Abstract
Morbid obesity (body mass index [BMI] ≥40 kg/m2) is a relative contraindication to liver transplantation (LT) at many transplant centers. The safety and efficacy of pre-LT bariatric surgery in morbidly obese LT candidates is unknown. Herein, we describe a cohort study of morbidly obese LT candidates who failed to achieve adequate weight loss through a medically supervised weight loss program and subsequently underwent sleeve gastrectomy (SG) at our institution. In total, 32 LT candidates with a median Model for End-Stage Liver Disease (MELD) score of 12 (interquartile range [IQR], 10–13) underwent SG. All LT candidates had a history of hepatic decompensation, but complications of liver disease were required to be well controlled at the time of SG. Median pre-SG BMI was 45.0 kg/m2 (IQR, 42.1–49.0 kg/m2). There were no perioperative deaths or liver-related morbidity. One patient experienced major perioperative morbidity secondary to a gastric leak, which was managed nonoperatively. Median weight loss at 6 and 12 months after SG was 22.0 kg (IQR, 18.9–26.8 kg) and 31.0 kg (IQR, 23.6–50.3 kg), respectively, corresponding to a percentage of excess body weight lost of 33.4% and 52.4%. Within 6 months after SG, 28 (88%) candidates were deemed eligible for LT. Our center’s experience highlights the potential option of SG in morbidly obese LT candidates with advanced liver disease who might otherwise be excluded from pursuing LT.
Obesity-related liver disease is the fastest growing indication for liver transplantation (LT) in the United States.(1) In LT recipients, obesity is associated with an increased incidence of wound infections and dehiscence, biliary and cardiopulmonary complications, and overall infection, and confers a higher risk of post-LT complications related to metabolic syndrome.(2,3) As such, morbid obesity (body mass index [BMI] ≥40 kg/m2) is considered a relative contraindication to LT at many transplant centers.(4,5)
The optimal approach for the morbidly obese LT candidate is not yet defined.(6) Although potential candidates are often referred to medically supervised weight loss programs that include dietary counseling, exercise programs, and behavioral therapies, these programs rarely result in adequate or sustained weight loss in morbidly obese patients.(7) Moreover, patients with decompensated liver disease may have difficulty adhering to intensive lifestyle interventions. Bariatric surgery is more effective than intensive lifestyle interventions for weight loss and improvement in obesi-ty-related comorbidities in the general population.(8) However, given that patients with cirrhosis are generally at a higher risk of complications and liver decompensation with any major surgical procedure,(9) there are limited data to support the feasibility and efficacy of bariatric surgery in the pre-LT setting.(10)
To address this problem, our institution developed a bariatric transplant clinical program that offers bariatric surgery, specifically laparoscopic sleeve gastrectomy (SG), to obese LT candidates. In this study, we first aimed to examine the safety and efficacy of SG in obese patients with advanced liver disease. Second, we aimed to determine whether SG improves rates of LT eligibility.
Patients and Methods
This is a retrospective cohort study of all adults (≥18 years of age) who were evaluated for LT at the University of California, San Francisco, between January 1, 2006 and June 1, 2016 and subsequently underwent SG for obesity. All LT candidates had failed to achieve adequate weight loss after completion of a medically supervised weight loss program, and all LT candidates met the National Institutes of Health (NIH) clinical criteria for bariatric surgery, defined as either class 2 obesity (BMI, 35 to <40 kg/m2) and an obesity-related comorbidity or class 3 obesity (BMI ≥40 kg/m2).(11) Obesity-related comorbidities included hypertension, dyslipidemia, type 2 diabetes mellitus, coronary heart disease, congestive heart failure, stroke, osteoarthritis, gallstones, sleep apnea, or history of cancer, as defined by the NIH clinical guidelines in bariatric surgery.(11) Our institution’s multidisciplinary bariatric transplant program is composed of a team of surgeons, hepatologists, dieticians, pharmacists, and nurse clinical coordinators, all of whom provide input regarding the selection of appropriate candidates for pre-LT SG.
