Summary
Background
Long non-coding RNA growth arrest-specific 5 (GAS5) is deregulated in many cancers because of its role in cell growth arrest and apoptosis. Additionally, GAS5 interacts with glucocorticoid receptor, making it a potential pharmacotranscription marker of glucocorticoid (GC) therapy. In this study, we aimed at analysing GAS5 expression in the remission induction therapy phase of childhood acute lymphoblastic leukemia (ALL), in which GCs are mandatorily used, and to correlate it with therapy response.
Methods
GAS5 expression was measured in peripheral blood mononuclear cells taken from 29 childhood ALL patients at diagnosis, on day 15 and day 33 of remission induction therapy using RT-qPCR methodology.
Results
Our results have shown interindividual differences in GAS5 expression at all time points. For each ALL patient, GAS5 expression was higher on day 15 in comparison to its level at diagnosis (p<0.0005). On day 33, the level of GAS5 expression decreased in comparison with day 15 (p<0.0005), but it was still significantly higher than at diagnosis for the majority of patients (p=0.001). Patients whose number of blasts on day 8 was below 100 per μL of peripheral blood had a higher GAS5 expression at diagnosis (p=0.016), and lower ratio day 15/diagnosis (p=0.009).
Conclusions
Our results suggest that the expression level of GAS5 could be a potential marker of therapy response in remission induction therapy of childhood ALL.
Keywords: childhood ALL, GAS5, glucocorticoid drugs, long non-coding RNA, pharmacotranscription
Kratak sadržaj
Uvod
Ekspresija duge nekodirajuće RNK GAS5 je izmenjena u mnogim kancerima zbog njene uloge u apoptozi i inhibiciji rasta ćelije. GAS5 interaguje sa glukokortikoidnim receptorom, što je čini potencijalnim farmakotranskripcionim markerom značajnim za glukokortikoidnu terapiju. Naš cilj u ovoj studiji je bio da analiziramo ekspresiju GAS5 tokom indukcione terapije kod dečje akutne limfoblastne leukemije (ALL), u kojoj se koriste glukokortikoidni lekovi, i da te rezultate povežemo sa odgovorom na terapiju.
Metode
Nivo ekspresije GAS5 u mononuklearnim ćelijama periferne krvi izolovanih od 29 dece obolelih od ALL, određen je metodologijom RT-qPCR, i to u momentu dijagnoze, 15. i 33. dana indukcione terapije.
Rezultati
Naši rezultati su pokazali da postoje interindividualne razlike u ekspresiji GAS5 kod pacijenata, i to u svim analiziranim tačkama. Kod svakog ALL pacijenta GAS5 ekspresija je 15. dana bila viša u odnosu na ekspresiju u momentu dijagnoze (p < 0,0005). Nivo ekspresije GAS5 je 33. dana bio niži u poređenju sa 15. danom (p < 0,0005), ali je i dalje bio značajno viši u odnosu na momenat dijagnoze kod većine pacijenata (p = 0,001). Pacijenti čiji je broj blasta u perifernoj krvi 8. dana bio ispod 100 po mikrolitru periferne krvi, imali su viši nivo ekspresije GAS5 (p = 0,016) i niži odnos ekspresija merenih 15. dana i u momentu dijagnoze (p = 0,009).
Zaključak
Naši rezultati ukazuju da bi nivo ekspresije GAS5 mogao da bude marker terapijskog odgovora u indukcionoj terapiji kod dece obolele od ALL.
Ključne reči: dečja ALL, duga nekodirajuća RNK, farmakotranskripcija, GAS5, glukokortikoidni lekovi
Introduction
Among pediatric malignancies, acute lymphoblastic leukemia (ALL) is the most common hematological and overall malignancy, contributing to 30% of diagnosed cancers and around 80% of all pediatric leukemias. Pediatric ALL is a malignancy with one of the highest cure rates, achieved by treating the patients with the internationally recognised treatment protocols like the Berlin-Frankfurt-Munster (BFM) protocol (1, 2, 3). Risk stratification of ALL is para mount in choosing the effective treatment strategy. Hence, risk stratification is already performed on the day of diagnosis according to the BFM protocol, as well as on other well-defined checkpoints, leading to adjustment of the therapy protocol accordingly.
