018.
- 6.Bize R, Johnson JA, Plotnikoff RC. Physical activity level and health-related quality of life in the general adult population: a systematic review. Prev Med. 2007;45(6):401–415. doi: 10.1016/j.ypmed.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 7.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 8.Hair B, Hayes S, Tse C, Bell M, Olshan A. Racial differences in physical activity among breast cancer surviviors: implications for breast cancer care. Cancer. 2014;120(14):2174–2182. doi: 10.1002/cncr.28630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valero-Elizondo J, Salami JA, Osondu CU, et al. Economic impact of moderate-vigorous physical activity among those with and without established cardiovascular disease: 2012 medical expenditure panel survey. J Am Heart Assoc. 2016;5(9):e003614. doi: 10.1161/JAHA.116.003614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janevic MR, McLaughlin SJ, Connell CM. Overestimation of physical activity among a nationally representative sample of underactive individuals with diabetes. Med Care. 2012;50(5):441–445. doi: 10.1097/MLR.0b013e3182422a52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eijsvogels TM, Thompson PD. Exercise is medicine: at any dose? Journal of American Medical Association. 2015;314(18):1915–1916. doi: 10.1001/jama.2015.10858. [DOI] [PubMed] [Google Scholar]
- 13.Naci H, Ioannidis JP. Comparative effectiveness of exercise and drug interventions on mortality outcomes: metaepidemiological study. The British Medical Journal. 2013;347:f5577. doi: 10.1136/bmj.f5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conn VS, Valentine JC, Cooper HM. Interventions to increase physical activity among aging adults: a meta-analysis. Ann Behav Med. 2002;24(3):190–200. doi: 10.1207/S15324796ABM2403_04. [DOI] [PubMed] [Google Scholar]
- 15.Gerritsen Jasper K W, Vincent Arnaud J P E. Exercise improves quality of life in patients with cancer: a systematic review and meta-analysis of randomised controlled trials. British Journal of Sports Medicine. 2015;50(13):796–803. doi: 10.1136/bjsports-2015-094787. [DOI] [PubMed] [Google Scholar]
- 16.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. Journal of American Medical Association. 2005;293(20):2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 17.Anderson L, Oldridge N, Thompson DR, Zwisler AD, Rees K, Martin N, Taylor RS. Exercise-based cardiac rehabilitation for coronary heart disease: Cochrane systematic review and meta-analysis. J Am Coll Cardiol. 2016;67(1):1–12. doi: 10.1016/j.jacc.2015.10.044. [DOI] [PubMed] [Google Scholar]
- 18.Umpierre D, Ribeiro PA, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. Journal of American Medical Association. 2011;305(17):1790–1799. doi: 10.1001/jama.2011.576. [DOI] [PubMed] [Google Scholar]
- 19.Hu G, Jousilahti P, Barengo NC, Qiao Q, Lakka TA, Tuomilehto J. Physical activity, cardiovascular risk factors, and mortality among Finnish adults with diabetes. Diabetes Care. 2005;28(4):799–805. doi: 10.2337/diacare.28.4.799. [DOI] [PubMed] [Google Scholar]
- 20.Pedersen BK, Saltin B. Exercise as medicine–evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. 2015;25(S3):1–72. doi: 10.1111/sms.12581. [DOI] [PubMed] [Google Scholar]
- 21.Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(1):4–10. doi: 10.1634/theoncologist.12-S1-4. [DOI] [PubMed] [Google Scholar]
- 22.Dishman RK. Increasing and maintaining exercise and physical activity. Behav Ther. 1991;22(3):345–378. doi: 10.1016/S0005-7894(05)80371-5. [DOI] [Google Scholar]
