Abstract
Here, we reported for the first time an increased expression of c-Met protein in primary cultures of human dermal and pulmonary fibroblasts of late passages. This suggests that c-Met could serve as an early marker of cellular senescence (CS). The levels of c-Met-related signaling proteins phospho-Akt and Stat3 were also increased in (pre)senescent fibroblasts. Considering the anti-apoptotic activity of Akt and the involvement of Stat3 in mediating the effects of proinflammatory cytokines, the findings of this study indicate that c-Met could contribute through its downstream targets or partners to at least two major phenotypical features of CS – resistance to apoptosis and senescence-associated secretory phenotype.
Keywords: cellular senescence, c-Met, Akt, Stat3, human dermal and pulmonary fibroblasts
Introduction
In the last years, the phenomenon of cellular senescence (CS) has been becoming a hot topic in biomedical research. Apart from being involved in tissue repair upon wound healing [1,2] and acute fibrosis [3,4], cell reprogramming [5], and tissue remodeling during embryonic development [6–10], CS has attracted lots of attention, first and foremost, due to its strong links with aging and age-related diseases [11,12]. Indeed, the growing body of evidence indicates that accumulation of senescent cells could be an important player in mechanisms of aging and late-onset pathologies [13–16]. Senescent cells are characterized by a stable cell growth arrest, enlarged, flattened shape of heterogenous morphology, resistance to apoptosis, secretion of a plethora of proinflammatory and ECM-modifying compounds, and expression of several molecular markers (e.g., p16INK4a, p21Cip1/Waf1, SA-β-gal, etc.) [17]. Yet, none of the existing molecular markers is exclusively indicative of CS, and there are ongoing attempts for reliable identification of senescent cells [18,19].
While searching for microRNAs with a potential involvement in CS, we observed a profound down-regulation of miR-199a-3p and miR-34a in pre-senescent human skin fibroblasts [20]. Among their experimentally validated targets is the hepatocyte growth factor receptor (MET), a well-known proto-oncogene encoding for the c-Met protein with tyrosine kinase activity [21]. Then, it would be reasonable to suggest that a decreased expression of miR-199a-3p and miR-34a in (pre)senescent cells could result in overexpression of c-Met. Indeed, elevated levels of c-Met were reported for a variety of tumors in which the members of miR-34 and miR-199 families are often silenced (reviewed by [22]). Not surprisingly, c-Met has extensively been studied in cancer research [21]. The studies on c-Met with regard to CS are fully absent.
The c-Met receptor can interact with a number of signaling proteins. As a result, this interaction may lead to induction of various signaling pathways (PI3K/AKT, JAK/STAT, etc.), thus explaining a wide range of c-Met biological activities including cell survival, migration and adhesion [21,23,24]. Of note, all of these activities could be relevant to CS.
This study was undertaken to evaluate whether the expression of c-Met is altered in the course of replicative CS, and if so, whether its levels could serve as a new marker of senescent cells. Also, to get insight into possible role of c-Met in CS, we determined the expression of its downstream targets Akt and Stat3.
RESULTS
Expression of c-Met protein in primary cultures of human fibroblasts
The patterns of cell growth and CS of primary cultures of human fibroblasts were similar to those described by us previously [25,26]. Briefly, fibroblasts (both dermal and pulmonary) of early passages (P.12-20) displayed a typical spindle-like shape, did not express SA-β-gal, and doubled their population each 48-72 h. In contrast, the fibroblast cultures of late passages (around P.35-40) had heterogenous morphotypes, with large cells of irregular shape, that were stained with CS marker SA-β-gal, and dramatically slowed down their growth (PDT 3-4 weeks) or ceased to divide. Altogether, this allows to characterize the cell cultures of late passages as pre- or senescent cultures.
