Abstract
Purpose:
Over 40% of newly diagnosed metastatic breast cancer patients are ≥ 70 years-old however this population is less likely to be represented in clinical trials. The objective of this study was to analyze PFS, dose reductions, dose delays and toxicity in a geriatric population receiving palbociclib in a non-trial setting.
Methods:
Patients with metastatic breast cancer receiving palbociclib in any line of therapy were identified from a cohort of 845 patients at a large academic institution. Dose delays, dose reductions, and toxicities were retrospectively extracted from the medical record. Data were analyzed using Fischer’s exact test for categorized variables and T test/Wilcoxon rank-sum test for continuous variables. PFS and OS were analyzed using the Kaplan Meier method.
Results:
605 patients who met eligibility criteria were included. 160 patients were ≥ 65 years-old and 92 patients were ≥ 70 years-old. Patients ≥ 70 had a significantly increased number of dose reductions (p=0.03) and dose delays (p=0.02) compared to the younger patients. There was no significant increase in toxicities, including neutropenic fever, infections, or hospitalizations, in the ≥ 70 cohort (p=0.3). The ≥ 70 cohort had a significantly improved PFS as compared to the younger cohort (p=0.02) however age was no longer a significant variable in the multivariate analysis.
Conclusions:
Palbociclib was well tolerated in the geriatric population and there was no difference in PFS between older and younger patients. These results are reassuring as palbociclib becomes the frontline standard of care therapy for patients.
Keywords: Metastatic breast cancer, CDK 4/6 inhibitors, Hormone receptor-positive breast cancer, Treatment toxicity
Introduction
Although cancer is commonly diagnosed in geriatric patients, this population is less likely to be represented in clinical trials due to comorbidities and poor performance status [1]. Furthermore, there may be concerns about polypharmacy in geriatric patients with chronic medical conditions [2]. As a result, the safety and efficacy data from large randomized clinical trials may not be generalizable to the general population [3]. There is a clear need for increased inclusion of geriatric patients in clinical trials and greater research efforts regarding efficacy and safety of emerging cancer treatments in this population [4].
Despite many recent advances in the field of oncology, metastatic breast cancer remains a significant cause of morbidity and mortality. There were an estimated 40,610 estimated deaths due to breast cancer in the United States in 2017 [5]. Over forty percent of newly diagnosed breast cancer patients are ≥ 70 years-old and the incidence is expected to increase with the aging population [6]. Hormone receptor-positive breast cancer remains the most common subtype of metastatic breast cancer and is commonly seen in older patients [7]. For many years, the standard therapy for these patients was endocrine therapy followed by chemotherapy when resistance occurred [8]. Recently, the development of cyclin-dependent kinase (CDK) 4/6 inhibitors has led to a changing landscape in metastatic hormone receptor-positive breast cancer [9].
Palbociclib is a small molecule inhibitor of CDK 4/6 which regulates cell cycle progression [10]. Early studies in human tumor xenografts showed inhibition of CDK 4/6 results in tumor reduction [10]. Further studies showed palbociclib inhibited growth of estrogen receptor-positive cells and increased sensitivity to endocrine therapy in previously hormone resistant cell lines [11]. Ultimately the FDA granted accelerated approval to palbociclib in February 2015 based on the PALOMA-1 study which showed an improvement in median progression free survival (PFS) with palbociclib plus letrozole over letrozole alone [12]. These findings were replicated in the larger phase 2 PALOMA-2 trial [13]. Palbociclib is now also approved for use with fulvestrant in the second line setting following the results of the PALOMA-3 trial which found an improved PFS with addition of palbociclib and fulvestrant when compared to fulvestrant alone [14, 15]. Palbociclib is the most widely used CDK 4/6 inhibitor and has the longest follow-up data available in clinical series.