The first part of the BFM protocol is the remission induction therapy. This phase lasts for 39 days, and its goal is to lower a number of lymphoblasts in the bone marrow and peripheral blood. There are three check points during the course of the remission induction therapy phase: day 8, day 15 and day 33. On day 8 of treatment, blast concentration in peripheral blood is measured, and a patient is put in the high-risk category if the number of blasts remains above 1000 per μL. Percentage of blasts in bone marrow on day 15 and 33 is also used for risk stratification, as a way of measuring the minimal residual disease (MRD). In the remission induction therapy, the patient is administered with prednisone, vincristine, daunorubicin, L-asparaginase and methotrexate intrathecally. Prednisone, a synthetic glucocorticoid (GC), is administrated throughout the remission induction therapy phase, and during the first 8 days, it is the only systemically administered therapeutic.
GCs are commonly used for the treatment of inflammatory, autoimmune diseases, pediatric leukemia and after transplantation (4). GCs are drugs which act through binding to the glucocorticoid receptor (GR) (5). GCs, when bound to the ligand binding domain (LBD) of GR, activate GR, the receptor dimerises and, through binding to the GC responsive elements in DNA, upregulate the expression of anti-inflammatory proteins, like lipocortin-1 and IκBα. On the other hand, it downregulates the expression of numerous cytokines, like IL-2, IL-4, IL-6, IL-10, etc. (6). GCs can also affect inflammation by binding to a key transcription factor in immune response and cytokine production, NF-κB, thus preventing immune response stimulation (7). Last, but not the least, GCs inhibit proliferation and promote apoptosis in immune cells (8). Due to these effects, GCs are essential in the remission induction therapy of any ALL protocol, since they are the most important drugs in lowering the number of lymphoblasts (9).
Even though the cure rate of ALL in childhood is high, around 15–20% of patients experience relapse which often leads to the lethal outcome (10). The mechanisms which contribute to an unfavourable outcome are still unclear. Implementation of pharmacogenomics in the treatment protocol could provide a solution to this problem, through therapy individualisation. Indeed, certain genetic variants are already used to guide therapy regimes of drugs used in ALL treatment. Recently, the expression level of certain RNAs has been associated with drug response, which means that measuring RNA expression could be used as a marker of drug response and could even guide therapy individualisation. If enough evidence is gathered for such RNAs, alongside pharmacogenetic markers, pharmacotranscription markers could also emerge.
Most of the genome contains transcripts which do not translate into proteins, the non-coding RNAs (ncRNAs). It was shown that there are significant interindividual differences in ncRNA expression and that some of them are involved in drug response (11). Pharmacotranscriptomics is a field which aims to create an adequate therapy for individuals based on transcriptome variations. When considering the transcriptome, numerous ncRNAs have shown to have aberrant expression or deregulation in diabetes (12), cancer (13, 14, 15) and other diseases. It has also been reported that ncRNAs are deregulated in leukemia (16, 17, 18). However, further inquiry into the role of ncRNAs in pediatric ALL is required.
Growth arrest-specific 5 (GAS5) is a long (more than 200 base pairs) non-coding RNA (lncRNA), which promotes apoptosis and halts the cell-cycle in T lymphocytes (8). Apart from its functional role as a growth arrest factor, GAS5 also imitates the glucocorticoid response element (GRE), a DNA sequence which is recognised by the DNA binding domain (DBD) of GR, acting as a decoy for GR, thus inhibiting GR’s actions (19). Thus, GAS5 is a possible prognostic and pharmacotranscription marker in childhood ALL.
Previously, GAS5 was studied in breast cancer (14), where its downregulation contributed to tumour growth and poor survival of patients. GAS5 was found deregulated in prostate cancer, and the cancer cells have shown an upregulation of GAS5, as well as resistance to androgen treatment (20). In pancreatic cancer, GAS5 was also downregulated (21). When it came to the pharmacotranscriptomic role of GAS5 in GC treatment, GAS5 was only studied in pediatric patients with inflammatory bowel disease (IBD) (22). In this study, GAS5 is recommended to be considered as a pharmacotranscription marker in pediatric IBD treatment. The pharmacotranscriptomic potential of GAS5 has not been studied in the context of pediatric leukemia.