All patients completed a formal LT evaluation and had no clear contraindications to LT other than obesity and obesity-related comorbidities. LT candidates with Child-Pugh score >9, Model for End-Stage Liver Disease (MELD) score ≥20, and/or severe coagulopathy (international normalized ratio [INR] >2.5) were excluded from SG evaluation. Patients with ascites not controlled by either medications or placement of a portosystemic shunt and/or hepatic encephalopathy not controlled by medications were also excluded from evaluation. All patients were required to undergo upper endoscopy within 12 months prior to bariatric surgery, and those patients with large esophageal varices and/or gastric varices were required to undergo endoscopic treatment of varices or the placement of a portosystemic shunt. However, the presence of portal hypertension with or without intra-abdominal varices was not considered an absolute contraindication to pre-LT bariatric surgery at our institution.
All patients underwent laparoscopic SG. SG was performed by mobilizing the greater curve of the stomach starting approximately 5–7 cm from the pylorus and continuing to the left diaphragmatic crus using ultrasonic coagulation shears. The stomach was stapled with 4.2-mm (antrum) and 3.5-mm (body and fundus) linear staplers around a 38-Fr bougie. Orogastric methylene blue was used to test for leaks. A clear liquid diet was routinely started on the first postoperative day. All patients received standard-of-care vitamin and mineral supplementation after SG, which includes vitamin B12, calcium and vitamin D, and a multivitamin.
Patient characteristics were collected in the preoperative setting (within 90 days prior to SG) and included the following: age, sex, race/ethnicity, liver disease etiology and complications, obesity-related comorbidities, and anthropometric and laboratory measurements. Perioperative outcomes were assessed and included estimated intraoperative blood loss, need for conversion to an open surgical approach, hospital length of stay, perioperative complications, liver-related morbidity, and all-cause mortality. Perioperative complications, liver-related morbidity, and all-cause mortality were defined as events occurring within 90 days after SG. Perioperative complications were classified and graded based on the Clavien-Dindo classification of surgical complications.(12) Liver-related morbidity was defined as the acute onset of ascites, spontaneous bacterial peritonitis, variceal hemorrhage, hepatic encephalopathy, or hepatorenal syndrome. Weight loss outcomes and the effect of SG on obesity-related comorbidities were examined at 3-month intervals up to 12 months after SG. Diabetes resolution was defined as random glycated hemoglobin A1c (HbA1c) <6.5% and/or random serum glucose <200 mg/dL without oral hypoglycemic agents or insulin therapy.(13) LT eligibility was determined based on assessment in our institution’s multidisciplinary transplant program. The institutional review board at the University of California, San Francisco, approved this research.
Results
PATIENT CHARACTERISTICS
Patient characteristics are listed in Table 1. Among the 32 adults who comprised our study cohort, the median age at the time of SG was 55 years (interquartile range [IQR] 50–61 years) and 23 (72%) were female. The majority of patients were Caucasian (59%) and Hispanic or Latino (31%). Median excess body weight at the time of SG was 70.3 kg (IQR, 58.9–84.2 kg). Median BMI at the time of SG was 45.0 kg/m2 (IQR, 42.1–49.0 kg/m2); 22% of patients had a BMI ≥50 kg/m2. All patients had at least 1 obesity-related co-morbidity, and 22% had 4 or more comorbidities. The most prevalent obesity-related comorbidities included hypertension (75%), osteoarthritis (63%), dyslipidemia (47%), and diabetes mellitus (44%).
TABLE 1.
