Significance
Long-duration spaceflight induces detrimental changes in human physiology due to microgravity. One example is a cephalic fluid shift. Here, we prospectively investigated the quantitative changes in cerebrospinal fluid (CSF) volume of the brain ventricular regions in space crew by means of a region of interest, observer-independent analysis on structural brain MRI scans. MRI scans were collected before the mission, shortly after and 7 mo after return to Earth. We found a significant increase in lateral and third ventricles at postflight and a trend to normalization at follow-up, but still significantly increased ventricular volumes. The observed spatiotemporal pattern of CSF compartment enlargement and recovery points to a reduced CSF resorption in microgravity as the underlying cause.
Keywords: spaceflight, microgravity, brain, ventricles, CSF
Abstract
Long-duration spaceflight induces detrimental changes in human physiology. Its residual effects and mechanisms remain unclear. We prospectively investigated the changes in cerebrospinal fluid (CSF) volume of the brain ventricular regions in space crew by means of a region of interest analysis on structural brain scans. Cosmonaut MRI data were investigated preflight (n = 11), postflight (n = 11), and at long-term follow-up 7 mo after landing (n = 7). Post hoc analyses revealed a significant difference between preflight and postflight values for all supratentorial ventricular structures, i.e., lateral ventricle (mean % change ± SE = 13.3 ± 1.9), third ventricle (mean % change ± SE = 10.4 ± 1.1), and the total ventricular volume (mean % change ± SE = 11.6 ± 1.5) (all P < 0.0001), with higher volumes at postflight. At follow-up, these structures did not quite reach baseline levels, with still residual increases in volume for the lateral ventricle (mean % change ± SE = 7.7 ± 1.6; P = 0.0009), the third ventricle (mean % change ± SE = 4.7 ± 1.3; P = 0.0063), and the total ventricular volume (mean % change ± SE = 6.4 ± 1.3; P = 0.0008). This spatiotemporal pattern of CSF compartment enlargement and recovery points to a reduced CSF resorption in microgravity as the underlying cause. Our results warrant more detailed and longer longitudinal follow-up. The clinical impact of our findings on the long-term cosmonauts’ health and their relation to ocular changes reported in space travelers requires further prospective studies.
Long-duration spaceflight is associated with several factors that can induce detrimental changes across many human physiological systems (1). An example hereof is the cephalic fluid shift that occurs immediately upon entering a microgravity environment because of the loss of hydrostatic pressures within the caudocranial fluid columns of the body (e.g., arterial and venous structures) (2). It has been suggested that this fluid shift is associated with ocular and visual acuity changes, a clinical syndrome that has been coined Visual Impairment Intracranial Pressure (VIIP) syndrome (3) and, more recently, redefined as Spaceflight-Associated Neuro-ocular Syndrome (SANS) (4). VIIP or SANS has been reported to present with globe flattening, choroidal folds, optic disk edema, and a hyperopic visual shift (see refs. 2 and 3 for an overview), with a potential risk of permanent visual acuity changes (5). The exact mechanisms underlying these visual changes related to long-duration spaceflight, however, are still unclear.
Previous studies have observed a cerebrospinal fluid (CSF) compartment enlargement, including the subarachnoid space, directly after long-duration spaceflight (6, 7). However, to date, no coherent imaging dataset and observer-independent analysis are available to quantify CSF volume changes longitudinally including a follow-up period longer than 1 mo after return to Earth.
In this prospective study, we therefore aimed to assess and quantify ventricular volume changes in cosmonauts and matched controls on a group level by means of an observer-independent region-of-interest analysis of CSF spaces derived from structural MRI brain scans before, shortly after, and 7 mo after long-duration spaceflight. We compared these findings together with other published analyses to try to unravel the underlying dynamics in CSF compartment changes.
Materials and Methods
Participants.
Data were prospectively collected between February 2014 and May 2018. Eleven Roscosmos cosmonauts were included in the study; all of them participated in a long-duration space mission to the International Space Station (ISS). In addition, 11 age-, gender-, and education-matched controls were also recruited. An overview of demographical data of both cohorts can be found in Table 1.
Table 1.
