Significance
In this report, we show that fibrinogen, identified by proteomics to be present in blood plasma extracellular vesicles (EVs), is sufficient and required for autoimmune-mediated spontaneous relapsing disease activity in a murine model of multiple sclerosis (MS). Unique to this model is that plasma EVs induced CD8-mediated disease. Analysis of human plasma EVs identified fibrinogen in MS patient samples, thereby providing a compelling translational association between our experimental findings and the perpetuation of CD8-mediated autoimmunity in human MS. Hence, these findings provide evidence for EVs as means by which to model an important aspect of spontaneous CD8+ T cell development related to autoimmunity in MS.
Keywords: autoimmunity, proteomics, EAE, T cell, relapse
Abstract
Extracellular vesicles (EVs) are emerging as potent mediators of intercellular communication with roles in inflammation and disease. In this study, we examined the role of EVs from blood plasma (pEVs) in an experimental autoimmune encephalomyelitis mouse model of central nervous system demyelination. We determined that pEVs induced a spontaneous relapsing−remitting disease phenotype in MOG35–55-immunized C57BL/6 mice. This modified disease phenotype was found to be driven by CD8+ T cells and required fibrinogen in pEVs. Analysis of pEVs from relapsing−remitting multiple sclerosis patients also identified fibrinogen as a significant portion of pEV cargo. Together, these data suggest that fibrinogen in pEVs contributes to the perpetuation of neuroinflammation and relapses in disease.
Successful translation of discovery research into clinical efficacy is often hampered by the authenticity of experimental animal models to faithfully recapitulate disease pathogenesis. For instance, study of the adaptive immune responses in experimental autoimmune encephalomyelitis (EAE) mouse models of multiple sclerosis (MS) has become a basis for our understanding and preclinical therapeutic development (1). However, the immunological phenotype of MS patients notably differs from mice in which immunization with myelin peptides induce robust T cell responses (2). Significant attention on CD4+ T cells in MS has been supported by both genome-wide association study-identified risk susceptibility genes for MS and the central role of CD4+ T cells in many EAE mouse models (3–5). However, the clinical disease course in human MS patients is not solely driven by CD4+ T cells, as interrogation of infiltrating lymphocyte subtypes in MS patients has revealed a predominance of CD8+ T cells over CD4+ T cells (6–8). The repertoire of CD8+ T cells in the cerebrospinal fluid of MS patients, and, to a lesser extent, in blood, reflects elevated levels of CD8+ T cells also identified in lesions within the CNS (9). These findings have led to important questions on the etiology of CD8+ T cell responses, their potential roles in mediating clinical exacerbations, and how to model this immune response in mice (10). The enrichment of CD8+ T cells in MS suggests a critical effector role for CD8+ T cells in this disease, but the inherent difficulty in modeling spontaneous CD8+ T cells in EAE models has hindered rigorous study of encephalitogenic CD8+ T cells in the context of autoimmunity and MS.
Extracellular vesicles (EVs) have emerged as potent mediators of immunity (11). EVs are nano-sized membrane particles released by cells that represent an evolutionarily conserved mode of intercellular communication (12). Interest in EVs has been stimulated by their emerging roles in disease and potential use as prognostic biomarkers (13). Interest in EVs is driven by recent experimental evidence demonstrating EV-directed intercellular communication contributes to cancer, inflammatory, neurological and autoimmune diseases (14). Hence, EVs represent a rapidly evolving field with potential to understand complex diseases. In particular, the innate cellular complexity and relative inaccessibility of the CNS offer the potential for EV biology to provide better understanding and insight to monitor inflammatory and degenerative changes (15). Plasma EVs (pEVs) have also been reported to reflect features of inflammation in human neurological diseases such as Alzheimer’s disease (16), Parkinson’s disease (17), and MS (18). However, how pEVs may contribute to the autoimmunity of MS has not been elucidated.
In this study, we assessed the functional impact of pEVs on neuroinflammation and clinical disability in a mouse model of CNS demyelination. We report that pEVs from naive C57BL/6 mice, when transferred into mice with active EAE, result in a distinct spontaneous relapsing−remitting EAE phenotype characterized by a prominent contribution of CD8+ T cells. Proteomic analysis identified a causal role for fibrinogen in pEVs inducing the unique clinical and immunological phenotype in this pEV−EAE model. We characterize the pEV−EAE approach as representing an authentic immunological system with utility for disease modeling and understanding the etiology of CD8+ T cell responses in disease relapses in MS which may have implications for translation of experimental findings to human patients.
Results
Plasma EVs Induce Spontaneous Relapses in EAE Mice.