Baseline Patient Characteristics at Time of SG
| Characteristic | Value (n = 32) |
|---|---|
| Age, years | 55 (50−61) |
| Sex, female | 23 (72) |
| Race/ethnicity | |
| Caucasian | 19 (60) |
| Hispanic or Latino | 10 (31) |
| African American | 2 (6) |
| Other | 1 (3) |
| Excess body weight, kg | 70.3 (58.9−84.2) |
| BMI, kg/m2 | 45.0 (42.1−49.0) |
| 35–39.9 kg/m2 | 4 (12.5) |
| 40–44.9 kg/m2 | 12 (37.5) |
| 45–49.9 kg/m2 | 9 (28) |
| ≥50 kg/m2 | 7 (22) |
| Number of obesity-related comorbidities | |
| 1 | 8 (25) |
| 2–3 | 17 (53) |
| ≥4 | 7 (22) |
| Obesity-related comorbidities | |
| Diabetes mellitus type 2 | 14 (44) |
| Hypertension | 24 (75) |
| Dyslipidemia | 15 (47) |
| Obstructive sleep apnea | 12 (38) |
| Osteoarthritis | 20 (63) |
| Child-Pugh classification | |
| A | 15 (48) |
| B | 17 (52) |
| Primary etiology of liver disease | |
| Hepatitis C virus | 15 (47) |
| Nonalcoholic fatty liver disease | 10 (31) |
| Alcohol | 3 (10) |
| Hepatitis B virus | 2 (6) |
| Other | 2 (6) |
| HCC* | 5 (16) |
| Laboratory MELD score | 12 (10−13) |
| Serum sodium, mEq/L | 137 (135−139) |
| Total bilirubin, mg/dL | 1.3 (1.0−2.4) |
| INR | 1.2 (1.1−1.4) |
| Serum creatinine, mg/dL | 0.9 (0.73−1.18) |
| Prior cirrhosis complications | |
| Ascites | 14 (44) |
| Hepatic encephalopathy | 12 (38) |
| Variceal bleed | 7 (22) |
| History of TIPS | 5 (16) |
| Serum albumin, g/dL | 3.3 (3.1−3.5) |
| Serum platelet count | 95 (79−141) |
NOTE: Data are given as n (%) or median (IQR). Prior cirrhosis complications are not mutually exclusive.
All patients with HCC were within Milan criteria at the time of sleeve gastrectomy.
Hepatitis C virus was the most common primary etiology of liver disease (47%), followed by nonalcoholic fatty liver disease (31%), alcohol (9%), and hepatitis B virus (6%). There were 5 (16%) patients who had hepatocellular carcinoma [HCC], all of which were within Milan criteria(14) at the time of SG. Median laboratory MELD score was 12 (IQR, 10–13; range, 7–18) at the time of SG, and approximately half of the patients (52%) were classified as Child-Pugh classification B. All patients had experienced a prior hepatic decompensation: 22% had a history of variceal hemorrhage, 44% had a history of ascites, and 38% had a history of hepatic encephalopathy. There were 5 (16%) patients who had previously undergone placement of a transjugular intrahepatic portosystemic shunt (TIPS). In 3 patients, this was performed for management of refractory ascites, and in 2 patients, this was performed for management of recurrent variceal hemorrhage.
OPERATIVE OUTCOMES, PERIOPERATIVE COMPLICATIONS, LIVER-RELATED MORBIDITY, AND ALL-CAUSE MORTALITY
Operative and perioperative outcomes are listed in Table 2. The median estimated blood loss was 50 mL (IQR, 50–100 mL). There were no conversions from a laparoscopic to an open procedure. Median length of hospital stay following SG was 3 days (IQR, 2–3 days, range 1–6 days). According to the Clavien-Dindo classification of surgical complications, 2 patients experienced grade 1 complications and 1 patient experienced a grade 2a complication. Grade 1 complications included renal insufficiency requiring albumin administration (not secondary to nausea/emesis or poor oral intake in the postoperative setting) and transient encephalopathy deemed secondary to medication effect and not hepatic decompensation, both lasting <36 hours. Both patients who experienced grade 1 complications were Child-Pugh class B at the time of SG. The patient with a grade 3a complication, who was Child-Pugh class A at the time of SG, experienced a gastric staple line leak due to an inadvertently retained orogastric tube. This was managed with percutaneous drain placement. There were no reoperations or perioperative deaths in the cohort.
TABLE 2.
Operative and Perioperative Outcomes With SG
| Characteristic | Value (n = 32) |
|---|---|
| Estimated blood loss, median (IQR), mL | 50 (50−100) |
| Conversion to open procedure, n | 0 |
| Hospital length of stay, median (IQR), days | 3 (2−3) |
| Reoperation, n | 0 |
| Perioperative morbidity, n | 3 |
| Major perioperative morbidity, n* | 1 |
| Liver-related morbidity, n | 0 |
| All-cause mortality, n | 0 |
NOTE: Perioperative outcomes were defined as those occurring within 90 days after SG.