As an oral therapy, palbociclib is generally well-tolerated and is an appealing option over cytotoxic chemotherapy [16]. One of the main side effects is neutropenia. Despite the high rates of neutropenia observed in the PALOMA studies, the incidence of neutropenic fever remained low. In the PALOMA-1 trial, the high incidence of neutropenia was not associated with serious infections [12]. Similarly, the rate of grade 3 and 4 neutropenia was 66.4% in the PALOMA-2 trial however the rate of febrile neutropenia was only 1.8% [13]. There was no significant difference of grade 3 or higher infections between patients receiving palbociclib and those receiving placebo [13]. Although the PALOMA-3 safety analysis found that neutropenia was the most common grade 3 (55%) and grade 4 (10%) adverse event, there was no difference in PFS among patients who had dose reductions or delays secondary to neutropenia [16].
However, these trials were composed largely of a younger population. The median age of the patients receiving palbociclib in the PALOMA-2 study was 62, and the majority of patients (59.2%) were younger than 65 years old [13]. The median age of the fulvestrant plus palbociclib cohort in the PALOMA-3 trial was 57 [15]. Although the rate of grade 3–4 neutropenia was not significantly higher for the cohort over 70 years-old in the PALOMA-3 trial, it is important to remember this cohort represented a smaller portion of the patients undergoing treatment [16].
In addition to being generally well tolerated on clinical trials, subgroup analysis of several trials have shown that the geriatric population has similar outcomes with palbociclib. In both the PALOMA-2 and PALOMA-3 trials, there was no difference in PFS in the subgroup analysis for the cohort of patients ≥ 65 years-old and the younger cohort [13, 15]. The older cohort in PALOMA-3 had a slightly improved hazard ratio of 0.35 compared to a hazard ratio of 0.44 in the younger cohort although this was not statistically significant [15].
Given the limited data in the older adult patient population, the FDA conducted a pooled analysis of patients treated with CDK 4/6 inhibitors on multiple registration trials which showed a trend towards improved PFS in the geriatric population compared to the younger cohort, although this was not statistically significant [17]. This study found more grade 3–4 events in the geriatric population, however the overall adverse event rate was low [17]. A recently published pooled analysis of the PALOMA trials evaluated outcomes and toxicities in patients ≥ 65 year-old and found that palbociclib was both well tolerated and significantly improved PFS in the older cohort of patients [18].
While these results are encouraging, these studies represent geriatric patients receiving palbociclib on clinical trial. Geriatric patients on clinical trial generally have superior performance statuses and less comorbidities than patients receiving treatment as standard of care [19]. It is therefore important to assess the efficacy and tolerance of palbociclib in a geriatric population receiving treatment as standard of care. In this study, we aimed to retrospectively examine toxicities and outcomes of a cohort of geriatric patients compared to a younger patient population receiving palbociclib in a non-trial setting.
Experimental/Materials and Methods
Patient Population
Patients receiving palbociclib in any line of therapy were identified from a cohort of patients treated at MD Anderson Cancer Center (MDACC) followed on a prospectively maintained database from 2015 to 2017. For the preliminary analysis of the association of dose reductions and dose delays with PFS, an initial cohort of 585 patients treated from January 2015 to February 2017 was used. This cohort was then expanded to 845 patients treated from January 2015 to October 2017 for the remainder of the analysis to include a larger number of patients for subsequent analyses. Patients over the age of 18 with a clinical diagnosis of metastatic hormone receptor-positive breast cancer were considered eligible and included in the study. Patients who received the majority of treatment at an outside hospital without documentation of therapy or on a clinical trial were excluded. Clinical, demographic, baseline labs, and recurrence data were collected via an IRB-approved protocol. Dose delays, dose reductions, and toxicities were recorded. A dose delay was defined as a temporary cessation of treatment for neutropenia or infectious complication. Early dose delays and reductions were defined as dosing events occurring during the first 2 cycles of palbociclib while late dosing events were defined as cycle 3 or later. The Charlson Comorbidity Index was calculated as per convention [20]. Clinical toxicities extracted from the medical record included neutropenic fevers, infections requiring antibiotic use, emergency room encounters or hospitalizations for infectious complications. Given the variable nature of recording additional less frequent toxicities, such as fatigue and nausea, these were not included in the analysis. Toxicity grade was not available for a significant number of patients and therefore not included in the analysis.
