Abstract
Background
Based on the extensive biological effects of melatonin (MLT), it is beneficial to increase the MLT content in the bodies of animals at a specific physiological stage. This study was conducted to investigate the effect of a diet supplemented with rumen-protected (RP) 5-hydroxytryptophan (5-HTP) on the pineal gland and intestinal tract MLT synthesis of sheep.
Material/Methods
Eighteen Kazakh sheep were assigned randomly to 3 diet groups: control group (CT, corn-soybean meal basal diet), CT+111 group (111 mg/kg BW RP 5-HTP), and CT+222 group (222 mg/kg BW RP 5-HTP). The gene expressions of aromatic amino acid decarboxylase (AADC), arylalkylamine N-acetyltransferase (AA-NAT), hydroxyindole-O-methyltransferase (HIOMT), monoamine oxidase A (MAOA), and the intermediates of MLT synthesis were observed from the pineal gland and intestinal tract by the reverse transcription (RT)-PCR method. The 5-HTP, 5-HT, N-acetylserotonin (NAS), MLT, and 5-hydroxyindole acetic acid (5-HIAA) contents in the pineal gland and intestinal tract were analyzed by ultra-high-performance liquid chromatography–tandem mass spectrometry.
Results
The study showed that the pineal gland HIOMT expression (P<0.05), MLT (P<0.05) and 5-HIAA (P<0.05) levels in the 222 mg/kg group significantly increased compared to those in the CT and CT+111 mg/kg groups. In addition, the AADC (P<0.01) and AA-NAT (P<0.05) gene expression levels in the duodenum and jejunum were increased by the supplementation of RP 5-HTP.
Conclusions
Rumen-protected 5-hydroxytryptophan promoted melatonin synthesis in the pineal gland and intestinal tract during the natural light period.
MeSH Keywords: 5-Hydroxytryptophan, Intestines, Melatonin, Pineal Gland, Rumen, Sheep
Background
It is generally believed that simultaneous changes in the reproductive cycle and the ambient photoperiod of sheep are achieved by N-acetyl-5-methoxytryptamine (melatonin; MLT) acting on the hypothalamic–pituitary–gonadal endocrine axis system. The duration of MLT secretion and the direction of change resulted in the seasonal reproduction of sheep. Melatonin is an indoleamine hormone synthesized by the pineal gland of the vertebrate, which has extensive biological functions [1]. Melatonin synthesis is fulfilled from its precursor tryptophan (Trp), after uptake by pinealocytes and enterochromaffin cells [2]. Tryptophan is converted to 5-hydroxytryptophan (5-HTP) by the enzyme tryptophan hydroxylase. The enzyme 5-hydroxytryptophan decarboxylase (5-HTPD) acts on 5-HTP to produce 5-hydroxytryptamine (5-HT), which, catalyzed by N-acetyl-transferase (NAT), is converted to N-acetylserotonin (NAS). The activity of 5-HT is increased during the nocturnal cycle. N-acetyl-serotonin is O-methylated by hydroxyindole-O-methyltransferase (HIOMT) to produce MLT [3]. The gastrointestinal tract deserves particular attention, with regard to MLT uptake and the extrapineal site of MLT biosynthesis. It is known that the presence of MLT in bacteria including Escherichia coli may suggest a contribution by intestinal bacteria to the high amounts of the indoleamine in the gut [1].
Based on the extensive biological effects of MLT, it is of positive significance to appropriately increase the content in animals. One way to regulate the MLT content in animals is to either administer MLT directly through intravenous injection or dietary supplementation. An alternative method is to supplement or inject animals with a precursor substance of MLT (including Trp, 5-HTP, etc.). Trp increases the plasma MLT content of monogastric animal, including chickens, rats [4], and mice [5]. In contrast, Trp loading in sheep was not an effective means of elevating blood 5-HT or MLT synthesis because of their lower tryptophan hydroxylase Michaelis constant (Km) [6]. However, the serum MLT was elevated in sheep injected with 5-HTP [7,8]. Thus, the 5-HTP content availability limits MLT synthesis in sheep. In some studies on ruminants, the addition of Trp or rumen-protected TRP in the diet did not significantly affect the plasma MLT content during lactation in the daytime and nighttime. An intraperitoneal injection of 500 mg/kg body weight (BW) Trp did not significantly increase the MLT content in sheep plasma; however, an intraperitoneal injection of 5-HTP significantly increased the MLT content in sheep plasma [9]. From this, Trp as a precursor could play a role in affecting MLT synthesis and may have different effects among species. Intraperitoneal injection of 5-HTP can affect the MLT content in sheep plasma, but it is unknown if the same effect is seen through gastrointestinal administration. Because rumen microorganisms generally have a degrading effect on amino acids, there are few research reports on whether 5-HTP or rumen-protected (RP) 5-HTP will degrade in sheep rumina. Most studies have focused on the MLT content in the blood or plasma, but a large amount of MLT can be synthesized in the pineal gland and intestinal tissue. Therefore, it is very necessary to study the comprehensive organ content distribution and mechanism system.
This study aimed to investigate the administration influence of 5-HTP on pineal gland and gut MLT synthesis during the natural light period. This experimental maneuver is widely used to evaluate sheep 5-HT production and subsequent increases in MLT concentration. Tryptophan hydroxylase is considered to be the rate-limiting enzyme of the 5-HT synthesis. Administration of the 5-HT precursor 5-HTP gives rise to MLT synthesis [10,11]. The gene expressions of aromatic amino acid decarboxylase (AADC), arylalkylamine N-acetyltransferase (AA-NAT), HIOMT, and monoamine oxidase A (MAOA), as well as the intermediates of MLT synthesis, were observed from the pineal gland and intestinal tract by the RT-PCR method. The 5-HTP, 5-HT, NAS, MLT, and 5-hydroxyindole acetic acid (5-HIAA) content analysis were determined by ultra-high-performance liquid chromatography–parallel reaction monitoring–mass spectrometry (UHPLC–PRM–MS) in the pineal gland and intestinal tract. We conclude that AADC, AA-NAT, and HIOMT of pineal gland and intestinal tract in sheep are usually unsaturated with substrate, and the diurnal induction of AADC, AA-NAT, and HIOMT are responsible for the dramatic increase in MLT concentration levels over the diurnal period. This provides a research basis for the technical method of regulating MLT synthesis in ruminants by adding 5-HTP to the diet.
Material and Methods
The L-5-HTP was purchased from Wuhan Yuancheng Gongchuang Technology Co. Ltd. (Wuhan, China). Additionally, RP L-5-HTP (containing 45% L-5-HTP) was purchased from Beijing Yahe Products Co. Ltd. (Beijing, China).
Animals and design
This study was conducted using 3-year-old Kazakh female sheep with an average body weight of 47.79±3.70 kg obtained from Xinjiang Huikang Animal Husbandry Biotechnology Co. Ltd., Wuhan, China. Eighteen sheep were assigned randomly to 3 diets (6 per group). The treatments groups included the control group (CT; corn-soybean meal basal diet), CT+111 group (111 mg/kg BW RP 5-HTP), and CT+222 group (222 mg/kg BW RP 5-HTP). The 5-HTP content in the CT+222 group was 2 times higher than that in the CT+111 group. The experimental period lasted for 25 days after the RP 5-HTP was added to the concentrate diet. The levels of RP 5-HTP were chosen based on a prior study [12]. Based on the differences in weight and breed, we set 2 levels (111 and 222 mg/kg BW) of supplementation. The animal care, handling, and sampling procedures were carried out according to the protocol approved by the Xinjiang Agricultural University Animal Care and Use Committee (2017004).
