Significance
Sperm exhibit dramatic evolutionarily divergent morphologies in almost all taxa. Some sexually reproductive species show polymorphisms in the sperm produced by single males. Here, we focused on Sex-lethal (Sxl), which is the master sex-determination gene in Drosophila melanogaster, and investigated its function in the lepidopteran insect Bombyx mori. Our genetic analyses revealed that Sxl is essential for the formation of anucleate nonfertile parasperm. It is not expected that Sxl would be involved in sperm polymorphisms. Yet, whereas many morphological observations and ecological surveys have been conducted on sperm polymorphisms, this paper identifies the gene involved in sperm polymorphisms. Moreover, we clearly demonstrate that parasperm of B. mori is necessary for sperm migration in female organs.
Keywords: Sex-lethal, sperm polymorphism, apyrene sperm, sex determination, Bombyx mori
Abstract
Sex is determined by diverse mechanisms and master sex-determination genes are highly divergent, even among closely related species. Therefore, it is possible that homologs of master sex-determination genes might have alternative functions in different species. Herein, we focused on Sex-lethal (Sxl), which is the master sex-determination gene in Drosophila melanogaster and is necessary for female germline development. It has been widely shown that the sex-determination function of Sxl in Drosophilidae species is not conserved in other insects of different orders. We investigated the function of Sxl in the lepidopteran insect Bombyx mori. In lepidopteran insects (moths and butterflies), spermatogenesis results in two different types of sperm: nucleated fertile eupyrene sperm and anucleate nonfertile parasperm, also known as apyrene sperm. Genetic analyses using Sxl mutants revealed that the gene is indispensable for proper morphogenesis of apyrene sperm. Similarly, our analyses using Sxl mutants clearly demonstrate that apyrene sperm are necessary for eupyrene sperm migration from the bursa copulatrix to the spermatheca. Therefore, apyrene sperm is necessary for successful fertilization of eupyrene sperm in B. mori. Although Sxl is essential for oogenesis in D. melanogaster, it also plays important roles in spermatogenesis in B. mori. Therefore, the ancestral function of Sxl might be related to germline development.
Sex is determined by multiple mechanisms, and the molecular functions of master sex-determination genes differ, even among closely related species (1). Therefore, these homologs may have completely different functions in different species. Herein, we investigated the functions of Sex-lethal (Sxl), which is a well-studied key sex-determination gene in Drosophila melanogaster (2, 3). In D. melanogaster, Sxl is the master sex-determination gene of soma and dosage-compensation pathways (4–6). Moreover, Sxl was required for the transition from stem cells to fully committed daughter cells in female D. melanogaster germ cells (7, 8). Sxl was also reportedly necessary for the cell-autonomous maintenance of female identity in germ cells (9). Although the sequence of Sxl is evolutionarily conserved, its function varies among insects. Specifically, the Sxl protein has moderately conserved N- and C-terminal regions and a well-conserved central region including two RNA recognition motifs (RRMs) (10). In nondrosophilid flies such as Ceratitis capitata and Musca domestica, Sxl homologs do not show sex-specific expression, and ectopic expression of these Sxl homologs in D. melanogaster (Dm-Sxl) do not show sex-specific functions (11, 12). Therefore, the functions of Sxl in sex determination appear to be limited to drosophilid flies, and the functions of highly evolutionary conserved Sxl homologs in other insects remain totally unknown. Sxl homologs are not involved in sex determination in Bombyx mori, and their functions remain totally unknown in this lepidopteran insect. In B. mori, sex determination and dosage compensation are regulated by the regulatory factors Feminizer (Fem) and Masculinizer (Masc) (13, 14). In females, the W chromosome-linked gene Fem yields a Fem PIWI-interacting RNA (piRNA) and the Fem piRNA–PIWI complex cleaves Masc mRNAs, but Masc mRNA is not cleaved in males (13). In other studies, mutation of the Sxl homolog in B. mori (Bm-Sxl) had no physiological or morphological effects on somatic sexual determinations (15). These results suggest that Bm-Sxl is not involved in sex determination cascades of B. mori.
The present data indicate that Bm-Sxl contributes to sperm polymorphisms. Sperm exhibit dramatic evolutionarily divergent morphologies in almost all taxa (16), and some sexually reproductive species show polymorphisms in sperm produced by single males. Sperm polymorphisms produce fertile and infertile sperm, and these are referred to as eusperm and parasperm, respectively. Sperm polymorphisms were described in snails as early as 1836 by von Siebold (17) and have subsequently been reported in invertebrates and vertebrates (18–20).
Many functions of parasperm have been described in previous studies. Among these, Higginson and Pitnick (19) summarize that parasperm contribute to (i) nonadaptive errors during spermatogenesis, (ii) the transport or capacitation of eusperm, (iii) enhanced fertilization in the presence of sperm competition, (iv) protection from spermicidal environments, (v) nutrient provision for females or other sperm, and (vi) the control of sex ratios. But these functions continue to lack convincing support in many species (18–20).