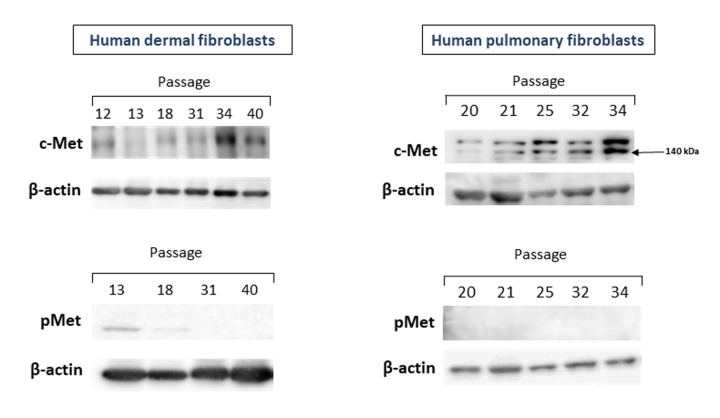
We first measured the levels of c-Met protein as well as its phosphorylated form (pMet) in primary cultures of human fibroblasts of various passages and different origin (dermal and pulmonary fibroblasts). As seen in Figure 1, both dermal (left panel) and pulmonary (right panel) fibroblasts displayed similar patterns of changes in c-Met protein levels during the course of replicative CS.
Figure 1.
The levels of c-Met and pMet proteins in primary cultures of human dermal (left panel) and pulmonary fibroblasts (right panel) of various passages. Immunoblots represent one of the 4 independent experiments.
The c-Met protein was hardly detectable in cell cultures of dermal fibroblasts of early passages (P.12-18), and this low level remained up to P.31. A marked increase in the c-Met level was observed in cell cultures of late passages (P.34-40), when the cell growth dramatically slowed down. In pulmonary fibroblast cultures, the level of c-Met started to gradually increase from P.25, with the peak in P.34. As in case of dermal fibroblasts, the c-Met protein levels coincided well with population doubling time (PDT), so that the longer PDT, the higher c-Met level. It should be noted that a clear elevation of c-Met levels was observed before reaching the full growth arrest. Yet, PDT of senescing cultures of the examined late passages was quite long (3-4 weeks) and most cells were stained with the CS marker SA-β-gal. In contrast to c-Met, its phosphorylated form pMet was either undetectable in cell cultures of both early and late passages (pulmonary fibroblasts) or was of a very low level in only early passages but not late ones (dermal fibroblasts) (Figure 1, left-lower panel).
Expression of Akt in primary cultures of human fibroblasts
One of the c-Met downstream pathways which could be related to CS is a PI3K/AKT pathway. With this in mind, we further examine whether Akt1/2/3 and its phosphorylated form pAkt undergo changes during replicative CS. As seen in Figure 2 (upper panel), no significant differences in the Akt protein level between fibroblast cultures of late and early passages were observed, while pAkt unexpectedly increased in fibroblast cultures of late passages (lower panel). This patterns of CS-related changes in Akt and pAkt were common for both dermal and pulmonary fibroblasts. As in the case of c-Met, the levels of pAkt tended to inversely correlate with the number of passages, i.e., the higher the passage number (and, accordingly, the lower cell growth rate), the higher the level of pAkt.
Figure 2.
The levels of Akt1/2/3 and pAkt proteins in primary cultures of human dermal (left panel) and pulmonary fibroblasts (right panel) of various passages. Immunoblots represent one of the 3 independent experiments.
Expression of Stat3 in primary cultures of human fibroblasts
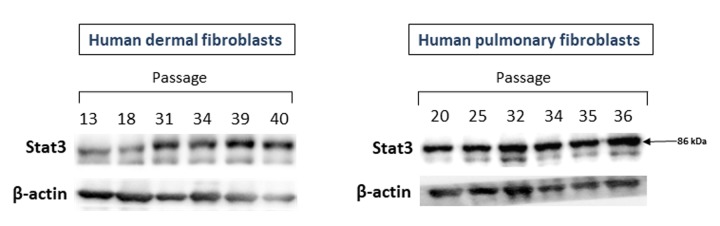
The c-Met target Stat3 could also be directly related to CS. Therefore, we examined the levels of Stat3 protein in fibroblast cultures of various passages. As seen in Figure 3, the expression of Stat3 protein increased in fibroblast cultures of late passages. This was particularly noted for dermal fibroblasts (left panel) and, to a lesser degree, for pulmonary fibroblasts (right panel).