The aim of this study is to provide an insight into the correlation between GAS5 expression levels and the clinical response of treatment during remission induction therapy phase in childhood ALL. We have measured GAS5 expression at three checkpoints of BFM protocol (at diagnosis, days 15 and 33), and correlated it with therapy response evaluated using BFM protocol parameters. In order to better characterise therapy response on day 8, we carried out additional analysis in which the cut-off value for therapy response was 100 blasts per μL in peripheral blood.
Materials and Methods
Subjects
Peripheral blood samples (n = 29) have been gathered from ALL pediatric patients from the University Children’s Hospital in Belgrade at diagnosis (day 0), on day 15 and day 33 of the remission induction therapy. The patients were diagnosed, stratified into risk groups and treated according to Berlin-Frankfurt-Munster protocols: BFM ALL IC-2002 and BFM ALL IC-2009. The therapy regimes of these two protocols did not differ throughout the remission induction therapy. Approval by the Ethics Committee of the University Children’s Hospital, University of Belgrade was obtained. Informed consent was obtained from each patient or patient’s parent or guardian. The principles of the Declaration of Helsinki were honoured during the entirety of the study’s course.
RNA isolation
Mononuclear cells were isolated from peripheral blood samples of childhood ALL patients using Ficoll-Paque Plus solution (GE Healthcare, Buckinghamshire, UK) and stored in TRI reagent solution (Ambion, TX, USA) at -80 °C. Total RNA was subsequently isolated according to manufacturer’s instruction and stored at -80 °C.
Measuring of GAS5 expression level
The level of GAS5 expression was assessed relative to GAPDH expression using the 2-ΔΔCt method. Normalised, median expression level before therapy was used as a calibrator to adjust the expression values of the other samples. To measure GAS5 expression relative to GAPDH expression, Hs03464472_m1 and Hs99999905_m1 Taqman gene expression assays were used (Applied Biosystems, Foster City, CA, USA) as well as KAPA Probe Fast qPCR Master Mix (Kapa Biosystems, MA, USA) on 7900HT Fast Real-Time PCR machine.
Statistical analysis
When analysing the correlation between continuous variables, the Spearman correlation coefficient was used (ρ). To measure the difference in GAS5 expression between different time points, Wilcoxon sign rank test was used. Association of clinical parameters of ALL patients and GAS5 expression level was assessed using the Mann-Whitney U test. The cut-off value of p=0.05 for statistical significance has been chosen. The SPSS software (IBM SPSS Statistics v.21) was the tool of choice for statistical analysis.
Results
Demographic and clinical characteristics of childhood ALL patients at diagnosis
Our sample contained 29 childhood ALL patients. There were 14 male patients (48.3%). The median age was 6.0 (interquartile range: 3.0–12.4) years. B-cell leukemia was present in 26 (89.65%) patients, while the rest were diagnosed with T-cell leukemia.
Expression of GAS5 at diagnosis, on day 15 and day 33 of remission induction therapy
First, we considered the level of GAS5 expression at diagnosis, on day 15 and day 33 irrespective of clinical characteristics of ALL patients. There were noted interindividual differences of GAS5 expression level among patients. Namely, at diagnosis, the median GAS5 expression level was 1.00 (interquartile
range 0.66–1.56). On day 15, the median GAS5 expression level was 6.94 (interquartile range 4.91–9.40). On day 33, the median GAS5 expression level was 1.75 (interquartile range 0.74–4.37).