Pathologic Assessment
Pathologic specimens were reviewed by designated breast pathologists at MDACC. Estrogen receptor and progesterone receptor status was determined by immunohistochemical analysis. Nuclear staining ≥1% for estrogen receptor and/or progesterone receptor was considered positive.
Outcome Measures and Statistical Analysis
The primary endpoint of the study was PFS, computed from date of initiation of palbociclib to date of progression, as assessed and documented clinically by the treating physician or radiographically. Secondary endpoints included dose reductions and dose delays. Overall survival data were collected as an exploratory endpoint. Data were analyzed using Fischer’s exact test for categorized variables and T-test/Wilcoxon rank-sum test for continuous variables. P values were not adjusted for multiple analyses as these were exploratory comparisons. PFS and OS were analyzed using the Kaplan-Meier method.
Results
Six hundred and five patients with metastatic hormone receptor-positive breast cancer who met eligibility criteria were analyzed. The baseline characteristics of this cohort are presented in Table 1. 160 patients were ≥ 65 years-old and 92 patients were ≥ 70 years-old at metastatic diagnosis. The median age of the cohort was 57 years-old. The majority of the patients were white (n=461, 76.2%) while the remaining patients were Hispanic (n=54, 8.9%), black (n=49, 8.1%) or other (n=41, 6.8%). The average Charlson score for the cohort was 6.5.
Table 1.
Patient Demographics and Clinical Characteristics (N=605).
| Variable | N (%) |
|---|---|
| Age at metastatic diagnosis (years) | |
| Median | 57 |
| Race | |
| White | 461 (76.2) |
| Black | 49 (8.1) |
| Hispanic | 54 (8.9) |
| Other | 41 (6.8) |
| Recurrent Disease | |
| Yes | 457 (75.5) |
| No (De novo) | 148 (24.5) |
| Prior Adjuvant Endocrine therapy | |
| No | 87 (19.0) |
| Yes | 370 (81.0) |
| Prior (Neo)adjuvant Chemotherapy | |
| No | 86 (18.8) |
| Yes | 371 (81.2) |
| Prior Adjuvant XRT | |
| No | 176 (38.5) |
| Yes | 281 (61.5) |
| Endocrine agent used in combination with palbociclib | |
| Letrozole | 352 (58.2) |
| Fulvestrant | 242 (40.0) |
| Other | 11 (1.8) |
| Metastatic Line | |
| Average | 2.4 |
| Charleson score | |
| Average | 6.5 |
| Neutropenic Fever | |
| No | 592 (97.9) |
| Yes | 13 (2.1) |
| Infection requiring antibiotic use | |
| No | 488 (80.7) |
| Yes | 117 (19.3) |
| ER/Hospitalization | |
| No | 542 (89.6) |
| Yes | 63 (10.4) |
| Use of GCSF | |
| No | 586 (96.9) |
| Yes | 19 (3.1) |
De novo presentation of metastatic disease at initial breast cancer diagnosis occurred in 148 (24.5%) patients, while 457 (75.5%) developed recurrence after early stage breast cancer. The majority of the recurrent patients had received prior adjuvant endocrine therapy (n=370, 81.0%). Of the patients with recurrent breast cancer, 137 patients (22.6%) had received neoadjuvant chemotherapy while 234 (38.7%) received adjuvant chemotherapy. 169 (49.0%) of the patients with recurrent disease had received prior radiation therapy (XRT) while 175 (50.7%) had not. The majority of the patients received palbociclib with letrozole (n=352, 58.2%), while 242 patients (40.0%) received palbociclib with fulvestrant. The majority of patients (N=283, 46.8%) received palbociclib first line while 125 patients (20.7%) received palbociclib second line and 197 (32.6%) received palbociclib in the third line setting or later.