General demographics and mission-related information for the cosmonauts and matched controls
| Cosmonauts | Controls | ||||
| Parameter | Mean | SD | Mean | SD | P value* |
| Age, y | 44.6 | 4.4 | 43.6 | 6.0 | 0.44 |
| Days to launch at preflight scan | 85 | 34 | |||
| Days after return at postflight scan | 9.9 | 2.9 | |||
| Scan interval,† d | 264 | 27 | 236 | 54 | 0.27 |
| Days after return at follow-up scan‡ | 214 | 45 | |||
| Prior space experience, d | 221 | 248 | |||
| Mission duration, d | 169 | 24 | |||
P value from Mann−Whitney u test between cosmonauts and matched controls.
Scan interval between preflight and postflight scan for cosmonauts and between two scans for matched controls.
Data from seven cosmonauts at follow-up. All other measures were obtained from data of 11 cosmonauts.
Each cosmonaut was scanned at least twice: once before launch (preflight) and once shortly after return to Earth (postflight). Seven cosmonauts could be scanned again at a third time point (follow-up), on average, 7 mo after their initial return to Earth. The matched control group was scanned twice, with a space mission-matched time interval (on average, 235 d) in between the two time points, on the same scanner to test for potentially confounding aging processes and scanner signal drift over time.
All participants, both cosmonauts and controls, provided a signed informed consent. All clinical investigations have been performed according to the principles expressed in the Declaration of Helsinki. This study was approved by the Institutional Review Board of Antwerp University Hospital (13/38/357), the European Space Agency (ESA) medical board, the Committee of Biomedicine Ethics of the Institute of Biomedical Problems of the Russian Academy of Sciences, and the Human Research Multilateral Review Board.
Data Acquisition.
We prospectively collected data on a 3T MRI scanner (Discovery MR750; GE Healthcare), located at the Federal Center for Treatment and Rehabilitation in Moscow, Russia, using a 16-channel head and neck array coil. For each scan at each time point, a high-resolution sagittal T1-weighted 3D fast spoiled gradient echo image was acquired (repetition time 7.90 ms; echo time 3.06 ms; inversion time 450 ms; voxel size 1 × 1 × 1 mm; flip angle 12°; field of view 240 mm, 180 slices; bandwidth 31.25 Hz). Data acquisition and protocol was identical for each time point and for cosmonauts and controls alike.
Data Quality Assurance and Preprocessing.
Data quality assurance (sample homogeneity), data preprocessing, and analysis were performed with the CAT12 toolbox (version 1363) by Christian Gaser and Robert Dahnke (Department of Psychiatry, University of Jena, Jena, Germany; http://www.neuro.uni-jena.de/cat) within the framework of Statistical Parametric Mapping SPM12 (8) (Version 7219) (https://www.fil.ion.ucl.ac.uk/spm/; Wellcome Department of Cognitive Neurology), using Matlab R2017b (Mathworks). In addition to verifying sample homogeneity within the CAT12 toolbox, a separate quality analysis of the structural data to detect acquisition artifacts and excessive head motion during the structural scanning was performed using MRI Quality Control tool (MRIQC) (9).
The data were processed using the longitudinal preprocessing option of CAT12, where spatial normalization parameters are calculated using an average image of the two time points. This was again applied to the first and the second and third images. Afterward, we derived tissue volume estimation in native space using the ventricle regions of interest (ROIs) from the Neuromorphometric atlas (provided by Neuromorphometrics, Inc.; http://neuromorphometrics.com). In total, the three ROIs applicable to the CSF space were included: lateral ventricle (a sum of left and right), third ventricle, and fourth ventricle. In addition, the sum of all ventricular volumes was calculated, yielding a total ventricular CSF volume measurement. These ROIs—which are defined in MNI template space—were transformed to native subject space for the actual volume calculations using the inverse nonlinear deformations needed to spatially normalize images to diffeomorphic anatomical registration through exponentiated lie algebra (DARTEL) template space with the geodesic shooting algorithm.
Visual Acuity Performance.
In each of the 11 cosmonauts, visual acuity scores were acquired before launch and 3 d after return from space for each eye individually as part of a different study not in sync with the MRI acquisitions. All tests were conducted in the morning. Subjects were presented with Sivtsev’s tables using the Rotta apparatus at 5 m distance. A score of 1 represents the healthy population norm, with higher scores corresponding to better visual acuity. The difference in visual acuity score between preflight and postflight was calculated, rendering negative values for visual acuity decreases at postflight compared with preflight.
Statistical Analysis.