Figure 3.
The levels of Stat3 proteins in primary cultures of human dermal (left panel) and pulmonary fibroblasts (right panel) of various passages. Immunoblots represent one of the 3 independent experiments.
DISCUSSION
In this study, we examined the expression of c-Met protein in fibroblast cultures of various passages, from early to late ones. The rationale was based on our recent finding, indicating that downregulation of several miRNAs could govern CS through overexpression of their targets, c-Met included (see Introduction). In line with this suggestion, we found for the first time an increased expression of c-Met protein in primary cultures of dermal and pulmonary fibroblasts during the course of replicative CS (Figure 1). Notably, in fibroblasts of early passages, the c-Met level was very low or undetectable, which is consistent with previously reported data [27,28]. The high c-Met protein levels were clearly evident in presenescent fibroblast cultures. This suggests that c-Met could serve as an early marker of CS. Two lines of observations indirectly support this notion. First, it was shown that both in vitro and in vivo, myofibroblasts (activated fibroblasts) express c-Met [29]. On the other hand, it was also found that senescent fibroblasts express the myofibroblast-specific marker α-SMA, thus connecting CS with myofibroblasts [25,30]. Second, transfection of normal dermal fibroblasts with the IL-1α gene whose overexpression is an initiation event in SASP development [31], resulted in high expression of c-Met mRNA [27]. Of note, the c-MET protein is expressed in a variety of cell types including epithelial, muscle, neuronal cells, etc [29,32]. Whether senescent cells other than dermal and pulmonary fibroblasts also express high levels of c-Met needs to be clarified.
As mentioned above, elevated levels of c-Met could be related to a decreased expression of miR-199a-3p and miR-34a. Downregulation of these microRNAs was consistently observed in cancer cells [22] and was also shown by us in (pre)senescent fibroblast cultures [20]. Another possibility for c-Met accumulation in both cancer and senescent cells could include its inadequate ubiquitination [33]. Whatever the case, it would be attractive to speculate that the proto-oncogene c-Met might induce CS by the well-known mechanism [34] of oncogene-induced cellular senescence.
Further complicating the cancer-CS relationships is a recently discovered HGF-associated mechanism by which the cancer cells may induce CS in normal cells that, in turn, form a cancer-promoting microenvironment [35,36]. However, whether c-Met mediates the CS-promoting effects of HGF has not yet been established.
In contrast to c-Met, we did not find any detectable passage-dependent elevation in its activated form pMet (Figure 1). It should however be noted that we determined only two (Tyr1234 and Tyr1235) of 14 sites of phosphorylation in c-Met that have thus far been identified [21]. Although phosphorylation of c-Met at Tyr1234 and Tyr1235 is believed to be a critical event in the c-Met kinase activity [37], the role of other phosphorylation sites cannot be excluded. Besides, activation of c-Met could be related to the production of its ligand—hepatocyte growth factor (HGF) [38,39]. Yet, the studies on HGF in CS are scarce and controversial. In early study by Miyazaki et al. (1998), an increased production of HGF by human embryonic lung fibroblasts of late passages vs. early passages was reported [40]. The authors also showed that the human skin fibroblasts derived from the old donors (80+ years) produced more HGF than the cells from the young and middle-aged donors. However, these findings were not confirmed by recent in vitro and in vivo studies on human skin fibroblasts. Qin et al. (2017) demonstrated that HGF mRNA was similarly expressed in the fibroblasts isolated from the skin of young (mean age 27±1 years) and aged (mean age 83±1.4 years) donors, as well as in fibroblast cultures of early passages and late passages (PDT of 45 days) [41]. Whatever the case, a possible role of HGF/c-Met axis in CS warrants further investigation.
c-Met receptor is a transmembrane protein which directly or indirectly interacts with numerous partners [37,https://thebiogrid.org/]. Among them, at least several could be relevant to CS. In this study, we focused on Akt and Stat3 proteins that are well known for their anti-apoptotic activity and mediating the effects of proinflammatory cytokines, respectively.