In lepidopteran insects, spermatogenesis results in the production of nucleated eupyrene sperm and anucleate nonfertile sperm, which are known as apyrene sperm (21, 22) (Fig. 1A) and are shorter than eupyrene sperm in B. mori (Fig. 1B). Eupyrene and apyrene sperm are produced in the same testicular follicles, but eupyrene spermatogenesis occurs before apyrene spermatogenesis (21, 22). Apyrene sperm may contribute to (i) nonadaptive errors during spermatogenesis, (ii) the transport or activation of eupyrene sperm for successful fertilization, (iii) enhanced fertilization success in the presence of sperm competition, and (iv) nutrients for females or eupyrene sperm (20). However, empirical evidence of apyrene sperm functions supports only two of these possibilities. Specifically, apyrene sperm protected male reproductive investments by delayed female remating in Pieris napi, indicating contributions to enhanced fertilization success in the presence of sperm competition (23). Other studies suggest that apyrene sperm are necessary for successful fertilization in B. mori (24, 25), supporting roles in transport or activation of eupyrene sperm for successful fertilization. However, it remains unknown how apyrene sperm might be involved in the fertilization process.
Fig. 1.
Dimorphic sperm production in lepidopteran insects. (A) Schematic representation of dimorphic sperm development. Nuclei of apyrene sperm bundles are enucleated during maturation. Nuclei are shown in blue. (B) Images of eupyrene and apyrene sperm; nuclear staining was performed using DAPI. (Scale bar, 100 μm.)
Herein, we show high expression of Bm-Sxl in spermatocytes. Subsequent genetic analyses using Sxl mutants revealed that Bm-Sxl is essential for apyrene sperm formation. Moreover, analyses of dysfunctional apyrene sperm mutants clearly demonstrated that apyrene sperm are necessary for eupyrene sperm migration from the bursa copulatrix to the spermatheca. Collectively, the present data indicate that the functions of Bm-Sxl in apyrene sperm formation and eupyrene sperm migration are necessary for male fertility.
Results and Discussion
Bm-Sxl is transcribed into the alternative splicing isoforms Bm-Sxl-L and Bm-Sxl-S (26). Bm-Sxl-L encodes eight exons and an open reading frame (ORF) for a protein of 336 amino acids. Bm-Sxl-S, in contrast, lacks the second exon and encodes an ORF that is truncated by 46 amino acids at the N terminus. We expressed Bm-Sxl-S and Bm-Sxl-L in D. melanogaster (SI Appendix, Supplementary Information Text). Ectopic expression of Bm-Sxl-L and Bm-Sxl-S led to sexual transformation (SI Appendix, Fig. S1A) and male-specific lethality (SI Appendix, Fig. S1B), respectively, in D. melanogaster. These sex-specific functions of Bm-Sxl and Dm-Sxl may indicate similar biochemical functions of proteins encoded by Bm-Sxl and Dm-Sxl. Sex determination and dosage compensation are regulated by the genes Fem and Masc in B. mori (13, 14), and mutation of Bm-Sxl had no physiological and morphological effects on somatic sexual determination (15). Moreover, temporal expression patterns of Bm-Sxl during embryogenesis were comparable between females and males in B. mori (SI Appendix, Fig. S2A). Together, these results suggest that Bm-Sxl does not regulate sex determination or dosage compensation in B. mori.
To predict the functions of Bm-Sxl in B. mori, we examined expression patterns of Bm-Sxl protein using an anti–Dm-Sxl antibody (SI Appendix, Materials and Methods and Fig. S3). In these experiments, Bm-Sxl was mainly expressed in adult testis (Fig. 2A). Expression patterns of Bm-Sxl-L and Bm-Sxl-S also differed during testis development. Bm-Sxl-L expression was observed at early stages in last instar larvae, whereas Bm-Sxl-S expression was initiated from a later stage, beginning in 4-d-old last instar larvae (SI Appendix, Fig. S2 B and C). As shown in Fig. 2B, pupal testes comprise the four testicular follicles, and these follicles are comprised of the following: spermatogonia, spermatocytes, spermatids, and sperm. Bm-Sxl was expressed in primary spermatocytes in the testis of fifth instar larvae and pupae (Fig. 2C and SI Appendix, Fig. S4).
Fig. 2.
Bm-Sxl expression. (A) Tissue distributions of Bm-Sxl protein in male and female adults. Western blotting was performed using a monoclonal anti–Dm-Sxl antibody. Protein aliquots of 15 μg were loaded into each lane. (B) Schematic of a testicular follicle at the pupal stage containing spermatogonia (red), spermatocytes (blue), spermatids (yellow), and sperm (green). (C) Localization of Bm-Sxl at a testicular follicle in a 4-d-old pupa; green, blue, and red signals indicate mitochondria (Mit.), Bm-Sxl (Sxl), and nuclei (Nuc.), respectively. (Scale bar, 500 μm.)
Next, we examined the subcellular localization of Bm-Sxl in the spermatocytes. Our results suggested the distinct subcellular localization between the Bm-Sxl-L and Bm-Sxl-S. In particular, immunohistochemical analyses of 1-d-old fifth instar larvae showed Bm-Sxl localized in the cytoplasms of spermatocytes (SI Appendix, Fig. S5 A–C), and Western blotting analyses confirmed that only the L isoform is expressed in 1-d-old fifth instar larvae (SI Appendix, Fig. S2B), indicating that the L isoform is localized in the cytoplasm of spermatocytes. Conversely, Bm-Sxl was mainly localized in nuclei of spermatocytes in 4-d-old pupae (SI Appendix, Fig. S5 D–F). Because the S isoform was mainly expressed in 4-d-old pupae (SI Appendix, Fig. S2C), it was localized in nuclei of spermatocytes. This distinct subcellular localization between Bm-Sxl isoforms implies that each isoform may have a different role in the spermatocytes.
