Abstract
Hydrogel surface properties can be modified to form bioactive interfaces to modulate the osteogenic differentiation of stem cells. In this work, a hydrogel made of gelatin methacrylamide (GelMA) and alginate was designed and tested as a scaffold to control stem-cell osteogenic differentiation. The hydrogel’s surface was treated with polydopamine (pDA) to create an adhesive layer for the adsorption of the osteoinductive drug dexamethasone (Dex). The presence of the pDA coating enhanced Dex adsorption and retention over 21 days. This effect resulted in a delay in the osteogenic differentiation of hASCs cultured on the hydrogel treated with a pDA layer.
Keywords: hydrogel coating, osteogenic differentiation, polydopamine, regenerative medicine, surface modification
Graphical Abstract
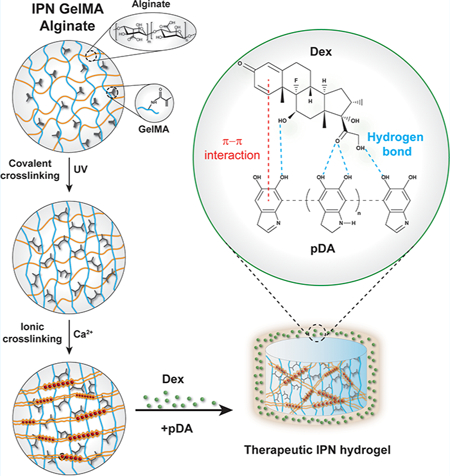
Hydrogels are hydrophilic polymeric networks characterized by high water content and tunable physical and mechanical properties. They are commonly designed to mimic the extracellular matrix (ECM) composition and to instruct stem-cell adhesion, proliferation, and differentiation.1,2 Based on this concept, hydrogels find applications as coatings to modulate the surface properties of biomedical implants or synthetic scaffolds that often lack the necessary biological cues to control stem-cell behavior. Such control can be achieved by binding bioactive molecules to create a local microenvironment on the hydrogels’ surface.3 The linking strategy to the hydrogel network needs to be properly designed to retain large amounts of biological cues without negatively affect their bioactivity. To achieve this goal, hydrogels can be modified following several strategies including chemical conjugation, layer-by-layer deposition of charged polymers, or the addition of a reactive layer of polydopamine (pDA) on the surface of the polymeric network.4 This last approach has been largely explored due to its set of advantages including stability, biocompatibility, and high adhesion to any material.5
pDA can be virtually adsorbed on any surface irrespective of their composition, size, and shape.6 Additionally, pDA incorporates many reactive groups such as catechol, ammine, and immine that can serve as starting points for further covalent modification.7 The thickness of the pDA layer can be controlled by modulating several parameters such as the concentration of the dopamine monomer, the time of polymerization, the type of buffer, and the pH of the reaction.8 The conventional method of pDA deposition consists of the oxidation method, in which a material is placed in contact with a solution of dopamine in an alkaline environment. Dopamine is first oxidized to dopamine-quinone, followed by intramolecular cyclization. The polymer skeleton of the pDA layer is based on the covalent bonds between the aryl rings, although physical interactions among indoles and catechol groups play a secondary role after the oligomerization of the primary polymeric network.9 The pDA binding layer establishes both covalent and physical bonds with drugs and peptides carrying thiol and amine groups, thus allowing the loading and retention of sufficient amount of therapeutic agents.10 Following this strategy, we aimed to design an osteogenic scaffold made of gelatin methacrylamide (GelMA) and alginate that can absorb and retain the model drug Dex to control stem-cell osteogenic differentiation. Our hypothesis is that the designed hydrogel can find application as a coating layer for synthetic scaffolds to generate an osteoinductive interface that controls osteogenic stem-cell differentiation. As the first part of our investigation, we identified the optimal hydrogel composition to mimic the main components of the ECM. To this end, both gelatin and alginate have been selected for the hydrogel synthesis. For instance, gelatin is a well-known polypeptide derived from the denaturation of collagen, which is naturally found in ECM.11 Gelatin can also be chemically modified to introduce reactive functional groups that allow the formation of covalently cross-linked hydrogels. The natural polysaccharide alginate was selected due to its intrinsic properties including water solubility, biocompatibility, and the possibility of forming physical cross-linking with divalent ions such as calcium.12,13
In this study, gelatin has been modified with methacrylamide groups (GelMA) to form photo-cross-linkable hydrogels (Figure 1A). The successful methacrylation was verified by 1H nuclear magnetic resonance (NMR) spectroscopy, which revealed the presence of the methacrylic groups. In particular, the signals at 5.4 and 5.7 ppm are relative to the protons of the double bond of the methacrylic group.14,15 As expected, these peaks were absent in the protonic spectra of gelatin (Figure S1). Further confirmation of gelatin methacrylation was obtained using the TNBSA free-amine assay, which allows for the quantification of free amine groups that did not react during the synthesis. The percent of methacrylation was found to be 85% ± 0.3, and this result is consistent with other similar reports describing GelMA functionalization.16
Figure 1.