Unexpectedly, the opposite of c-Met picture was observed for Akt which is activated via interaction of c-Met with PI3K, directly or by forming a protein complex with GAB1 [37]. While there were no significant differences in the Akt protein level between fibroblast cultures of late and early passages, the levels of its active form pAkt was markedly increased in (pre)senescent fibroblasts (Figure 2). It should be noted that apart from c-Met, several other signaling pathways (e.g., EGF/EGFR, INS/IGF-1) could also activate the Akt protein [43]. Of note, among the major downstream effectors of Akt is a serine/threonine kinase mTOR [44], known to be strongly associated with CS and aging (for recent review see [45]). The levels of another c-Met target, Stat3 protein, a member of signal transducers and activators of transcription (JAK/STAT) pathway, also increased in fibroblast cultures of late passages (Figure 3).
In line with our findings, demonstrating the increase in the levels of pAktSer473 and Stat3 in (pre)senescent human dermal and pulmonary fibroblasts, are the most recently obtained evidence of an increased expression and/or activation of Akt and Stat3 both in replicative and stress-induced CS. These observations are summarized in Table 1 and together with our data suggest that the abovementioned changes in Akt and Stat3 are typical for senescent cells of various types.
Table 1. Evidence for the involvement of Akt and Stat3 in cellular senescence.
| Cells | Type of CS | Changes in activity/expression | Reference |
| IMR90 human lung fibroblasts | Replicative CS H2O2-induced CS | Increased Akt-1 and p-Akt-1 levels in senescent cells | [48] |
| Human vascular smooth muscle cells (VSMCs) | Replicative CS | Increased p-Akt level in senescent cells | [49] |
| EJ p53-null human bladder cancer cells | Replicative CS p53-induced CS | Increased p-Akt (pS473 and pT308) protein level in senescent cells | [46] |
| TIG3 human fibroblasts | Replicative CS IL-6-induced or soluble IL-6Rα- induced CS |
Stat3 was constitutively activated in senescent cells (both with or without exogenous IL-6/ IL-6Rα stimulation) | [50] |
| Human umbilical vein endothelial cells (HUVECs) | TNFα-induced CS | Increased p-Stat1 and p-Stat3 levels in senescent cells | [51] |
| IPF-derived lung fibroblasts | Replicative CS | Hyperphosphorylation of Stat3 in IPF-derived lung fibroblasts with features of CS | [52] |
Apart from their “canonical” functions, Akt and Stat3 could be linked to CS by other activities. For example, a recent study by Kim et al. (2017) suggests that Akt activation is crucial not only for promoting cell survival but also for induction of SASP [46]. On the other hand, binding of non-phosphorylated Stat3 (but not pStat3!) to regulatory regions of pro-apoptotic genes with subsequent inhibition of their expression, results in an increased resistance to apoptosis [47]. The latter could be also promoted through the c-Met partner BAG1 (BCL2 Associated Athanogene 1), which enhances the anti-apoptotic effects of Bcl2 (GeneCards – Human Gene Database; https://www.genecards.org/). In the model of stress-induced premature CS, we found an increased BAG1 protein level in senescent dermal fibroblasts vs. “young” cultures (data not shown).
In summary, c-Met seems to be mechanistically linked to CS and could serve as a marker of CS. Considering the anti-apoptotic and SASP-related activities of Akt and Stat3, the findings of this study indicate that c-Met could contribute through its downstream targets or partners to at least two major phenotypical features of CS – resistance to apoptosis and senescence-associated secretory phenotype (SASP). The role of c-Met and related proteins in CS appears to be an important point for further investigation.