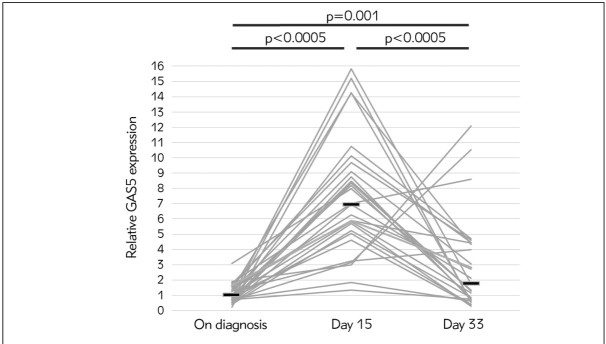
Our results have shown that for each ALL patient, GAS5 expression was higher on day 15 in comparison to its level at diagnosis (Wilcoxon sign rank test, p<0.0005). On day 33, the level of GAS5 expression decreased for all except 4 patients (Wilcoxon sign rank test, p<0.0005). Still, the level of GAS5 expression on day 33 was higher than at diagnosis for the majority of patients (Wilcoxon sign rank test, p=0.001) (Figure 1).
Figure 1.
Relative GAS5 expression measured in mononuclear cells at diagnosis (day 0), on day 15 and day 33 in 29 ALL patients. Each grey line connects relative GAS5 expression measured in one ALL patient. Dark horizontal lines denote median values of relative GAS5 expression at each time point.
As stated above, the level of GAS5 expression increased between day 15 and day 33 of the treatment for 4 patients only. We further analysed this result by comparing the clinical and demographic characteristics of this subgroup of patients with the rest of ALL patients. All 4 patients were male, B-cell leukemia and stratified into the high-risk category. Moreover, in this subgroup of 4 patients, there was a higher percentage of blasts in bone marrow on day 15 in comparison to the rest of ALL patients (Mann-Whitney test, p=0.051).
No correlation was observed between age or gender and the level of GAS5 expression at any time point of therapy.
Correlation of GAS5 expression with clinical characteristics of patients before therapy
We analysed the correlation of GAS5 expression and patients’ clinical parameters before therapy. The number of leukocytes per μL of peripheral blood on the day of diagnosis is important for prognosis and further treatment considerations. Our results showed a statistical trend towards a negative correlation between the level of GAS5 expression at diagnosis and the number of leucocytes per μL of peripheral blood at diagnosis (Spearman’s correlation, p=0.069, ρ=-0.342).
Correlation between GAS5 expression levels at diagnosis and on days 15 and 33 and response to therapy
We considered the level of GAS5 expression at diagnosis in correlation with clinical parameters indicative of therapy response. The lower number of blasts (below 100 per μL of peripheral blood) on day 8 of therapy was associated with higher GAS5 levels at diagnosis (Mann-Whitney test, p=0.016, Figure 2).
Figure 2.
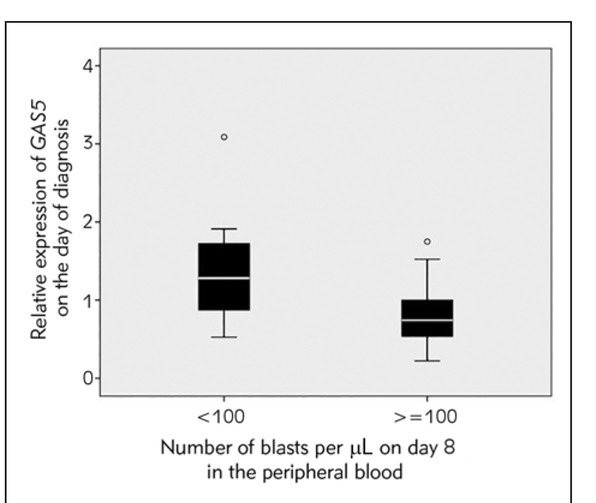
Boxplot chart showing relative expression level of GAS5 in patients’ mononuclear cells on the day of diagnosis in patients with an absolute number of blasts per μL of peripheral blood on day 8 below 100 and no less than 100 (Mann-Whitney test, p=0.016).
We also considered the ratio between the expression levels of GAS5 on day 15 and at diagnosis. ALL patients had a significantly higher ratio when the number of blasts on day 8 of therapy was above 100 (Mann-Whitney test, p = 0.009, Figure 3).
Figure 3.