Overall, the rate of toxicity from palbociclib for the cohort was low. Neutropenic fever occurred in 2.1% of all patients (n=13), while 19.3% (n=117) of the patients had a documented infection requiring antibiotic use and 63 patients (10.4%) had a documented emergency room visit or hospitalization secondary to an infectious complication. When toxicity was analyzed in relation to age, there was no significant difference between younger patients and older patients, using age cut-offs of ≥ 65 years-old or ≥ 70 years-old.
Factors associated with age using the cut-off of 65 and 70 are presented in Tables 2 and 3, respectively. When using both age cut-offs, older adults were significantly more likely to have early dose reductions, as well as dose delays, including both early and late delays. The older cohorts had significantly more dose reductions as well. Amongst a smaller initial cohort of 585 patients used for the initial analysis of association between PFS and dose reductions and dose delays, 344 patients met eligibility criteria. The patients experiencing dose reductions had a significantly longer PFS (unadjusted HR 0.7, P=0.01) although the adjusted HR was not found to be significant (HR 0.7, P=0.07). Patients experiencing dose delays had a significantly longer PFS (unadjusted HR 0.7, P=0.006) however the adjusted HR was again not found to be significant (HR 0.8, P=0.07).
Table 2.
Factors associated with age ≥ 65.
| Age < 65 (N=445) | Age ≥ 65 (N=160) | P value | |
|---|---|---|---|
| De novo Metastatic Disease | 0.4 | ||
| Yes | 113 (25.4) | 35 (21.9) | |
| No | 332 (74.6) | 125 (78.1) | |
| Prior Therapy for Metastatic Disease | <0.05 | ||
| None | 194 (43.6) | 89 (55.6) | |
| Hormonal | 90 (20.2) | 34 (21.3) | |
| Chemotherapy | 161 (36.2) | 37 (23.1) | |
| Early Dose Reductions | <0.05 | ||
| Yes | 91 (20.4) | 51 (31.9) | |
| No | 346 (77.8) | 106 (66.3) | |
| Late Dose Reductions | 0.3 | ||
| Yes | 74 (16.6) | 32 (20.0) | |
| No | 363 (81.6) | 125 (78.1) | |
| Number of Dose Reductions | <0.05 | ||
| 0 | 287 (64.5) | 91 (56.9) | |
| 1 | 123 (27.6) | 39 (24.4) | |
| 2 | 33 (7.4) | 29 (18.1) | |
| Dose Delays | <0.05 | ||
| Yes | 159 (35.7) | 82 (51.3) | |
| No | 286 (64.3) | 78 (48.8) | |
| Metastatic Line | <0.05 | ||
| Mean | 2.6 | 1.8 | |
| Median (range) | 2 (1–16) | 1 (1–11) | |
| Charlson Comorbidity Index | <0.0001 | ||
| Mean | 6.4 | 6.9 | |
| Toxicity | 0.7 | ||
| No | 350 (78.7) | 123 (76.9) | |
| Yes | 95 (21.3) | 37 (23.1) | |
| Heart Disease | <0.0001 | ||
| No | 426 (95.7) | 135 (84.4) | |
| Yes | 18 (4.0) | 24 (15.0) | |
| Kidney Disease | <0.0001 | ||
| No | 302 (67.9) | 71 (44.4) | |
| Yes | 143 (32.1) | 89 (55.6) |
Table 3.
Factors associated with age ≥ 70.