To test for differences in these regional brain ventricular volumes between the preflight, the postflight, and the follow-up measurements of the cosmonauts, we fitted a linear mixed model with time point as fixed effect and individual as random effect. In case the fixed effect of time was significant, a post hoc analysis with Tukey correction was carried out. Furthermore, we tested the interaction effect of group (cosmonauts and controls) and time (preflight and postflight) using linear mixed models. Group, time, and the interaction effect were set as fixed effects, while individual was set as random effect. A post hoc analysis was carried out if the interaction effect was significant, to look at which comparison(s) drove the significant interaction effect. An alpha level of 0.017 was used for the significance of the fixed effects, corrected for multiple testing. These statistical analyses were performed using JMP software (JMP, Version 13; SAS Institute Inc.).
Descriptive statistics were used to characterize the study samples. For the cosmonauts, the relationship between total brain ventricular volume change (preflight vs. postflight) and mission duration, age at launch, previous space experience, total intracranial volume, and change in visual acuity scores (preflight vs. postflight) was investigated by means of a nonparametric Kendall’s Tau correlation analysis. Also, a Wilcoxon signed rank test was performed to statistically compare postflight with preflight visual acuity data for each eye separately. Finally, nonparametric Mann−Whitney u tests were used to compare the differences in age and scan interval between the cosmonauts and matched controls. These statistical analyses were performed with SPSS version 24.0 (IBM Corp.).
Results
Linear mixed model analysis revealed a significant effect of time on the ventricular volume measurements in the cosmonauts for the lateral ventricle, third ventricle, and total ventricular volume (all P < 0.0001). There was no significant effect of time on the volume of the fourth ventricle for the cosmonauts.
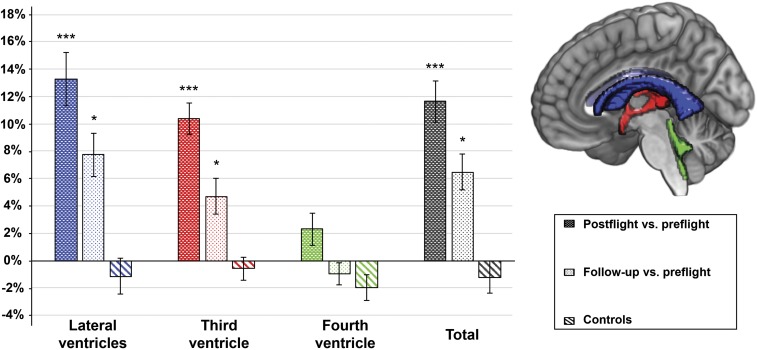
Post hoc analyses in the cosmonaut group revealed a significant difference between preflight and postflight values in the lateral ventricle (mean % change ± SE = 13.3 ± 1.9; P < 0.0001), third ventricle (mean % change ± SE = 10.4 ± 1.1; P < 0.0001), and total ventricular volume (mean % change ± SE = 11.6 ± 1.5; P < 0.0001), with higher volumes at postflight. Comparison between postflight and follow-up showed a significant difference for the third ventricle (mean % change ± SE = −5.7 ± 0.4; P = 0.0002) and total ventricular volume (mean % change ± SE = −3.9 ± 1.0; P = 0.0104). Finally, comparison between preflight and follow-up revealed significant differences for the lateral ventricle (mean % change ± SE = 7.7 ± 1.6; P = 0.0009), the third ventricle (mean % change ± SE = 4.7 ± 1.3; P = 0.0063), and total ventricular volume (mean % change ± SE = 6.4 ± 1.3; P = 0.0008). These results are summarized in Fig. 1, which shows the percentage volume difference for each ventricular compartment. In addition, the interaction effect of group and time was significant for the lateral ventricle (cosmonauts mean % change ± SE = 13.3 ± 1.9; controls mean % change ± SE = −1.2 ± 1.3; P = 0.0001), third ventricle (cosmonauts mean % change ± SE = 10.4 ± 1.1; controls mean % change ± SE = −0.6 ± 0.9; P < 0.0001), fourth ventricle (cosmonauts mean % change ± SE = 2.3 ± 1.2; controls mean % change ± SE = −2.0 ± 1.0; P = 0.0102), and total ventricular volume (cosmonauts mean % change ± SE = 11.6 ± 1.5; controls mean % change ± SE = −1.2 ± 1.2; P < 0.0001). Post hoc analyses revealed that the significant interaction effect was driven by the volume change between preflight and postflight measurements in cosmonauts for third, lateral, and total ventricular volume (P < 0.0001). For the fourth ventricle, none of the post hoc tests revealed significance.