The present data indicate that Bm-Sxl may be involved in spermatogenesis. In B. mori, meiotic divisions for eupyrene sperm mainly occur during the early fifth instar, whereas meiosis for apyrene sperm starts before the spinning stage, which corresponds with 5- to 6-d-old fifth instar larvae in this strain and continues during the pupal stage. High expression of Bm-Sxl-S was observed from immediately before the spinning stage (SI Appendix, Fig. S2B), corresponding well with the developmental stage for the transition from eupyrene to apyrene spermatogenesis. Apyrene sperm are considered an apomorphic trait in advanced Lepidoptera. Moreover, expression patterns of Sxl in five representative lepidopteran species from different families showed that Sxl-S is mainly expressed at the pupal stage (i.e., the apyrene sperm formation period) (SI Appendix, Fig. S6). Therefore, Sxl-S is likely involved in apyrene sperm formation.
To investigate the functions of Bm-Sxl in spermatogenesis, we generated Bm-Sxl mutants using transcription activator-like effector nuclease (TALEN) constructs targeting coding sequences in exon 2 of the Bm-Sxl-L–specific region (TALEN 1-2) and targeting the RRM motif (TALEN 3-4) that is common to both Bm-Sxl-L and Bm-Sxl-S (Fig. 3A). Subsequently, we generated three Bm-Sxl-L deletion mutants (Sxl-LΔ13/Sxl-LΔ13, Sxl-LΔ9/Sxl-LΔ9, and Sxl-LΔ6/Sxl-LΔ6) that were induced by TALEN 1-2. The 13-bp deletion in Sxl-LΔ13 is a frameshift mutation, whereas the 9-bp (Sxl-LΔ9) and 6-bp (Sxl-LΔ6) deletions truncate the protein by three and two amino acids, respectively. All of these mutants were fertile, and no aberrant phenotypes were observed in comparisons with control (+/+) insects (SI Appendix, Fig. S7A and Table S1A). Hence, Bm-Sxl-L is likely dispensable for spermatogenesis and survival, or has some redundancy in the presence of Bm-Sxl-S. As for the mutants targeting RRM1, which is highly conserved in insects (SI Appendix, Fig. S7B), a 3-bp insertion (SxlIn3/SxlIn3) and a 6-bp deletion (SxlΔ6/SxlΔ6) were induced by TALEN 3-4 (Fig. 3B). Crosses of the three genotypes (+/+, +/SxlIn3, and SxlIn3/SxlIn3) revealed that only SxlIn3/SxlIn3 males have complete sterility (Fig. 3 C–E and SI Appendix, Table S1B). Because the same phenotype was also observed in SxlΔ6/SxlΔ6 mutants (SI Appendix, Fig. S7C and Table S1C), we conclude that mutations of RRM in Bm-Sxl cause male-specific sterility.
Fig. 3.
Bm-Sxl mutants show male-specific sterility. (A) Design of TALEN target sites; arrowheads indicate target sites of TALENs. The filled box is the ORF. The blue boxes indicate RRM-type RNA binding domains. TALEN 1-2 targeted the sequence of Bm-Sxl-L–specific exon 2. TALEN 3-4 targeted the sequence of RRM1. (B) Nucleotide sequences around TALEN target sites; deletions of 13 bp (Sxl-LΔ13), 9 bp (Sxl-LΔ9), and 6 bp (Sxl-LΔ6) were generated using TALEN 1-2. Deletion of 6 bp (SxlΔ6) and insertion of 3 bp (SxlIn3) was achieved using TALEN 3-4. The red underline indicates the recognition sequence of TALENs. Lowercase letters indicate nonprotein encoding sequences; capital letters indicate protein-encoding regions. (C) Eggs laid by a +/+ female crossed with a +/+ male; almost all eggs were fertilized (pale to deep purple). (Scale bar, 1 cm.) (D) Eggs laid by a SxlIn3/SxlIn3 female crossed with a SxlIn3/SxlIn3 male; no eggs were fertilized (yellowish). (Scale bar, 1 cm.) (E) Fertility of SxlIn3 mutants. Fertility was evaluated as the ratio of fertilized eggs to the total number of eggs (see Materials and Methods). The details of all fertility experiments are shown in the SI Appendix, Table S1B. n, number of crosses; error bars show SDs.
Approximately 85% of SxlΔ6/SxlΔ6 homo mutants died before hatching (SI Appendix, Supplementary Information Text, Fig. S7 D and E, and Table S2). Thus, we mainly used SxlIn3/SxlIn3 males in subsequent analyses. No external morphological abnormalities of the testis were observed in SxlIn3/SxlIn3 mutants. Because high Bm-Sxl expression in germ cells (Fig. 2C and SI Appendix, Figs. S4 and S5) suggests functions in spermatogenesis, we investigated the morphology of eupyrene and apyrene sperm.