MATERIALS AND METHODS
Cell cultures
Primary cultures of human dermal and pulmonary fibroblasts (obtained from ScienCell, Carlsbad, CA, US) were grown under standard conditions (37 °C, 5% CO2) in Dulbecco’s modified Eagles medium (DMEM), supplemented with 10% fetal bovine serum, 1% L-glutamine, and 1% penicillin/streptomycin. All products for cell cultures were from Biological Industries, Beit Haemek, Israel. The cultures were inspected every day under inverted phase-contrast microscope (Primo Vert, Zeiss) and cells were passaged 1:2 upon 75–80% confluence. The number and concentration of viable cells were calculated using Trypan blue exclusion assay. Replicative CS was achieved by serial passaging. The cells were defined as pre-senescent or senescent, based on a dramatic inhibition of cell proliferation or cell growth arrest, respectively; typical CS morphology, and expression of the CS marker SA-β-gal. The SA-β-gal assay was carried out using the Sigma Aldrich SA-β-galactosidase detection kit (GALS), according to the manufacturer’s protocol, with subsequent visualization using the Primo Vert microscope.
Preparation of cell lysates
Cells were washed with ice-cold PBS and scraped into ice-cold lysis buffer with phosphatase inhibitor cocktail (Stratech Scientific Ltd., CAT# B15001-BIT). Lysates were centrifuged at 14,000 rpm for 10 min at 4 °C, and supernatants were collected. Total protein concentration in each sample was determined using Bradford protein assay (Bio-Rad Laboratories, CAT# 5000006). Quantified samples were then analyzed by Western blotting.
Western blotting
Equal amounts of protein were separated on 10% SDS-polyacrylamide gels, and electrophoretically transferred into PVDF membranes (Bio-Rad, #1704157). Membranes were blocked for 1 h in 5% BSA in Tris-buffered saline (TBS)-Tween and then incubated with primary antibodies in 5% BSA TBS-Tween. The following primary antibodies were used (all from Cell Signaling Technology): Met (D1C2) Rabbit mAb (#8198), phospho-Met (Tyr1234/1235) Rabbit mAb (#3077), pan-Akt (C67E7) Rabbit mAb (#4691), phospho-Akt (Ser473) (D9E) Rabbit mAb (#4060), and Stat3 (D3Z2G) Rabbit mAb (#12640). Membranes were then incubated with rabbit horseradish peroxidase (HRP)-conjugated secondary antibodies (1:20,000, GE Healthcare) diluted in blocking solution. Protein-antibody complexes were detected by chemiluminescence with ECL supernova (Cyanagen), and images were captured with the Azure C300 Chemiluminescent Western Blot Imaging System, Azure Biosystems.
ACKNOWLEDGEMENTS
The authors thank Mrs. Anna Knyazer for her valuable assistance in the manuscript preparation.
Abbreviations
- α-SMA
α-smooth muscle actin
- BSA
bovine serum albumin
- CS
cellular senescence
- HGF
hepatocyte growth factor
- PDT
population doubling time
- SA-β-gal
senescence-associated β-galactosidase
- SASP
senescence-associated secretory phenotype
Footnotes
CONFLICTS OF INTEREST: The authors declare no conflicts of interest.
FUNDING: This study was supported by the Fund in Memory of Dr. Amir Abramovich (to VEF), the Israel-America Foundation (to VEF), the Israel Science Foundation (ISF 700/16; to ME) and the Concern Foundation (#7895; to ME).