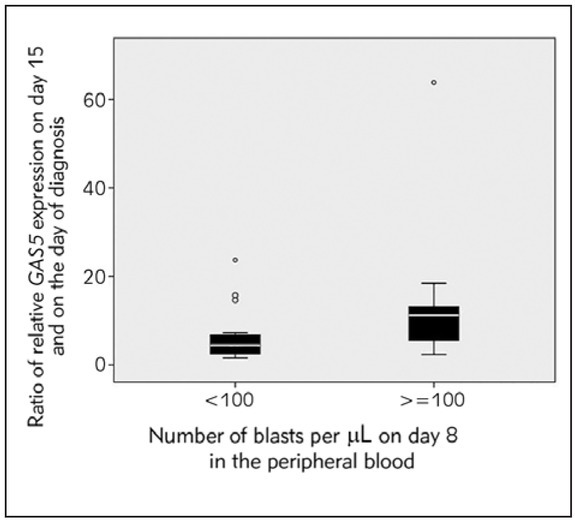
Boxplot chart showing the ratio of GAS5 expression in patients’ mononuclear cells on day 15 and at diagnosis in patients with an absolute number of blasts per μL of peripheral blood on day 8 below 100 and no less than 100 (Mann-Whitney test, p=0.009).
Expression of GAS5 at any time point was not associated with MRD on day 15 or 33 of remission induction therapy.
Discussion
Among transcriptomic markers of hematological malignancies, lncRNA GAS5 has come to the special attention of researchers and medical practitioners.
There is sporadic data on the expression of GAS5 in hematological malignancies. It was found that GAS5 was overexpressed in pediatric B-cell precursor acute lymphoblastic leukemia, specifically in high hyperdiploid (HeH) and t(1;19)(q23;p13)-positive cases (23). However, it is essential to underline that GAS5 gene is located on chromosome 1q and that its increased expression level could be a consequence of a gene dosage effect in subtypes of childhood ALL characterised by a gain of 1q through duplication or an unbalanced translocation.
In another study, the influence of GAS5 rs55829688 (T > C) variant on GAS5 expression in peripheral blood mononuclear cells (PBMC) of acute myeloid leukemia patients was demonstrated. GAS5 rs55829688 CC genotype showed increased GAS5 expression (24).
This is the first study that analysed the correlation between GAS5 expression and therapy response in childhood ALL. We have observed that GAS5 expression levels were GC treatment-dependent. More precisely, we have detected an increased level of expression of GAS5 on day 15 (continuous administration of GCs) compared to the day before the GC therapy started. We have also detected that the level of GAS5 expression significantly decreased on day 33 (GC dose significantly reduced). Changes in the expression level of GAS5 according to the dosage of GCs were observed previously in HeLa and LoVo cells (22). Although both untreated Hela (human cervical carcinoma) and LoVo (colorectal cancer) cell lines had similar GAS5 expression levels, upregulation of GAS5 was observed in LoVo cell line after GC treatment, while in HeLa cells a downregulation was detected (22). It is evident that the expression of GAS5 is not the same in different tissues or diseases (25). Since we have studied GAS5 expression in PBMC, we aimed to compare our results with similar ones. There are limited reports on GAS5 expression in PBMC treated with GCs. One of them was in vitro study of GAS5 expression in PBMC of healthy blood donors treated with GCs, in which interindividual differences were reported (26).
Interestingly, our results indicated that in all childhood ALL patients, GAS5 expression was increased after administration of GCs. It is known that GCs induce apoptosis in lymphoid cells (27) through the signalling pathway in which the cellular inhibitor of apoptosis 2 (cIAP2) is involved (28, 29, 30). Overexpression of cIAP2 results in inhibition of apoptosis. The cIAP2 gene promoter contains GREs to which GR binds and activates the gene’s transcription (28, 29). It has been shown that GAS5 hampers GR binding to cIAP2’s GREs, thus preventing cIAP2’s transcription (31). The conclusion of this study is that the increased level of GAS5 is associated with induction of apoptosis. We presume that the increased level of GAS5 expression in the initial phase of therapy, detected in all patients in our study, reflects the therapeutic effect of GCs on leukemia cells through induction of apoptosis. The signalling path way down-stream from GAS5 to cell death proteases caspase 3, 7, and 9 has been investigated and relatively elucidated (30, 31, 32). However, the upstream part of the pathway, leading from GCs binding to the LBD of GR, to GAS5, has not been studied yet. There fore, the molecular mechanism of GAS5 transcriptional induction by GCs in leukemia cells, detected in our study, needs to be clarified in future.
