Abstract
Iris yellow spot virus (IYSV) from the genus Tospovirus, family Peribunyaviridae, reduces yield in several crops, especially Allium spp. IYSV is primarily transmitted by onion thrips (Thrips tabaci), but little is known about how IYSV impacts the biology of its principal vector. In a controlled experiment, the effect of IYSV on the lifespan and fecundity of onion thrips was examined. Larvae were reared on IYSV-infected onions until pupation. Individual pupae were confined until adults eclosed, and the lifespan and total progeny produced per adult were monitored daily. Thrips were tested for the virus in reverse-transcriptase polymerase chain reaction using specific primers to confirm the presence of IYSV. Results indicated that 114 and 35 out of 149 eclosing adults tested positive (viruliferous) and negative (nonviruliferous) for IYSV, respectively. The viruliferous adults lived 1.1–6.1 d longer (average of 3.6 d) than nonviruliferous adults. Fecundity of viruliferous and nonviruliferous onion thrips was similar with 2.0 ± 0.1 and 2.3 ± 0.3 offspring produced per female per day, respectively. Fecundity for both viruliferous and nonviruliferous thrips also was significantly positively correlated with lifespan. These findings suggest that the longer lifespan of viruliferous onion thrips adults may allow this primary vector of IYSV to infect more plants, thereby exacerbating IYSV epidemics.
Keywords: onion thrips, Iris yellow spot virus, Tospovirus, lifespan, fecundity
Viruses from the genus Tospovirus, family Peribunyaviridae are economically significant plant viruses responsible for annual yield losses of many agronomic crops (Pappu et al. 2009). These viruses are transmitted by thrips (Thysanoptera), which acquire the virus during larval development and remain viruliferous until death. Tospoviruses are persistent and propagative in their vector, replicating within the thrips midgut and associated digestive organs including the salivary glands (Wijkamp et al. 1993, Birithia et al. 2013). Like many plant viruses, tospoviruses alter the biology and behavior of their insect vectors. Studies have documented positive and negative effects of these viruses on their thrips vectors (DeAngelis et al. 1993, Stumpf and Kennedy 2005, Stafford et al. 2011, Shrestha et al. 2012, Stafford-Banks et al. 2014). However, tospovirus infection tends to increase vector fitness. For example, viruliferous Frankliniella spp. typically have more offspring and longer life spans than those not infected (Maris et al. 2004, Stumpf and Kennedy 2005, Shrestha et al. 2012, Ogada et al. 2013, Zheng et al. 2014, Keough et al. 2016).
Most research on tospoviruses and thrips has focused on describing the relationship between Frankliniella spp. and tomato spotted wilt virus (e.g., DeAngelis et al. 1993, Stumpf and Kennedy 2005, Stafford et al. 2011, Shrestha et al. 2012, Stafford-Banks et al. 2014) although interactions with other important thrips vectors have been examined (Birithia et al. 2013, Chen et al. 2014, Keough et al. 2016). Iris yellow spot virus (IYSV) from the genus Tospovirus significantly reduces yield in Allium crops (Pozzer et al. 1999, Gent et al. 2006). Onion thrips (Thysanoptera: Thripidae) (Thrips tabaci Lindeman) is the primary vector of IYSV, but there is limited information on the impact of IYSV on the fitness of onion thrips. Some studies have indicated that IYSV infection does not impact the reproduction or mortality of thrips when monitored for the first week after eclosion (Inoue et al. 2010, Birithia et al. 2013); however, no studies have examined the long-term effects of IYSV on the total lifespan and production of progeny of onion thrips. Knowledge of the impact that IYSV has on the lifespan and fecundity of onion thrips could provide better insight into the epidemiology of IYSV in Allium crops. For example, a longer lifespan and increased fecundity of viruliferous thrips or both could accelerate the spread of IYSV, thereby increasing IYS disease in agricultural systems. The purpose of this study was to examine the effect of IYSV infection on the lifespan and fecundity of onion thrips. We predicted that viruliferous thrips would positively benefit from IYSV infection by living longer and producing more offspring.