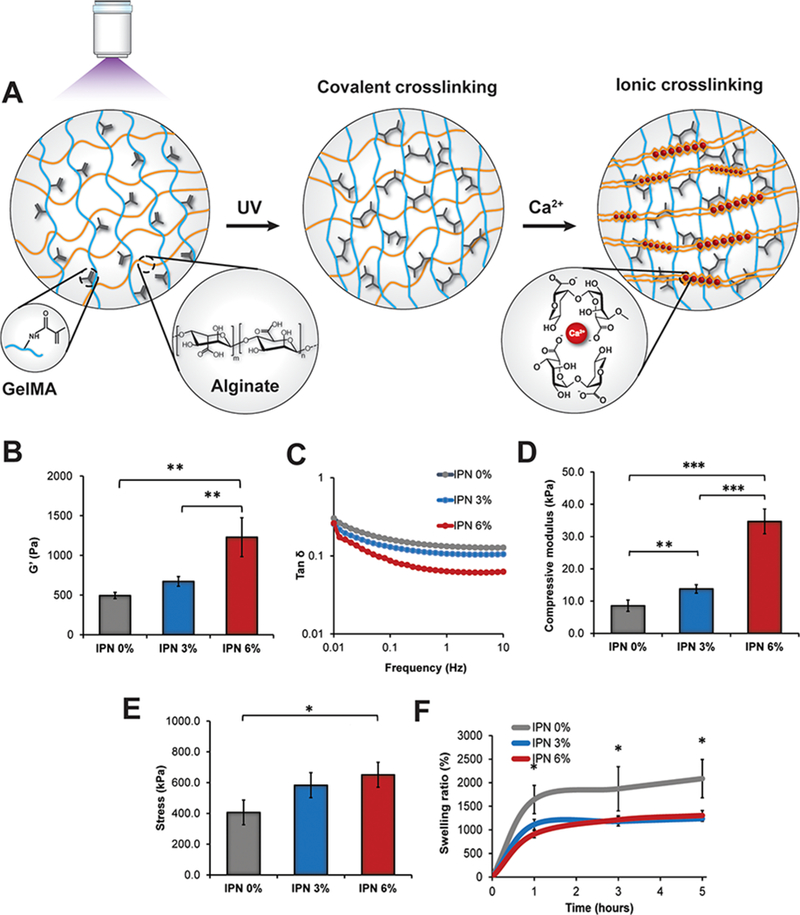
Hydrogel coating characterization. (A) Schematic representing the hydrogel composition. Gelatin methacrylamide (GelMA) was UV- cross-linked followed by the physical cross-linking of alginate with calcium ions. On the right, the typical egg-box model representing the interactions between the polymeric chains and calcium ions. (B) Storage modulus (G’) values at 1 Hz for the different groups containing several GelMA concentrations 0, 3, and 6% w/v. The concentration of alginate (1% w/v) was kept constant in all of the systems investigated. (C) Tan δ values of the samples in the range of frequencies from 0.01 up to 10 Hz. (D) Compressive modulus values calculated from the slope of the stress—strain curves in the region of 10% of the strain. (E) Maximum stress values obtained from the stress—strain curves of the different groups at the point of breakage. (F) Swelling profiles of the various samples showing a decrease in the swelling ratio dependent on the concentration of GelMA in the hydrogels. The results are reported as a mean plus or minus standard deviation (n = 5). Single asterisks indicate p < 0.05, double asterisks indicate p < 0.01, and triple asterisks indicate p < 0.001.