Besides our attempt to elaborate the mechanism of GAS5 participation in the GC therapeutic effect, our study also pointed out to the potential role of GAS5 expression as a pharmacotranscription marker in the remission induction therapy of childhood ALL.
We have observed that the expression level of GAS5 at diagnosis correlated with the number of leukocytes per μL of peripheral blood. The higher the GAS5 expression level was, the lower the number of leukocytes was. Additionally, patients with a higher expression level of GAS5 at diagnosis had a lower number of blasts in peripheral blood on day 8 of therapy. These findings point out the contribution of GAS5 to apoptosis in leukemia. We propose that GAS5 expression could be included in the pharmacotranscriptomic algorithm in the initial phase of remission induction therapy of childhood ALL patients since the level of GAS5 expression predicts GC response on day 8.
On the contrary, we have observed a correlation between the ratio of GAS5 expression level on day 15/diagnosis and the number of blasts in peripheral blood on day 8. The higher the ratio, the higher the number of blasts, indicating poor therapeutic response associated with an increase of GAS5 expression in later phases of therapy. A possible mechanism of these clinical manifestation related to GAS5 is that GAS5, imitating GRE, acts as a decoy for GR, binds it and prevents its action (19).
It is difficult to predict the role of GAS5 in leukemia due to its roles both in apoptosis and cell-cycle arrest (8). Additionally, a dual role of GAS5 in opposing cellular processes is even more prominent upon GC administration. Therefore, our results suggest that during remission induction therapy of childhood ALL, the antiapoptotic effect of GAS5, mediated through its GR-binding, becomes dominant. However, it is necessary to emphasise that in the later course of the remission induction therapy, not only GCs are administered, but also other drugs. The GAS5 expression may also be influenced by those drugs.
This is the first study of pharmacotranscriptomic potential of GAS5 in childhood ALL. We find that our study lacks the GAS5 expression level data on day 8 since that is an important checkpoint of BFM protocol. Further studies on a larger cohort of childhood ALL patients are needed to corroborate our findings.
Acknowledgement
This work was supported by Ministry of Education, Science and Technological Development, Republic of Serbia (Grant No. III41004).
List of abbreviations
- ALL
acute lymphoblastic leukemia
- cIAP2
cellular inhibitor of apoptosis 2
- GC
glucocorticoid
- GR
glucocorticoid receptor
- GAS5
growth arrest-specific 5
- IBD
inflammatory bowel disease
- LBD
ligand binding domain
- lncRNA
long non-coding RNA
- MRD
minimal residual disease
- PBMC
peripheral blood mononuclear cells.
Footnotes
Conflict of interest
Conflict of interest statement: The authors stated that they have no conflicts of interest regarding the publication of this article.
References
- 1.Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet. 2008;371:1030–43. doi: 10.1016/S0140-6736(08)60457-2. [DOI] [PubMed] [Google Scholar]
- 2.Schrappe M, Reiter A, Zimmermann M, Harbott J, Ludwig WD, Henze G. Long-term results of four consecutive trials in childhood all performed by the ALL-BFM study group from 1981 to 1995. Leukemia 2000; 14:2205–22. doi: 10.1038/sj.leu.2401973. [DOI] [PubMed] [Google Scholar]
- 3.Stary J, Zimmermann M, Campbell M, Castillo L, Dibar E, Donska S. Intensive chemotherapy for childhood acute lymphoblastic leukemia: Results of the randomized intercontinental trial ALL IC-BFM 2002. J Clin Oncol 2014; 32:174–84. doi: 10.1200/JCO.2013.48.6522. [DOI] [PubMed] [Google Scholar]