Materials and Methods
Plant and Thrips Collection
Onion transplants (cv. ‘Bradley’) exhibiting typical IYSV symptoms, including straw-colored diamond-shaped lesions, were collected from an onion field in Elba, NY. All plants collected were similar in size (approximately six leaves and weighed 60 ± 10 g). Plants were free of any additional plant diseases and not treated with any insecticides or fungicides. All plants were collected early in the onion growing season (10 June 2017), when onion thrips populations are typically low to absent; therefore, infection likely occurred prior to transplantation. After collection, onion plants were transported to Cornell AgriTech in Geneva, NY, cleaned with ethanol to remove any thrips that might have been on the plants and then placed singly into thrips-proof cages (‘2120F’, BioQuip, Rancho Dominguez, CA) with a damp paper towel on the bottom of the cage. Plants were monitored for 14 d to ensure no thrips larvae emerged. These were considered our source onion plants. Onion thrips adults used in this study were acquired from a laboratory colony originally established from individuals collected from a non-IYSV-infected onion field in Elba, NY in 2017. All subsequent thrips generations were reared on cabbage, which is not a host plant for IYSV (Smith et al. 2011).
Thrips and Data Collection
Approximately 25–30 adults from the laboratory colony were placed on the source onion plants and caged on the plants in a controlled environment and maintained at 25 ± 1 °C with 60 ± 5% relative humidity and a photoperiod of 16 h light and 8 h dark. Adults laid eggs and larvae developed on these onions until pupation.
Pupae were removed from the cages and then placed singly into Falcon dishes (150 × 25 mm; Falcon, item #353025, BD, Franklin Lakes, NJ) containing a single cabbage leaf disc (5 cm diameter, ~6 cm3 volume). Cabbage is a highly desired host for onion thrips, but not a host for IYSV. Therefore, cabbage was an ideal food source and ovipositional medium for onion thrips in our study.
Observations of adult lifespan and fecundity began as soon as adults eclosed and thrips were monitored every 24 h until death. Because this was a thelytokous population of onion thrips, all individuals were female and reproduced parthenogenetically (= referred to as mother from here on). At 5-d intervals, cabbage discs in each dish were examined for larvae. Mothers were transferred to new falcon dishes containing a new cabbage disk every 5 d. Progeny were counted on each disk, and then summed to determined total progeny per mother. Mothers were deemed ‘alive’ if they moved when observed or gently prodded with a paintbrush tip. If a mother died, she was placed into a 0.5 ml centrifuge tube and stored at −80°C until tested for IYSV with reverse-transcriptase polymerase chain reaction (RT–PCR) to determine her vector status. Any mother that died within the first 24 h of observation was excluded from analysis, which resulted in a total of 149 thrips mothers monitored and tested for IYSV in this study.
IYSV Testing
All thrips (n = 149) were tested for the IYSV nucleoprotein (N) gene using RT–PCR and total RNA isolated from individual thrips using modified procedures from the Omega MicroElute RNA Kit (Omega Bio-Tek, Norcross, GA), as previously described (Leach et al. 2018).
The diagnostic primers used to detect IYSV were IYSV-N402F 5′-ACTCACCAATGTCTTCAAC-3′ and IYSV-N402R 5′-GGCTT CCTCTGGTAAGTGC-3′, which were designed from the N gene of several IYSV isolates collected in New York (Leach et al. 2018). Primers ThMCOI-F 5′-CGGGAACGGGATGAACAG-3′ and ThMCOI-R 5′-GGTCCCCTCCCC CTCTA-3′ (designed in the mitochondrial cytochrome oxidase subunit I gene sequence, GenBank accession no. DQ228494) were used in a multiplex RT–PCR to confirm the nature of onion thrips and ensure quality of RNA extracts. Nonviruliferous thrips, which were reared exclusively on cabbage, were included in RT–PCR testing to protect against false negatives. RT–PCR was carried out with the Qiagen one-step kit in a final volume of 12.5 µl and the following thermal cycling conditions: 50°C for 30 min (one cycle), 95°C for 15 min followed by 40 cycles of 94°C for 30s, 50°C for 1 min, 72°C for 1 min, and a final extension at 72°C for 10 min (BioRad ThermalCycler). RT–PCR products (402 bp for IYSV and 325 bp for onion thrips) were stained with GelRed (Biotium, Hayward, CA) following electrophoresis on 1.5% agarose gels, and then imaged using ultra-violet illumination.