GelMA was photochemically cross-linked and used as the primary network component, and its concentration was varied from 3% to 6% w/v. However, GelMA hydrogels possess poor mechanical properties because they are too fragile and cannot be used alone as a coating for biomedical implants.17–20 To overcome this issue, high-molecular-weight alginate (196 kDa) was introduced and cross-linked using calcium ions during the second step of the hydrogel preparation (Figure 1A). GelMA, alginate, and the photoinitiator were mixed in a ratio of 5:4:1. To reach a concentration of alginate 1% w/v, a stock solution of alginate of 2.5% w/v (0.1 Pa.s) was mixed with GelMA to obtain a homogeneous polymeric mixture with a viscosity of 0.06 Pa.s at room temperature. This value is comparable to the one reported in a similar study (0.08 Pa s), in which GelMA 4.5% was mixed with low-molecular-weight alginate (33 kDa) at the concentration of 4% w/v.21
Such a high concentration (4% w/v) could not be used in combination with GelMA in this study because the corresponding mixture was difficult to mix due to the high viscosity of the stock solution of alginate (Figure S2). For this reason, alginate amount was kept constant in all the samples prepared. The designed hydrogel represents an example of an interpenetrating polymer network (IPN) that displays enhanced physical and mechanical properties compared to hydrogels made of single polymeric networks.19,22 The presence of both covalent and ionic interactions is a successful strategy to reinforce the mechanical properties of hydrogels.22 The concentration of calcium chloride was tested in the range of 1 to 10 mM to design hydrogels with different degree of physical cross-linking. Frequency sweep tests of the hydrogels were carried out to evaluate this effect. At concentrations higher than 1 mM, a significant increase in the value of G’ at 1 Hz was observed, which is indicative of a more-cross-linked hydrogel (Figure S3). However, cytotoxic effects were observed for concentrations above 1 mM as demonstrated by in vitro biocompatibility studies investigating the morphology and proliferation of human umbilical endothelial cells (HUVECs) (Figure S4). For this reason, 1 mM was kept constant as the optimal concentration of calcium for all of the groups investigated.
GelMA concentration was the primary factor that mainly influenced the final mechanical properties of the systems tested. The increase in GelMA concentration enabled the formation of stiffer gels with a higher compressive modulus. Specifically, the values of the storage modulus G’ at 1 Hz increased from 490 ± 40 Pa in the gels made only of alginate at 1% w/v (IPN 0%) to 1230 ± 230 Pa in the sample containing GelMA at 6% w/v (IPN 6%). However, no significant improvement was found when GelMA at 3% w/v (IPN 3%) was mixed with alginate (Figure 1B). Similarly, the value of tan δ, which represents the ratio between the loss modulus G’’ and the storage modulus G’, was significantly reduced only in the group with the higher GelMA concentration, suggesting the formation of a stiffer hydrogel (Figure 1C). All of the tested gels displayed values of tan δ lower than 1, which is representative of a highly cross-linked network.
Comparable results were obtained from the compressive studies in which an increase in the compressive modulus from 8.6 ± 1.7 to 34.7 ± 3.8 kPa was observed in the IPN 6% group. This change in the elastic modulus is in accordance with the rheological results mentioned above (Figure 1D). Additionally, the presence of GelMA at the concentration of 6% w/v determined a significant change in the maximum stress value from 406.4 ± 79.9 to 650.9 ± 80.9 kPa for the IPN 6% group (Figure 1E). However, the IPN 6% samples displayed a lower mechanical resistance when subjected to a strain higher than 10%. Specifically, strain-sweep analysis of the IPN 6% group showed an increase in the slope of tan 8 for low values of strain (45–50%) that is reflective of a less-elastic network (Figure S5). Based on these results, the concentration of GelMA was not increased further to avoid the formation of much-stiffer hydrogels that would have been too brittle to be used as coatings for synthetic scaffolds. Finally, the inclusion of GelMA decreased the value of swelling ratio compared with the gels made only of Alg 1% irrespective of the concentration of GelMA used. No significant difference was observed according to the amount of GelMA included into the IPN network (Figure 1F). Based on these findings, the IPN 6% system was selected as the model gel coating due to its higher mechanical resistance and was further tested in this project.
Aside from the optimization of the gel mechanical properties, it was important to create a sticky pDA layer on the surface of the hydrogel. The designed IPN was coated with a layer of pDA by soaking the samples into different solutions of dopamine ranging from 0.5 to 2.0 mg/mL in Tris-HCl buffer (pH 8.5). The mechanism of pDA oxidation and polymerization is summarized (Figure 2A). The hydrogel developed a brown color due to the oxidation of dopamine and corresponding polymerization to pDA (Figure 2B). To investigate whether the coating was biocompatible, hASCs were seeded on the hydrogels with (+pDA) and without the coating (−pDA). pDA has been evaluated for its cytotoxicity, and several studies have shown that pDA does not hinder the viability or proliferation of a variety of mammalian cells including fibroblasts, osteoblasts, neurons, and endothelial cells.23 Additionally, pDA coating can be helpful in promoting cell adhesion and proliferation mainly due to the covalent linking of serum extracellular proteins present in the media.24,25 In this study, hASCs were able to adhere and proliferate on the surface of the hydrogels in both pDA and −pDA groups, displaying a spindle-like morphology after 48 h of culture, as shown by immunofluorescence staining (Figure 2C). No significant change in the internal pore size distribution was determined by the adsorption of pDA. SEM images of hydrogels’ cross-sections demonstrated an inter-connected porous network with an average pore size of around 94.8 ± 19.4 and 96.2 ± 22.2 μm for the −pDA and the +pDA groups, respectively (Figure S6). However, these values were obtained from the hydrogels in their freeze-dried state, and a more-accurate estimation of the pore size should be carried out on the gels in their swollen state. An average pore size of almost 100 μm is suitable to promote the exchange of fluids and cell viability, although a larger pore size up to 500 μm is required to promote cell migration throughout the polymeric network.26
Figure 2.