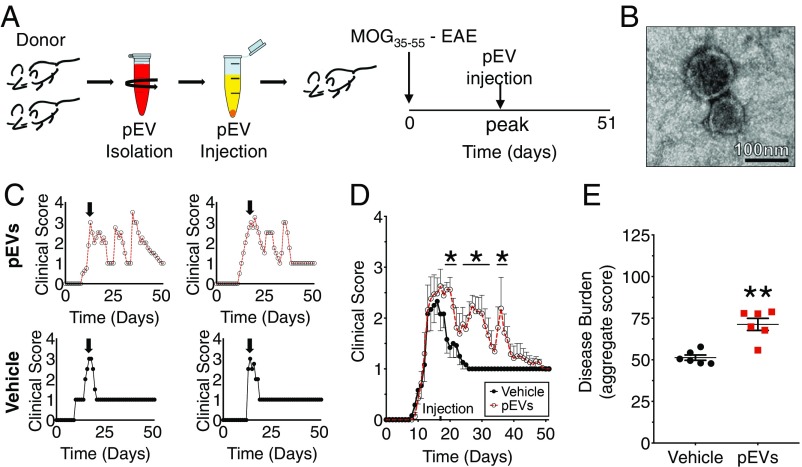
EAE immunization in mice on the C57BL/6 background induces a robust and consistent monophasic clinical disease course mediated by a prominent CD4+ lymphocyte activation (10, 19). The pEVs isolated from stored red blood cell units have been found to bind to monocytes and induce proinflammatory cytokines, which boosts CD4+ and CD8+ T cell responses in vitro (20). Additionally, EVs are capable of augmenting preexisting inflammatory responses in vivo (21). Whether and how pEVs can augment the neuroinflammatory events in EAE have not been previously examined. To determine whether pEVs can augment the inflammatory response in EAE mice, we first isolated and confirmed the presence of EVs from mouse blood plasma. The purity of isolated pEVs was verified by electron microscopy (EM), an approach to validate EVs we have used previously (22) (Fig. 1B). To determine the effect pEVs have on the EAE disease course, MOG35–55 EAE immunized mice received a single injection of pEVs at the time of peak clinical disability (Fig. 1A). We found that pEV-injected MOG35–55 EAE mice developed a worsened pattern of EAE (Fig. 1E) with clinical disability characterized by spontaneous relapsing/remitting (R/R) that were not observed in control EAE subjects (Fig. 1 C and D). These findings are consistent with a previous report that permutations of MOG35–55 EAE in C57BL/6 mice can evoke a relapsing−remitting disease phenotype (23).
Fig. 1.
The pEVs induced a spontaneous relapsing−remitting phenotype in wild-type C57BL/6 mice during MOG35–55-induced EAE. (A) The pEVs isolated from donor mouse plasma (confirmed by transmission EM shown in B) or vehicle were administered (i.v.) to EAE mice at peak clinical illness (arrow). (Scale bar in B: 100 nm.) (C) Representative clinical EAE disease courses in pEV and vehicle-treated mice. (D) Grouped treatment effect over 51-d experimental period of vehicle (n = 6) and pEV-treated MOG35–55 EAE (n = 8) mice. (E) Comparison of disease burden (aggregate score) between vehicle-treated (n = 6) and pEV-treated MOG35–55 EAE (n = 6) mice. (Values are mean ± SEM, where *P < 0.05 is calculated by two-way ANOVA with uncorrected Fisher’s LSD post hoc test; *P < 0.05 is nonparametric Mann−Whitney u test.) Grouped data are the result of three independent experiments. Reproduced clinical data can be found in SI Appendix, Fig. S1 A and B.
pEVs Induce CD8+ T Cells.
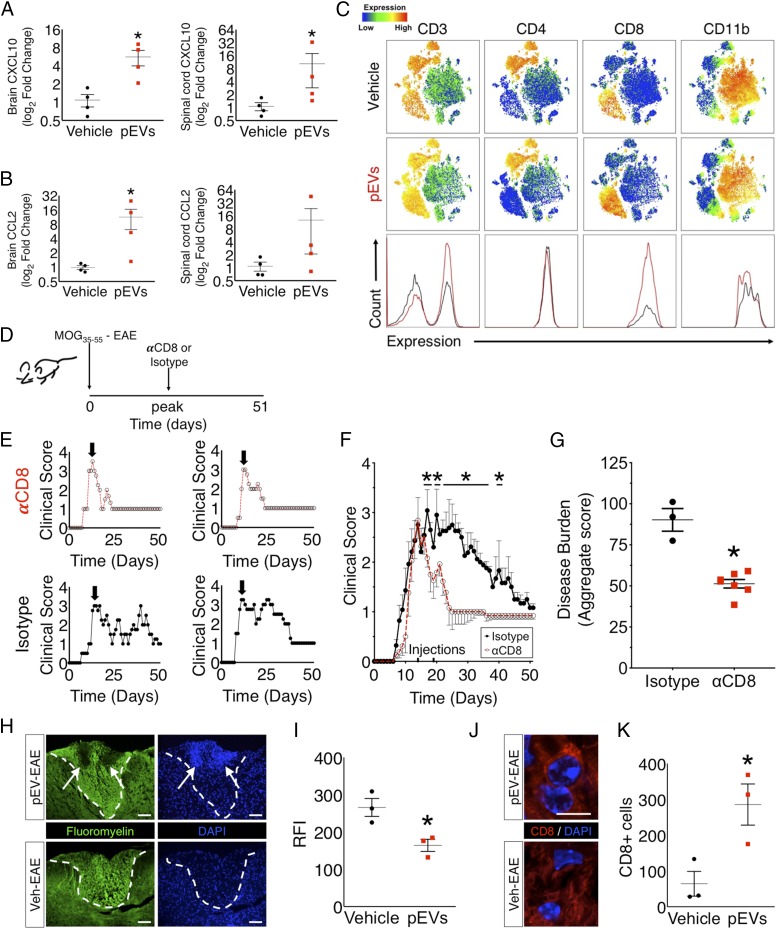
To determine whether and how pEVs evoked neuroinflammatory changes within the CNS of EAE mice, we next analyzed expression of the chemokines that are known to attract T cells into the CNS, Ccl2 and Cxcl10, in brain and spinal cord tissues of R/R EAE (pEV-administered) mice during a first-relapse event. Ccl2 and Cxcl10 levels were found to be increased in the brain during relapses in pEV-treated R/R mice (Fig. 2A). However, analysis of spinal cord tissues from R/R EAE mice revealed that only Cxcl10 was found to be significantly increased (Fig. 2B). Since these two chemokines are known to promote T cell and macrophage recruitment into the CNS (24), we examined the composition of the immune cell infiltrates in the spinal cords of pEV-administered EAE mice during a relapse. To explore this question, we applied an unbiased survey of immune cells by using flow cytometry by time of flight (CyTOF) and applied T-distributed stochastic neighbor embedding analysis of all labeled CNS immune cells. This approach identified a specific increase in CD3+/CD8+ T cells in pEV-administered, R/R EAE mice (Fig. 2C) during an active relapse. Since autoimmunity in MOG35–55 EAE mice is typically driven by CD4+ T cells (25), we next established the contribution of