- 23.Mullen SP. Perceptions of change and certainty regarding the self-as-exerciser: a multistudy report. Journal of Sport and Exercise Psychology. 2011;33(5):710–733. doi: 10.1123/jsep.33.5.710. [DOI] [PubMed] [Google Scholar]
- 24.von Gruenigen VE, Courneya KS, Gibbons HE, Kavanagh MB, Waggoner SE, Lerner E. Feasibility and effectiveness of a lifestyle intervention program in obese endometrial cancer patients: a randomized trial. Gynecol Oncol. 2008;109(1):19–26. doi: 10.1016/j.ygyno.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry. 2002;59(10):877–883. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- 27.Pedersen BK, Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports. 2006;16(S1):3–63. doi: 10.1111/j.1600-0838.2006.00520.x. [DOI] [PubMed] [Google Scholar]
- 28.Wong JN, McAuley E, Trinh L. Physical activity programming and counseling preferences among cancer survivors: a systematic review. Int J Behav Nutr Phys Act. 2018;15(1):48. doi: 10.1186/s12966-018-0680-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kampshoff CS, Jansen F, van Mechelen W, May AM, Brug J, Chinapaw MJ, Buffart LM. Determinants of exercise adherence and maintenance among cancer survivors: a systematic review. Int J Behav Nutr Phys Act. 2014;11(1):80. doi: 10.1186/1479-5868-11-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daly J, Sindone AP, Thompson DR, Hancock K, Chang E, Davidson P. Barriers to participation in and adherence to cardiac rehabilitation programs: a critical literature review. Prog Cardiovasc Nurs. 2002;17(1):8–17. doi: 10.1111/j.0889-7204.2002.00614.x. [DOI] [PubMed] [Google Scholar]
- 31.Allen NA. Social cognitive theory in diabetes exercise research: an integrative literature review. The Diabetes Educator. 2004;30(5):805–819. doi: 10.1177/014572170403000516. [DOI] [PubMed] [Google Scholar]
- 32.Yang Z, Scott CA, Mao C, Tang J, Farmer AJ. Resistance exercise versus aerobic exercise for type 2 diabetes: a systematic review and meta-analysis. Sports Med. 2014;44(4):487–499. doi: 10.1007/s40279-013-0128-8. [DOI] [PubMed] [Google Scholar]
- 33.Anderson L, Taylor RS. Cardiac rehabilitation for people with heart disease: an overview of Cochrane systematic reviews. Int J Cardiol. 2014;177(2):348–361. doi: 10.1016/j.ijcard.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 34.Dalal HM, Zawada A, Jolly K, Moxham T, Taylor RS. Home based versus Centre based cardiac rehabilitation: Cochrane systematic review and meta-analysis. Br Med J. 2010;340:b5631. doi: 10.1136/bmj.b5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gokal K, Wallis D, Ahmed S, Boiangiu I, Kancherla K, Munir F. Effects of a self-managed home-based walking intervention on psychosocial health outcomes for breast cancer patients receiving chemotherapy: a randomised controlled trial. Support Care Cancer. 2016;24(3):1139–1166. doi: 10.1007/s00520-015-2884-5. [DOI] [PubMed] [Google Scholar]
- 36.Huang HP, Wen FH, Tsai JC, et al. Adherence to prescribed exercise time and intensity declines as the exercise program proceeds: findings from women under treatment for breast cancer. Support Care Cancer. 2015;23(7):2061–2071. doi: 10.1007/s00520-014-2567-7. [DOI] [PubMed] [Google Scholar]
- 37.Cadmus LA, Salovey P, Yu H, Chung G, Kasl S, Irwin ML. Exercise and quality of life during and after treatment for breast cancer: results of two randomized controlled trials. Psycho-Oncology: Journal of the Psychological, Social and Behavioral Dimensions of Cancer. 2009;18(4):343–352. doi: 10.1002/pon.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Courneya KS, Sellar CM, Stevinson C, et al. Randomized controlled trial of the effects of aerobic exercise on physical functioning and quality of life in lymphoma patients. Proc Am Soc Clin Oncol. 2009. [DOI] [PubMed]