Statistical Analysis
The lifespan of each mother and numbers of her offspring were analyzed using R statistical software and packages ‘lme4’ (Bates et al. 2015) and ‘emmeans’ (Lenth et al. 2016). Lifespan data were analyzed with a normal distribution, and fecundity data (number of progeny and progeny per day) were analyzed using a Poisson distribution. Vector status (viruliferous or nonviruliferous) was treated as a fixed effect, individual thrips nested by individual source plant (specific onion plant that the mother was originally reared on as a larva) as the random effect, and a weight term was included to correct for the differing sample sizes between status groups. Differences within each analysis were compared using a one-way ANOVA. Differences within each analysis were compared using least square means (P < 0.05).
Results
All symptomatic plants used in this experiment yielded onion thrips that tested both positive and negative for IYSV. Many of the mothers tested positive for IYSV (77%; n = 114) and were considered viruliferous, whereas the remainder did not (23%; n= 35) and were considered nonviruliferous. Vector status significantly impacted the lifespan of the onion thrips mothers (P = 0.02459, F1,144 = 5.02; Table 1). Viruliferous adults lived for 20.2 ± 1.6 d, which was 1.1–6.2 d longer (average of 3.6 d) than nonviruliferous adults (16.6 ± 0.9 d). Differences in survival were observed early in the data collection, as 28.6 ± 0.9% of nonviruliferous thrips died within the first 5 d, which was significantly greater than the percentage of viruliferous thrips that died at that point (19.3 ± 0.4%) (P < 0.001, F1,144 = 132.4).
Table 1.
Mean lifespan and fecundity of adult onion thrips infected (viruliferous) and not infected (nonviruliferous) with iris yellow spot virus
| Vector status | n | Mean lifespan (days) ± SE* | Mean progeny (emerged larvae) ± SE* | Mean progeny per day ± SE* |
|---|---|---|---|---|
| Viruliferous | 114 | 20.2 ± 1.6a | 40.4 ± 6.9a | 2.0 ± 0.1a |
| Nonviruliferous | 35 | 16.6 ± 0.9b | 38.2 ± 3.2a | 2.3 ± 0.3a |
Females were monitored daily until death, and total progeny per female counted. Thrips infected with IYSV were confirmed with RT–PCR.
*Significant values determined by LSMEANS at a 0.05 significance level.
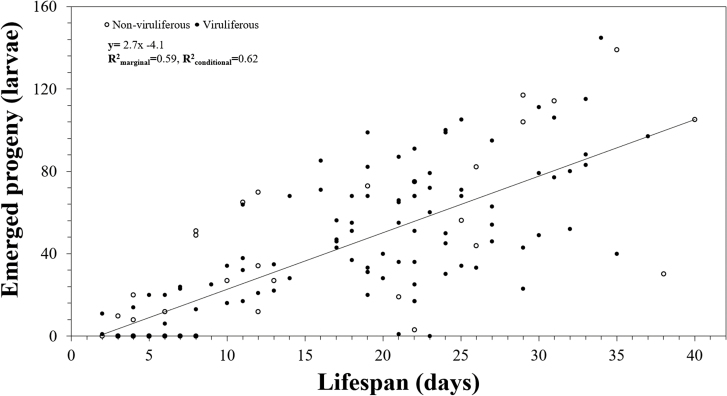
Fecundity of viruliferous and nonviruliferous thrips was similar (P > 0.05) (Table 1). Viruliferous thrips produced an average of 40.4 ± 6.9 offspring per female and nonviruliferous produced an average of 38.2 ± 3.2 offspring per female. The number of larvae produced per day was not significantly different between mothers who were or were not viruliferous, as both groups produced approximately two larvae per day (Table 1). Most thrips produced the greatest number of progeny between 7 and 21 d from adult emergence. Fecundity of thrips from both groups also was significantly positively correlated with lifespan (P < 0.001, F1,144 = 595.1; Fig. 1).