CD8+ T cells to R/R EAE mice by immunodepletion of CD8+ cells from R/R mice (Fig. 2D). Administration of anti-CD8 antisera was found to prevent the relapsing activity phenotype in response to pEV administration in treated mice (Fig. 2 E and F) and reduced the severity of clinical disability (Fig. 2I). Anti-CD8 antisera did not affect disease in control EAE mice not given pEVs (SI Appendix, Fig. S4 B and C), nor did an Ig-matched control antisera change disease exacerbation activity in treated R/R mice (Fig. 2 D and E). Hence, pEVs induced a robust CD8+ T cell response which was required for the relapsing−remitting phenotype in treated EAE mice.
Fig. 2.
The pEVs induce a CD8+ T cell response responsible for the development of a spontaneous relapsing phenotype in MOG35–55 EAE. (A) Expression of chemokines Cxcl10 (n = 4) and Ccl2 (n = 4) was significantly up-regulated at time of a spontaneous relapse in the brains of pEV R/R mice. (B) Expression of the chemokine Cxcl10 (n = 4) was significantly up-regulated at time of a spontaneous relapse in the spinal cords of pEV R/R mice with no difference in expression of Ccl2 (n = 4). (C) CyTOF analysis of spinal cords of pEV R/R mice at time of a spontaneous relapse identified a unique CD8+ T cell population. (D) EAE in pEV-treated MOG35–55 EAE mice also given αCD8 function blocking antibody or isotype antisera, and (E) representative clinical disease courses in individual treated mice, and (F) plotted as group (αCD8; n = 8; isotype control, n = 7). (G) Comparison of disease burden (aggregate score) between pEV-treated MOG35–55 EAE mice given αCD8 function blocking antibody (n = 6) or isotype antisera (n = 3). (H) Histology for myelin (fluoromyelin) and nuclei (DAPI) in the dorsal column of pEV−EAE and vehicle-treated (Veh EAE) mice collected at the same time as the first relapse in pEV-treated animals show robust cellular infiltrates that (I) colocalized with enhanced demyelinated lesions in pEV-treated mice. (Scale bar in H: 100 μm.) (J) Representative CD8+ immune cells in pEV-treated animals (Scale bar in J: 6 μm) and (K) quantification of these cells indicated elevated numbers of CD8+ T cells within demyelinated lesion areas in the spinal cords of pEV-treated mice. (Values in F are mean ± SEM, where *P < 0.05 calculated by two-way ANOVA with uncorrected Fisher’s LSD post hoc test; in A, B, and G, *P < 0.05, Mann−Whitney nonparametric u test; in I and K, *P < 0.05 Welch’s t test.) Grouped qPCR and clinical data are the result of samples collected from three independent experiments. Reproduced clinical data can be found in SI Appendix, Fig. S3 A and B.
Histological analysis of spinal cord tissues at the time of the first relapse in pEV-treated EAE mice and time-matched control EAE subjects identified extensive mononuclear cell infiltrates within the white matter of pEV-treated mice that were not observed in control-treated mice (Veh EAE) (Fig. 2H and SI Appendix, Fig. S2A). Our CyTOF findings indicated that CD8+CD3+ represented the majority of CD8+ cells in the spinal cords of pEV-treated mice (SI Appendix, Fig. S2B). Immunohistochemical characterization of these cellular infiltrates in association with demyelinated lesions revealed an abundance of CD8+ T cells (Fig. 2 J and K), CD4+ T cells (SI Appendix, Fig. S2C), and CD68+ macrophages (SI Appendix, Fig. S2D) in the infiltrates of pEV-treated subjects at higher frequencies than observed in vehicle-treated animals. Analysis of neurofilament M in the dorsal column of pEV and vehicle-treated animals also determined that there were no differences in axon numbers at this time point between treatment groups (SI Appendix, Fig. S2E).
Fibrinogen Mediates Relapse-Inducing Action of pEVs.