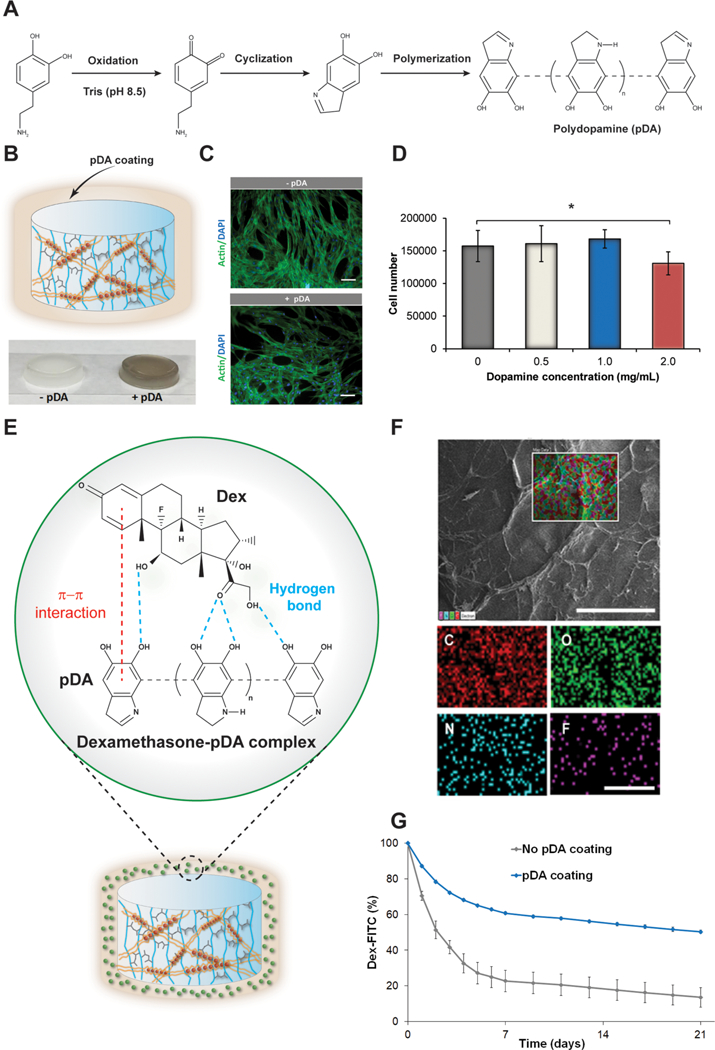
Biocompatibility of the pDA coating and dexamethasone adsorption. (A) Schematic indicating the pDA layer formation carried out in TRIS buffer at pH = 8.5. (B) Representation of the pDA coating surrounding the gel. On the bottom, pictures displaying the hydrogels with and without pDA coating. (C) Actin/DAPI fluorescent staining of hASCs grown for 48 h on the hydrogels with and without the pDA coating (1 mg/ mL). Scale bar: 100 μm. (D) MTS results of hASCs grown on the hydrogels’ surface with different concentration of dopamine compared to the sample without any coating. The results are reported as the mean ± standard deviation (n = 5). Single asterisks indicate p < 0.05. (E) Schematic indicating the main physical interactions between the pDA layer and dexamethasone. (F) SEM image of IPN hydrogels after dopamine coating and adsorption with dexamethasone. Scale bar: 500 μm. On the bottom, elementary maps displaying the presence of carbon, oxygen, nitrogen, and fluorine on the surface of the hydrogels. Scale bar: 250 μm. (G) Graph displaying the percentage of Dex-FITC desorbed from the hydrogel for 21 days. The results are reported as a mean plus or minus standard deviation (n = 10). Single asterisks indicate p < 0.05.