Diets and feeding
The sheep were housed in an open-sided barn in individual pens (1.0×1.5 m). The powder concentrate was fed at a concentration of 1.0% of BW daily and divided into 2 equal meals at 0730 hour and 1930 hour. Hay and fresh water were provided ad libitum. The formulation and nutrition levels of the powder concentrate, and nutrition levels of hay are shown in Table 1.
Table 1.
Composition and nutrient levels of diet (DM basis) %.
| Items | Control group | CT+111 | CT+222 |
|---|---|---|---|
| Concentrate supplement* | 27.07 | 27.41 | 27.81 |
| Corn silage | 24.92 | 24.48 | 25.51 |
| Alfalfa hay | 23.43 | 23.48 | 22.78 |
| Wheat straw | 24.58 | 24.63 | 23.90 |
| Total | 100.00 | 100.00 | 100.00 |
| Nutrient levels | |||
| DM | 93.83 | 93.81 | 92.80 |
| Ash | 9.83 | 9.82 | 9.80 |
| CP | 12.20 | 12.24 | 12.29 |
| NDF | 56.87 | 56.76 | 56.70 |
| ADF | 34.72 | 34.62 | 34.52 |
| Ca | 0.99 | 0.99 | 0.99 |
| P | 0.28 | 0.28 | 0.28 |
One kg of concentrate supplement contained the following: corn 0.44 kg, oat 0.16 kg, barley 0.15 kg, soybean meal 0.20 kg, CaHPO4 0.03 kg, salt 0.01 kg, premix 0.01 kg.
The premix provided the following per kg of the concentrate supplement: VA 480 IU, VB1 816 mg, VB2 333 mg, VB6 49 mg, VD 70 U, VE 21333 IU, Pantothenic acid20 mg, nicotinamide485 mg, Cu (as copper sulfate) 11 mg, Fe (as ferrous sulfate) 35 mg, Mn (as manganese sulfate) 33 mg, Zn (as zinc sulfate) 31 mg, I (as potassium iodide) 2 mg, Se (as sodium selenite) 6 mg, Co (as cobalt chloride) 1 mg.
Sample preparation
On the 25th day of the experiment, blood was collected from the jugular vein at 0600 hour (after morning feeding) and centrifuged at 1200 g for 15 minutes at 4°C. The plasma was stored at −20°C. After blood sampling, sheep were sacrificed by an intravenous injection of Somlethal (20 mL; Med-Tech Inc., Elwood, KS, USA). The pineal glands were collected within 2–5 minutes, weighed, and frozen immediately in tubes with liquid nitrogen. Then, the body was longitudinally dissected through the ventral body surface, and the mucosal layers of the duodenum, jejunum, ileum, cecum, and colon samples were quickly scraped from the underlying muscular layers with a razor blade and immediately frozen in liquid nitrogen. The rumen fluid was filtered through a nylon bag, then collected into a tube and stored in liquid nitrogen.
Plasma 5-HTP, Trp, 5-HT, and MLT content analysis
The total plasma tryptophan was assayed through the fluorometric method [13]. Then, 100 mL of plasma was deproteinized with 900 mL of 70 mL/L HClO4, centrifuged at 2500 g for 15 minutes at 4°C, and stored at −70°C. Absorbance detection at 280 nm was used to assay tryptophan; the mobile phase for tryptophan measurement contained 100 mL/L methanol. The concentration of 5-HTP in the plasma was determined as described previously method [14]. The plasma (750 mL) was mixed with 4 mL of acidified butanol (4 mL of 70% HClO4 per L of 1-butanol) and centrifuged at 1900 g at 4°C. Subsequently, the precipitate was removed, and the supernatant was mixed with 6 mL of n-hexane and centrifuged at 5600 g at 4°C to obtain 400 mL of the aqueous phase (containing 5-HTP), which was used for column chromatography. The organic phase was discarded. Then, 25 mL of the extracted plasma was used for column chromatography, with the column buffer at 70°C, and a flow rate of 0.3 mL/minute. The effluent from the column was reacted with fluorogenic reagent (per L: 3 mmol phthaldialdehyde, 20 mg FC-134, and 150 mg Brij-35, all in 9 mol/L HCl). This reagent was prepared daily, maintained in the dark at 70°C for 8 minutes before measuring the fluorescence.
The plasma serotonin levels were analyzed using a commercial ELISA kit (IBL, Hamburg, Germany). The intra- and inter-assay coefficients of variation (CVs) were 3.2–6.2% and 6.9–14.9%, respectively. The MLT concentration in plasma was estimated in duplicate aliquots of 100 μL of plasma by radioimmunoassay [15]. The sensitivity of the assay was 4 pg/mL. The inter- and intra-assay CVs, estimated from plasma pools every 100 unknown samples, were 2.2% and 13.6%, respectively.
Pineal gland and intestinal mucosa AADC, 5-HT, NAS, MLT, and 5-HIAA mRNA expression analysis
Total RNA was extracted from the pineal gland and intestinal mucosa of Kazakh sheep using TRIzol Reagent (Tiangen, Beijing, China), following the manufacturer’s instructions. The extracted RNA was immediately used for cDNA synthesis with a First Strand cDNA synthesis kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. The complete open reading frames (ORFs) and partial up/downstream noncoding regions of the AADC, AA-NAT, HIOMT, and MAOA genes were respectively amplified using Trans Start FastPfu DNA Polymerase (Trans Gen Biotech, Beijing, China). The specific primers, which were designed by Primer Premier 5.0 software, were added the same restriction sites (XhoI) according to the sequences of AADC, AA-NAT, HIOMT, and MAOA in Ovis aries obtained from the National Center for Biotechnology Information. The primers contained XhoI restriction enzyme sites.
Total RNA was extracted and immediately reverse-transcribed using the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA, USA). The RT-PCR reactions consisted of a PrimeScriptTM RT reagent Kit with gDNA Eraser (Perfect Real Time) (10 μL; TaKaRa Bio Group, Dalian, China), 10 mM forward and reverse primers, 4 μL template and ddH2O were added up to a total volume of 20 μL. The procedure was as follows: 95°C for 15 minutes, 55 cycles of 95°C for 10 seconds, 58°C for 20 seconds, 72°C for 20 seconds, and a melting curve of 45°C–99°C, increasing in increments of 0.1°C every 1 second. Normalization was performed using the housekeeping gene GAPDH as a control. The relative mRNA expression was calculated by the 2−ΔΔCt method. The primer sequences are listed in Table 2.
Table 2.
Real-time PCR primer sequences.
| Gene symbol | Primer sequence 5′-3′ | Amplicon size (bp) | GeneBank accession numbers |
|---|---|---|---|
| AADC | AGGCCTATATCCGCAAGCAC TCTCAGGGAACAAGGGACCA |
200 | XM_004007693 |
| AA-NAT | ACGATTCCGTTCGAGAACCTCG AACCCATCAGCCCGTTGTGC |
150 | NC_000962.3 |
| HIOMT | TCTACAGGTCGGAGGACGAA CATCGTGGGTACAGGGACAC |
175 | KC290950 |
| MAOA | TATGTAGGCCCGACCCAGAA TGTTATCCATGGTCCGCCAC |
196 | XM_012158476 |
| GAPDH | GGTGAAGGTCGGTGTGAACG CTCGCTCCTGGAAGATGGTG |
233 | M32599 |
Pineal gland and intestinal mucosa 5-HTP, 5-HT, NAS, MLT, and 5-HIAA content analysis
For metabolite extraction, an aliquot of each individual sample was precisely weighed and transferred to an Eppendorf tube. After the addition of 1000 μL of extract solvent (precooled at −20°C, acetonitrile–methanol–water, 2: 2: 1 [V/V/V]), the samples were vortexed for 30 seconds, homogenized at 45 Hz for 4 minutes, and sonicated for 5 minutes in an ice-water bath. The homogenate and sonicate cycles were repeated twice, followed by incubation at −20°C for 1 hour and centrifugation at 4000 g and 4°C for 15 minutes. A 100 μL aliquot of the clear supernatant was used for UHPLC–MS/MS analysis.