As shown in Fig. 4 A and B, both eupyrene and apyrene sperm were observed as bundles in the testis before the peristaltic squeezing stage of early pupal development (27, 28). Moreover, no differences in eupyrene sperm bundles were observed between control (+/+) and mutant insects (+/SxlIn3 and SxlIn3/SxlIn3) (Fig. 4 A, C, and E). During the late stage of spermatogenesis, accurate apyrene sperm bundles, which are shorter than eupyrene sperm bundles, were located spherically with nuclei in the middle region (27, 28) in +/+ and +/SxlIn3 (Fig. 4 B and D). In contrast, accurate apyrene sperm bundles were not observed in SxlIn3/SxlIn3 mutants. Instead, apyrene-like sperm bundles in SxlIn3/SxlIn3 were as short as accurate apyrene sperm bundles but had nuclei scattered with sickle or ellipsoid shapes in the middle to anterior-half regions of sperm bundles (Fig. 4F). These nuclear shapes were observed during +/+ eupyrene spermatogenesis (27) (SI Appendix, Fig. S8 H and O). Considering that accurate apyrene sperm bundles were not observed in SxlIn3/SxlIn3 mutants, the spermatogenesis of apyrene sperm failed in SxlIn3/SxlIn3 mutants.
Fig. 4.
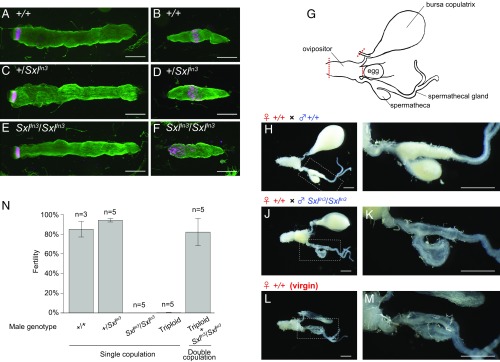
Analyses of Sxl In3/Sxl In3 mutants demonstrate that Sxl is essential for apyrene sperm formation. (A–F) Fluorescence microscopic images of (A, C, and E) eupyrene, (B and D) apyrene, and (F) apyrene-like sperm bundles from 4- to 5-d-old pupa with immunofluorescence staining for tubulin (green) and nuclei (magenta). (Scale bar, 100 μm.) (G–M) Semen in the bursa copulatrix and spermatheca. (G) Schematic of female reproductive organs; the dotted red line indicates the site where the incision was made. (H, J, and L) Bursa copulatrix and spermatheca from +/+ females crossed with +/+ (H) and SxlIn3/SxlIn3 (J) males, and those of an unmated virgin +/+ female (L). (Scale bar, 1 mm.) (I, K, and M) Magnified view of the area marked by the dotted box in H, J and L, respectively. (Scale bar, 1 mm.) (N) Fertility in double-copulation experiments; mating was performed with +/+ females. Fertility was determined as the ratio of fertilized eggs to the total number of eggs laid from a single pair or from double-copulation mating experiments (see Materials and Methods). Fertility details are shown in SI Appendix, Table S3. n, number of crosses; error bars indicate SD.
Sperm from +/+ and SxlIn3/SxlIn3 were ejaculated into the bursa copulatrix (Fig. 4 H and J). We observed sperm motility isolated from the bursa copulatrix. Eupyrene sperm of both +/+ and SxlIn3/SxlIn3 males showed motility (Movies S1 and S2). Apyrene sperm of +/+ males isolated from the bursa copulatrix showed high motility (Movie S3), whereas we could not detect any apyrene sperm of SxlIn3/SxlIn3 (Movie S4). Sperm from SxlIn3/SxlIn3 males were ejaculated into the bursa copulatrix, but no semen appeared in the spermatheca (Fig. 4K). These observations are not consistent with the migration of +/+ eupyrene spermatozoa (Fig. 4I). Hence, if failure of sperm migration in Sxl mutants was caused by dysfunction of apyrene sperm, addition of functional apyrene sperm may rescue fertility. To test this, we performed double-copulation experiments to rescue from dysfunctions of apyrene sperm (24) using triploid male silkworms that produce dysfunctional eupyrene and functional apyrene sperms (29). Single females copulated initially with a triploid male and then with a SxlIn3/SxlIn3 male, and subsequently laid eggs with normal fertility (82.1 ± 14.0%, mean ± SD; n = 5). However, almost no fertilized eggs were obtained from crosses of single +/+ females and single SxlIn3/SxlIn3 or triploid males (Fig. 4N and SI Appendix, Table S3). These results indicate that the loss of function of apyrene sperm in SxlIn3/SxlIn3 mutants results in defects of eupyrene sperm migration from the bursa copulatrix to the spermatheca (SI Appendix, Supplementary Information Text). Together, our results suggest that apyrene sperm are necessary for eupyrene sperm migration from the bursa copulatrix to the spermatheca.
The present data suggest that Bm-Sxl mutations cause mild embryonic lethality (SI Appendix, Supplementary Information Text and Fig. S7 D and E). Thus, we analyzed in-frame Bm-Sxl mutants with mutations corresponding with 1-aa insertions or 2-aa deletions in the RRM. These mutants showed male-specific sterility (Fig. 3E and SI Appendix, Fig. S7C). Considering that mutants with Bm-Sxl-L deletions were fertile (SI Appendix, Fig. S7A) and aberrant phenotypes were not observed in Bm-Sxl-L deletion mutants, the present male sterility was probably caused by mutations in Bm-Sxl-S.
Abnormal shapes of apyrene-like sperm bundle nuclei were observed in Sxl mutants (Fig. 4F). These nuclei formed sickle or ellipsoid shapes (SI Appendix, Fig. S8 H and L) that were similar to those observed during normal eupyrene spermatogenesis (ref. 27 and SI Appendix, Fig. S8O). Hence, in-frame mutations in the RRM region may cause incomplete shifts from eupyrene to apyrene spermatogenesis.