No significant change in cell number was observed between the control and the test groups in all of the ranges of concentrations investigated except for 2 mg/mL (Figure 2D). For this reason, a concentration of dopamine equal to 1 mg/ mL was chosen for further testing. The hydrogels reacted with dopamine due to the presence of amine and thiol groups in the lysine and cysteine residues of the gelatin backbone, as reported in other studies.27
The pDA strategy has been investigated to link peptides and drugs on the surface of other synthetic and natural scaffolds. To create an osteoinductive hydrogel coating, the hydrogel presenting a pDA layer was treated with Dex, which is a model drug to induce osteogenic differentiation. Dex can interact with pDA by π–π interactions and establish hydrogen bonding with the oxidized layer of dopamine (Figure 2E). Hydrogel surfaces coated with pDA and Dex were analyzed using EDX analysis, which provides the visual mapping of the major elements present on the surface of the hydrogels (Figure 2F). Specifically, the adsorption of Dex was confirmed by the detection of fluorine, which is present in the chemical structure of the drug (Table S1). To quantify the amount of Dex linked on the hydrogels, samples were soaked in a solution of Dex-FITC, and the fluorescence of the unbound drug was measured before and after the adsorption. The binding efficiency of Dex-FITC was higher in the +pDA group (81.2% ± 1.5) compared with the −pDA hydrogels (68.5% ± 2.5) after 24 h of soaking. Additionally, the pDA layer enabled a greater retention of the drug over a period of 21 days (Figure 2G). Precisely, after a week only 39.3% ± 0.9 of the drug was desorbed from the surface of the +pDA group compared to 77.3% ± 6.0 from the −pDA hydrogels. This result can be mainly attributed to the potential interaction between the drug and the binding layer of pDA. Dex-FITC was selected instead of DEX due to the interference caused by the photoinitiator in the UV absorbance region of DEX. The pDA layer enabled greater adsorption and retention of DEX-FITC, and the difference was mainly observed in the first week of study. However, after the first week, dexamethasone was desorbed at the same rate in both hydrogels up to 21 days.
As for the last step, we investigated whether the difference in dexamethasone adsorption and release from the IPN 6% hydrogels with and without a pDA layer was able to modulate the osteogenic differentiation of hASCs. To prove this concept, we tested two groups labeled as (pDA/Dex) and (Dex), which represent the samples treated with pDA and dexamethasone (0.1 mM) or the drug only at the same concentration. Moreover, we also tested whether the pDA coating had any effect in promoting osteogenic differentiation of hASCs. To verify this, we included two additional groups in which hASCs were cultured in osteogenic media on gels with and without a pDA layer.
Alkaline phosphatase (ALP) expression was evaluated at 7 and 14 days based on our previous study, demonstrating that the maximum expression of the ALP gene in hASCs is observed after 14 days of culture in osteogenic medium.28 Staining of ALP revealed a more-significant presence of the enzyme in the hASCs cultured on the Dex group compared to the system of pDA/Dex at day 7. This difference may be due to the larger desorption of the drug observed from the hydrogel that did not possess a pDA layer, which resulted in a higher presence of dexamethasone in the media. This effect was not evident after 14 days of culture probably because the drug is getting released at the same rate in both groups (Dex and pDA/Dex) after the first week. (Figure 3A). Similarly, a higher expression of ALP was quantified after 7 days of differentiation in the Dex group compared to the pDA/Dex sample, suggesting that the desorption of the drug dexamethasone was faster in the group without the pDA layer. The presence of pDA did not significantly influence the process of osteogenic differentiation at both days 7 and 14, which was found to be similar in both groups (Ctrl (+) and Ctrl (+) pDA; Figure 3B). Further confirmation of the modulation in osteogenic differentiation of hASCs due to the different profile of desorption of Dex was provided by assessing the amount of intracellular calcium formation. Calcium was assessed at 14 and 21 days as a later marker of osteogenic differentiation. Both alizarin red staining and calcium quantification displayed a higher calcium deposition in the Dex group compared to the pDA/Dex after 14 days. This difference was not present at day 21, at which point the two groups displayed similar calcium content (Figure 3C,D). Finally, the addition of a pDA layer did not enhance the production of calcium at both time points compared with the gels without any treatment (Ctrl (+) and Ctrl (+) pDA). These results are in accordance with the ALP findings described above.
Figure 3.