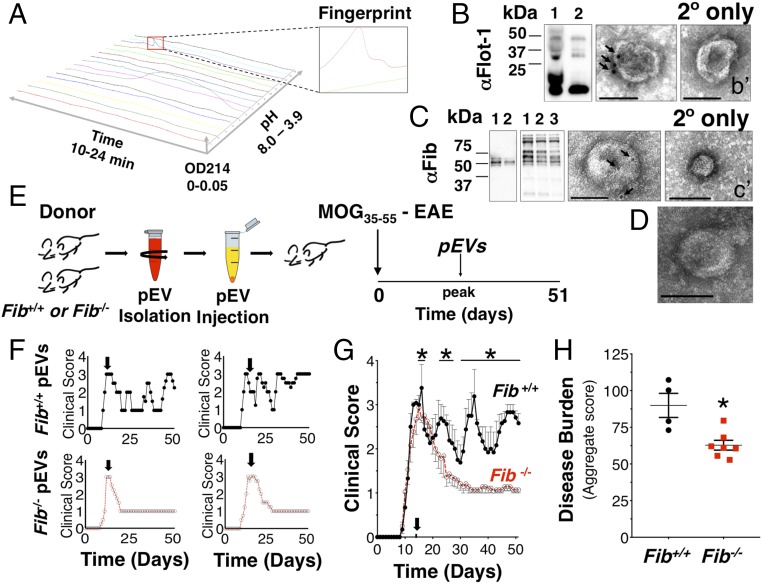
Proteomic analysis of the pEVs was performed using the 2D protein fractionation platform (PF-2D; Fig. 3A). Proteome laboratory identifications of enriched fractions were interrogated by liquid chromatography (LC) with tandem mass spectrometry. These fractions identified a limited number of candidate factors (SI Appendix, Table S1), which included fibrinogen alpha chain (FGA). As a candidate pEV factor, fibrinogen was of particular interest because fibrinogen deposition occurs with vascular leakage and promotes T cell recruitment, CNS demyelination, and axonal damage (26). Immunoblotting and EM confirmed pEV expression of the EV marker, flotillin-1 (Fig. 3B), and colocalization of fibrinogen in the pEVs (Fig. 3C). To determine whether fibrinogen was a necessary cargo of pEVs to induce R/R in MOG35–55 EAE mice, pEVs were collected from fibrinogen knockout (Fib−/−) mice and wild-type littermate (Fib+/+) controls (Fig. 3 D and E). When the Fib−/− pEVs were administered to MOG35–55 EAE mice, these mice did not develop any change in typical EAE course (Fig. 3 F and G), while administration of Fib+/+ pEVs induced the R/R clinical phenotype with disease exacerbation as observed with other wild-type pEV donor blood samples (Fig. 3 F and G), which was reflected by the increased disease burden (Fig. 3H). These data determined that fibrinogen in pEVs was required to induce a pattern of spontaneous relapsing−remitting activity in EAE mice.
Fig. 3.
The pEV fibrinogen is necessary to induce a spontaneous relapsing−remitting phenotype in wild-type C57BL/6 mice during MOG35–55-induced EAE. (A) Topographic plot of proteomic (PF-2D) analysis of mouse pEVs and identified fractions for tandem mass spectrometry peptide identification. Immunoblotting (Left) and immuno-EM (Right) confirmed EV marker (B) flotillin-1 (48 kDa) and (C) blood coagulation factor fibrinogen alpha (63.5 kDa), beta (56 kDa), and gamma (47 kDa) chain expression on pEVs as confirmed by plasma-isolated murine fibrinogen immunoblotting. (b′ and c′) Secondary-only immuno-EM of pEVs. (D) Electron micrograph of pEVs from plasma of Fib−/− mice confirmed intact EV morphology. (E) Clinical effect of pEVs isolated from Fib+/+ or Fib−/− mouse plasma when injected (i.v.) into MOG35–55 EAE mice at peak clinical illness. (F) Representative clinical disease course of individual MOG35–55 EAE mice administered Fib+/+ or Fib−/− pEVs. (G) Grouped treatment effect data of Fib+/+ (n = 6) and Fib−/− (n = 9) pEV-treated mice. (H) Comparison of disease burden (aggregate score) between MOG35–55 EAE mice administered Fib+/+ (n = 4) or Fib−/− (n = 7) pEVs. (Scale bars: 100 nm.) Arrows indicate α-fibrinogen and α-flotillin-1 gold particles. (Values are mean ± SEM, where *P < 0.05 is calculated by two-way ANOVA with uncorrected Fisher’s LSD post hoc test; *P < 0.05 is nonparametric Mann−Whitney u test.) Fib+/+ denotes wild-type littermate mice, and Fib−/− denotes fibrinogen knock-out mice. Grouped data are the result of two independent experiments. Reproduced clinical data can be found in SI Appendix, Fig. S5 A and B.
Fibrinogen Is Present in pEVs from MS Patients.