Stock solutions were individually prepared by dissolving or diluting each standard substance to give a final concentration of 10 mmol/L. An aliquot of each stock solution was transferred to a 10 mL flask to form a mixed working standard solution. A series of calibration standard solutions were then prepared by stepwise dilution of the mixed standard solution.
For the analysis of UHPLC–PRM–MS, UHPLC separation was carried out using a 1290 Infinity series UHPLC System (Agilent Technologies, Palo Alto, CA, USA), equipped with a Waters ACQUITY UPLC BEH C18 column (100×2.1 mm, 1.7 μm). The mobile phase A was 0.1% formic acid in water, and mobile phase B was acetonitrile. The elution gradient is shown in Table 3. The column temperature was set at 40°C. The autosampler temperature was set at 4°C and the injection volume was 1 μL.
Table 3.
UHPLC gradient.
| Time | Mobile phase A 0.1% formic acid | Mobile phase B ACN | Flow rate(mL/min) |
|---|---|---|---|
| 0.0 min | 95% | 5% | 0.40 |
| 1.5 min | 95% | 5% | 0.40 |
| 6.0 min | 60% | 40% | 0.40 |
| 7.0 min | 5% | 95% | 0.40 |
| 8.8 min | 5% | 95% | 0.40 |
| 9.0 min | 95% | 5% | 0.40 |
| 12.0 min | 95% | 5% | 0.40 |
A Q Exactive™ Focus mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) was applied for assay development. The typical ion source parameters were as follows: spray voltage=+3500/−3100 V, sheath gas (N2) flow rate=40, aux gas (N2) flow rate=15, sweep gas (N2) flow rate=0, aux gas (N2) temperature=350°C, capillary temperature=320°C.
The PRM parameters for each of the targeted analytes were optimized by injecting the standard solutions of the individual analyte into the API source of the mass spectrometer. Under the optimized PRM parameters, the production that showed the highest sensitivity and selectivity was selected for quantitative monitoring (Table 4). The additional product ions were used to verify the identity of the target analyte.
Table 4.
PRM parameter optimization.
| Compound name | Prec Ion | Prod Ion | CE (V) | Start (min) | End (min) | Polarity |
|---|---|---|---|---|---|---|
| 5-HTP | 221.09207 | 204.06496 | 10 | 0.10 | 2.23 | Positive |
| 5-HT | 177.10224 | 160.07530 | 10 | 0.10 | 2.23 | Positive |
| NAS | 219.11280 | 160.07530 | 10 | 2.24 | 4.37 | Positive |
| MLT | 233.12845 | 174.09098 | 10 | 4.38 | 12.00 | Positive |
| 5-HIAA | 192.06552 | 146.05972 | 10 | 2.24 | 4.37 | Positive |
For calibration curves, calibration solutions were subjected to UHPLC–PRM–MS/MS analysis using the methods as described. Table 4 summarizes the results for the calibration curves, where y is the peak areas for the analyte, and x is the concentration (nmol/L). The least squares method was used for the regression fitting. A 1/x weighting factor was applied to the curve fitting, since it provided the highest accuracy and correlation coefficient (R2). The level was excluded from the calibration if the signal-to-noise ratio (S/N) was close to or below 20, or the accuracy of calibration was not within 80–120%. Detailed calibration curves for individual analytes are shown in Table 5.
Table 5.
Quantitative parameters of the target compounds.
| Compound name | LLOD (nmol/L) | LLOQ (nmol/L) | R2 |
|---|---|---|---|
| 5-HTP | 2.4 | 4.9 | 0.9983 |
| 5-HT | 4.9 | 9.8 | 0.9990 |
| NAS | 0.3 | 0.6 | 0.9977 |
| MLT | 0.6 | 1.2 | 0.9960 |
| 5-HIAA | 9.8 | 19.5 | 0.9992 |
Limits of detection (LODs) and limits of quantitation (LOQs) were determined. The calibration standard solution was diluted stepwise, with a dilution factor of 2. These standard solutions were subjected to UHPLC–PRM–MS analysis. The S/N was used to determine the lower limits of detection (LLODs) and lower limits of quantitation (LLOQs). The LLODs and LLOQs were defined as the analyte concentrations that led to peaks with S/Ns of 3 and 10, respectively, according to the US FDA guidelines for bioanalytical method validation.
The precision of the quantitation was measured as the relative standard deviation (RSD), determined by injecting analytical replicates of quality control (QC) samples. The accuracy of quantitation was measured as the analytical recovery of the QC samples determined. The percent recovery was calculated as [mean observed concentration/spiked concentration]×100% (Table 6).
Table 6.
The recoveries and standard relative deviation of the samples.
| Compound name | Concentration (nmol/L) | Recovery | RSD |
|---|---|---|---|
| 5-HTP | 500 | 96.4% | 1.5% |
| 5-HT | 500 | 95.3% | 3.5% |
| NAS | 500 | 108.2% | 4.5% |
| MLT | 500 | 100.7% | 4.7% |
| 5-HIAA | 500 | 94.5% | 2.1% |
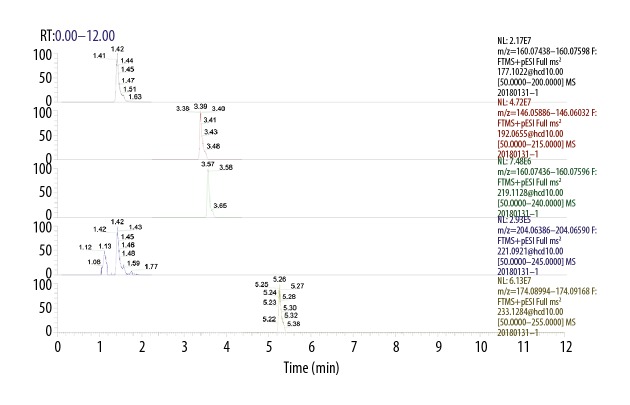
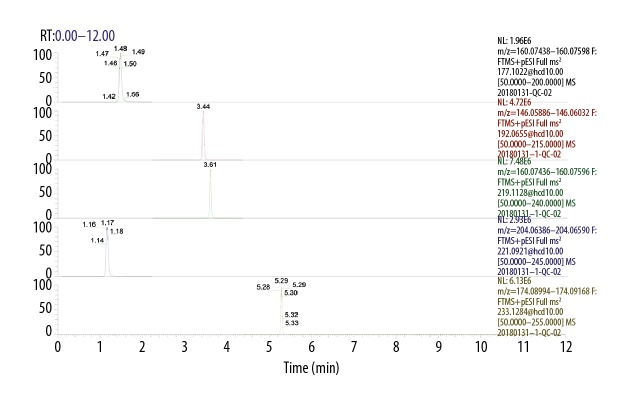
UHPLC separation are shown in Figures 1 and 2, which show the extracted ion chromatographs (EICs) from a standard solution (Figure 1) and a sample of the targeted analytes under the optimal conditions (Figure 2). As seen from the figures: all of the analytes showed excellent peak shapes; the baseline separations were obtained, and 5-HIAA, NAS, and 5-HTP were detected in most samples, while serotonin was only detected in several samples.