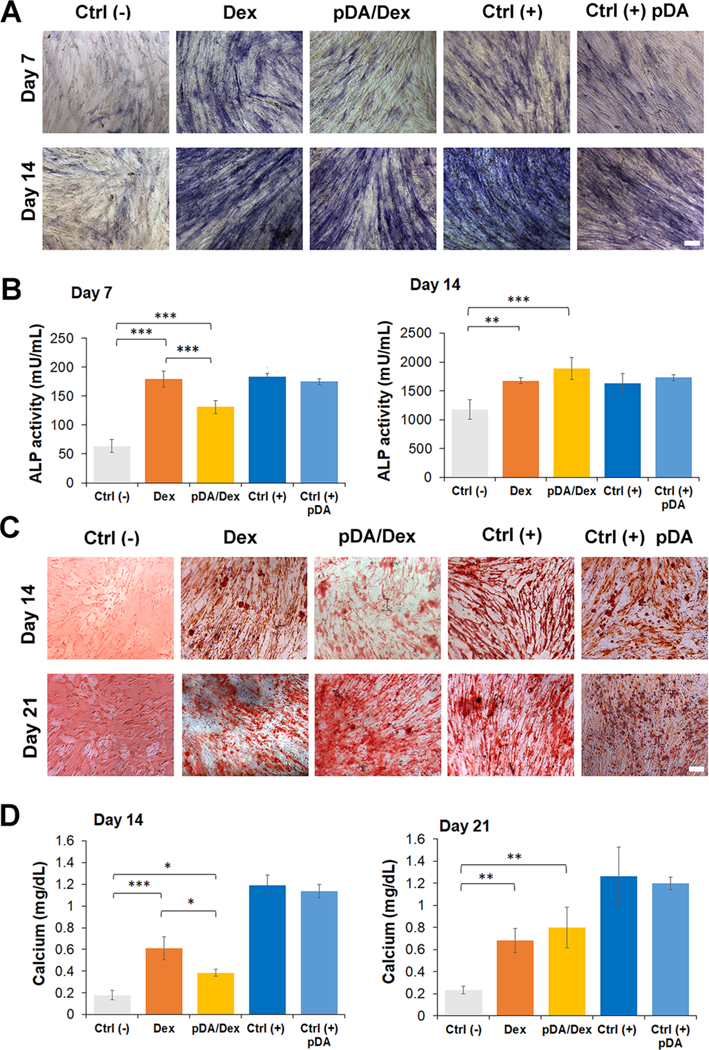
Differentiation of hASCs cultured on gels coated with pDA and treated with dexamethasone. (A) Alkaline phosphatase staining of hASCs seeded on the different gels at day 7 and day 14. A total of five groups were tested. The Ctrl (–) group indicates hASCs cultured on gel without any treatment in culture medium without any osteogenic factor. The Dex group represents hASCs cultured on gel soaked with Dex 0.1 mM in non-osteogenic medium. The pDA/Dex group indicates hASCs cultured on gel precoated with a layer of pDA and soaked afterwards with Dex 0.1 mM. Cells were cultured in the same condition as the Dex group. The Ctrl (+) group represents hASCs cultured on gel without any treatment in osteogenic medium. The Ctrl (+) pDA group displays hASCs cultured on gel precoated with a layer of pDA and cultured in osteogenic medium. Scale bar: 100 μm. (B) ALP quantification at days 7 and 14 for the different groups (n = 5). (C) Alizarin red staining of calcium deposited by hASCs seeded on the different groups at days 14 and 21. Scale bar: 100 μm. (D) Quantification of calcium in the different samples after differentiation of hASCs for 14 and 21 days. (n = 5). The results are reported as a mean plus or minus standard deviation. (n = 5). Single asterisks indicate p < 0.05, double asterisks indicate p < 0.01, and triple asterisks indicate p < 0.001.
Finally, to prove the applicability of the designed IPN hydrogel as a coating for synthetic scaffolds, IPN 6% gels were used to coat prefabricated polycaprolactone (PCL) constructs (Figure S7A). The polymeric solution could be efficiently used to soak the PCL scaffold. After the process of UV irradiation, the gel firmly adhered to the surface due to the physical interaction (hydrogen bonds and ion–dipole interaction) between GelMA/Alg mixture and the carbonyl groups of PCL. The hydrogel coating was dyed green to show a higher contrast between the white scaffold and the colorless gel. SEM images displayed the homogeneous hydrogel coating on the surface of the scaffold with a layer surrounding the filaments of the PCL construct (Figure S7B). However, further characterization is required to test the stability of the hydrogels overtime, which is an essential parameter to assess whether the gel can be employed as a biomaterial coating to promote bone regeneration.
Additionally, the polymeric mixture of alginate and GelMA could also be bioprinted into defined 3D structures to support and guide cell adhesion and blood vessel formation. Attempts in this direction have been made by fabricating a bioink made of alginate at a low molecular weight and GelMA that could support cell adhesion and proliferation.21 Moreover, the gelatin alginate IPN system has also found applicability as a template to form a sacrificial polymeric network that can be used to design fiber-based 3D structures with great potential in tissue engineering applications.29 Overall, a hydrogel made of GelMA and alginate has been fabricated, creating an IPN network with improved mechanical properties compared with the single networks made only of GelMA. The hydrogel surface was modified with pDA, which did not affect hASCs’ morphology and ability to proliferate. Additionally, the pDA binding layer enabled the favorable adsorption of the osteoinductive drug Dex. The treated hydrogel displayed a higher binding and retention of Dex over time compared with the uncoated samples that released almost 80% of the drug after a week. Dex was efficiently retained in the +pDA hydrogel during the first week of study leading to a delay in the osteogenic differentiation of hASCs. This strategy could be extended to modulate the adsorption and retention of other types of molecules including growth factors that are required in different stages of the process of bone repair as in the case of BMP2 or BMP7 that do not need to be delivered in the initial inflammatory phase.30 However, to extend the applicability of the designed system for enhancing the process of bone regeneration in vivo, further optimization is essential to defining the type and dose of growth factors required and test whether their biological activity is preserved after the process of adsorption on the pDA layer.