- 39.Segal RJ, Reid RD, Courneya KS, et al. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J Clin Oncol. 2009;27(3):344–351. doi: 10.1200/JCO.2007.15.4963. [DOI] [PubMed] [Google Scholar]
- 40.Al-Majid S, Wilson LD, Rakovski C, Coburn JW. Effects of exercise on biobehavioral outcomes of fatigue during cancer treatment: results of a feasibility study. Biological Research for Nursing. 2015;17(1):40–48. doi: 10.1177/1099800414523489. [DOI] [PubMed] [Google Scholar]
- 41.Courneya KS, McKenzie DC, Mackey JR, et al. Moderators of the effects of exercise training in breast cancer patients receiving chemotherapy. Cancer. 2008;112(8):1845–1853. doi: 10.1002/cncr.23379. [DOI] [PubMed] [Google Scholar]
- 42.Dodd MJ, Cho MH, Miaskowski C, et al. A randomized controlled trial of home-based exercise for cancer-related fatigue in women during and after chemotherapy with or without radiation therapy. Cancer Nurs. 2010;33(4):245–257. doi: 10.1097/NCC.0b013e3181ddc58c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duijts SF, van Beurden M, Oldenburg HS, et al. Efficacy of cognitive behavioral therapy and physical exercise in alleviating treatment-induced menopausal symptoms in patients with breast cancer: results of a randomized, controlled, multicenter trial. J Clin Oncol. 2012;30(33):4124–4133. doi: 10.1200/JCO.2012.41.8525. [DOI] [PubMed] [Google Scholar]
- 44.Giallauria F, Gentile M, Chiodini P, et al. Exercise training reduces high mobility group box-1 protein levels in women with breast cancer: findings from the DIANA-5 study. Monaldi Arch Chest Dis. 2015;82(2):61–67. doi: 10.4081/monaldi.2014.45. [DOI] [PubMed] [Google Scholar]
- 45.Nikander R, Sievänen H, Ojala K, et al. Effect of a vigorous aerobic regimen on physical performance in breast cancer patients—a randomized controlled pilot trial. Acta Oncol. 2007;46(2):181–186. doi: 10.1080/02841860600833145. [DOI] [PubMed] [Google Scholar]
- 46.Pickett M, Mock V, Ropka ME, Cameron L, Coleman M, Podewils L. Adherence to moderate-intensity exercise during breast cancer therapy. Cancer Pract. 2002;10(6):284–292. doi: 10.1046/j.1523-5394.2002.106006.x. [DOI] [PubMed] [Google Scholar]
- 47.Shang J, Wenzel J, Krumm S, Griffith K, Stewart K. Who will drop out & who will drop in, exercise adherence in a RCT among patients receiving active cancer treatment. Cancer Nurs. 2012;35(4):312–322. doi: 10.1097/NCC.0b013e318236a3b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Courneya K, Friedenreich C, Quinney H, Fields A, Jones L, Fairey A. A randomized trial of exercise and quality of life in colorectal cancer survivors. European Journal of Cancer Care. 2003;12(4):347–357. doi: 10.1046/j.1365-2354.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- 49.Lian XQ, Zhao D, Zhu M, et al. The influence of regular walking at different times of day on blood lipids and inflammatory markers in sedentary patients with coronary artery disease. Prev Med. 2014;58:64–69. doi: 10.1016/j.ypmed.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 50.Li X, Xu S, Zhou L, Li R, Wang J. Home-based exercise in older adults recently discharged from the hospital for cardiovascular disease in China: randomized clinical trial. Nurs Res. 2015;64(4):246–255. doi: 10.1097/NNR.0000000000000102. [DOI] [PubMed] [Google Scholar]
- 51.Salvetti XM, Oliveira JA, Servantes DM, Vincenzo de Paola AA. How much do the benefits cost? Effects of a home-based training programme on cardiovascular fitness, quality of life, programme cost and adherence for patients with coronary disease. Clin Rehabil. 2008;22(10–11):987–996. doi: 10.1177/0269215508093331. [DOI] [PubMed] [Google Scholar]