Figure 1.
The extracted ion chromatograms (EICs) of the standard solution.
Figure 2.
The extracted ion chromatograms (EICs) of the rumen fluid sample.
Table 5 lists the resulting LLODs and LLOQs: the LLODs were between 0.3–9.8 nmol/L, and LLOQs were between 0.6–19.5 nmol/L for all analytes. The R2 values of regression fitting were above 0.9960 for all the analytes, indicating a good quantitative relationship between the MS responses and concentrations, which was satisfactory for targeted metabolomics analysis (Table 5).
Table 6 lists the analytical recoveries and RSDs of the QC samples, with 11 technical replicates. The recoveries determined were 94.5–108.2% for all the analytes, with all RSDs below 4.7% (n=11). The analysis metrics indicated that this method allowed an accurate quantitation of the targeted metabolites in the biological sample in the concentration range described. The quantification results are shown in Table 7. The final concentration in nmol/L equals the calculated concentration (already multiplied by the dilution factor). The metabolite concentration (in ng/g) equals the final amount of the metabolite in the sample divided by the mass of the sample. The abbreviation “NF” means that the targeted metabolite was not found in the corresponding samples, and “detected” means that the targeted metabolites were detected but not quantified, and the concentrations were below the LLOQs.
Table 7.
Effect of diet supplemented with different levels of RPT 5-HTP on 5-HTP, 5-HT, NAS, MLT, 5-HIAA content in sheep pineal gland (n=6 nmol/pineal gland).
| Items | 5-HTP | 5-HT | NAS | MLT | 5-HIAA |
|---|---|---|---|---|---|
| Control group | 1.24±0.15 | 15.09±0.42aA | 0.56±0.04cB | 0.28±0.02cB | 26.06±3.23B |
| CT+111 | 1.32±0.15 | 8.90±0.41cB | 0.68±0.02aA | 0.32±0.01bB | 29.68±1.50AB |
| CT+222 | 1.34±0.11 | 10.31±0.90bB | 0.62±0.02bAB | 0.51±0.02aA | 35.80±3.50A |
In the same column, values with no letter or the same letter superscripts mean significant different (P>0.05), while with different small letter superscripts mean significant different (P<0.05), and with different capital letter superscripts mean significant difference (P<0.01).
Statistical analyses
The data were presented as the means ± standard deviation and were analyzed by analysis of variance (ANOVA) followed by Duncan’s test using SPSS 18.0 statistical software (IBM, America). Results with P<0.05 were considered statistically different and those with P<0.01 were considered significantly statistically different.
Results
Effect of diet supplemented with different levels of RP 5-HTP on the plasma 5-HTP and MLT concentrations in sheep
It was found that 25 days of pretreatment with RP 5-HTP (111 or 222 mg/kg BW) produced a 1.1–1.4-fold increase in the accumulation of 5-HTP, 5-HT and MLT in the plasma (Table 8), and this increase showed difference in the CT+222 group (P<0.05). The concentrations of Trp in the plasma were found to be unchanged during a time period of 6 hours after RP 5-HTP (111 or 222 mg/kg) treatment.
Table 8.
Effect of diet supplemented with different levels of RPT 5-HTP on plasma 5-HTP, MLT in sheep.
| Items | Control group | CT+111 | CT+222 |
|---|---|---|---|
| 5-HTP (nmol/L) | 1076.33±101.03B | 1336.97±139.54AB | 1481.72±207.78A |
| TRP (nmol/L) | 38037.25±5873.42 | 41864.12±12843.12 | 37163.21±4172.35 |
| 5-HT (nmol/L) | 1556.01±187.27B | 1738.00±252.01AB | 2211.10±279.20A |
| MLT (nmol/L) | 0.27±0.04B | 0.31±0.04AB | 0.36±0.04A |
In the same row, values with no letter or the same letter superscripts mean significant different (P>0.05), while with different small letter superscripts mean significant different (P<0.05), and with different capital letter superscripts mean significant difference (P<0.01).
Effect of diet supplemented with different levels of RP 5-HTP on the AADC, AA-NAT, HIOMT, and MAOA mRNA expression levels in the sheep pineal gland
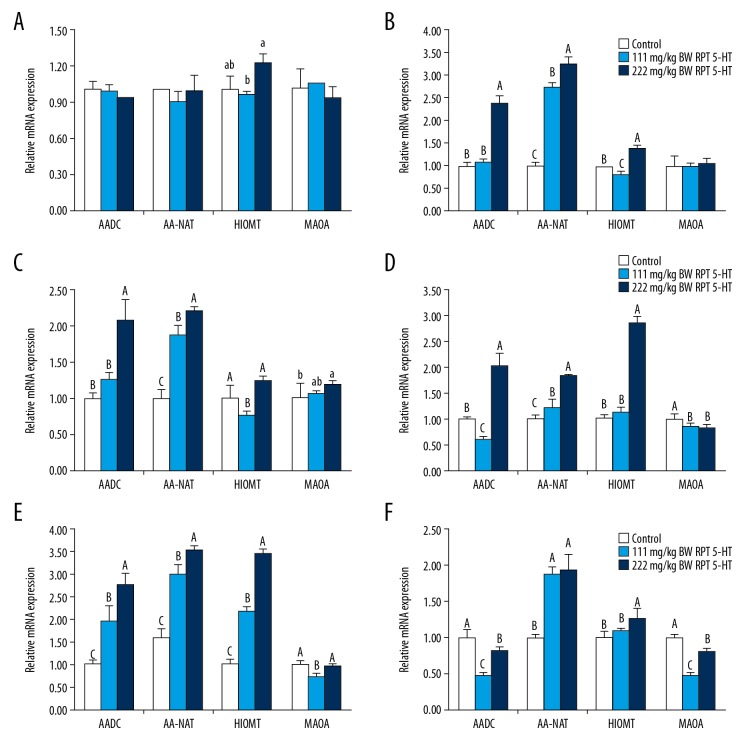
The RT-PCR results showed that the expression of HIOMT mRNA in the CT+222 group was significantly higher than that in the CT group (Figure 3; P<0.05). However, no differences in the expressions of AADC, AA-NAT, and MAOA mRNA were found among the CT, CT+111, and CT+222 groups (P>0.05).
Figure 3.
Aromatic amino acid decarboxylase (AADC), arylalkylamine N-acetyltransferase (AA-NAT), hydroxyindole-O-methyl transferase (HIOMT), and monoamine oxidase A (MAOA) mRNA expression levels in the pineal gland and intestinal mucosa of sheep. Provided diet supplemented with 111 or 222 mg/kg BW RP 5-HTP. (A) Pineal gland; (B) Duodenum; (C) Jejunum; (D) Ileum; (E) Cecum; (F) Colon. Values with no letter or the same letter superscripts mean significant different (P>0.05), while with different small letter superscripts mean significant different (P<0.05), and with different capital letter superscripts mean significant difference (P<0.01).
Effect of diet supplemented with different levels of RP 5-HTP on the 5-HTP, 5-HT, NAS, MLT, and 5-HIAA contents in the sheep pineal gland
The RP 5-HTP (111 or 222 mg/kg) treatment did not markedly alter the pineal gland 5-HTP concentration (Table 7, P>0.05). The 5-HT contents in the CT+111 group (8.90±0.41 nmol/g) and CT+222 group (10.31±0.90 nmol/g) were lower than that in the CT group (15.09±0.42 nmol/g, P<0.01), whereas the MLT and 5-HIAA concentrations in the pineal gland were increased by increasing the levels of RP 5-HTP.