Fig. 1.
Percentage volume change for each ventricular CSF compartment, as well as total ventricular volume change given for comparisons postflight vs. preflight (dark colored bar) and follow-up vs. preflight (light colored bar). The percentage volume difference between the two time points for the controls is also given (diagonal stripes). The bar colors represent each ventricular ROI, as also indicated on the sagittal slice. A positive value indicates an increase in ventricular volume at postflight compared with preflight or at follow-up compared with preflight. A negative value indicates a decrease in ventricular volume at postflight compared with preflight or at follow-up compared with preflight. ***P < 0.0001 (Bonferroni-corrected) and *P < 0.017 (Bonferroni-corrected), from post hoc tests of the linear mixed model. Bars indicate SE.
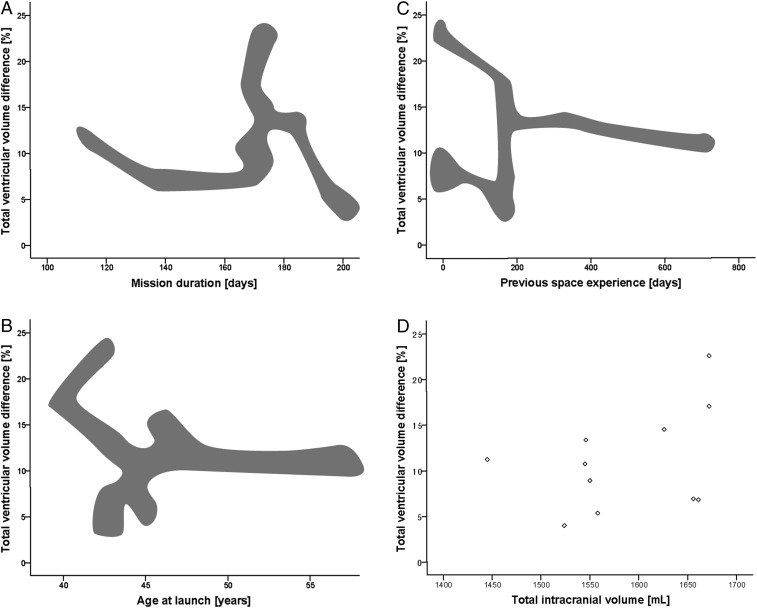
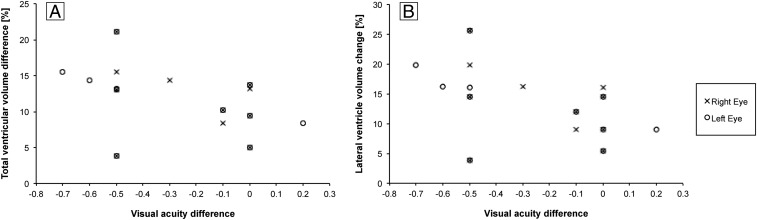
The correlation analyses showed no significant relationship between the percentage of total ventricular volume change and mission duration, age at launch, previous space experience, total intracranial volume (Fig. 2), and change in visual acuity scores (Fig. 3). However, the lateral ventricular volume change was significantly correlated with visual acuity change in the left eye only (r = −0.517; P = 0.035), showing greater ventricular volume increases for greater visual acuity loss (Fig. 3). Veiled original data (except for total intracranial volume) for each potential correlation analysis are shown in Fig. 2 to depict the distribution pattern.
Fig. 2.
Scatter plots showing the relationship between total relative brain ventricular volume change for preflight and postflight (in 11 cosmonauts) with respect to (A) mission duration (in days), (B) age at launch (in years), (C) previous space experience (in days), and (D) total intracranial volume (in milliliters). No significant correlations were found. Results in A, B, and C are blurred to guarantee anonymity of the cosmonauts, given the fact that most of the information is publicly available. The masking cloud follows the actual data distribution pattern, but is modified to disguise individual data points.
Fig. 3.
Scatter plots showing the relationship between (A) total and (B) lateral relative brain ventricular volume change for preflight and postflight (in 11 cosmonauts) with respect to visual acuity difference. No significant correlation was found for total ventricular volume difference and visual acuity difference, while a marginally significant correlation was found for lateral ventricular volume change and visual acuity difference for the left eye only.