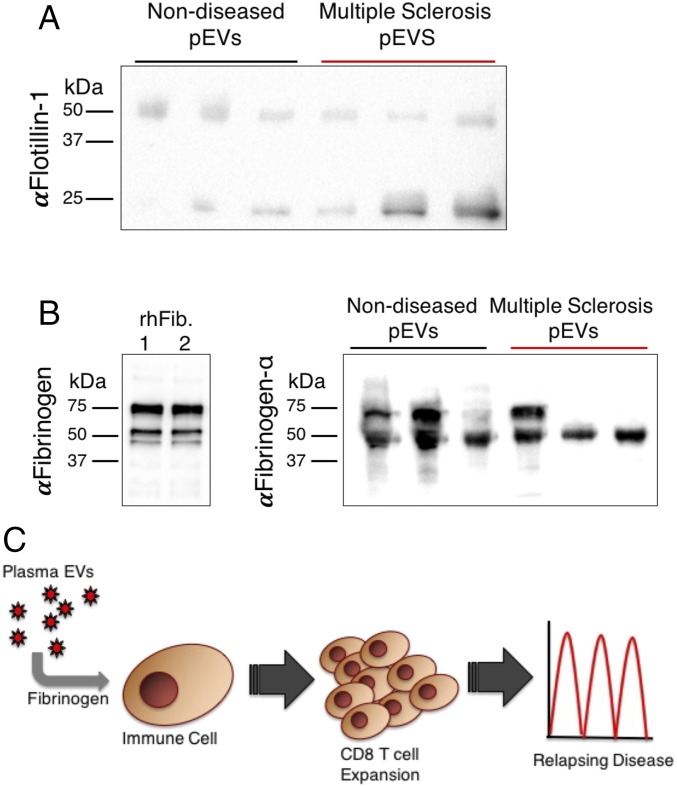
To determine whether pEVs from human blood, including in MS patients, also contained fibrinogen, we performed a proteomic analysis of pEVs from nondiseased subjects and patients with relapsing-remitting MS (RRMS). LC-tandem mass spectrometry of the pEVs also identified fibrinogen in human pEVs (SI Appendix, Table S2). We validated isolation of pEVs and confirmed the presence of fibrinogen in these pEVs by immunoblotting using plasma-isolated human fibrinogen as a positive control (Fig. 4 A and B). No apparent differences in fibrinogen peptide fragments were identified by tandem mass spectrometry when comparing RRMS with control pEV samples. Together, these data support our experimental findings that the presence of fibrinogen in pEVs of RRMS patients may contribute to the inflammatory differences underlying R/R disease activity.
Fig. 4.
Western blot analysis of pEVs from RRMS patient samples identified the presence of fibrinogen. Immunoblotting confirmed EV marker (A) flotillin-1 and (B) FGA on human pEVs (n = 3 per condition) as confirmed by plasma-isolated human fibrinogen. (C) Proposed model for pEV fibrinogen promoting CD8+ T cell-mediated relapses and inflammation associated with CNS demyelination.
Discussion
In this report, we demonstrate a mechanism to induce and study encephalitogenic CD8+ T cells in the widely used MOG35–55 EAE mouse model of MS. We show that pEVs administered to mice with EAE at the time of peak clinical disease resulted in the development of a unique spontaneous phenotype of relapsing−remitting disease—a clinical feature not generally observed in this MOG35–55 EAE mouse model. Using a top-down proteomics approach to interrogate pEVs, we identified fibrinogen as the component of pEVs responsible for inducing CD8+ T cell-mediated spontaneous relapses.
CD8+ T cells are the predominant T cell subtype found within active lesions in RRMS patients, which are posited to be the primary cellular mediators of CNS injury during relapse events (27, 28). Despite this, the most widely studied mouse model of MS, MOG35–55 EAE, induces a prominent CD4+ T cell-driven disease (1). While this model has provided important insights into the immunology of MS, the paucity of CD8+ T cells has limited the detailed exploration on their role as a salient feature of the disease in humans (7). Our study applied CyTOF analysis to identify which immune cells in pEV-treated MOG35–55 EAE mice contributed to the relapsing phenotype. This unbiased approach identified CD8+ T cells that, we subsequently determined by immunoblocking, were responsible for the R/R phenotype in pEV-treated mice. These data further strengthen the argument for CD8+ T cells as a driver of relapsing−remitting disease.
Widespread elevated expression of MHC class I antigens in active lesions in acute and chronic MS (29, 30) suggests a pervasive role for cytotoxic CD8+ T cells in the MS brain that ostensibly could be studied more efficiently using this R/R EAE system. Our findings on pathogenic CD8+ immune cells in this spontaneous R/R EAE model contrasts with previous work by others which reports potential beneficial actions of MOG35–55-specific CD8+ T cells as immunosuppressive through regulation of CD4+ T cells (31, 32). However, our results are consistent with work by others which demonstrated a pathogenic potential of effector CD8+ T cells in a myelin basic protein model of EAE in C3HeB mice (33). Our histological findings indicate the presence of CD8+ T cells within areas of demyelination. This may represent oligodendrocyte-specific autoreactive CD8+ T cells which have been found to lyse oligodendrocytes in vitro (34). However, CD8+ T cells can also cause demyelination through secretion of toxic factors, such as lymphotoxin, that are also damaging to oligodendrocytes (35, 36). One consistent issue reported in many previous studies examining CD8+ cells in EAE models is the low frequency of CD8+ T cells (25), whereas we found that, with pEV administration, there was a robust difference in CD8+ T cells that was found to mediate the relapsing−remitting disease activity. This difference in EAE phenotype following pEV administration likely reflects a preferential activation of CD8+ precursors through a yet to be defined mechanism. In this context, our data would place pEVs, and fibrinogen as a proximal driver of encephalitogenic CD8+ T cells, in this disease model (Fig. 4C). Future studies will be needed to resolve the identities and specificities of the CD8+ cell population(s), such as CD8+ populations other than CD3+ T cells (SI Appendix, Fig. S3A), as well as the modes of their initiation and expansion, to resolve their extent of their contributions to promoting autoimmunity.