Effect of diet supplemented with different levels of RP 5-HTP on the AADC, AA-NAT, HIOMT, and MAOA mRNA expression levels in the sheep intestinal mucosa
Figure 3 shows the mRNA abundance for the main enzymes involved in MLT synthesis. The levels of intestinal mucosa AADC, AA-NAT, HIOMT, and MAOA mRNA expression were in response to treatment with RP 5-HTP (111 or 222 mg, Figure 3). Rumen-protected 5-HTP (111 or 222 mg/kg BW) showed significant effects on the AADC mRNA expression in the duodenum, jejunum, and cecum in the CT+222 group compared to those in the CT group and the CT+111 group (P<0.01), whereas the AADC mRNA abundance in the ileum and colon in the CT+111 group was lower than that in the CT group and CT+222 groups (P<0.01). The addition of RP 5-HTP (111 or 222 mg/kg BW) resulted in a significant upregulation of AA-NAT mRNA expression in all of the intestinal segments (Figure 3). After treatment with 222 mg/kg BW RP 5-HTP, the HIOMT mRNA abundance in the intestinal mucosa was upregulated (P<0.01). In contrast, the HIOMT mRNA expressions in the duodenum and jejunum decreased significantly compared to those in the CT group (P<0.01). The ileum and colon MAOA mRNA expressions showed decreases in both the CT+111 group and CT+222 groups (P<0.01).
Effect of diet supplemented with different levels of RP 5-HTP on the 5-HTP, 5-HT, NAS, MLT, 5-HIAA contents levels in the sheep intestinal mucosa
Table 9 shows the concentrations of 5-HTP and its metabolites involved in MLT synthesis. Rumen-protected 5-HTP, particularly at a higher dose (222 mg/kg), increased the concentrations of intestinal mucosa 5-HTP and NAS. Levels of 5-HTP and 5-HT in the rumen and duodenum were increased with increasing RP 5-HTP (P<0.05), but the MLT concentration was reduced with increasing RP 5-HTP. The ileum and colon mucosa 5-HT contents in the CT+111 group and CT+222 group were lower than those in the CT group. In contrast, the 5-HT concentrations in the jejunum and cecum in the CT+222 group were higher than those in the CT group (P<0.01). The MLT contents in the jejunum, cecum, and colon in the CT+111 group and CT+222 group were higher than those in the CT group (P>0.05), and the ileum MLT concentration remarked extremely level after treatment with 111 mg RP 5-HTP. Except for in the colon, RP 5-HTP (111 or 222 mg/kg) did not influence the intestinal mucosa 5-HIAA concentration (Table 9).
Table 9.
Effect of diet supplemented with different levels of RPT 5-HTP on 5-HTP, 5-HT, NAS, MLT, 5-HIAA in sheep intestinal mucosa (n=6 nmol/g intestinal mucosa (wet weight)).
| Intestinal segment | Grouping | 5-HTP | 5-HT | NAS* | MLT | 5-HIAA |
|---|---|---|---|---|---|---|
| Rumen | Control group | 0.10±0.01C | 0.30±0.03B | 49.34±3.39 | 15.05±1.11a | 3.05±0.28 |
| CT+111 | 0.76±0.11B | 0.37±0.01AB | 52.45±4.78 | 13.59±0.42ab | 3.29±0.20 | |
| CT+222 | 1.35±0.13A | 0.83±0.02A | 49.94±4.57 | 12.84±1.38b | 3.08±0.06 | |
| Duodenum | Control group | 0.11±0.02C | 1.59±0.22B | 10.14±0.89C | 23.46±1.64A | 1.03±0.07A |
| CT+111 | 1.19±0.10B | 1.60±0.09B | 13.18±1.16B | 19.16±1.10B | 0.74±0.08B | |
| CT+222 | 3.03±0.12A | 2.38±0.31A | 16.93±1.61A | 19.66±1.31B | 0.69±0.18B | |
| Jejunum | Control group | 0.17±0.01B | 5.91±0.08C | 2.71±0.28B | 20.04±2.22 | 2.25±0.43 |
| CT+111 | 0.33±0.16AB | 5.98±0.45B | 3.84±0.18A | 20.69±1.86 | 1.82±0.36 | |
| CT+222 | 0.47±0.06A | 6.66±0.26A | 3.56±0.12A | 21.36±1.98 | 2.31±0.59 | |
| Ileum | Control group | 0.22±0.02B | 3.75±0.51A | 2.71±0.03b | 3.46±0.36B | 2.16±0.56 |
| CT+111 | 0.26±0.10B | 2.19±0.45B | 4.41±0.15a | 4.97±0.39A | 2.36±0.44 | |
| CT+222 | 0.48±0.09A | 2.51±0.47B | 3.15±0.32ab | 3.54±0.26B | 2.38±0.29 | |
| Cecum | Control group | 0.11±0.02b | 20.16±2.49B | 2.56±0.37b | 7.40±0.65B | 2.27±0.38 |
| CT+111 | 0.11±0.02b | 20.94±1.32B | 2.93±0.44ab | 8.07±0.56AB | 2.20±0.24 | |
| CT+222 | 0.17±0.03a | 30.68±3.09A | 3.18±0.16a | 8.99±0.94A | 2.14±0.38 | |
| Colon | Control group | 0.14±0.05 | 23.98±3.23A | 1.72±0.16cB | 10.08±1.07 | 3.95±0.54A |
| CT+111 | 0.15±0.02 | 13.81±2.81B | 3.01±0.03aA | 10.84±0.63 | 2.72±0.30B | |
| CT+222 | 0.19±0.05 | 20.85±0.55A | 2.19±0.32bB | 10.89±0.65 | 3.93±0.49A |
pmol/g intestinal mucosa(rumen fliud).
In the same column, values with no letter or the same letter superscripts mean significant different (P>0.05), while with different small letter superscripts mean significant different (P<0.05), and with different capital letter superscripts mean significant difference (P<0.01).
Discussion
There are few reports on the distribution of 5-HTP, 5-HT, NAS, 5-HIAA, and MLT in the plasma, pineal gland, and intestinal tract of sheep so far. The results of our study demonstrated that sheep plasma 5-HTP was influenced by RP 5-HTP (Table 8). These results were in accordance with those of Lanis et al. [16] and Waclawikova and El Aidy [17], who demonstrated serum 5-HTP changes in humans after orally administered 5-HTP. Moreover, 5-HT is a secondary product of 5-HTP, and increased sheep plasma 5-HTP levels helped to elevate their plasma 5-HT concentration. This is also in accordance with a report in which the plasma 5-HT content of patients with depression increased after treatment with 5-HTP [18]. Namboodiri et al. first reported that a 5-HTP injection (20 or 200 mg/kg) caused a statistically significant increase in plasma MLT after 2–5 hours [19]. Our study confirmed that RP 5-HTP administration elevated the plasma MLT 1.17–1.35-fold after 6 hours in the morning (Table 8). While an injection of 5-HTP can directly make it into the bloodstream, oral administration of RP 5-HTP might have delayed effects, and the 5-HTP plasma might therefore be cleared in the liver and kidneys [20]; thus, the synthetic MLT precursor content in sheep might be reduced. Trp is not only an important indirect precursor of MLT, but also plays an important biological role in vertebrates [21–23]. The present study indicated that RP 5-HTP administration did not affect the plasma Trp content in sheep. Intestinal absorption of 5-HTP does not require the presence of a transport molecule [24], whereas Trp is absorbed by amino acid transporters [25]. Therefore, it is suggested that 5-HTP absorption was not affected by the presence of other amino acids.