| Age < 70 (N=513) | Age ≥ 70 (N=92) | P value | |
|---|---|---|---|
| De novo Metastatic Disease | 0.4 | ||
| Yes | 129 (25.1) | 19 (20.7) | |
| No | 384 (74.9) | 73 (79.3) | |
| Prior Therapy for Metastatic | <0.0001 | ||
| None | 224 (43.7) | 59 (64.1) | |
| Hormonal | 106 (20.7) | 18 (19.6) | |
| Chemotherapy | 183 (35.7) | 15 (16.3) | |
| Early Dose Reductions | <0.05 | ||
| Yes | 107 (20.9) | 35 (38.0) | |
| No | 397 (77.4) | 55 (59.8) | |
| Late Dose Reductions | 0.6 | ||
| Yes | 88 (17.2) | 18 (19.6) | |
| No | 416 (81.1) | 72 (78.3) | |
| Number of Dose Reductions | <0.0001 | ||
| 0 | 330 (64.3) | 48 (52.2) | |
| 1 | 139 (27.1) | 23 (25.0) | |
| 2 | 41 (8.0) | 21 (22.8) | |
| Dose Delays | <0.0001 | ||
| Yes | 189 (36.8) | 52 (56.5) | |
| No | 324 (63.2) | 40 (43.5) | |
| Metastatic Line | <0.05 | ||
| Mean | 2.5 | 1.7 | |
| Median (range) | 2 (1–16) | 1 (1–6) | |
| Charlson Comorbidity Index | <0.0001 | ||
| Mean | 6.4 | 6.9 | |
| Toxicity | 0.3 | ||
| No | 405 (78.9) | 68 (73.9) | |
| Yes | 108 (21.1) | 24 (26.1) | |
| Heart Disease | <0.0001 | ||
| No | 487 (94.9) | 74 (80.4) | |
| Yes | 25 (4.9) | 17 (18.5) | |
| Kidney Disease | <0.0001 | ||
| No | 341 (66.5) | 32 (34.8) | |
| Yes | 172 (33.5) | 60 (65.2) |
Older patients were also more likely to receive palbociclib as frontline treatment (mean metastatic line 1.7 versus 2.5 for cohort ≥ 70, p<0.05 and mean metastatic line 1.8 versus 2.6 for cohort ≥ 65, p<0.05). Older adults were less likely to have received prior chemotherapy and/or hormonal therapy for metastatic disease. Older patients were more likely to have more comorbidities, as evidence by significantly higher Charlson Comorbidity Index as well as higher prevalence of heart disease and kidney disease, compared to the younger cohort. Toxicity, including neutropenic fever, infections and hospitalizations, were not significantly different between cohorts.
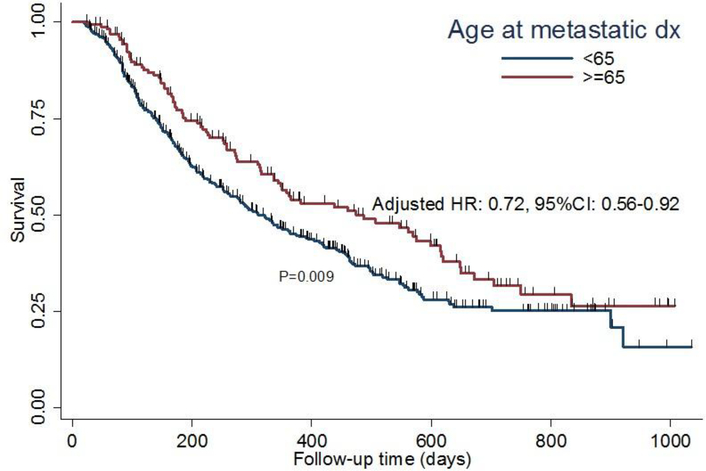
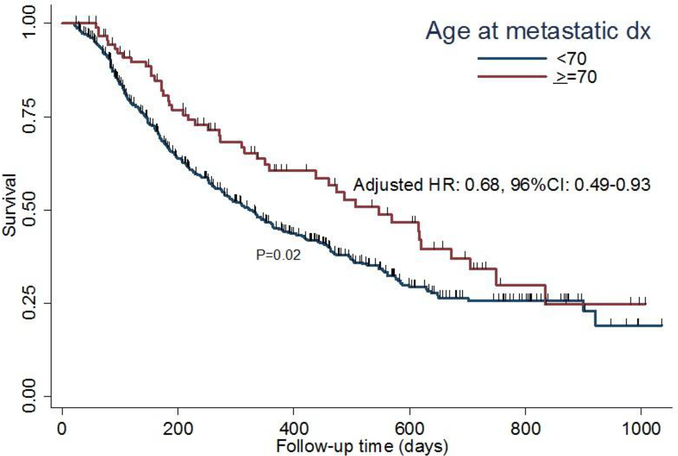
The primary endpoint of PFS for the geriatric patients using an age cut-off of 65 and 70 are presented in Figures 1 and 2, respectively. The ≥ 70 cohort had a significantly improved PFS as compared to the younger cohort (p=0.02). This was also true when an age cut-off of ≥ 65 was used (p=0.009). Median PFS for the cohort was 344 days. In the multivariable analyses for factors associated with progression, tumor grade, metastatic line and presence of liver metastasis were found to be significant (Table 4). Age was no longer significant for PFS.