Major morbidity was defined as Clavien-Dindo surgical complication grade of >2.
No patients experienced liver-related morbidity in the perioperative period. Trends in MELD score following SG are shown in Fig. 1. Median laboratory MELD score at 3 and 6 months after SG was 12 (IQR 8–16) and 11 (IQR 9–19), respectively. Among the 32 patients in the cohort, 5/32 (16%) had an increase in MELD score at 6 months after SG with a mean MELD score increase of 3 (range 2–6). The remainder of patients had either a stable or improved MELD score at 6 months after SG.
FIG. 1.
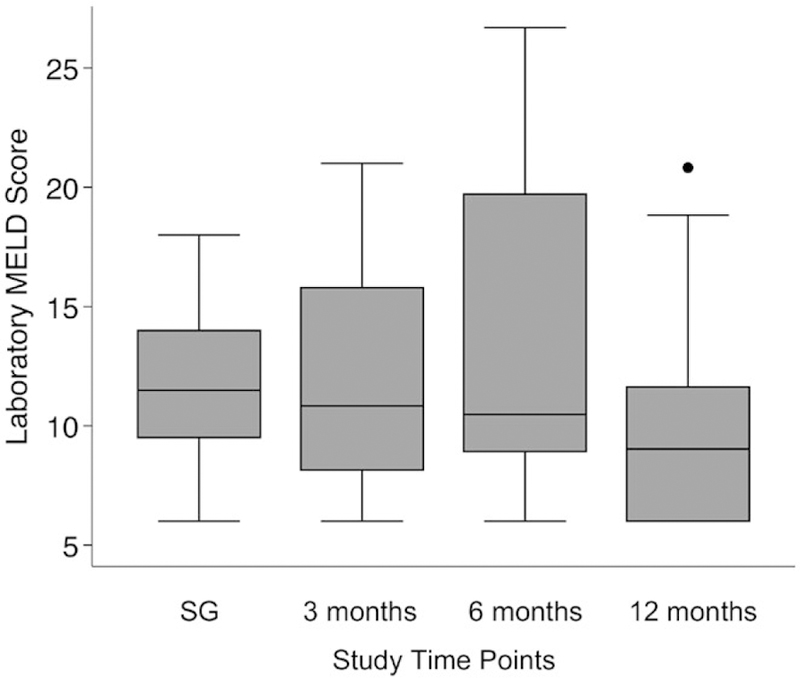
MELD score at time points after pre-LT SG.
WEIGHT LOSS AND IMPROVEMENT IN OBESITY-RELATED COMORBIDITIES AFTER SG
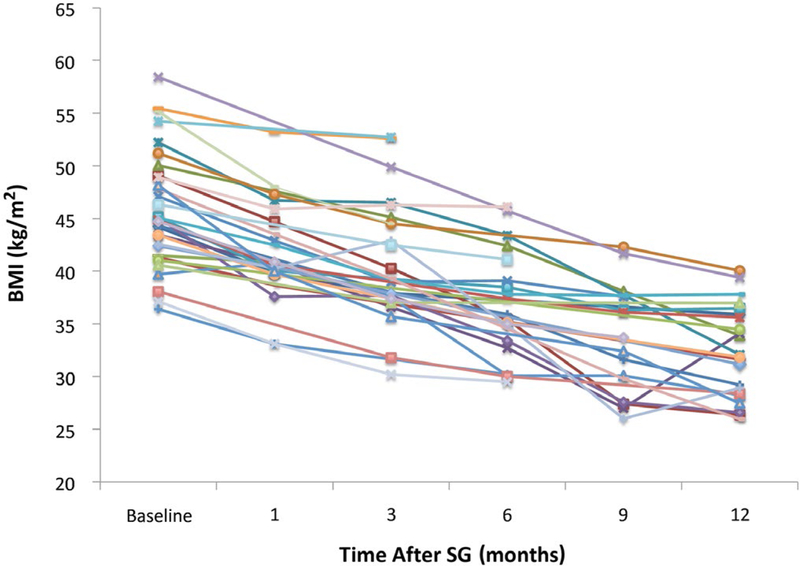
Weight loss outcomes after SG are shown in Table 3. Among the 24 patients with follow-up to at least 12 months after SG, the median percentage of excess body weight lost was 52.4%, which correlated to an absolute weight loss of 31 kg. The median BMI at 12 months after SG was 32.9 kg/m2, which represented an absolute reduction of 11.3 kg/m2 in BMI. BMI trends for each patient are shown in Fig. 2. At 6 and 12 months following SG, 59% and 81% of patients had reached a BMI of <40 kg/m2 (Fig. 3).