Supplementary Material
ACKNOWLEDGMENTS
A.P. acknowledges an investigator grant provided by the Institutional Development Award (IDeA) from the National Institute of General Medical Sciences (NIGMS) of the NIH award no. P20GM103638 and the Umbilical Cord Matrix Project fund from the State of Kansas. K.R. acknowledges the undergraduate scholarship program supported by NIH/NIGMS Kansas IDeA Network of Biomedical Research Excellence (K-INBRE) P20 GM103418. Dr. Prem Thapa of the University of Kansas Microscopy and Analytical Imaging Laboratory is acknowledged for his constant assistance with SEM microscopy and EDX analysis.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsami.8b05200.
Full details of the experimental procedure. Images showing 1H NMR spectra of gelatin and GelMA, viscosity measurements, frequency- and strain-sweep profiles, the effect of calcium concentration, MTS data on cell viability and morphology SEM images, quantification of pore size average, and PCL scaffold coating. A table providing the elementary composition of the surface in the different hydrogels. (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Ahmed EM Hydrogel: Preparation, characterization, and applications: A review. Journal of Advanced Research 2015, 6 (2), 105–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Schuurman W; Levett PA; Pot MW; van Weeren PR; Dhert WJ; Hutmacher DW; Melchels FP; Klein TJ; Malda J Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs. Macromol. Biosci. 2013, 13 (5), 551–561. [DOI] [PubMed] [Google Scholar]
- (3).Pacelli S; Basu S; Whitlow J; Chakravarti A; Acosta F; Varshney A; Modaresi S; Berkland C; Paul A Strategies to develop endogenous stem cell-recruiting bioactive materials for tissue repair and regeneration. Adv. Drug Delivery Rev. 2017, 120, 50–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Pacelli S; Manoharan V; Desalvo A; Lomis N; Jodha KS; Prakash S; Paul A Tailoring biomaterial surface properties to modulate host-implant interactions: implication in cardiovascular and bone therapy. J. Mater. Chem. B 2016, 4 (9), 1586–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Liu Y; Ai K; Lu L Polydopamine and its derivative materials: synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114 (9), 5057–5115. [DOI] [PubMed] [Google Scholar]
- (6).Lee H; Dellatore SM; Miller WM; Messersmith PB Mussel-inspired surface chemistry for multifunctional coatings. Science (Washington, DC, U. S.) 2007, 318 (5849), 426–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Liebscher J; Mrowczynski R; Scheidt HA; Filip C; Hadade ND; Turcu R; Bende A; Beck S Structure of polydopamine: a never-ending story? Langmuir 2013, 29 (33), 10539–10548. [DOI] [PubMed] [Google Scholar]
- (8).Bernsmann F; Ball V; Addiego F; Ponche A; Michel M; Gracio J. J. d. A.; Toniazzo V; Ruch D Dopamine-Melanin Film Deposition Depends on the Used Oxidant and Buffer Solution. Langmuir 2011, 27 (6), 2819–2825. [DOI] [PubMed] [Google Scholar]
- (9).Hong S; Na YS; Choi S; Song IT; Kim WY; Lee H Non-Covalent Self-Assembly and Covalent Polymerization Co-Contribute to Polydopamine Formation. Adv. Funct. Mater. 2012, 22 (22), 4711–4717. [Google Scholar]
- (10).Lee H; Rho J; Messersmith PB Facile Conjugation of Biomolecules onto Surfaces via Mussel Adhesive Protein Inspired Coatings. Adv. Mater. (Weinheim, Ger.) 2009, 21 (4), 431–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Nichol JW; Koshy ST; Bae H; Hwang CM; Yamanlar S; Khademhosseini A Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31 (21), 5536–5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Lee KY; Mooney DJ Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37 (1), 106–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Berger J; Reist M; Mayer JM; Felt O; Peppas NA; Gurny R Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57 (1), 19–34. [DOI] [PubMed] [Google Scholar]
- (14).Van Den Bulcke AI; Bogdanov B; De Rooze N; Schacht EH; Cornelissen M; Berghmans H Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 2000, 1 (1), 31–38. [DOI] [PubMed] [Google Scholar]