REFERENCES
- 1.Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge RM, Vijg J, Van Steeg H, Dollé ME, Hoeijmakers JH, de Bruin A, Hara E, Campisi J. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 2014; 31:722–33. 10.1016/j.devcel.2014.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jun JI, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol. 2010; 12:676–85. 10.1038/ncb2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008; 134:657–67. 10.1016/j.cell.2008.06.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sagiv A, Burton DG, Moshayev Z, Vadai E, Wensveen F, Ben-Dor S, Golani O, Polic B, Krizhanovsky V. NKG2D ligands mediate immunosurveillance of senescent cells. Aging (Albany NY). 2016; 8:328–44. 10.18632/aging.100897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mosteiro L, Pantoja C, de Martino A, Serrano M. Senescence promotes in vivo reprogramming through p16INK4a and IL-6. Aging Cell. 2018; 17:17. 10.1111/acel.12711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davaapil H, Brockes JP, Yun MH. Conserved and novel functions of programmed cellular senescence during vertebrate development. Development. 2017; 144:106–14. 10.1242/dev.138222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lorda-Diez CI, Garcia-Riart B, Montero JA, Rodriguez-León J, Garcia-Porrero JA, Hurle JM. Apoptosis during embryonic tissue remodeling is accompanied by cell senescence. Aging (Albany NY). 2015; 7:974–85. 10.18632/aging.100844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muñoz-Espín D, Cañamero M, Maraver A, Gómez-López G, Contreras J, Murillo-Cuesta S, Rodríguez-Baeza A, Varela-Nieto I, Ruberte J, Collado M, Serrano M. Programmed cell senescence during mammalian embryonic development. Cell. 2013; 155:1104–18. 10.1016/j.cell.2013.10.019 [DOI] [PubMed] [Google Scholar]
- 9.Storer M, Mas A, Robert-Moreno A, Pecoraro M, Ortells MC, Di Giacomo V, Yosef R, Pilpel N, Krizhanovsky V, Sharpe J, Keyes WM. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell. 2013; 155:1119–30. 10.1016/j.cell.2013.10.041 [DOI] [PubMed] [Google Scholar]
- 10.Zhao XX, An XL, Zhu XC, Jiang Y, Zhai YH, Zhang S, Cai NN, Tang B, Li ZY, Zhang XM. Inhibiting transforming growth factor-β signaling regulates in vitro maintenance and differentiation of bovine bone marrow mesenchymal stem cells. J Exp Zool B Mol Dev Evol. 2018; 330:406–16. 10.1002/jez.b.22836 [DOI] [PubMed] [Google Scholar]
- 11.Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013; 75:685–705. 10.1146/annurev-physiol-030212-183653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013; 153:1194–217. 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He S, Sharpless NE. Senescence in Health and Disease. Cell. 2017; 169:1000–11. 10.1016/j.cell.2017.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Magalhães JP, Passos JF. Stress, cell senescence and organismal ageing. Mech Ageing Dev. 2018; 170:2–9. 10.1016/j.mad.2017.07.001 [DOI] [PubMed] [Google Scholar]
- 15.Muñoz-Espín D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. 2014; 15:482–96. 10.1038/nrm3823 [DOI] [PubMed] [Google Scholar]
- 16.Yanai H, Fraifeld VE. The role of cellular senescence in aging through the prism of Koch-like criteria. Ageing Res Rev. 2018; 41:18–33. 10.1016/j.arr.2017.10.004 [DOI] [PubMed] [Google Scholar]
- 17.Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007; 8:729–40. 10.1038/nrm2233 [DOI] [PubMed] [Google Scholar]