This research has given some insight into the changes in the pineal gland 5-HTP metabolism and expression of MLT synthase. It appeared that the RP 5-HTP-induced elevation of plasma MLT was due to the increased synthesis, because the pineal gland NAS, MLT, and HIOMT mRNA expressions were all increased after RP 5-HTP administration (Table 7, Figure 3). The changes were readily apparent with a larger dose of RP 5-HTP (222 mg/kg). One study reported that a 5-HTP injection (20 or 200 mg/kg) produced a marked increase in the pineal gland 5-HT, NAS, MLT contents and HIOMT activity, while no change was observed in the pineal gland indoleamine N-acetyltransferase (NAT) activity [26], which was consistent with our study finding. This finding indicated that RP 5-HTP administration did not indirectly increase sympathetic stimulation of the pineal gland by the action of some site along the suprachiasmatic nucleus–pineal pathway. No increases in NAT and 5-HT (an enzyme known to be under neural control) were noted (Table 7), which suggests that substrate (5-HT) availability might limit the synthesis of MLT in sheep. However, the unchanged AADC, AA-NAT, and MAOA expressions might be due to the time of year; this study was conducted in the summer, and long light periods can affect the regulation of enzyme activity in the pineal gland by RP 5-HTP.
Melatonin synthesis has been found in different sites; a major source is the gastrointestinal tract (GIT), where it is essentially synthesized by intestinal enterochromaffin cells [27]. The MLT level in the GIT organs exceeds its nighttime peak in the pineal gland at 400-fold [28]. In this study, MLT concentrations were measured in the plasma and gastrointestinal mucosa of sheep. The mucosa levels profoundly exceeded those of plasma, and was consistent with previous studies in chickens [29], pigs [30], mice [31], rats [32], pigs and cows [33]. A much higher MLT concentration was detected in the GIT than that in plasma (Table 9). This again could be attributed to the fact that MLT plays a role in many vitally important physiological processes in the gut [34].
Different species show different MLT concentrations in the GIT. In the polygastric cow, high concentrations of MLT were observed in the mucosa of the rumen, reticulum, omasum, and abomasum. Conversely, high MLT concentrations were found in the cecum and colon mucosa of pigs. In addition, the jejunum had the lowest MLT contents in both pigs and cows [35]. In this sheep study, MLT was the lowest in the ileum mucosa and highest in the duodenum mucosa (Table 9). The presence of different microbes in the individual segments of bovine and porcine GIT may account for the much lower MLT values in the GIT anterior segment of pigs compared to those of cows [36]. However, the pattern of MLT distribution in the gut was still quite different between sheep and cows, although they are both ruminants. There were several reasons for this. First, very high interindividual differences were encountered in previous experiments [37,38]. Second, there were individual variations in the content of digesta, which can influence local concentrations of MLT [38]; in addition, the amount of intestinal digesta in cows was much greater than that in sheep. For the sampling time, the cows were fed ad libitum, and the bovine intestinal mucosa were collected between 0800–1000 hour. However, sheep were fed at 0730 hour, and the intestinal mucosa were collected 6 hours after the morning feeding.
Many studies have shown a substrate effect in the regulation of MLT synthase in animals. In our study, the gene expressions of AADC and AA-NAT in the duodenum, jejunum, and cecum were significantly upregulated (Figure 3). In addition, the 5-HTP and NAS levels in the intestinal mucosa also increased (Table 9). These results confirmed that the direct administration of RP 5-HTP was an efficient and reliable approach for the regulation of AADC and AA-NAT in sheep. However, we found that the gene expressions of HIOMT and MLT in the duodenum and jejunum mucosa of the CT+111 group were lower than those in the CT group. As HIOMT is the enzyme principally responsible for converting NAS to MLT, these results indicated that HIOMT limited MLT synthesis; this further validated that HIOMT was the crucial enzyme in MLT synthesis [39]. It has also been shown that the duodenum mucosa is an acidic environment, and is constantly exposed to a vast array of microbes and food antigens [40], which may limit the regulation of HIOMT by RP 5-HTP. Monoamine oxidase A catalyzed the conversion of 5-HT to 5-HIAA [41]. In our study, the gene expression of MAOA in the jejunum, ileum, cecum, and colon of the CT+111 group was significantly reduced, making more 5-HT metabolites to NAS, further providing more precursors for MLT synthesis.
Until now, the GIT has not been considered as a possible contributor of extrapineal sites of MLT synthesis to circulating levels in higher vertebrates [42,43]. However, the amount of MLT found in the GIT was reported to be much higher than in the pineal gland, and the gut made a significant contribution to circulating MLT [37]. The gut has been identified as a major source of elevated plasma MLT concentrations after Trp administration and circulating MLT level changes induced by feeding regime [44].
In this study, the MLT concentration in the sheep pineal gland increased, while the gene expressions of AADC, AA-NAT, and MAOA did not have a statistically significant increase (Figure 3). Moreover, the gene levels in the intestinal mucosa increased (Figure 3), but the MLT did not increase in the duodenum, jejunum, and colon. However, the plasma MLT levels of RP 5-HTP exhibited significant differences from those in the CT group. These results showed that the increased plasma MLT level might be derived from the gut. The majority of studies have reported MLT levels in the plasma as measured by radioimmunoassay and gas chromatography–mass spectrometry in pinealectomized chicks [45], rats [46], sheep [47], and Syrian hamsters [48]. Daytime MLT levels were found to be almost unaffected by the removal of the pineal gland [49–51]; only the nighttime rise of plasma MLT was prevented. This was taken as evidence that the nocturnal increase in plasma MLT was derived from the pineal gland. Finally, evidence has also been presented that circadian MLT rhythm persists (with a less pronounced elevation at nighttime) in the plasma of pinealectomized birds [12,52,53] and rats [54]. The MLT produced by the pineal gland and gut might play different roles in sheep, and there might also be differences in maintaining the MLT content in the plasma. The mechanisms of these differences are currently unknown, and will be investigated in the future.
Conclusions
Rumen-protected 5-HTP induced MLT synthase expression in the intestinal tract of sheep, and the concentration of MLT synthesis intermediates were also increased in this study. However, the MLT synthase expression levels in the sheep pineal gland did not increase. The increased plasma MLT might be derived from the intestinal tract, and MLT synthesized can maintain the MLT concentration in sheep plasma during the daytime. This study provides a scientific basis for elucidating the role of MLT in the regulation of intestinal motility and tissue health maintenance in ruminants, such as sheep.