- (15).Yue K; Trujillo-de Santiago G; Alvarez MM; Tamayol A; Annabi N; Khademhosseini A Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Rizwan M; Peh GSL; Ang H-P; Lwin NC; Adnan K; Mehta JS; Tan WS; Yim EKF Sequentially-crosslinked bioactive hydrogels as nano-patterned substrates with customizable stiffness and degradation for corneal tissue engineering applications. Biomaterials 2017, 120, 139–154. [DOI] [PubMed] [Google Scholar]
- (17).Weng L; Gouldstone A; Wu Y; Chen W Mechanically Strong Double Network Photocrosslinked Hydrogels from N, N- Dimethylacrylamide and Glycidyl Methacrylated Hyaluronan. Biomaterials 2008, 29 (14), 2153–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Wu W; Ni Q; Xiang Y; Dai Y; Jiang S; Wan L; Liu X; Cui W Fabrication of a photo-crosslinked gelatin hydrogel for preventing abdominal adhesion. RSC Adv. 2016, 6 (95), 92449–92453. [Google Scholar]
- (19).Naficy S; Kawakami S; Sadegholvaad S; Wakisaka M; Spinks GM Mechanical properties of interpenetrating polymer network hydrogels based on hybrid ionically and covalently crosslinked networks. J. Appl. Polym. Sci. 2013, 130 (4), 2504–2513. [Google Scholar]
- (20).Gong JP Why are double network hydrogels so tough? Soft Matter 2010, 6 (12), 2583–2590. [Google Scholar]
- (21).Colosi C; Shin SR; Manoharan V; Massa S; Costantini M; Barbetta A; Dokmeci MR; Dentini M; Khademhosseini A Microfluidic Bioprinting of Heterogeneous 3D Tissue Constructs Using Low-Viscosity Bioink. Adv. Mater. (Weinheim, Ger.) 2016, 28 (4), 677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Myung D; Waters D; Wiseman M; Duhamel P-E; Noolandi J; Ta CN; Frank CW Progress in the development of interpenetrating polymer network hydrogels. Poiym. Adv. Technoi. 2008, 19 (6), 647–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Ku SH; Ryu J; Hong SK; Lee H; Park CB General functionalization route for cell adhesion on non-wetting surfaces. Biomateriais 2010, 31 (9), 2535–2541. [DOI] [PubMed] [Google Scholar]
- (24).Luo R; Tang L; Zhong S; Yang Z; Wang J; Weng Y; Tu Q; Jiang C; Huang N In Vitro Investigation of Enhanced Hemocompatibility and Endothelial Cell Proliferation Associated with Quinone-Rich Polydopamine Coating. ACS Appi. Mater. Interfaces 2013, 5 (5), 1704–1714. [DOI] [PubMed] [Google Scholar]
- (25).Cheng Y-L; Chen Y-W; Wang K; Shie M-Y Enhanced adhesion and differentiation of human mesenchymal stem cell inside apatite-mineralized/poly(dopamine)-coated poly(?-caprolactone) scaffolds by stereolithography. J. Mater. Chem. B 2016, 4 (38), 6307–6315. [DOI] [PubMed] [Google Scholar]
- (26).Liao X; Lu S; Zhuo Y; Winter C; Xu W; Li B; Wang Y Bone Physiology, Biomaterial and the Effect of Mechanical/Physical Microenvironment on MSC Osteogenesis: A Tribute to Shu Chien’s 80th Birthday. Ceil. Mol. Bioeng. 2011, 4 (4), 579–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Wang Z; Chen L; Wang Y; Chen X; Zhang P Improved Cell Adhesion and Osteogenesis of op-HA/PLGA Composite by Poly(dopamine)-Assisted Immobilization of Collagen Mimetic Peptide and Osteogenic Growth Peptide. ACS Appi. Mater. Interfaces 2016, 8 (40), 26559–26569. [DOI] [PubMed] [Google Scholar]
- (28).Pacelli S; Maloney R; Chakravarti AR; Whitlow J; Basu S; Modaresi S; Gehrke S; Paul A Controlling Adult Stem Cell Behavior Using Nanodiamond-Reinforced Hydrogel: Implication in Bone Regeneration Therapy. Sci Rep. 2017, 7 (1), 6577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Tamayol A; Najafabadi AH; Aliakbarian B; Arab-Tehrany E; Akbari M; Annabi N; Juncker D; Khademhosseini A Hydrogel templates for rapid manufacturing of bioactive fibers and 3D constructs. Adv. Heaithcare Mater. 2015, 4 (14), 2146–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).El Bialy I; Jiskoot W; Reza Nejadnik M Formulation, Delivery and Stability of Bone Morphogenetic Proteins for Effective Bone Regeneration. Pharm. Res. 2017, 34 (6), 1152–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