- 18.Biran A, Zada L, Abou Karam P, Vadai E, Roitman L, Ovadya Y, Porat Z, Krizhanovsky V. Quantitative identification of senescent cells in aging and disease. Aging Cell. 2017; 16:661–71. 10.1111/acel.12592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evangelou K, Lougiakis N, Rizou SV, Kotsinas A, Kletsas D, Muñoz-Espín D, Kastrinakis NG, Pouli N, Marakos P, Townsend P, Serrano M, Bartek J, Gorgoulis VG. Robust, universal biomarker assay to detect senescent cells in biological specimens. Aging Cell. 2017; 16:192–97. 10.1111/acel.12545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tacutu R, Budovsky A, Yanai H, Fraifeld VE. Molecular links between cellular senescence, longevity and age-related diseases - a systems biology perspective. Aging (Albany NY). 2011; 3:1178–91. 10.18632/aging.100413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maroun CR, Rowlands T. The Met receptor tyrosine kinase: a key player in oncogenesis and drug resistance. Pharmacol Ther. 2014; 142:316–38. 10.1016/j.pharmthera.2013.12.014 [DOI] [PubMed] [Google Scholar]
- 22.Giglio S, Vecchione A. c-Met and miRs in Cancer. Biomedicines. 2015; 3:32–44. 10.3390/biomedicines3010032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003; 4:915–25. 10.1038/nrm1261 [DOI] [PubMed] [Google Scholar]
- 24.Sagi Z, Hieronymus T. The Impact of the Epithelial-Mesenchymal Transition Regulator Hepatocyte Growth Factor Receptor/Met on Skin Immunity by Modulating Langerhans Cell Migration. Front Immunol. 2018; 9:517. 10.3389/fimmu.2018.00517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yanai H, Shteinberg A, Porat Z, Budovsky A, Braiman A, Ziesche R, Fraifeld VE, Fraifeld VE. Cellular senescence-like features of lung fibroblasts derived from idiopathic pulmonary fibrosis patients. Aging (Albany NY). 2015; 7:664–72. 10.18632/aging.100807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yehuda S, Yanai H, Priel E, Fraifeld VE. Differential decrease in soluble and DNA-bound telomerase in senescent human fibroblasts. Biogerontology. 2017; 18:525–33. 10.1007/s10522-017-9688-6 [DOI] [PubMed] [Google Scholar]
- 27.Kawaguchi Y, Harigai M, Hara M, Fukasawa C, Takagi K, Tanaka M, Tanaka E, Nishimagi E, Kamatani N. Expression of hepatocyte growth factor and its receptor (c-met) in skin fibroblasts from patients with systemic sclerosis. J Rheumatol. 2002; 29:1877–83. [PubMed] [Google Scholar]
- 28.Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, Olsson I, Edlund K, Lundberg E, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015; 347:1260419. 10.1126/science.1260419 [DOI] [PubMed] [Google Scholar]
- 29.Tokunou M, Niki T, Eguchi K, Iba S, Tsuda H, Yamada T, Matsuno Y, Kondo H, Saitoh Y, Imamura H, Hirohashi S. c-MET expression in myofibroblasts: role in autocrine activation and prognostic significance in lung adenocarcinoma. Am J Pathol. 2001; 158:1451–63. 10.1016/S0002-9440(10)64096-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hecker L, Logsdon NJ, Kurundkar D, Kurundkar A, Bernard K, Hock T, Meldrum E, Sanders YY, Thannickal VJ. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci Transl Med. 2014; 6:231ra47. 10.1126/scitranslmed.3008182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orjalo AV, Bhaumik D, Gengler BK, Scott GK, Campisi J. Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc Natl Acad Sci USA. 2009; 106:17031–36. 10.1073/pnas.0905299106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Organ SL, Tsao MS. An overview of the c-MET signaling pathway. Ther Adv Med Oncol. 2011. (Suppl ); 3:S7–19. 10.1177/1758834011422556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nat Rev Cancer. 2012; 12:89–103. 10.1038/nrc3205 [DOI] [PubMed] [Google Scholar]
- 34.Courtois-Cox S, Jones SL, Cichowski K. Many roads lead to oncogene-induced senescence. Oncogene. 2008; 27:2801–09. 10.1038/sj.onc.1210950 [DOI] [PubMed] [Google Scholar]
- 35.Mikuła-Pietrasik J, Uruski P, Pakuła M, Maksin K, Szubert S, Woźniak A, Naumowicz E, Szpurek D, Tykarski A, Książek K. Oxidative stress contributes to hepatocyte growth factor-dependent pro-senescence activity of ovarian cancer cells. Free Radic Biol Med. 2017; 110:270–79. 10.1016/j.freeradbiomed.2017.06.015 [DOI] [PubMed] [Google Scholar]