Fig 1.
Kaplan-Meier survival plot demonstrating association between age and PFS in patients ≥ 65
Fig 2.
Kaplan-Meier survival plot demonstrating association between age and PFS in patients ≥ 70
Table 4.
Multivariable analyses Cox model for the factors associated with progression.
| HR | P | 95% CI | ||
|---|---|---|---|---|
| Tumor grade | ||||
| I/II | referent | |||
| III | 1.52 | 0.001 | 1.18 | 1.97 |
| Metastatic Line | ||||
| 1st | referent | |||
| >1st | 1.96 | <0.001 | 1.56 | 2.46 |
| Liver mets | ||||
| No | referent | |||
| Yes | 1.84 | <0.001 | 1.47 | 2.31 |
The overall survival (OS) data, which served as an exploratory endpoint, remain immature. At the time of analysis, there were 83 deaths recorded in the overall cohort, 17 deaths in the ≥ 65 years-old cohort and 11 deaths in the ≥ 70 years-old cohort. At this time, there was no difference in OS in either cohort (age ≥70 p=0.4, age ≥ 65 p=0.9). Median OS follow up time for the cohort was 882 days.
Discussion
CDK 4/6 inhibitors add significant benefit to endocrine therapy in the treatment of metastatic hormone receptor-positive breast cancer and are now often used as first and second line treatment [9]. In addition to adding clinical benefit, CDK 4/6 inhibitors are generally well tolerated. Neutropenia was found to be the most common side effect of palbociclib in patients receiving palbociclib on clinical trial [12–15]. Neutropenia remained the most common side effect among all sub-groups in the safety analysis of the PALOMA-1 trial21. The mechanism of neutropenia occurs through cell-cycle arrest, as opposed to apoptosis, which occurs with traditional chemotherapy regimens [22]. Preclinical studies showed that the neutropenia due to CDK 4/6 inhibitors is reversible [23]. As a result, the neutropenia secondary to palbociclib is associated with less febrile neutropenia and mortality than chemotherapy induced neutropenia [24]. Similar to the PALOMA trials, this study found that the rate of neutropenic fever remained low.
As CDK 4/6 inhibitors may be used frequently in geriatric patients, it is important to evaluate CDK 4/6 inhibitors’ safety and efficacy in this population. The recent FDA pooled analysis found a low overall adverse event rate in the geriatric cohort however there was more grade 3–4 events in the geriatric population [17]. This current study aimed to identify clinically relevant toxicities and reassuringly found the rates of neutropenic fever, infections and hospitalizations were not higher in the geriatric cohort.
Although CDK 4/6 inhibitors are generally well tolerated, they are associated with side effects and ensuring that geriatric patients derive meaningful benefit from these drugs is essential. Previous subgroup analysis from the PALOMA trials as well as the recent FDA pooled analysis have shown no difference between PFS between the geriatric and younger cohort of patients [12–15, 17]. In the single variate analysis, the geriatric cohort was found to have improved PFS compared to the younger cohort when both age ≥ 65 and ≥ 70 cut-offs were used. However, the multivariate analysis found no difference in PFS between the cohorts, indicating there are likely underlying confounding variables which account for the PFS benefit found in the initial single variate analysis. The geriatric cohort was more likely to receive palbociclib earlier in their treatment course where patients often derive greater absolute benefit and may account for these differences. Overall, it is reassuring that patients receiving CDK 4/6 inhibitors as standard of care equally benefitted.