TABLE 3.
Weight Loss Outcomes After SG*
| Time After SG | Absolute Weight Loss, kg | Percentage of Excess Body Weight Loss | Absolute BMI Reduction, kg/m2 |
|---|---|---|---|
| 1 month (n = 25) | 9.8 (7.2−12.6) | 15.0 (10.2−19.2) | 3.8 (2.6−4.4) |
| 3 months (n = 27) | 16.6 (11.8−21.7) | 23.0 (16.8−29.5) | 6.3 (3.8−7.3) |
| 6 months (n = 22) | 22.0 (18.9−26.8) | 33.4 (27.9−45.5) | 8.0 (7.3−9.7) |
| 9 months (n = 19) | 30.4 (26.3−46.4) | 44.4 (34.8−62.5) | 12.0 (8.8−16.6) |
| 12 months (n = 24) | 31.0 (23.6−50.3) | 52.4 (36.9−63.3) | 11.3 (9.2−18.5) |
All results are reported as median (IQR).
FIG. 2.
BMI trends for each patient after pre-LT SG.
FIG. 3.
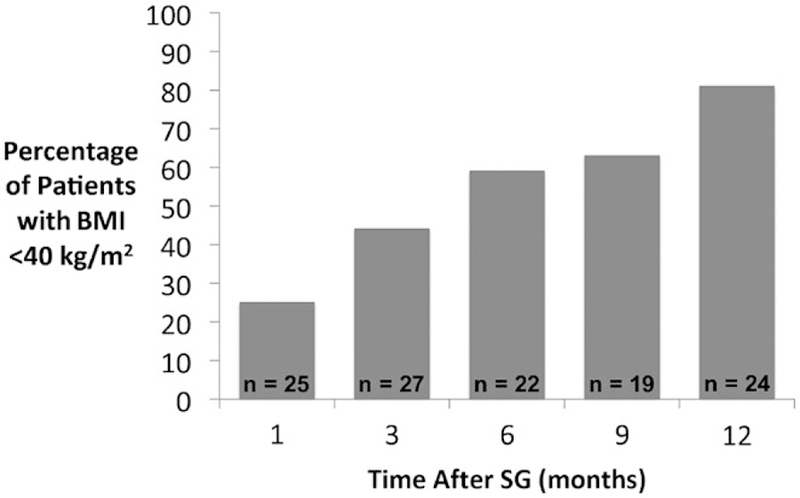
Percentage of patients who achieved a BMI <40 kg/m2 at time points after SG.
Among the 14 patients with diabetes, 5 (36%) patients had complete resolution of diabetes and 9 (64%) patients had improvement in diabetes within 12 months following SG. Mean HbA1c reduction at 12 months was 1.5%. Among the 24 patients with hypertension, 20 (83%) had reduction in antihypertensive medication use within 12 months after SG. No patients experienced either inability to tolerate oral intake or nutritional deficiencies with routine postoperative supplementation after SG.
ELIGIBILITY FOR LT AFTER SG
There were 28 (88%) patients who were deemed eligible to be actively listed for LT at our institution within 6 months following SG. Of the 4 patients who were not eligible for active listing, 3 patients either transferred care to another transplant center or did not return for follow-up with our transplant program. The remaining ineligible patient experienced postoperative gastric leak with additional complications precluding LT.