Fig. 1.
Relationship between an onion thrips mother’s lifespan and her total progeny (number offspring over lifespan) produced for mothers that were either viruliferous with iris yellow spot virus or were not viruliferous. Each data point represents a single mother and the total number of her progeny. A total of 149 thrips were monitored in this study, 114 were viruliferous and 35 nonviruliferous.
Discussion
Onion thrips were positively impacted by IYSV infection, as viruliferous thrips lived almost four days longer than those not infected. Our results contrast from previous studies that reported no significant effect of IYSV infection on onion thrips mortality, reproduction or development (Inoue et al. 2010, Birithia et al. 2013). Inoue et al. (2010) reported that onion thrips mortality, development, and reproduction were not significantly different between groups feeding on infected and healthy tissue and noted that IYSV-exposed thrips had numerically higher mortalities than unexposed thrips. Similarly, Birithia et al. (2013) found no significant difference in the mortality rates between onion thrips feeding on IYSV-infected tissue and healthy tissue (virus-free). The difference between our results and those mentioned above may be methodological. Previous studies did not confirm the vector status of thrips tested. Rather, there was an assumption that the thrips would be viruliferous after feeding for 16 h on IYSV-positive plant tissue (Inoue et al. 2010, Birithia et al. 2013). However, acquisition of tospoviruses by thrips can vary (Bautista et al. 1995, Hunter et al. 1995, Srinivasan et al. 2012, Chitturi et al. 2015). Variable virus acquisition may confound experimental results as numbers of nonviruliferous thrips may be underestimated, thereby reducing the likelihood of finding significant differences between treatment groups. In our trial, we observed that only 77% of thrips acquired IYSV after feeding on symptomatic plants during larval development; thus, larval feeding on IYSV-infected onion plants did not guarantee IYSV infection. Therefore, it was important to confirm the vector status of each thrips to correctly associate effects of a tospovirus infection with onion thrips reproduction and mortality.
Another difference in methodology between our study and those described in Birithia et al. (2013) and Inoue et al. (2010) was that onion thrips was reared on fabaceous hosts including soybean (Glycines max) and snow pea (Pisum sativum var. saccharatum). These two species are suboptimal hosts of T. tabaci compared with Alliums and Brassicas (Doederlein et al. 1993, Lewis et al. 1997). Therefore, it is possible that host plant quality may significantly impact the effect of tospoviruses on adult thrips biology, thereby masking differences in lifespan between viruliferous and nonviruliferous adults.
In our study, viruliferous thrips lived longer. This may increase the rate of IYSV spread in onion fields. While there are no studies that have documented the daily movement of an individual thrips over time, studies have shown that populations of adult thrips are very mobile (Smith et al. 2015). Thrips move readily both within and between plants (Lewis 1997) and are known to disperse long distances, in some cases hundreds of kilometers under the right environmental conditions (Laughlin 1977, Lewis 1997). Studies in New York onion fields (Elba, NY) showed that onion thrips tended to disperse short distances, but some engage in long-distance dispersal (Smith et al. 2015). A longer lifespan may provide adults with more time to disperse and feed on multiple host plants, thereby increasing the number of plants infected with IYSV, and consequently accelerating epidemics.