Statistical comparison of postflight versus preflight visual acuity scores revealed a significant decrease postflight for both the left eye [median difference (median absolute deviation, MAD) = −0.3 (0.3); P = 0.023] and the right eye [median difference (MAD) = −0.1 (0.1); P = 0.016]. However, the visual acuity scores postflight did not fall below the clinical norm of 1.0.
No significant differences in ventricular volume were found in any of the ROIs for the control group over time or between cosmonauts and controls at any time point.
Discussion
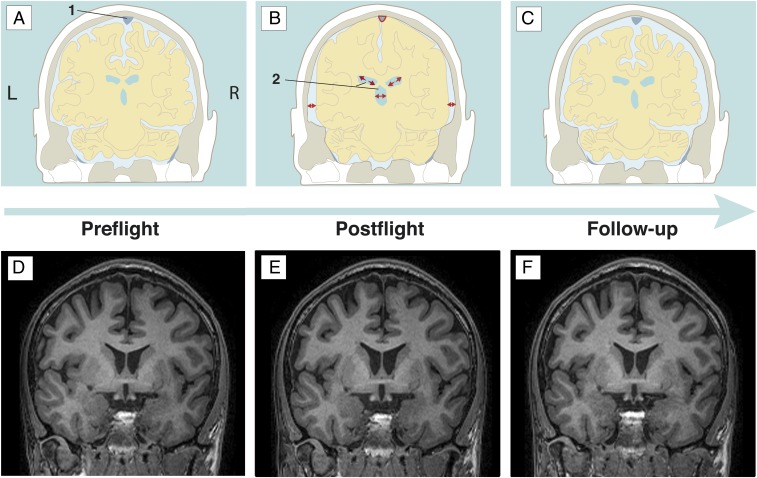
In summary, we found an increased ventricular CSF volume after spaceflight in supratentorial ventricular structures (i.e., lateral and the third ventricles), while the infratentorial fourth ventricle was not significantly enlarged. At follow-up, 7 mo after return from space, ventricular volume compared with preflight still had not completely returned to baseline levels, with still significant volume increases for the lateral and third ventricles. A schematic overview of our current findings together with previously established or observed CSF compartment changes can be found in Fig. 4. Our results indicate that a longitudinal and homogeneous within-cohort approach seems to be the most sensitive to investigate the effects of long-term exposure to microgravity in humans.
Fig. 4.
Overview of the changes occurring in the subarachnoid and intracerebral CSF spaces [including the superior sagittal sinus (area 1) and the ventricles (area 2) of the cosmonauts across the different time points. (A−C) Schematic coronal visualization, taking together the current findings as well as an overview of previously described changes in previous studies of long-duration space travelers (6, 7). (D−F) Exemplary individual raw data on similar coronal slices, from which, especially, the ventricular enlargement is visible to the untrained, naked eye. (A and D) Baseline status, i.e., the preflight situation. (B and E) Postflight situation (on average, 9 d after returning to Earth). Cerebral ventricular enlargement, widening of the subarachnoid CSF space around the temporal and parietal lobes (7), and a compression of the superior sagittal sinus (6, 7) and a narrower longitudinal fissure can be noted. (C and F) Illustrations of the situation at follow-up (on average, 7 mo after returning to Earth). They show a partial normalization of ventricular CSF volumes and rewidening of the superior sagittal sinus.
Previously, we reported alterations in total gray matter, white matter, and CSF tissue volumes after long-duration spaceflight (7). In addition, we found a progressive enlargement of the subarachnoid CSF space in cosmonauts after their return from space (7). Another MRI study in ISS astronauts also observed a ventricular enlargement after spaceflight in combination with a narrowing of the central sulcus (6). Upon visual inspection, the same study noted an upward brain shift in astronauts after long-duration spaceflight (6), a phenomenon that has also been described during head-down bed rest, a spaceflight analog (10). This upward shift of the brain could lead to a compression of the superior sagittal sinus and Pachioni’s granulations, two structures responsible for the majority of CSF resorption (11). Marginally elevated pressure levels in the cranial venous structures from the cephalad fluid shift or an obstruction at or near the superior sagittal sinus over longer periods of time could result in a generally reduced CSF resorption.