Morphological differences between eupyrene and apyrene spermatozoa are not visible in spermatogonia but appear in primary spermatocytes and become obvious at the meiotic metaphase (30). Because Bm-Sxl-S is expressed in primary spermatocytes (Fig. 2C), which have the potential to differentiate into apyrene or eupyrene sperm (Fig. 1A), Bm-Sxl-S may regulate the shift from eupyrene to apyrene spermatogenesis in primary spermatocytes.
Sxl mutants with dysfunctional apyrene sperm showed male-specific sterility (Fig. 3E), and fertility was restored after addition of functional apyrene sperm (Fig. 4N), suggesting that apyrene sperm is necessary for fertilization in B. mori. Moreover, we found that eupyrene sperm of Sxl mutants were ejaculated into the bursa copulatrix (Fig. 4J and Movie S2), but no eupyrene sperm appeared in the spermatheca (Fig. 4K). These results indicate that apyrene sperm are necessary for eupyrene sperm migration from the bursa copulatrix to the spermatheca. In recent years, delayed female remating has been a dominant explanation for apyrene sperm function in lepidopteran insects (20, 31). In agreement, storage of large numbers of apyrene sperm in the spermatheca prevented remating in the green-veined white (P. napi) (23). In contrast, our results clearly indicate that apyrene sperm are essential for fertilization in B. mori. However, because the delayed female remating hypothesis cannot apply to monandrous species (31), this cannot be the only function of apyrene sperm in lepidopteran insects. Accordingly, the present study emphasizes the importance of nonfertile sperm in fertilization.
Although the sequence of Sxl is evolutionarily conserved, the sex-determining functions of Sxl are not common to all insects (10–12). The Sxl gene is involved in meiotic recombination of female germ cells and in differentiation and proliferation during oogenesis in D. melanogaster (8, 32, 33). In addition, the Sxl homolog in the phorid fly Megaselia scalaris is expressed in gonadal tissues, but is not expressed in somatic tissues of adult flies (34). Bopp et al. (32) suggested that the germline function of Sxl may be widely conserved, as indicated by expression of Sxl in germ cells from nondrosophilid flies; this is unlike the sex-determining function in D. melanogaster. Our data indicate that Sxl is involved in apyrene sperm formation in B. mori. Although the roles of Sxl are female specific in D. melanogaster and male specific in B. mori, these functions are present in germ cells of both species. Therefore, the ancestral function of Sxl may contribute to germline development in insects.
Materials and Methods
B. mori Strains.
Several strains of B. mori were used in this study. The sex-linked black egg strain (No. 459) was used in analyses of Bm-Sxl expression patterns in embryos, the N4 strain was used in analyses of Bm-Sxl expression patterns in larvae and adults, and the Daizo strain was used in analyses of TALEN-induced mutations. Triploid B. mori was obtained as described previously (24) by mating tetraploid females with diploid males. Briefly, tetraploid individuals were induced by low temperature treatment at −10 °C for 24 h. F1 eggs from the progeny of re9 females and Tw1 males were used for tetraploid induction, and rs (sex-linked chocolate, red egg, and striped; sch/sch, re/re, and pS/pS) males were used to produce triploids.
Lepidopteran Insects.
Antheraea yamamai was provided by Z. Kajiura, Shinshu University, Ueda, Japan, and Agrius convolvuli was provided by K. Shirai, Shinshu University, Ueda, Japan. Mythimna separata was provided by T. Tanaka, Nagoya University, Nagoya, Japan, and Papilio protenor was purchased from Eiko-Kagaku. Testes proteins were extracted from 5-d-old fifth instar larvae and 7-d-old pupa of the species A. yamamai, whereas 3-d-old fifth instar larvae and 8-d-old pupa were from the species A. convolvuli, 2-d-old sixth instar larvae and 0-d-old pupa were from the species M. separata, and 1-d-old fifth instar larvae and 0-d-old pupa were generated from the species P. protenor.
Western Blotting Analyses.
Before Western blotting analyses, tissues were treated with SDS sample buffer containing 6.25 mM Tris-HCl, 1% SDS, 2% mercaptoethanol, and 10% glycerol for 5 min at 100 °C. Total protein quantities were determined using the Bradford Bio-Rad protein assay (Bio-Rad) according to the manufacturer’s protocols. Subsequently, 15-μg aliquots of total protein lysates were loaded into each lane, and proteins were separated by electrophoresis on separating SDS polyacrylamide gels (containing 12.5% acrylamide and stacking gels containing 6% acrylamide) and then transferred to nitrocellulose membranes (Toyo Roshi). Membranes were blocked with 5% skim milk, Tris-buffered saline, and 0.1% Tween 20 and then probed with primary and peroxidase-conjugated secondary antibodies. Immunoreactive bands were visualized by exposing Hyperfilm ECL (Amersham Pharnacia Biotech). Anti–Dm-Sxl monoclonal antibody [(M114); Developmental Studies Hybridoma Bank] was diluted 1:2,000 for use as the primary antibody. M114 was deposited into the Developmental Studies Hybridoma Bank by P. Schedl. Peroxidase-conjugated anti-goat IgG (Zymed) was diluted 1:5,000 for use as the secondary antibody.