Among the 28 patients deemed eligible for active listing, listing was deferred in 7 patients because of low MELD laboratory score (“being too well”); 2 of these 7 patients were Child-Pugh class B at the time of SG. The remaining 21 patients were actively listed for LT at our institution, and of these, 14 underwent LT (including all 5 patients with HCC who received MELD exceptions). Median time between SG and LT was 22 months (IQR, 14–88 months), and median MELD laboratory score at the time of LT was 15 (IQR 12–28). Among the 7 patients who were listed and did not undergo LT, 2 were later delisted because of low MELD score; 2 were delisted for nonobesity-related reasons (nonadherence and psychosocial issues); 2 patients died secondary to complications of advanced liver disease prior to receiving a LT offer; and 1 remains active on the waiting list.
Discussion
As a result of the obesity epidemic, the number of obese patients with end-stage liver disease undergoing evaluation for LT is expected to rise. Our institution’s experience with SG in the pretransplant setting yields several important findings pertinent to the management of obese LT candidates. This study suggests that SG in appropriately selected patients is technically feasible, has acceptable complication rates, and results in significant and sustained weight loss.
A few small studies have reported on the safety and efficacy of bariatric surgery in patients with cirrhosis. However, in the majority of these studies, cirrhosis was unidentified prior to bariatric surgery, and thus, most patients had well-compensated liver disease.(15–18) Just over 50% of the patients in our cohort were classified as Child-Pugh B, and all patients had a prior history of liver decompensation. However, it was required that these liver complications were well controlled at the time of bariatric surgery. Despite the severity of liver disease in our patient cohort, there was no perioperative mortality or liver-related morbidity. The major morbidity rate in our patient cohort was 3% (1 patient with a gastric staple line leak) and similar to the major morbidity rate with laparoscopic SG in the general bariatric surgery patient population, which ranges from 2.1% to 5.6%.(19–21) We also noted similar reoperation rates (0% versus 3%) and mean length of hos-pitalization (2.4 versus 3.0 days) in our patient cohort as compared with published data of outcomes in the overall bariatric surgery population.(19–21)
Importantly, SG yielded significant sustained weight loss associated with improvement or resolution of obesity-related comorbidities in the majority of patients. By 12 months after SG, the absolute reduction in BMI in our cohort was 11.3 kg/m2, which compares to a BMI reduction of 11.9 kg/m2 in the general bariatric surgery population.(19) Excellent weight loss outcomes in our cohort resulted in high (88%) rates of eligibility for active listing for LT.
Laparoscopic SG was chosen as the weight loss procedure in our patient cohort. There were no adjustments to the SG operative technique in this patient population, as compared with the general bariatric population, other than the need for more careful hemostasis intraoperatively. SG has several advantages, particularly in a patient population with advanced liver disease, when compared with other bariatric surgery techniques. First, SG has been shown to require less operative time and confers lower perioperative risks when compared with Roux-en-Y gastric bypass.(22) Second, SG does not compromise endoscopic evaluation of the upper gastrointestinal tract, including maintaining direct access to the biliary tract should biliary complications arise after LT. Finally, given that SG is a restrictive bariatric surgery technique, there is less risk of malabsorption of both nutrients and medications.
An alternative strategy in morbidly obese LT candidates is to perform SG at the time of LT.(23,24) Potential benefits of a combined operation include a single operation and recovery period in addition to avoiding the risk of complications or liver-related morbidity in the pre-LT period, which could delay or prevent LT. Moreover, LT candidates deemed “too sick” for pre-LT SG—particularly those with high MELD score and hepatic decompensation—may derive a benefit from a combined procedure, given that pre-LT SG would not be appropriate in these candidates. We chose a staged approach, using SG in the pretransplant period in those LT candidates with a low MELD score and well-controlled complications of liver disease (ie, those early in their pretransplant course), for several reasons. First, we hypothesized that sustained weight loss after SG may reduce the risk for further hepatic decompensation and potentially even negate the need for LT alto-gether in some candidates. Second, in those candidates who proceed to LT, SG prior to LT versus concurrent with LT may lessen the risk of staple-line complications, which could be precipitated by high-dose steroid exposure after LT.(25) Additionally, we postulated that rapid weight loss in the posttransplant period resulting from a combined SG and LT procedure could make immunosuppressive management more challenging. Finally, as compared with simultaneous SG and LT, patients who undergo pre-LT SG may have lower risk of intolerance to oral intake immediately after LT, which is especially important given that the optimization of nutritional intake is an integral component of immediate post-LT care.(26) Further investigation is needed to determine the optimal timing of SG in morbidly obese LT candidates, but an individualized approach will likely be necessary.