Fibrinogen is known to be a critical component of the blood coagulation cascade that is cleaved into fibrin to stabilize the formation of blood clots. Interestingly, due to a unique molecular structure, binding sites on fibrinogen for receptors expressed by CNS cells, such as astrocytes, oligodendrocytes, and microglia, also mediate binding with other proteins (e.g., TGFβ and Aβ) that have been found to regulate key nervous system functions (26, 34). Our data support accumulating evidence that now point to fibrinogen as an important disease-related trigger for the development of an encephalitogenic adaptive immune response associated with CNS demyelination with striking features similar to those observed in human MS (24, 26). This result is consistent with previous findings that fibrinogen-deficient plasma, when injected into the brain parenchyma, did not induce an encephalitogenic T cell response compared with fibrinogen-rich plasma (24). That pEV fibrinogen was identified as the source of CD8+ T cell responses in R/R EAE mice was consistent with prior observations on the action of pEVs in other disease models. For instance, in cancer, MHC class I and MHC class II complexes on EVs have been shown to carry tumor or pathogenic peptides that can stimulate CD4+ or CD8+ T cells directly, as well as indirectly through interactions with antigen-presenting cells (APCs) (14). Additionally, EVs can transfer both antigens and signals to APCs to promote their activation into immunogenic APCs (15). That pEV fibrinogen contributed to the development of CD8+ T cell immunity suggests that fibrinogen, as a key cargo of EVs, may be important in APC function during autoimmunity, and fibrinogen may facilitate this action in some way. While we have determined that pEV fibrinogen can evoke CD8+ T cells when administered to EAE mice, this experimental manipulation does not convey any information regarding the natural changes in pEVs or their cargo during the course of EAE or MS. While future studies can expand upon these findings, the precise mechanism by which pEV-associated fibrinogen achieves this effect is unresolved. Nevertheless, based on these findings, we would also postulate that pEV-associated fibrinogen in MS may contribute to HLA class I linkage disequilibrium and increased susceptibility for autoimmunity associated with this disease (37).
EV biology is a rapidly growing field of study. EVs are membrane-bound organelles that are released from tissues containing a diverse array of molecular cargo (e.g., RNA, protein, and metabolites) (38). EVs have been identified in many different biofluids, including blood plasma. The functional relevance of the release of EVs into circulation in the broad contexts of health and disease is an area of continuing investigation. Our data suggest that pEV functions are at least partially reliant upon the EV proteome. Previous studies have shown RNA and even protein transfer can occur as a consequence of intercellular communication mediated by EVs (38). Of note is that EVs are known to retain markers of their cells of origin, which suggests that future studies may identify subpopulations from specific cell types that contribute unique or critical signals to the overall EV population. Given the diverse potential effector functions of EVs on the activity of the immune cells, our findings do provide evidence that pEVs can influence autoimmune responses in a model of MS. Moreover, our analysis of pEVs from healthy human donors and from MS patients would suggest that, in patients, the presence of fibrinogen in pEVs supports our experimental findings that it is not the pEVs per se but their activity in combination with inflammation that elicits profoundly different outcomes. While fibrinogen itself is known to promote autoimmunity (24), the unique aspect of fibrinogen associated with pEVs may also offer insights into how EVs contribute to immunity. For instance, we speculate that posttranslational modifications of peptides which have been implicated as potential drivers of autoimmunity in other models (39) may contribute to the potential for EV-associated peptides to promote autoimmunity and immune-mediated pathology in MS.
In summary, our findings highlight the potential influence of CD8+ T cells in mediating autoimmunity in EAE mice. Our elucidation of pEV fibrinogen-induced CD8-driven immunity in EAE provides unique insights into plausible mechanisms by which endogenous blood factors can drive encephalitogenic T cell responses in MS. With continued study, we consider this pEV−EAE model a means by which to elucidate the etiology of relapse activity in human RRMS (29, 33).
Materials and Methods
Animals.
Mice used in this study included wild-type male C57BL/6 (strain #000664; The Jackson Laboratory) and Fib−/−mice (40) (kindly provided by Jay Degen, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH). All procedures involving animals were conducted with prior approval from the Institutional Animal Care and Use Committees at the University of Connecticut School of Medicine and the University of California, San Francisco in accordance with guidelines set forth by the National Research Council of the National Academies Guide for the Care and Use of Laboratory Animals.
EAE was induced in wild-type C57BL/6 mice (8 wk to 12 wk old) as described in SI Appendix, Extended Materials and Methods. Also see SI Appendix, Extended Materials and Methods for additional details, including experimental antibody treatments.
Blood Collection and pEV Isolation.