IYSV infection may have additional impacts on onion thrips biology. Indeed, other studies have identified many effects of tospoviruses on thrips biology and ecology including increased development time, changes in probing behaviors, and differences in dietary preferences (Stumpf and Kennedy 2005, Stafford et al. 2011, Stafford-Banks et al. 2014, Zheng et al. 2014). Further studies are needed with larger sample sizes, and different IYSV isolates and thrips populations to fully evaluate the impact of IYSV on these aspects of onion thrips biology.
Acknowledgments
We would like to thank Molly Cappiello who patiently counted and (gently) prodded our thrips. Thank you to Dr. Erika Mudrak (Cornell University, Department of Statistics) who assisted with statistical analyses. This study was funded by the New York State Onion Research and Development Program.
References Cited
- Bates D., Maechler M., Bolker B., and Walker S.. . 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67: 1–48. [Google Scholar]
- Bautista R. C., Mau R. F. L., Cho J. J., and Custer D. M.. . 1995. Potential of tomato spotted wilt Tospovirus plant hosts in Hawaii as virus reservoirs for transmission by Frankliniella occidentalis (Thysanoptera: Thripidae). Phytopath. 85: 953–958. [Google Scholar]
- Birithia R., Subramanian S., Pappu H. R., Muthomi J., and Narla R. D.. . 2013. Analysis of Iris yellow spot virus replication in vector and non-vector thrips species. Plant Pathol. 62: 1407–1414. [Google Scholar]
- Chen W. T., Tseng C. H., and Tsai C. W.. . 2014. Effect of watermelon silver mottle virus on the life history and feeding preference of Thrips palmi. PLoS One 9: e102021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitturi A., Riley D., Nischwitz C., Gitaitis R., and Srinivasan R.. . 2015. Thrips settling, oviposition and IYSV distribution on onion foliage. J. Econ. Entomol. 108: 1164–1175. [DOI] [PubMed] [Google Scholar]
- Deangelis J. D., Sether D. M., and Rossignol P. A.. . 1993. Survival, development, and reproduction in western flower Thrips (Thysanoptera: Thripidae) exposed to impatiens necrotic spot virus. J. Environ. Ent. 22: 1308–1312. [Google Scholar]
- Doederlein T. A., and Sites R. W.. . 1993. Host plant preferences of Frankliniella occidentalis and Thrips tabaci (Thysanoptera: Thripidae) for onions and associated weeds on the Southern high plains. J. Econ. Ent. 86: 1706–1713. [Google Scholar]
- Gent D. H., du Toit L. J., Fichtner S. F., Mohan S. K., Pappu H. R., and Schwartz H. F.. . 2006. Iris yellow spot virus: an emerging threat to onion bulb and seed production. Plant Dis. 90: 1468–1480. [DOI] [PubMed] [Google Scholar]
- Hunter W. B., Hsu H. T., and Lawson R. H.. . 1995. A novel method for Tospovirus acquisition by thrips. Phytopath. 85: 480–483. [Google Scholar]
- Inoue T., Murai T., and Natsuaki T.. . 2010. An effective system for detecting Iris yellow spot virus transmission by Thrips tabaci. Plant Pathol. 59: 422–428. [Google Scholar]
- Keough S., Han J., Shuman T., Wise K., and Nachappa P.. . 2016. Effects of Soybean vein necrosis virus on life history and host preference of its vector, Neohydatothrips variabilis and evaluation of vector status of Frankliniella tritici and F. fusca. Econ. Ent. 110: 133–141. [DOI] [PubMed] [Google Scholar]
- Laughlin R. 1977. The gum tree thrips (Isoneurothrips australis Bagnall). Survival at different temperatures and humidities and its relation to capacity for dispersal. Austral. J. Ecol. 2: 391–398. [Google Scholar]