- 36.Mikuła-Pietrasik J, Uruski P, Matuszkiewicz K, Szubert S, Moszyński R, Szpurek D, Sajdak S, Tykarski A, Książek K. Ovarian cancer-derived ascitic fluids induce a senescence-dependent pro-cancerogenic phenotype in normal peritoneal mesothelial cells. Cell Oncol (Dordr). 2016; 39:473–81. 10.1007/s13402-016-0289-1 [DOI] [PubMed] [Google Scholar]
- 37.Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol. 2010; 11:834–48. 10.1038/nrm3012 [DOI] [PubMed] [Google Scholar]
- 38.Boissinot M, Vilaine M, Hermouet S. The Hepatocyte Growth Factor (HGF)/Met Axis: A Neglected Target in the Treatment of Chronic Myeloproliferative Neoplasms? Cancers (Basel). 2014; 6:1631–69. 10.3390/cancers6031631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Silva DM, Roy A, Kato T, Cecchi F, Lee YH, Matsumoto K, Bottaro DP. Targeting the hepatocyte growth factor/Met pathway in cancer. Biochem Soc Trans. 2017; 45:855–70. 10.1042/BST20160132 [DOI] [PubMed] [Google Scholar]
- 40.Miyazaki M, Gohda E, Kaji K, Namba M. Increased hepatocyte growth factor production by aging human fibroblasts mainly due to autocrine stimulation by interleukin-1. Biochem Biophys Res Commun. 1998; 246:255–60. 10.1006/bbrc.1998.8593 [DOI] [PubMed] [Google Scholar]
- 41.Qin Z, Worthen CA, Quan T. Cell-size-dependent upregulation of HGF expression in dermal fibroblasts: impact on human skin connective tissue aging. J Dermatol Sci. 2017; 88:289–97. 10.1016/j.jdermsci.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 42.Chatr-Aryamontri A, Oughtred R, Boucher L, Rust J, Chang C, Kolas NK, O’Donnell L, Oster S, Theesfeld C, Sellam A, Stark C, Breitkreutz BJ, Dolinski K, Tyers M. The BioGRID interaction database: 2017 update. Nucleic Acids Res. 2017; 45:D369–79. 10.1093/nar/gkw1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ersahin T, Tuncbag N, Cetin-Atalay R. The PI3K/AKT/mTOR interactive pathway. Mol Biosyst. 2015; 11:1946–54. 10.1039/C5MB00101C [DOI] [PubMed] [Google Scholar]
- 44.Martini M, De Santis MC, Braccini L, Gulluni F, Hirsch E. PI3K/AKT signaling pathway and cancer: an updated review. Ann Med. 2014; 46:372–83. 10.3109/07853890.2014.912836 [DOI] [PubMed] [Google Scholar]
- 45.Weichhart T. mTOR as Regulator of Lifespan, Aging, and Cellular Senescence: A Mini-Review. Gerontology. 2018; 64:127–34. 10.1159/000484629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim YY, Jee HJ, Um JH, Kim YM, Bae SS, Yun J. Cooperation between p21 and Akt is required for p53-dependent cellular senescence. Aging Cell. 2017; 16:1094–103. 10.1111/acel.12639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fathi N, Rashidi G, Khodadadi A, Shahi S, Sharifi S. STAT3 and apoptosis challenges in cancer. Int J Biol Macromol. 2018; 117:993–1001. 10.1016/j.ijbiomac.2018.05.121 [DOI] [PubMed] [Google Scholar]
- 48.Hong S, Kim MM. IGFBP-3 plays an important role in senescence as an aging marker. Environ Toxicol Pharmacol. 2018; 59:138–45. 10.1016/j.etap.2018.03.014 [DOI] [PubMed] [Google Scholar]
- 49.Tan P, Wang YJ, Li S, Wang Y, He JY, Chen YY, Deng HQ, Huang W, Zhan JK, Liu YS. The PI3K/Akt/mTOR pathway regulates the replicative senescence of human VSMCs. Mol Cell Biochem. 2016; 422:1–10. 10.1007/s11010-016-2796-9 [DOI] [PubMed] [Google Scholar]
- 50.Kojima H, Inoue T, Kunimoto H, Nakajima K. IL-6-STAT3 signaling and premature senescence. JAK-STAT. 2013; 2:e25763. 10.4161/jkst.25763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kandhaya-Pillai R, Miro-Mur F, Alijotas-Reig J, Tchkonia T, Kirkland JL, Schwartz S. TNFα-senescence initiates a STAT-dependent positive feedback loop, leading to a sustained interferon signature, DNA damage, and cytokine secretion. Aging (Albany NY). 2017; 9:2411–35. 10.18632/aging.101328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Waters DW, Blokland KE, Pathinayake PS, Burgess JK, Mutsaers SE, Prele CM, Schuliga M, Grainge CL, Knight DA. Fibroblast senescence in the pathology of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2018; 315:L162–72. 10.1152/ajplung.00037.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]