Whole blood from naïve C57BL/6 mice was collected while mice were under deep isoflurane anesthesia, using a 1-mL syringe that had been flushed with 0.5 M EDTA (Fisher Scientific). Whole blood was collected from confirmed Fib−/− and Fib+/+ littermates and was coded for genotype and blinded before pEVs isolation and EAE experiments. Injected isolated pEVs into any single recipient were equal to the blood volume of one mouse’s (1.46 mL) whole blood. Each experimental subject was considered an independent experiment, as the donor pEVs were not pooled or shared across subjects, meaning that the sera from multiple unique donors were collected to provide the pEVs for a single individual EAE recipient.
CyTOF.
Spinal cords were obtained from vehicle or pEV-injected MOG35–55 EAE mice during an active relapse. Spinal cords were digested with a collagenase/DNase solution, and lymphocytes were isolated using a 70%/40% percoll gradient. Each sample (n = 4) was labeled with Cell-ID cisplatin to identify live and dead cells and subsequently barcoded with Cell-ID Pd barcoding kit [all mass cytometry (CyTOF) reagents are from Fluidigm].
Immunohistochemistry was performed as described in SI Appendix, Extended Materials and Methods.
EM was performed as described in SI Appendix, Extended Materials and Methods.
Proteomics.
Please see SI Appendix, Extended Materials and Methods.
Human Blood Samples.
This study was approved by the Office for the Protection of Research Subjects at University of Illinois at Chicago and the Department of Defense Human Research Protection Office. Plasma samples were obtained from a total of three patients with MS and three healthy, nondiseased controls, after patient consent (SI Appendix, Table S3).
Western blot analysis was performed as described in SI Appendix, Extended Materials and Methods.
Quantitative real-time PCR was performed as described in SI Appendix, Extended Materials and Methods.
Statistical Analysis.
Data were analyzed by two-way ANOVA with uncorrected Fisher’s least significant difference (LSD) post hoc test, Welch’s t test, and nonparametric Mann−Whitney u test as indicated, using GraphPad Prism version 7 for MAC OS X (GraphPad Software). Differences were considered significant when P < 0.05. Data are presented as mean ± SEM.
Supplementary Material
Acknowledgments
We thank Brittany E. Knight for her expertise in blood collection. We thank the Electron Microscopy and Flow Cytometry core facilities for their expert guidance. We thank the Mass Spectrometry (MS) & Proteomics Resource of the W. M. Keck Foundation Biotechnology Resource Laboratory at Yale University. This work was supported by National Institutes of Health (NIH) Grants R21NS087578 (to S.J.C. and A.T.V.), and R56NS099359 (to S.J.C.), NIH Shared Instrumentation Grant NIH ODOD018034 (Yale School of Medicine), National Institute of Neurological Diseases and Stroke Grant R35NS097976 (to K.A.), National Institute of Allergy and Infectious Disease Grant T32AI007334-29 (to A.S.M.), Race to Erase MS Young Investigator Award and American Heart Association Scientist Development Grant (to J.K.R.), and National Multiple Sclerosis Society Postdoctoral Fellowship FG-1708-28925 (to A.S.M.).
Footnotes
Conflict of interest statement: K.A. is a scientific cofounder of MedaRed, Inc. Her interests are managed by the Gladstone Institutes in accordance with its conflict of interest policy.
This article is a PNAS Direct Submission.
Data deposition: The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository, https://www.ebi.ac.uk/pride/ (dataset identifier PXD013630).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1816911116/-/DCSupplemental.
References
- 1.Steinman L, Zamvil SS (2006) How to successfully apply animal studies in experimental allergic encephalomyelitis to research on multiple sclerosis. Ann Neurol 60:12–21. [DOI] [PubMed] [Google Scholar]
- 2.Johnson TA, Jirik FR, Fournier S (2010) Exploring the roles of CD8+ T lymphocytes in the pathogenesis of autoimmune demyelination. Semin Immunopathol 32:197–209. [DOI] [PubMed] [Google Scholar]
- 3.Hellberg S, et al. (2016) Dynamic response genes in CD4+ T cells reveal a network of interactive proteins that classifies disease activity in multiple sclerosis. Cell Rep 16:2928–2939. [DOI] [PubMed] [Google Scholar]
- 4.Blankenhorn EP, et al. (2011) Genetics of experimental allergic encephalomyelitis supports the role of T helper cells in multiple sclerosis pathogenesis. Ann Neurol 70:887–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sawcer S, et al. ; International Multiple Sclerosis Genetics Consortium; Wellcome Trust Case Control Consortium 2 (2011) Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 476:214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salou M, Nicol B, Garcia A, Laplaud DA (2015) Involvement of CD8+ T cells in multiple sclerosis. Front Immunol 6:604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaskow BJ, Baecher-Allan C (2018) Effector T cells in multiple sclerosis. Cold Spring Harb Perspect Med 8:a029025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinha S, Boyden AW, Itani FR, Crawford MP, Karandikar NJ (2015) CD8+ T-cells as immune regulators of multiple sclerosis. Front Immunol 6:619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salou M, et al. (2015) Expanded CD8 T-cell sharing between periphery and CNS in multiple sclerosis. Ann Clin Transl Neurol 2:609–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gold R, Linington C, Lassmann H (2006) Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain 129:1953–1971. [DOI] [PubMed] [Google Scholar]