- Leach A., Fuchs M., Harding R., Schmidt-Jeffris R., and Nault B. A.. . 2018. Importance of transplanted onions contributing to late-season iris yellow spot virus epidemics in New York. Plant Dis. 102: 1264–1272. [DOI] [PubMed] [Google Scholar]
- Lenth R. V. 2016. Least-squares means: the R package lsmeans. J. Stat. Softw. 69: 1–33. [Google Scholar]
- Lewis T. 1997. Thrips as crop pests. CAB International, New York, NY. [Google Scholar]
- Maris P. C., Joosten N. N., Goldbach R. W., and Peters D.. . 2004. Tomato spotted wilt virus infection improves host suitability for its vector Frankliniella occidentalis. Phytopathology 94: 706–711. [DOI] [PubMed] [Google Scholar]
- Ogada P. A., Maiss E., and Poehling H. M.. . 2013. Influence of tomato spotted wilt virus on performance and behaviour of western flower thrips (Frankliniella occidentalis). J. Appl. Entomol. 137: 488–498. [Google Scholar]
- Pappu H. R., Jones R. A., and Jain R. K.. . 2009. Global status of tospovirus epidemics in diverse cropping systems: successes achieved and challenges ahead. Virus Res. 141: 219–236. [DOI] [PubMed] [Google Scholar]
- Pozzer L., Bezerra I. C., Kormelink R., Prins M., Peters D., Resende R. O., and de Ávila A. C.. . 1999. Characterization of a tospovirus isolate of iris yellow spot virus associated with a disease in onion fields in Brazil. Plant Dis. 83: 345–350. [DOI] [PubMed] [Google Scholar]
- Shrestha A., Srinivasan R., Riley D. G., and Culbreath A. K.. . 2012. Direct and indirect effects of a thrips-transmitted Tospovirus on the preference and fitness of its vector, Frankliniella fusca. Entomol. Exp. Appl. 145: 260–271. [Google Scholar]
- Smith E. A., Ditommaso A., Fuchs M., Shelton A. M., and Nault B. A.. . 2011. Weed hosts for onion thrips (Thysanoptera: Thripidae) and their potential role in the epidemiology of Iris yellow spot virus in an onion ecosystem. Environ. Entomol. 40: 194–203. [Google Scholar]
- Smith E. A., Fuchs M., Shields E. J., and Nault B. A.. . 2015. Long-distance dispersal potential for onion thrips (Thysanoptera: Thripidae) and Iris yellow spot virus (Bunyaviridae: Tospovirus) in an Onion Ecosystem. Environ. Entomol. 44: 921–930. [DOI] [PubMed] [Google Scholar]
- Srinivasan R., Sundaraj S., Pappu H. R., Diffie S., Riley D. G., and Gitaitis R. D.. . 2012. Transmission of Iris yellow spot virus by Frankliniella fusca and Thrips tabaci (Thysanoptera: Thripidae). J. Econ. Entomol. 105: 40–47. [DOI] [PubMed] [Google Scholar]
- Stafford C. A., Walker G. P., and Ullman D. E.. . 2011. Infection with a plant virus modifies vector feeding behavior. Proc. Natl. Acad. Sci. USA 108: 9350–9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford-Banks C. A., Yang L. H., McMunn M. S., and Ullman D. E.. . 2014. Virus infection alters the predatory behavior of an omnivorous vector. Oikos. 123: 1384–1390. [Google Scholar]
- Stumpf C. F., and Kennedy G. G.. . 2005. Effects of tomato spotted wilt virus isolates, host plants, and temperature on survival, size, and development time of Frankliniella occidentalis. Entomol. Exp. Appl. 123: 139–147. [Google Scholar]
- Wijkamp I., van Lent J., Kormelink R., Goldbach R., and Peters D.. . 1993. Multiplication of tomato spotted wilt virus in its insect vector, Frankliniella occidentalis. J. Gen. Virol. 74 (Pt 3): 341–349. [DOI] [PubMed] [Google Scholar]
- Zheng X., Zhang J., Chen Y., Dong J., and Zhang Z.. . 2014. Effects of Tomato Zonate Spot Virus infection on the development and reproduction of its vector Frankliniella occidentalis (Thysanoptera: Thripidae). Fla. Entomol. 97: 549–555. [Google Scholar]