We hypothesize that the impact we see on the supratentorial regions is an indicator for the structural compliance and CSF reserve capacity of these ventricles acting as buffers in healthy adults. This might be considered a preliminary coping mechanism in space that acts as an intermediate overflow zone for the reduced CSF resorption. Furthermore, a previous study by Kramer et al. (12) found that astronauts with an increased CSF maximum systolic velocity did not show visual symptoms, suggesting an increased craniospinal compliance in these astronauts and thus a potential “negative” risk factor to VIIP. This could be attributed to a larger compensatory reserve capacity in CSF space [e.g., by a greater compliance and elasticity of the ventricles (13)] for the group of astronauts presenting without ophthalmic changes. This further suggests a potential link between these brain ventricular and CSF changes and VIIP/SANS symptoms. Furthermore, the hampered CSF resorption could subsequently lead to slightly elevated intracranial pressure akin to prodromal idiopathic intracranial hypertension and result in papilledema and loss of visual acuity (6), both known features of VIIP/SANS (3, 4). Recently, a modeling study has suggested that VIIP/SANS symptoms are mainly caused by the upward brain shift (14), and it has been put forward that CSF plays a primary role in the formation of VIIP (15). Lastly, the periventricular white matter changes previously reported in astronauts (16) might be a direct consequence of transependymal water diffusion from the enlarged ventricles into periventricular structures, due to pressure peaks in a backed up CSF circulation.
The previously reported and mentioned upward shift of the brain with or without a concurrent potential cerebral venous hypertension as a result of the cephalic fluid shift could be the underlying cause. Due to the overall restrictions to cosmonaut access, we were only able to conduct the postflight scan, on average, 10 d after return. Theoretically, the observed changes may already be partially reversed by the readaptation taking place immediately after landing back on Earth. We might therefore actually underestimate the degree to which CSF circulation might be perturbed. As such, an important gap in data collection the first few days after spaceflight currently remains. The exact temporal dynamics remain to be determined.
Our correlation analysis between the visual acuity data and the CSF volume changes revealed an association of lateral ventricular volume changes with visual acuity difference in the left eye. The greater the ventricular expansion, the more visual acuity was decreased postflight. However, no statistically significant result was observed for any other ventricular compartment or for the visual acuity change in the right eye. Please note that the visual acuity data were acquired 1 wk earlier than the MRI scan. Unfortunately, visual acuity data were not available at follow-up. We strongly encourage future studies to follow up this probable link using a coherent dataset, additional supporting measurements, and a higher sample size.
Furthermore, we assessed whether the ventricular volume enlargement would return back to preflight baseline levels at a 7-mo follow-up time point. Here, our results indicate that the observed structural changes are indeed subject to a long, yet incomplete, recovery process as shown in our data. At the same time, we already know that VIIP/SANS also shows a long recovery time, as concluded by Mader et al (5). They found prolonged symptoms of VIIP and elevated intracranial pressure values in seven astronauts and a decline period of symptoms up to 19 mo after spaceflight (5). Our current data corroborate these conclusions and suggest an overlap between brain ventricular volume changes and VIIP/SANS. In addition, one study has previously implemented a follow-up time point 1 mo after return from space for five astronauts (16). Here, an increase in ventricular volume after spaceflight was also seen in a qualitative assessment, which also appeared to be only partially normalized after 1 mo (16). The presented findings suggest the necessity for even longer follow-up intervals for neuroimaging data to reveal the complete course of the observed changes. We therefore see a need for a detailed neurological, ophthalmological, and neuroradiological evaluation from preflight to more than a year after landing for any long-duration space traveler, potentially in combination with inflight examinations (e.g., transtemporal and transocular ultrasound). A specific example hereof could be the assessment of CSF hydrodynamics by means of MRI, to quantify the physiological response to a long-term exposure to microgravity and to optimize countermeasures and risk mitigation for the potential clinical consequences of this phenomenon. In addition, risk factors, such as age, body mass index, head size, gender, CSF turnover rate, and craniospinal compliance (12), should be assessed to determine a specific individual’s likelihood to develop VIIP/SANS.
In summary, our results warrant further and more detailed longitudinal follow-up on Earth, potentially in combination with inflight examinations, to determine the precise temporal dynamics of the observed circulatory changes in the CSF system and within the cerebral venous vasculature downstream. The clinical impact of our findings on the long-term cosmonauts’ health and their relation to VIIP/SANS requires further prospective studies. Determining the underlying cause and then identifying and applying countermeasures seems pivotal for space crew health and for the success of future interplanetary missions.