- 52.Gary RA, Cress ME, Higgins MK, Smith AL, Dunbar SB. Combined aerobic and resistance exercise program improves task performance in patients with heart failure. Arch Phys Med Rehabil. 2011;92(9):1371–1381. doi: 10.1016/j.apmr.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guimaraes GV, de Barros Cruz LG, Fernandes-Silva MM, Dorea EL, Bocchi EA. Heated water-based exercise training reduces 24-hour ambulatory blood pressure levels in resistant hypertensive patients: a randomized controlled trial (HEx trial) Int J Cardiol. 2014;172(2):434–441. doi: 10.1016/j.ijcard.2014.01.100. [DOI] [PubMed] [Google Scholar]
- 54.Houle J, Doyon O, Vadeboncoeur N, Turbide G, Diaz A, Poirier P. Innovative program to increase physical activity following an acute coronary syndrome: randomized controlled trial. Patient Educ Couns. 2011;85(3):e237–e244. doi: 10.1016/j.pec.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 55.Kitzman DW, Brubaker PH, Morgan TM, Stewart KP, Little WC. Exercise training in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. Circ Heart Fail. 2010;3(6):659–667. doi: 10.1161/CIRCHEARTFAILURE.110.958785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee SF, Pei D, Chi MJ, Jeng C. An investigation and comparison of the effectiveness of different exercise programmes in improving glucose metabolism and pancreatic β cell function of type 2 diabetes patients. Int J Clin Pract. 2015;69(10):1159–1170. doi: 10.1111/ijcp.12679. [DOI] [PubMed] [Google Scholar]
- 57.Church TS, Blair SN, Cocreham S, et al. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. J Am Med Assoc. 2010;304(20):2253–2262. doi: 10.1001/jama.2010.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dela F, von Linstow ME, Mikines KJ, Galbo H. Physical training may enhance β-cell function in type 2 diabetes. American Journal of Physiology-Endocrinology and Metabolism. 2004;287(5):E1024–E1031. doi: 10.1152/ajpendo.00056.2004. [DOI] [PubMed] [Google Scholar]
- 59.Balducci S, Vulpiani MC, Pugliese L, et al. Effect of supervised exercise training on musculoskeletal symptoms and function in patients with type 2 diabetes: the italian diabetes exercise study. Acta Diabetol. 2014;51(4):647–654. doi: 10.1007/s00592-014-0571-5. [DOI] [PubMed] [Google Scholar]
- 60.Negri C, Bacchi E, Morgante S, et al. Supervised walking groups to increase physical activity in type 2 diabetic patients. Diabetes Care. 2010;33(11):2333–2335. doi: 10.2337/dc10-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nicolucci A, Balducci S, Cardelli P, et al. Relationship of exercise volume to improvements of quality of life with supervised exercise training in patients with type 2 diabetes in a randomised controlled trial: the italian diabetes and exercise study (IDES) Diabetologia. 2012;55(3):579–588. doi: 10.1007/s00125-011-2425-9. [DOI] [PubMed] [Google Scholar]
- 62.Sigal RJ, Kenny GP, Boulé NG, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147(6):357–369. doi: 10.7326/0003-4819-147-6-200709180-00005. [DOI] [PubMed] [Google Scholar]
- 63.Nam S, Dobrosielski DA, Stewart KJ. Predictors of exercise intervention dropout in sedentary individuals with type 2 diabetes. Journal of Cardiopulmonary Rehabilitation and Prevention. 2012;32(6):370–378. doi: 10.1097/HCR.0b013e31826be485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tessier D, Ménard J, Fülöp T, et al. Effects of aerobic physical exercise in the elderly with type 2 diabetes mellitus. Arch Gerontol Geriatr. 2000;31(2):121–132. doi: 10.1016/S0167-4943(00)00076-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.