As shown in Fig. 2A and SI Appendix, Fig. S2, two bands of 38 and 34 kDa were detected in B. mori. Molecular masses of Bm-Sxl-L and Bm-Sxl-S proteins that were estimated from the amino acid sequences (37.5 and 32.2 kDa) corresponded well with these two bands.
Immunohistological Observations of Testes.
Tissues were fixed in solutions containing 4% paraformaldehyde, 30 mM NaPO4, 100 mM NalO4, and 75 mM lysine (PLP) for 1 h on ice. After fixation, PLP solutions were sequentially substituted with 12%, 15%, and 18% sucrose in HBSS buffer containing 8 mg/mL NaCl, 0.4 mg/mL KCl, 0.25 mg/mL glucose, 0.03 mg/mL Na2HPO4, and 0.6 mg/mL Hepes. Tissues were frozen in O.C.T. Compound (Sakura Finetechnical) and then sectioned at a thickness of 20 μm using a Leica CM1950 cryostat (Leica Biosystems). Sections were fixed in 4% paraformaldehyde/PBS for 15 min to stabilize antigens, washed three times with PBS, incubated in methanol at −20 °C for 30 min, and washed again in PBS. Sections were blocked with 5% skim milk/PBS for 1 h, washed with PBS, and then treated overnight at 4 °C with anti–Dm-Sxl monoclonal antibody diluted at 1:200 in 5% skim milk/PBS. After washing in PBS three times, sections were treated for 1 h at 37 °C using 1:400 Cy 5-conjugated AffiniPure F(ab′)2 fragment donkey anti-mouse IgG (H+L) (Jackson ImmunoResearch) with 2.5 μg/mL propidium iodide and 1 mg/mL RNase. Sections were then washed three times in PBS and were treated for 1 h at room temperature with 100 nM Mito Tracker Green FM (Molecular Probes) diluted in 5% skim milk/PBS. Sections were finally washed three times in PBS and then covered and mounted with VECTASHIELD (Vector Laboratories) and observed using an LSM 510 confocal laser microscope (Carl Zeiss).
Observations of Sperm.
Sperm from the bursa copulatrix were smeared on coated MAS slide glasses (Matsunami Glass) and fixed with 4% paraformaldehyde in PBS for 30 min. Sperm nuclei were stained with 50 ng/mL DAPI dissolved in PBS for 30 min. Stained sperm were observed using an LSM 510 microscope (Carl Zeiss), and images were captured using an NY-D5500 camera (Nikon).
Immunohistological Observations of Sperm Bundles.
Sperm bundles were immunohistologically observed according to a previously described method with some modifications (35). Briefly, spermatids and sperm bundles from excised testes of pupae were smeared on coated MAS slide glasses (Matsunami Glass) and were doubly fixed in solutions containing 4% paraformaldehyde, 8 mM NaOH, 7 mM NaCl, and 0.05% Tween 20 in PBS for 30 min and then in acetone at −20 °C for 10 min. Mouse monoclonal antibody against human alpha-tubulin (CLT9002; Cederlane) was used for cross-hybridization to silkworm alpha-tubulin. This antibody and carboxytetramethylrhodamine-conjugated rabbit anti-mouse IgG (AnaSpec) were applied in sequence to slide glasses, and sperm nuclei were stained with 1 μg/mL DAPI in solution containing 20.8 mM 1,4-diazabicyclo [2.2.2] octane, 20 mM Tris-HCl (pH 8.0), and 90% glycerol. Stained specimens were observed using a Leica DM6000B fluorescence microscope (Leica Microsystems).
Generation of Bm-Sxl Mutants.
Bm-Sxl mutants were generated using TALENs as described previously (36, 37). Briefly, TALEN mRNAs (200 ng/µL for left and right TALEN mRNA) were injected into preblastoderm embryos of the Daizo strain. Targets sites of TALENs are indicated in Fig. 3 A and B. Primers used for screening mutants are listed in SI Appendix, Table S4.
Genotyping of Sxl Mutants.
Genotyping of SxlΔ6/SxlΔ6 and SxlIn3/SxlIn3 mutants was performed using PCR. Primers pairs for PCR are listed in the SI Appendix, Table S4 and were designed to amplify TALEN targeted regions. Template DNA was extracted from adult legs, adult heads, or larval haemonymphs using GenCheck (FASMAC) according to the manufacturer’s instructions. Subsequently, PCR was performed with 0.5-μL aliquots of DNA template and AmpliTaq Gold 360 (Applied Biosystems) or TaKaRa ExTaq (TaKaRa) according to the manufacturers’ protocols. Amplified PCR products were electrophoresed on 4% agarose gels, and size differences of amplified products between +/+ and mutant were determined.
Assessments of Fertility.
Fertilized eggs are easily distinguished from nonfertilized eggs by the presence of pigmentation and embryogenesis. Diapause eggs, which are pigmented, and nondiapause eggs that had progressed to embryogenesis, were regarded as fertilized eggs. Fertility was evaluated as the ratio of fertilized eggs to total numbers of eggs laid by single females.
Image Processing.
All figures and movies were produced using Adobe Photoshop CS6 (Adobe Systems) and Adobe Illustrator CS6 (Adobe Systems). Linear adjustments of contrast, brightness, and color were applied consistently across entire images. In figures that were assembled from multiple photographs, separate parts are indicated by white lines.