- 11.Chen G, et al. (2018) Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 560:382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tkach M, Théry C (2016) Communication by extracellular vesicles: Where we are and where we need to go. Cell 164:1226–1232. [DOI] [PubMed] [Google Scholar]
- 13.van Niel G, D’Angelo G, Raposo G (2018) Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 19:213–228. [DOI] [PubMed] [Google Scholar]
- 14.Robbins PD, Morelli AE (2014) Regulation of immune responses by extracellular vesicles. Nat Rev Immunol 14:195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson AG, et al. (2016) Extracellular vesicles in neurodegenerative disease–Pathogenesis to biomarkers. Nat Rev Neurol 12:346–357. [DOI] [PubMed] [Google Scholar]
- 16.Goetzl EJ, et al. (2015) Altered lysosomal proteins in neural-derived plasma exosomes in preclinical Alzheimer disease. Neurology 85:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi M, et al. (2014) Plasma exosomal α-synuclein is likely CNS-derived and increased in Parkinson’s disease. Acta Neuropathol 128:639–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sáenz-Cuesta M, Osorio-Querejeta I, Otaegui D (2014) Extracellular vesicles in multiple sclerosis: What are they telling us? Front Cell Neurosci 8:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellerman KE, Powers JM, Brostoff SW (1988) A suppressor T-lymphocyte cell line for autoimmune encephalomyelitis. Nature 331:265–267. [DOI] [PubMed] [Google Scholar]
- 20.Danesh A, et al. (2014) Exosomes from red blood cell units bind to monocytes and induce proinflammatory cytokines, boosting T-cell responses in vitro. Blood 123:687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hezel MEV, Nieuwland R, Bruggen RV, Juffermans NP (2017) The ability of extracellular vesicles to induce a pro-inflammatory host response. Int J Mol Sci 18:E1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willis CM, et al. (2017) A refined bead-free method to identify astrocytic exosomes in primary glial cultures and blood plasma. Front Neurosci 11:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berard JL, Wolak K, Fournier S, David S (2010) Characterization of relapsing-remitting and chronic forms of experimental autoimmune encephalomyelitis in C57BL/6 mice. Glia 58:434–445. [DOI] [PubMed] [Google Scholar]
- 24.Ryu JK, et al. (2015) Blood coagulation protein fibrinogen promotes autoimmunity and demyelination via chemokine release and antigen presentation. Nat Commun 6:8164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frausto RF, Crocker SJ, Eam B, Whitmire JK, Whitton JL (2007) Myelin oligodendrocyte glycoprotein peptide-induced experimental allergic encephalomyelitis and T cell responses are unaffected by immunoproteasome deficiency. J Neuroimmunol 192:124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petersen MA, Ryu JK, Akassoglou K (2018) Fibrinogen in neurological diseases: Mechanisms, imaging and therapeutics. Nat Rev Neurosci 19:283–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huseby ES, Huseby PG, Shah S, Smith R, Stadinski BD (2012) Pathogenic CD8 T cells in multiple sclerosis and its experimental models. Front Immunol 3:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neumann H, Medana IM, Bauer J, Lassmann H (2002) Cytotoxic T lymphocytes in autoimmune and degenerative CNS diseases. Trends Neurosci 25:313–319. [DOI] [PubMed] [Google Scholar]
- 29.Babbe H, et al. (2000) Clonal expansions of CD8+ T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J Exp Med 192:393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friese MA, Fugger L (2005) Autoreactive CD8+ T cells in multiple sclerosis: A new target for therapy? Brain 128:1747–1763. [DOI] [PubMed] [Google Scholar]
- 31.York NR, et al. (2010) Immune regulatory CNS-reactive CD8+T cells in experimental autoimmune encephalomyelitis. J Autoimmun 35:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen ML, Yan BS, Kozoriz D, Weiner HL (2009) Novel CD8+ Treg suppress EAE by TGF-beta- and IFN-gamma-dependent mechanisms. Eur J Immunol 39:3423–3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huseby ES, et al. (2001) A pathogenic role for myelin-specific CD8+ T cells in a model for multiple sclerosis. J Exp Med 194:669–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jurewicz A, Biddison WE, Antel JP (1998) MHC class I-restricted lysis of human oligodendrocytes by myelin basic protein peptide-specific CD8 T lymphocytes. J Immunol 160:3056–3059. [PubMed] [Google Scholar]
- 35.Buckle GJ, Hollsberg P, Hafler DA (2003) Activated CD8+ T cells in secondary progressive MS secrete lymphotoxin. Neurology 60:702–705. [DOI] [PubMed] [Google Scholar]
- 36.Jewtoukoff V, Lebar R, Bach MA (1989) Oligodendrocyte-specific autoreactive T cells using an alpha/beta T-cell receptor kill their target without self restriction. Proc Natl Acad Sci USA 86:2824–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Link J, et al. (2012) Importance of human leukocyte antigen (HLA) class I and II alleles on the risk of multiple sclerosis. PLoS One 7:e36779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shah R, Patel T, Freedman JE (2018) Circulating extracellular vesicles in human disease. N Engl J Med 379:958–966. [DOI] [PubMed] [Google Scholar]
- 39.Caprariello AV, et al. (2018) Biochemically altered myelin triggers autoimmune demyelination. Proc Natl Acad Sci USA 115:5528–5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suh TT, et al. (1995) Resolution of spontaneous bleeding events but failure of pregnancy in fibrinogen-deficient mice. Genes Dev 9:2020–2033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.