- 4.Swartz SL, Dluhy RG. Corticosteroids: Clinical pharmacology and therapeutic use. Drugs. 1978;16:238–55. doi: 10.2165/00003495-197816030-00006. [DOI] [PubMed] [Google Scholar]
- 5.Newton R. Molecular mechanisms of glucocorticoid action: What is important. Thorax. 2000;55:603. doi: 10.1136/thorax.55.7.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ebrahimi-Rad M, Khatami S, Ansari S, Jalylfar S, Valadbeigi S, Saghiri R. Adenosine deaminase 1 as a biomarker for diagnosis and monitoring of patients with acute lymphoblastic leukemia. J Med Biochem. 2018;37:128–33. doi: 10.1515/jomb-2017-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ratman D, Vanden Berghe W, Dejager L, Libert C, Tavernier J, Beck IM. How glucocorticoid receptors modulate the activity of other transcription factors: A scope beyond tethering. Mol Cell Endocrinol. 2013;380:41–54. doi: 10.1016/j.mce.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 8.Mourtada-Maarabouni M, Hedge VL, Kirkham L, Farzaneh F, Williams GT. Growth arrest in human t-cells is controlled by the non-coding RNA growth-arrest-specific transcript 5 (gas5) J Cell Sci. 2008;121:939–46. doi: 10.1242/jcs.024646. [DOI] [PubMed] [Google Scholar]
- 9.Milosevic G, Kotur N, Krstovski N, Lazic J, Zukic B, Stankovic B, Janic D, Katsila T, Patrinos PG, Pavlovic S, Dokmanovic L. Variants in TPMT, ITPA, ABCC4 and ABCB1 henes as predictors of 6-mercaptopurine induced toxicity in children with acute lymphoblastic leukemia. J Med Biochem. 2018;37:320–7. doi: 10.1515/jomb-2017-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ko RH, Ji L, Barnette P, Bostrom B, Hutchinson R, Raetz E. Outcome of patients treated for relapsed or refractory acute lymphoblastic leukemia: A therapeutic advances in childhood leukemia consortium study. J. Clin Oncol. 2010;28:648–54. doi: 10.1200/JCO.2009.22.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen QN, Wei CC, Wang ZX, Sun M. Long non-coding RNAs in anti-cancer drug resistance. Oncotarget. 2017;8:1925–36. doi: 10.18632/oncotarget.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carter G, Miladinovic B, Patel AA, Deland L, Mastorides S, Patel NA. Circulating long noncoding RNA GAS5 levels are correlated to prevalence of type 2 diabetes mellitus. BBA clinical. 2015;4:102–7. doi: 10.1016/j.bbacli.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W, Huang K, Wen F, Cui G, Guo H, Zhao S. Genetic variation of lncRNA GAS5 contributes to the development of lung cancer. Oncotarget. 2017;8:91025–29. doi: 10.18632/oncotarget.19955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mourtada-Maarabouni M, Pickard MR, Hedge VL, Farzaneh F, Williams GT. GAS5, a non-protein-coding RNA, controls apoptosis and is downregulated in breast cancer. Oncogene. 2009;28:195–208. doi: 10.1038/onc.2008.373. [DOI] [PubMed] [Google Scholar]
- 15.Wen Q, Liu Y, Lyu H, Xu X, Wu Q, Liu N. Long Non-coding RNA GAS5, which acts as a tumor suppressor via microRNA 21, regulates cisplatin resistance expression in cervical cancer. Int J Gynecol Cancer. 2017;27:1096–108. doi: 10.1097/IGC.0000000000001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calin GA, Liu CG, Ferracin M, Hyslop T, Spizzo R, Sevignani C. Ultraconserved regions encoding ncRNAs are altered in human leukemias and carcinomas. Cancer Cell. 2007;12:215–29. doi: 10.1016/j.ccr.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 17.Lammens T, Durinck K, Wallaert A, Speleman F, Van Vlierberghe P.. Long non-coding RNAs in leukemia: Biology and clinical impact. Curr Opin Hematol. 2017;24:353–8. doi: 10.1097/MOH.0000000000000354. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Zhu B, Huang Z, Chen L, He Z, Zhang H. microRNAs as biomarkers in leukemia. Stem Cell Investigation. 2014;1:11. doi: 10.3978/j.issn.2306-9759.2014.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garabedian MJ, Logan SK. Glucocorticoid receptor DNA binding decoy is a gas. Science signaling. 2010;3:pe5. doi: 10.1126/scisignal.3108pe5. [DOI] [PubMed] [Google Scholar]