Supplementary Material
Acknowledgments
We thank D. J. Emlen for critically reading the manuscript; T. Ando, S. Morita, T. Konagaya, and Y. Chikami for discussions; T. Kadowaki and J. F. Ferveur for providing fly strains; and Z. Kajiura, K. Shirai, and T. Tanaka for providing lepidopteran insects. We thank the National Institute of Agrobiological Science for providing silkworm strain No. 459; the National BioResource Project of Japan for providing A. yamamai and for their technical support; the Laboratory of Applied Molecular Entomology at Hokkaido University for supplying silkworm strains re9, Tw1, and rs and for polyploid induction by Y. Yamada; the Developmental Studies Hybridoma Bank maintained by the University of Iowa for the monoclonal antibody M114; and the Model Plant Research Facility at the National Institute for Basic Biology (NIBB) BioResource Center for their technical support. Confocal images using Fluoview FV1000 were acquired at the Spectrography and Bioimaging Facility, NIBB Core Research Facilities. This work was supported by the Japanese Government Grants-in-Aid for Scientific Research (KAKENHI) Grant JP16H01260 (to T.N.) and the Japan Society for the Promotion of Science KAKENHI Grant JP17J05973 (to H.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1820101116/-/DCSupplemental.
References
- 1.Bachtrog D, et al. Tree of Sex Consortium Sex determination: Why so many ways of doing it? PLoS Biol. 2014;12:e1001899. doi: 10.1371/journal.pbio.1001899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gempe T, Beye M. Function and evolution of sex determination mechanisms, genes and pathways in insects. BioEssays. 2011;33:52–60. doi: 10.1002/bies.201000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bopp D, Saccone G, Beye M. Sex determination in insects: Variations on a common theme. Sex Dev. 2014;8:20–28. doi: 10.1159/000356458. [DOI] [PubMed] [Google Scholar]
- 4.Bell LR, Maine EM, Schedl P, Cline TW. Sex-lethal, a Drosophila sex determination switch gene, exhibits sex-specific RNA splicing and sequence similarity to RNA binding proteins. Cell. 1988;55:1037–1046. doi: 10.1016/0092-8674(88)90248-6. [DOI] [PubMed] [Google Scholar]
- 5.Kelley RL, Wang J, Bell L, Kuroda MI. Sex lethal controls dosage compensation in Drosophila by a non-splicing mechanism. Nature. 1997;387:195–199. doi: 10.1038/387195a0. [DOI] [PubMed] [Google Scholar]
- 6.Salz HK. Sex determination in insects: A binary decision based on alternative splicing. Curr Opin Genet Dev. 2011;21:395–400. doi: 10.1016/j.gde.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashiyama K, Hayashi Y, Kobayashi S. Drosophila Sex lethal gene initiates female development in germline progenitors. Science. 2011;333:885–888. doi: 10.1126/science.1208146. [DOI] [PubMed] [Google Scholar]
- 8.Chau J, Kulnane LS, Salz HK. Sex-lethal enables germline stem cell differentiation by down-regulating Nanos protein levels during Drosophila oogenesis. Proc Natl Acad Sci USA. 2012;109:9465–9470. doi: 10.1073/pnas.1120473109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shapiro-Kulnane L, Smolko AE, Salz HK. Maintenance of Drosophila germline stem cell sexual identity in oogenesis and tumorigenesis. Development. 2015;142:1073–1082. doi: 10.1242/dev.116590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Traut W, Niimi T, Ikeo K, Sahara K. Phylogeny of the sex-determining gene Sex-lethal in insects. Genome. 2006;49:254–262. doi: 10.1139/g05-107. [DOI] [PubMed] [Google Scholar]
- 11.Meise M, et al. Sex-lethal, the master sex-determining gene in Drosophila, is not sex-specifically regulated in Musca domestica. Development. 1998;125:1487–1494. doi: 10.1242/dev.125.8.1487. [DOI] [PubMed] [Google Scholar]
- 12.Saccone G, et al. The Ceratitis capitata homologue of the Drosophila sex-determining gene Sex-lethal is structurally conserved, but not sex-specifically regulated. Development. 1998;125:1495–1500. doi: 10.1242/dev.125.8.1495. [DOI] [PubMed] [Google Scholar]
- 13.Kiuchi T, et al. A single female-specific piRNA is the primary determiner of sex in the silkworm. Nature. 2014;509:633–636. doi: 10.1038/nature13315. [DOI] [PubMed] [Google Scholar]
- 14.Sakai H, et al. Transgenic expression of the piRNA-resistant Masculinizer gene induces female-specific lethality and partial female-to-male sex reversal in the silkworm, Bombyx mori. PLoS Genet. 2016;12:e1006203. doi: 10.1371/journal.pgen.1006203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu J, et al. Bombyx mori P-element somatic inhibitor (BmPSI) is a key auxiliary factor for silkworm male sex determination. PLoS Genet. 2017;13:e1006576. doi: 10.1371/journal.pgen.1006576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pitnick S, Hosken DJ, Birkhead TR. Sperm morphological diversity. In: Birkhead TR, Hosken DJ, Pitnick S, editors. Sperm Biology: An Evolutionary Perspective. Academic Press; Oxford: 2008. pp. 69–149. [Google Scholar]