Our study has limitations. This is a single-center retrospective study with a modest number of patients. Moreover, all SG procedures were performed by 1 attending surgeon (A.M.P.). Ultimately, additional questions need to be addressed to further define the role of bariatric surgery in patients with end-stage liver disease. These considerations include delineating both the appropriate timing and type of bariatric surgery procedure, in addition to refining patient selection criteria in order to identify those patients who would derive the greatest benefit with the least risk of harm. Moreover, we must examine whether pre-LT SG leads to improvement in wait-list outcomes and/or longterm graft function and overall survival, as compared with patients who achieve weight loss with nonsurgical therapies prior to LT. Nevertheless, our center’s experience highlights the potential option of SG in obese patients with advanced liver disease who might other-wise be excluded from pursuing LT.
Acknowledgments
This study was supported in part by NIH T32 DK060414 (to S.R.S.).
Abbreviations:
- BMI
body mass index
- HbA1c
hemoglobin A1c
- HCC
hepatocellular carcinoma
- INR
international normalized ratio
- IQR
interquartile range
- LT
liver transplantation
- MELD
Model for End-Stage Liver Disease
- MELD-Na
Model for End-Stage Liver Disease–sodium
- NIH
National Institutes of Health
- SG
sleeve gastrectomy
- TIPS
transjugular intrahepatic portosystemic shunt
REFERENCES
- 1).Carter D, Dieterich DT, Chang C. Nonalcoholic fatty liver disease/nonalcoholic steatohepatitis in liver transplantation. Clin Liver Dis 2018;22:213–227. [DOI] [PubMed] [Google Scholar]
- 2).Spengler EK, O’Leary JG, Te HS, Rogal S, Pillai AA, Al-Osaimi A, et al. Liver transplantation in the obese cirrhotic patient. Transplantation 2017;101:2288–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Barone M, Viggiani MT, Losurdo G, Principi M, Leandro G, Di Leo A. Systematic review with meta-analysis: postoperative complications and mortality risk in liver transplant candidates with obesity. Aliment Pharmacol Ther 2017;46:236–245. [DOI] [PubMed] [Google Scholar]
- 4).Martin P, DiMartini A, Feng S, Brown R Jr, Fallon M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology 2014;59:1144–1165. [DOI] [PubMed] [Google Scholar]
- 5).Secunda K, Gordon EJ, Sohn MW, Shinkunas LA, Kaldjian LC, Voigt MD, Levitsky J. National survey of provider opinions on controversial characteristics of liver transplant candidates. Liver Transpl 2013;19:395–403. [DOI] [PubMed] [Google Scholar]
- 6).Diwan TS, Rice TC, Heimbach JK, Schauer DP. Liver trans-plantation and bariatric surgery: timing and outcomes. Liver Transpl 2018;24:1280–1287. [DOI] [PubMed] [Google Scholar]
- 7).Ochner CN, Puma LM, Raevuori A, Teixeira J, Geliebter A. Effectiveness of a prebariatric surgery insurance-required weight loss regimen and relation to postsurgical weight loss. Obesity 2010;18:287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database Syst Rev 2014;8:CD003641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Friedman LS. The risk of surgery in patients with liver disease. Hepatology 1999;29:1617–1623. [DOI] [PubMed] [Google Scholar]
- 10).Lin MY, Tavakol MM, Sarin A, Amirkiai SM, Rogers SJ, Carter JT, Posselt AM. Laparoscopic sleeve gastrectomy is safe and efficacious for pretransplant candidates. Surg Obes Relat Dis 2013;9:653–658. [DOI] [PubMed] [Google Scholar]
- 11).Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004;292:1724–1737. [DOI] [PubMed] [Google Scholar]