- 20.Pickard MR, Mourtada-Maarabouni M, Williams GT. Long non-coding RNA GAS5 regulates apoptosis in prostate cancer cell lines. Biochim Biophys Acta. 2013;1832:1613–23. doi: 10.1016/j.bbadis.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Lu X, Fang Y, Wang Z, Xie J, Zhan Q, Deng X. Downregulation of GAS5 increases pancreatic cancer cell proliferation by regulating CDK6. Cell Tissue Res. 2013;354:891–6. doi: 10.1007/s00441-013-1711-x. [DOI] [PubMed] [Google Scholar]
- 22.Lucafo M, Di Silvestre A, Romano M, Avian A, Antonelli R, Martelossi S. Role of the long non-coding RNA growth arrest-specific 5 in glucocorticoid response in children with inflammatory bowel disease. Basic Clin Pharmacol Toxicol. 2018;122:87–93. doi: 10.1111/bcpt.12851. [DOI] [PubMed] [Google Scholar]
- 23.Gunnarsson R, Dilorenzo S, Lundin-Strom KB, Olsson L, Biloglav A, Lilljebjorn H. Mutation, methylation, and gene expression profiles in dup(1q)-positive pediatric b-cell precursor acute lymphoblastic leukemia. Leukemia. 2018;32:2117–25. doi: 10.1038/s41375-018-0092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan H, Zhang DY, Li X, Yuan XQ, Yang YL, Zhu KW. Long non-coding RNA GAS5 polymorphism predicts a poor prognosis of acute myeloid leukemia in Chinese patients via affecting hematopoietic re constitution. Leuk. Lymphoma. 2017;58:1948–57. doi: 10.1080/10428194.2016.1266626. [DOI] [PubMed] [Google Scholar]
- 25.Xin Y, Zeng L. Long non-coding RNA growth arrest-specific transcript 5 in tumor biology. Oncol Lett. 2015;10:1953–58. doi: 10.3892/ol.2015.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lucafo M, De Iudicibus S, Di Silvestre A, Pelin M, Candussio L, Martelossi S. Long noncoding RNA GAS5: A novel marker involved in glucocorticoid response. Curr Mol Med. 2015;15:94–9. doi: 10.2174/1566524015666150114122354. [DOI] [PubMed] [Google Scholar]
- 27.Frankfurt O, Rosen S T. Mechanisms of glucocorticoid-induced apoptosis in hematologic malignancies: Updates. Curr Opin Oncol. 2004;16:553–63. doi: 10.1097/01.cco.0000142072.22226.09. [DOI] [PubMed] [Google Scholar]
- 28.Hong S-Y, Yoon W-H, Park J-H, Kang S-G, Ahn J-H, Lee TH. Involvement of two NF-kb binding elements in tumor necrosis factor a-, CD40-, and epstein-barr virus latent membrane protein 1-mediated induction of the cellular inhibitor of apoptosis protein 2 gene. J Biol Chem. 2000;275:18022–28. doi: 10.1074/jbc.M001202200. [DOI] [PubMed] [Google Scholar]
- 29.Webster JC, Huber RM, Hanson RL, Collier PM, Haws TF, Mills JK. Dexamethasone and tumor necrosis factor-alpha act together to induce the cellular inhibitor of apoptosis-2 gene and prevent apoptosis in a variety of cell types. Endocrinology. 2002;143:3866–74. doi: 10.1210/en.2002-220188. [DOI] [PubMed] [Google Scholar]
- 30.Wen L-P, Madani K, Fahrni J, Duncan SR, Rosen G. Dexamethasone inhibits lung epithelial cell apoptosis induced by IFN-gamma and Fas. Journal. 1997;273:L921–9. doi: 10.1152/ajplung.1997.273.5.L921. [DOI] [PubMed] [Google Scholar]
- 31.Kino T, Hurt DE, Ichijo T, Nader N, Chrousos GP. Non-coding RNA GAS5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Science signaling. 2010;3:ra8. doi: 10.1126/scisignal.2000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deveraux QL, Reed JC. Iap family proteins – suppressors of apoptosis. Genes Dev. 1999;13:239–52. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]