Footnotes
Source of support: The study was supported by the National Natural Science Foundation of China (grant number 31760681)
References
- 1.Reiter RJ, Mayo JC, Tan DX, et al. Melatonin as an antioxidant: Under promises but over delivers. J Pineal Res. 2016;61(3):253–78. doi: 10.1111/jpi.12360. [DOI] [PubMed] [Google Scholar]
- 2.Gupta S, Haldar C. Photoperiodic modulation of local melatonin synthesis and its role in regulation of thymic homeostasis in Funambulus pennanti. Gen Comp Endocrinol. 2016;239:40–49. doi: 10.1016/j.ygcen.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Pandey SK, Haldar C, Vishwas DK, et al. Synthesis and in vitro evaluation of melatonin entrapped PLA nanoparticles: An oxidative stress and T-cell response using golden hamster. J Biomed Mater Res A. 2015;103(9):3034–44. doi: 10.1002/jbm.a.35441. [DOI] [PubMed] [Google Scholar]
- 4.Mendes C, Lopes AM, do Amaral FG, et al. Adaptations of the aging animal to exercise: Role of daily supplementation with melatonin. J Pineal Res. 2013;55(3):229–39. doi: 10.1111/jpi.12065. [DOI] [PubMed] [Google Scholar]
- 5.Tian Y, Yabuki Y, Moriguchi S, et al. Melatonin reverses the decreases in hippocampal protein serine/threonine kinases observed in an animal model of autism. J Pineal Res. 2014;56(1):1–11. doi: 10.1111/jpi.12081. [DOI] [PubMed] [Google Scholar]
- 6.Miller SL, Yan EB, Castillo-Melendez M, et al. Melatonin provides neuroprotection in the late-gestation fetal sheep brain in response to umbilical cord occlusion. Dev Neurosci. 2005;27(2–4):200–10. doi: 10.1159/000085993. [DOI] [PubMed] [Google Scholar]
- 7.Byeon Y, Park S, Kim YS, et al. Microarray analysis of genes differentially expressed in melatonin-rich transgenic rice expressing a sheep serotonin N-acetyltransferase. J Pineal Res. 2013;55(4):357–63. doi: 10.1111/jpi.12077. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Chai M, Tian X, et al. Effects of melatonin on superovulation and transgenic embryo transplantation in small-tailed han sheep (Ovis aries) Neuro Endocrinol Lett. 2013;34(4):294–301. [PubMed] [Google Scholar]
- 9.Sugden D, Namboodiri M, Klein D, et al. Ovine pineal indoles: Effects of L-tryptophan or L-5-hydroxytryptophan administration. J Neurochem. 1985;44(3):769–72. doi: 10.1111/j.1471-4159.1985.tb12881.x. [DOI] [PubMed] [Google Scholar]
- 10.Ates-Alagoz Z, Buyukbingol Z, Buyukbingol E. Synthesis and antioxidant properties of some indole ethylamine derivatives as melatonin analogs. Pharmazie. 2005;60(9):643–47. [PubMed] [Google Scholar]
- 11.Ettaoussi M, Peres B, Errazani A, et al. Synthesis and pharmacological evaluation of dual ligands for melatonin (MT1/MT2) and serotonin 5-HT2C receptor subtypes (II) Eur J Med Chem. 2015;90:822–33. doi: 10.1016/j.ejmech.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 12.Markus RP, Fernandes PA, Kinker GS, et al. Immune-pineal axis – acute inflammatory responses coordinate melatonin synthesis by pinealocytes and phagocytes. Br J Pharmacol. 2018;175(16):3239–50. doi: 10.1111/bph.14083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curzon G, Kantamaneni BD, Tricklebank MD. A comparison of an improved o-phthalaldehyde fluorometric method and high pressure liquid chromatography in the determination of brain 5-hydroxyindoles of rats treated with L-tryptophan and p-chlorophenyl-alanine. Br J Pharmacol. 1981;73(2):555–61. doi: 10.1111/j.1476-5381.1981.tb10455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keszthelyi D, Troost FJ, Jonkers DM, et al. Visceral hypersensitivity in irritable bowel syndrome: evidence for involvement of serotonin metabolism – a preliminary study. Neurogastroenterol Motil. 2015;27(8):1127–37. doi: 10.1111/nmo.12600. [DOI] [PubMed] [Google Scholar]
- 15.Huang YL, Liang XB, Qian LQ, et al. Effects of Kaixin Powder on melatonin receptor expression and (125)I-Mel binding affinity in a rat model of depression. Chin J Integr Med. 2015;21(7):507–15. doi: 10.1007/s11655-014-1787-x. [DOI] [PubMed] [Google Scholar]
- 16.Lanis JM, Alexeev EE, Curtis VF, et al. Tryptophan metabolite activation of the aryl hydrocarbon receptor regulates IL-10 receptor expression on intestinal epithelia. Mucosal Immunol. 2017;10(5):1133–44. doi: 10.1038/mi.2016.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waclawikova B, El Aidy S. Role of microbiota and tryptophan metabolites in the remote effect of intestinal inflammation on brain and depression. Pharmaceuticals (Basel) 2018;11(3) doi: 10.3390/ph11030063. pii: E63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharbatoghli M, Rezazadeh Valojerdi M, et al. The relationship between seminal melatonin with sperm parameters, DNA fragmentation and nuclear maturity in intra-cytoplasmic sperm injection candidates. Cell J. 2015;17(3):547–53. doi: 10.22074/cellj.2015.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Namboodiri MA, Sugden D, Klein DC, Mefford IN. 5-hydroxytryptophan elevates serum melatonin. Science. 1983;221(4611):659–61. doi: 10.1126/science.6867734. [DOI] [PubMed] [Google Scholar]
- 20.Abdel Moneim AE, Ortiz F, Leonardo-Mendonca RC, et al. Protective effects of melatonin against oxidative damage induced by Egyptian cobra (Naja haje) crude venom in rats. Acta Trop. 2015;143:58–65. doi: 10.1016/j.actatropica.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Nagashima S, Yamashita M, Tojo C, et al. Can tryptophan supplement intake at breakfast enhance melatonin secretion at night? J Physiol Anthropol. 2017;36(1):20. doi: 10.1186/s40101-017-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delgadillo JA, Velez LI, Flores JA. Continuous light after a long-day treatment is equivalent to melatonin implants to stimulate testosterone secretion in Alpine male goats. Animal. 2016;10(4):649–54. doi: 10.1017/S1751731115002177. [DOI] [PubMed] [Google Scholar]
- 23.Oyama T, Nagai R, Fujimoto M, et al. Development of a fully-automated on-line oxidation column-switching HPLC system for the determination of endogenous melatonin in human clinical samples. Anal Sci. 2015;31(11):1129–35. doi: 10.2116/analsci.31.1129. [DOI] [PubMed] [Google Scholar]
- 24.Tossou MC, Liu H, Bai M, et al. Effect of high dietary tryptophan on intestinal morphology and tight junction protein of weaned pig. Biomed Res Int. 2016;2016 doi: 10.1155/2016/2912418. 2912418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruera O, Sances G, Leston J, et al. Plasma melatonin pattern in chronic and episodic headaches: Evaluation during sleep and waking. Funct Neurol. 2008;23(2):77–81. [PubMed] [Google Scholar]