- 12).Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Shoar S, Saber AA. Long-term and midterm outcomes of laparoscopic sleeve gastrectomy versus Roux-en-Y gastric bypass: a systematic review and meta-analysis of comparative studies. Surg Obes Relat Dis 2017;13:170–180. [DOI] [PubMed] [Google Scholar]
- 14).Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693–699. [DOI] [PubMed] [Google Scholar]
- 15).Shimizu H, Phuong V, Maia M, Kroh M, Chand B, Schauer PR, Brethauer SA. Bariatric surgery in patients with liver cirrhosis. Surg Obes Relat Dis 2013;9:1–6. [DOI] [PubMed] [Google Scholar]
- 16).Dallal RM, Mattar SG, Lord JL, Watson AR, Cottam DR, Eid GM, et al. Results of laparoscopic gastric bypass in patients with cirrhosis. Obes Surg 2004;14:47–53. [DOI] [PubMed] [Google Scholar]
- 17).Mosko JD, Nguyen GC. Increased perioperative mortality following bariatric surgery among patients with cirrhosis. Clin Gastroenterol Hepatol 2011;9:897–901. [DOI] [PubMed] [Google Scholar]
- 18).Pestana L, Swain J, Dierkhising R, Kendrick ML, Kamath PS, Watt KD. Bariatric surgery in patients with cirrhosis with and without portal hypertension: a single-center experience. Mayo Clin Proc 2015;90:209–215. [DOI] [PubMed] [Google Scholar]
- 19).Hutter MM, Schirmer BD, Jones DB, Ko CY, Cohen ME, Merkow RP, Nguyen NT. First report from the American College of Surgeons Bariatric Surgery Center Network: laparoscopic sleeve gastrectomy has morbidity and effectiveness positioned between the band and the bypass. Ann Surg 2011;254:410–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Melissas J, Stavroulakis K, Tzikoulis V, Peristeri A, Papadakis JA, Pazouki A, et al. Sleeve gastrectomy vs Roux-en-Y Gastric bypass. data from IFSO-European Chapter Center of Excellence Program. Obes Surg 2017;27:847–855. [DOI] [PubMed] [Google Scholar]
- 21).Lager CJ, Esfandiari NH, Subauste AR, Kraftson AT, Brown MB, Cassidy RB, et al. Roux-En-Y Gastric bypass vs. sleeve gastrectomy: balancing the risks of surgery with the benefits of weight loss. Obes Surg 2017;27:154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Zhang Y, Wang J, Sun X, Cao Z, Xu X, Liu D, et al. Laparoscopic sleeve gastrectomy versus laparoscopic Roux-en-Y gastric bypass for morbid obesity and related comorbidities: a meta-analysis of 21 studies. Obes Surg 2015;25:19–26. [DOI] [PubMed] [Google Scholar]
- 23).Heimbach JK, Watt KD, Poterucha JJ, Ziller NF, Cecco SD, Charlton MR, et al. Combined liver transplantation and gastric sleeve resection for patients with medically complicated obesity and end-stage liver disease. Am J Transplant 2013;13:363–368. [DOI] [PubMed] [Google Scholar]
- 24).Zamora-Valdes D, Watt KD, Kellogg TA, Poterucha JJ, Di Cecco SR, Francisco-Ziller NM, et al. Long-term outcomes of patients undergoing simultaneous liver transplantation and sleeve gastrectomy. Hepatology 2018;68:485–495. [DOI] [PubMed] [Google Scholar]
- 25).Kaplan JA, Schecter SC, Rogers SJ, Lin MYC, Posselt AM, Carter JT. Expanded indications for bariatric surgery: should patients on chronic steroids be offered bariatric procedures? Surg Obes Relat Dis 2017;13:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Major P, Stefura T, Małczak P, Wysocki M, Witowski J, Kulawik J, et al. Postoperative care and functional recovery after laparoscopic sleeve gastrectomy vs. laparoscopic Roux-en-Y gastric bypass among patients under ERAS protocol. Obes Surg 2018;28:1031–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]