Acknowledgments
We thank all cosmonauts and volunteers for their participation. We thank Jitka Annen for feedback on the draft version of the manuscript. This work was supported by ESA Grant ISLRA 2009-1062, Russian Science Foundation Grant N14-25-00167, Belgian Science Policy Prodex, the Research Foundation Flanders (FWO Vlaanderen) (to A.V.O. and B.J.), French Speaking Community Concerted Research Action Grant ARC-06/11-340, German Federal Ministry of Education and Research Grant 01 EO 0901 (to P.z.E. and R.M.R.), and Zonta International Amelia Earhart Fellowship 2016−2017 (to A.V.O.). B.J. is a Postdoctoral Fellow for FWO Vlaanderen; S.L. is Research Director at the Fonds de la Recherche Scientifique.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1820354116/-/DCSupplemental.
References
- 1.Clément G. (2011) Fundamentals of Space Medicine (Springer, Dordrecht, The Netherlands: ), 2nd Ed. [Google Scholar]
- 2.Nelson ES, Mulugeta L, Myers JG (2014) Microgravity-induced fluid shift and ophthalmic changes. Life (Basel) 4:621–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tarver W, Otto C (2012) NASA’s spaceflight visual impairment intracranial pressure (VIIP) risk: Clinical correlations and pathophysiology. Aerospace Medicine Grand Rounds (NASA, Washington, DC: ). [Google Scholar]
- 4.Lee AG, Mader TH, Gibson CR, Brunstetter TJ, Tarver WJ (2018) Space flight-associated neuro-ocular syndrome (SANS). Eye (Lond) 32:1164–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mader TH, et al. (2011) Optic disc edema, globe flattening, choroidal folds, and hyperopic shifts observed in astronauts after long-duration space flight. Ophthalmology 118:2058–2069. [DOI] [PubMed] [Google Scholar]
- 6.Roberts DR, et al. (2017) Effects of spaceflight on astronaut brain structure as indicated on MRI. N Engl J Med 377:1746–1753. [DOI] [PubMed] [Google Scholar]
- 7.Van Ombergen A, et al. (2018) Brain tissue-volume changes in cosmonauts. N Engl J Med 379:1678–1680. [DOI] [PubMed] [Google Scholar]
- 8.Ashburner J, Friston KJ (2000) Voxel-based morphometry–The methods. Neuroimage 11:805–821. [DOI] [PubMed] [Google Scholar]
- 9.Esteban O, et al. (2017) MRIQC: Advancing the automatic prediction of image quality in MRI from unseen sites. PLoS One 12:e0184661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts DR, et al. (2010) Cerebral cortex plasticity after 90 days of bed rest: Data from TMS and fMRI. Aviat Space Environ Med 81:30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakka L, Coll G, Chazal J (2011) Anatomy and physiology of cerebrospinal fluid. Eur Ann Otorhinolaryngol Head Neck Dis 128:309–316. [DOI] [PubMed] [Google Scholar]
- 12.Kramer LA, et al. (2015) MR-derived cerebral spinal fluid hydrodynamics as a marker and a risk factor for intracranial hypertension in astronauts exposed to microgravity. J Magn Reson Imaging 42:1560–1571. [DOI] [PubMed] [Google Scholar]
- 13.Masoumi N, et al. (2013) 2D computational fluid dynamic modeling of human ventricle system based on fluid-solid interaction and pulsatile flow. Basic Clin Neurosci 4:64–75. [PMC free article] [PubMed] [Google Scholar]
- 14.Shinojima A, Kakeya I, Tada S (2018) Association of space flight with Problems of the brain and eyes. JAMA Ophthalmol 136:1075–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alperin N, Bagci AM (2018) Spaceflight-induced visual impairment and globe deformations in astronauts are linked to orbital cerebrospinal fluid volume increase. Acta Neurochir Suppl 126:215–219. [DOI] [PubMed] [Google Scholar]
- 16.Alperin N, Bagci AM, Lee SH (2017) Spaceflight-induced changes in white matter hyperintensity burden in astronauts. Neurology 89:2187–2191. [DOI] [PubMed] [Google Scholar]