- 17.von Siebold CT. Fernere Beobachtungen uber die Spermatozoen der wirbellosen Tiere. Müller’s Archiv Anat Physiol Wiss Med. 1836;1:232–265. German. [Google Scholar]
- 18.Hayakawa Y. Parasperm: Morphological and functional studies on nonfertile sperm. Ichthyol Res. 2007;54:111–130. [Google Scholar]
- 19.Higginson DM, Pitnick S. Evolution of intra-ejaculate sperm interactions: Do sperm cooperate? Biol Rev Camb Philos Soc. 2011;86:249–270. doi: 10.1111/j.1469-185X.2010.00147.x. [DOI] [PubMed] [Google Scholar]
- 20.Swallow JG, Wilkinson GS. The long and short of sperm polymorphisms in insects. Biol Rev Camb Philos Soc. 2002;77:153–182. doi: 10.1017/s1464793101005851. [DOI] [PubMed] [Google Scholar]
- 21.Friedländer M. Control of the eupyrene-apyrene sperm dimorphism in Lepidoptera. J Insect Physiol. 1997;43:1085–1092. doi: 10.1016/s0022-1910(97)00044-9. [DOI] [PubMed] [Google Scholar]
- 22.Friedländer M, Seth RK, Reynolds SE. Eupyrene and apyrene sperm: Dichotomous spermatogenesis in Lepidoptera. Adv Insect Physiol. 2005;32:206–308. [Google Scholar]
- 23.Cook PA, Wedell N. Non-fertile sperm delay female remating. Nature. 1999;397:486. [Google Scholar]
- 24.Sahara K, Kawamura N. Double copulation of a female with sterile diploid and polyploid males recovers fertility in Bombyx mori. Zygote. 2002;10:23–29. doi: 10.1017/s0967199402002046. [DOI] [PubMed] [Google Scholar]
- 25.Sahara K, Takemura Y. Application of artificial insemination technique to eupyrene and/or apyrene sperm in Bombyx mori. J Exp Zool A Comp Exp Biol. 2003;297:196–200. doi: 10.1002/jez.a.10250. [DOI] [PubMed] [Google Scholar]
- 26.Niimi T, et al. Molecular cloning and chromosomal localization of the Bombyx Sex-lethal gene. Genome. 2006;49:263–268. doi: 10.1139/g05-108. [DOI] [PubMed] [Google Scholar]
- 27.Sahara K, Kawamura N. Roles of actin networks in peristaltic squeezing of sperm bundles in Bombyx mori. J Morphol. 2004;259:1–6. doi: 10.1002/jmor.10168. [DOI] [PubMed] [Google Scholar]
- 28.Kawamura N, Yamashiki N, Saitoh H, Sahara K. Peristaltic squeezing of sperm bundles at the late stage of spermatogenesis in the silkworm, Bombyx mori. J Morphol. 2000;246:53–58. doi: 10.1002/1097-4687(200011)246:2<53::AID-JMOR1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 29.Kawamura N, Yamashiki N, Saitoh H, Sahara K. Significance of peristaltic squeezing of sperm bundles in the silkworm, Bombyx mori: Elimination of irregular eupyrene sperm nuclei of the triploid. Zygote. 2001;9:159–166. doi: 10.1017/s0967199401001174. [DOI] [PubMed] [Google Scholar]
- 30.Matsuda Y, Yamashiki N. Microtubule dynamics and distribution of γ-tubulin in male germ cells of Bombyx mori (Lepidoptera) J Insect Biotechnol Sericol. 2007;76:113–120. [Google Scholar]
- 31.Konagaya T, Idogawa N, Watanabe M. Motility and dynamics of eupyrene and apyrene sperm in the spermatheca of the monandrous swallowtail butterfly Byasa alcinous. Physiol Entomol. 2016;41:185–192. [Google Scholar]
- 32.Bopp D, Schütt C, Puro J, Huang H, Nöthiger R. Recombination and disjunction in female germ cells of Drosophila depend on the germline activity of the gene Sex-lethal. Development. 1999;126:5785–5794. doi: 10.1242/dev.126.24.5785. [DOI] [PubMed] [Google Scholar]
- 33.Wang Z, Lin H. Sex-lethal is a target of Bruno-mediated translational repression in promoting the differentiation of stem cell progeny during Drosophila oogenesis. Dev Biol. 2007;302:160–168. doi: 10.1016/j.ydbio.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sievert V, Kuhn S, Traut W. Expression of the sex determining cascade genes Sex-lethal and doublesex in the phorid fly Megaselia scalaris. Genome. 1997;40:211–214. doi: 10.1139/g97-030. [DOI] [PubMed] [Google Scholar]
- 35.Kawamura N, Sahara K. In vitro cultivation of spermatocysts to matured sperm in the silkworm Bombyx mori. Dev Growth Differ. 2002;44:273–280. doi: 10.1046/j.1440-169x.2002.00641.x. [DOI] [PubMed] [Google Scholar]
- 36.Daimon T, Kiuchi T, Takasu Y. Recent progress in genome engineering techniques in the silkworm, Bombyx mori. Dev Growth Differ. 2014;56:14–25. doi: 10.1111/dgd.12096. [DOI] [PubMed] [Google Scholar]
- 37.Takasu Y, et al. Efficient TALEN construction for Bombyx mori gene targeting. PLoS One. 2013;8:e73458. doi: 10.1371/journal.pone.0073458. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.