- 26.Wen H, Feng L, Jiang W, et al. Dietary tryptophan modulates intestinal immune response, barrier function, antioxidant status and gene expression of TOR and Nrf 2 in young grass carp (Ctenopharyngodon idella) Fish Shellfish Immunol. 2014;40(1):275–87. doi: 10.1016/j.fsi.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Haque R, Chong NW, Ali F, et al. Melatonin synthesis in retina: cAMP-dependent transcriptional regulation of chicken arylalkylamine N-acetyltransferase by a CRE-like sequence and a TTATT repeat motif in the proximal promoter. J Neurochem. 2011;119(1):6–17. doi: 10.1111/j.1471-4159.2011.07397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leston J, Harthe C, Mottolese C, et al. Is pineal melatonin released in the third ventricle in humans? A study in movement disorders. Neurochirurgie. 2015;61(2–3):85–89. doi: 10.1016/j.neuchi.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Adamska I, Marhelava K, Walkiewicz D, et al. All genes encoding enzymes participating in melatonin biosynthesis in the chicken pineal gland are transcribed rhythmically. J Physiol Pharmacol. 2016;67(4):521–30. [PubMed] [Google Scholar]
- 30.Garcia-Gil FA, Albendea CD, Escartin J, et al. Melatonin prolongs graft survival of pancreas allotransplants in pigs. J Pineal Res. 2011;51(4):445–53. doi: 10.1111/j.1600-079X.2011.00908.x. [DOI] [PubMed] [Google Scholar]
- 31.He C, Wang J, Zhang Z, et al. Mitochondria synthesize melatonin to ameliorate its function and improve mice oocyte’s quality under in vitro conditions. Int J Mol Sci. 2016;17(6) doi: 10.3390/ijms17060939. pii: E939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Juszczak M, Wolak M, Bojanowska E, et al. The role of melatonin membrane receptors in melatonin-dependent oxytocin secretion from the rat hypothalamo-neurohypophysial system – an in vitro and in vivo approach. Endokrynol Pol. 2016;67(5):507–14. doi: 10.5603/EP.a2016.0035. [DOI] [PubMed] [Google Scholar]
- 33.Carvajal JC, Gomez-Esteban MB, Carbajo S, et al. Melatonin-like immunoreactivity in the pineal gland of the cow: An immunohistochemical study. Histol Histopathol. 2004;19(4):1187–92. doi: 10.14670/HH-19.1187. [DOI] [PubMed] [Google Scholar]
- 34.Konturek SJ, Konturek PC, Brzozowska I, et al. Localization and biological activities of melatonin in intact and diseased gastrointestinal tract (GIT) J Physiol Pharmacol. 2007;58(3):381–405. [PubMed] [Google Scholar]
- 35.Bulc M, Lewczuk B, Prusik M, et al. The foetal pig pineal gland is richly innervated by nerve fibres containing catecholamine-synthesizing enzymes, neuropeptide Y (NPY) and C-terminal flanking peptide of NPY, but it does not secrete melatonin. Histol Histopathol. 2013;28(5):633–46. doi: 10.14670/HH-28.633. [DOI] [PubMed] [Google Scholar]
- 36.Bubenik GA, Ayles HL, Friendship RM, et al. Relationship between melatonin levels in plasma and gastrointestinal tissues and the incidence and severity of gastric ulcers in pigs. J Pineal Res. 1998;24(1):62–66. doi: 10.1111/j.1600-079x.1998.tb00367.x. [DOI] [PubMed] [Google Scholar]
- 37.Velarde E, Cerda-Reverter JM, Alonso-Gomez AL, et al. Melatonin-synthesizing enzymes in pineal, retina, liver, and gut of the goldfish (Carassius): mRNA expression pattern and regulation of daily rhythms by lighting conditions. Chronobiol Int. 2010;27(6):1178–201. doi: 10.3109/07420528.2010.496911. [DOI] [PubMed] [Google Scholar]
- 38.Al-Ghoul WM, Abu-Shaqra S, Park BG, et al. Melatonin plays a protective role in postburn rodent gut pathophysiology. Int J Biol Sci. 2010;6(3):282–93. doi: 10.7150/ijbs.6.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rath MF, Coon SL, Amaral FG, et al. Melatonin synthesis: acetylserotonin o-methyltransferase (asmt) is strongly expressed in a subpopulation of pinealocytes in the male rat pineal gland. Endocrinology. 2016;157(5):2028–40. doi: 10.1210/en.2015-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mukherjee S, Maitra SK. Gut melatonin in vertebrates: Chronobiology and physiology. Front Endocrinol (Lausanne) 2015;6:112. doi: 10.3389/fendo.2015.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song GH, Gwee KA, Moochhala SM, et al. Melatonin attenuates stress-induced defecation: Lesson from a rat model of stress-induced gut dysfunction. Neurogastroenterol Motil. 2005;17(5):744–50. doi: 10.1111/j.1365-2982.2005.00653.x. [DOI] [PubMed] [Google Scholar]
- 42.Anderson G, Vaillancourt C, Maes M, et al. Breastfeeding and the gut-brain axis: Is there a role for melatonin? Biomol Concepts. 2017;8(3–4):185–95. doi: 10.1515/bmc-2017-0009. [DOI] [PubMed] [Google Scholar]
- 43.Pal PK, Hasan KN, Maitra SK. Gut melatonin response to microbial infection in carp Catla catla. Fish Physiol Biochem. 2016;42(2):579–92. doi: 10.1007/s10695-015-0161-7. [DOI] [PubMed] [Google Scholar]
- 44.Velarde E, Alonso-Gomez AL, Azpeleta C, et al. Melatonin effects on gut motility are independent of the relaxation mediated by the nitrergic system in the goldfish. Comp Biochem Physiol A Mol Integr Physiol. 2011;159(4):367–71. doi: 10.1016/j.cbpa.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 45.Nogueira RC, Sampaio LFS. Eye and heart morphogenesis are dependent on melatonin signaling in chick embryos. J Exp Biol. 2017;220(Pt 20):3826–35. doi: 10.1242/jeb.159848. [DOI] [PubMed] [Google Scholar]
- 46.Mortezaee K, Sabbaghziarani F, Omidi A, et al. Therapeutic value of melatonin post-treatment on CCl4-induced fibrotic rat liver. Can J Physiol Pharmacol. 2016;94(2):119–30. doi: 10.1139/cjpp-2015-0266. [DOI] [PubMed] [Google Scholar]
- 47.Luridiana S, Mura MC, Daga C, et al. Melatonin treatment in spring and reproductive recovery in sheep with different body condition score and age. Anim Reprod Sci. 2015;160:68–73. doi: 10.1016/j.anireprosci.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 48.Chakir I, Dumont S, Pevet P, et al. Pineal melatonin is a circadian time-giver for leptin rhythm in Syrian hamsters. Front Neurosci. 2015;9:190. doi: 10.3389/fnins.2015.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tan DX, Manchester LC, Reiter RJ. CSF generation by pineal gland results in a robust melatonin circadian rhythm in the third ventricle as an unique light/dark signal. Med Hypotheses. 2016;86:3–9. doi: 10.1016/j.mehy.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 50.Prusik M, Lewczuk B, Ziolkowska N, et al. Regulation of melatonin secretion in the pineal organ of the domestic duck – an in vitro study. Pol J Vet Sci. 2015;18(3):635–44. doi: 10.1515/pjvs-2015-0082. [DOI] [PubMed] [Google Scholar]
- 51.Franklin M, Hlavacova N, Babic S, et al. Pineal melatonin in a sub-chronic tryptophan depletion female rat model of treatment-resistant depression. Pharmacopsychiatry. 2015;48(4–5):e3. doi: 10.1055/s-0035-1555824. [DOI] [PubMed] [Google Scholar]
- 52.Semenenko S, Tymofiychuk I, Boreyko L, et al. Peculiairities of melatonin effect on chonorhytmic organization of kidney acid-regulating function influenced by nitrogen monoxide synthesis blockade under conditions of pineal gland hypofunction. Georgian Med News. 2017;(271):117–22. [PubMed] [Google Scholar]
- 53.Munoz MF, Arguelles S, Cano M, et al. Aging and oxidative stress decrease pineal elongation factor 2: In vivo protective effect of melatonin in young rats treated with cumene hydroperoxide. J Cell Biochem. 2017;118(1):182–90. doi: 10.1002/jcb.25624. [DOI] [PubMed] [Google Scholar]
- 54.Aynali G, Naziroglu M, Celik O, et al. Modulation of wireless (2.45 GHz)-induced oxidative toxicity in laryngotracheal mucosa of rat by melatonin. Eur Arch Otorhinolaryngol. 2013;270(5):1695–700. doi: 10.1007/s00405-013-2425-0. [DOI] [PubMed] [Google Scholar]