Dose reductions and dose delays due to neutropenia are common in patients receiving palbociclib and are recommended for patients experiencing grade 3 and 4 neutropenia [25]. This study also found that dose reductions and delays were common in patients receiving palbociclib outside of a clinical trial and were higher in the geriatric cohort. In the PALOMA-3 trial, dose reductions were not associated with significantly decreased PFS [16]. This study found that in patients receiving palbociclib as standard of care, patients with dose reductions and delays had a longer PFS than those without dose reductions and delays however this difference was no longer significant when adjusting for underlying variables. The initial superior PFS of patients with dose reductions and delays is likely explained by underlying confounding variables and differences between the two cohorts, which is a limitation of retrospective analyses. Given the high rates of dose delays and reductions in the geriatric cohort, it is reassuring that the PFS was not negatively affected in these patients.
There are several additional limitations of the current study. Analysis of dose reductions, dose delays and toxicities relied on physician and patient reporting and may be underestimated due to incomplete records, such as care being received partially at an outside institution. Another limitation is the potential selection bias of patients receiving treatment at a large academic tertiary care center who may be more fit than patients seen in a community oncology practice.
A strength of the current study is that patients receiving palbociclib as standard of care were analyzed. This population is generally less fit than patients on clinical trials and may be more generalizable. The geriatric cohort in this study had significantly more comorbidities than the younger cohort, which supports this. Furthermore, the geriatric cohort was also found to have increased dose reductions and delays, which also indicates a less fit population.
Overall, this study reassuringly finds that geriatric patients with more comorbidities receiving palbociclib as standard of care tolerate palbociclib and had similar benefit. As use of palbociclib as standard of care becomes more common, future larger retrospective studies are warranted to continue to analyze tolerance and efficacy of palbociclib in a geriatric population.
Acknowledgments
Funding: Dr. Clifton receives research support from T32 CA009666-24.
Footnotes
Compliance with Ethical Standards:
Conflict of Interest: The following authors report conflicts of interest:
Dr. Jennifer Litton-advisory boards and consulting for Pfizer (both uncompensated); research funding for clinical trials from Pfizer.
Dr. Meghan Karuturi-consulting for Pfizer
Dr. Debu Tripathy-Novartis-clinical trial support (support paid to the institution), consultant, consulting for Pfizer
Dr. Clifton, Dr. Min, Jaime Kimmel do not report any conflicts of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: The Institutional Review Board approved this study; informed consent requirement was waived given the retrospective study design.
References
- 1.Herrera AP, Snipes SA, King DW, Torres-Vigil I, Goldberg DS, Weinberg AD. Disparate Inclusion of Older Adults in Clinical Trials: Priorities and Opportunities for Policy and Practice Change. American Journal of Public Health. 2010;100(Suppl 1):S105–S112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shenoy P, Harugeri A. Elderly patients’ participation in clinical trials. Perspectives in Clinical Research. 2015;6(4):184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell AP, Harrison MR, George DJ, et al. Clinical trial subjects compared to “real world” patients: Generalizability of renal cell carcinoma trials. Journal of Clinical Oncology. 2014;32:6510–6510. [Google Scholar]
- 4.Watts G Why the exclusion of older people from clinical research must stop. BMJ. 2012;344:e3445. [DOI] [PubMed] [Google Scholar]
- 5.American Cancer Society Cancer Facts & Figures 2017. Supplemental Data. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breastcancer-facts-and-figures/breast-cancer-facts-and-figures-2017-2018.pdf.2017 [accessed 12 January 2018]
- 6.Mariotto AB, Etzioni R, Hurlbert M, et al. Estimation of the Number of Women Living with Metastatic Breast Cancer in the United States. Cancer Epidemiol Biomarkers Prev. 2017;26(6):809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106(5):dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reinert T, Barrios CH. Optimal management of hormone receptor positive metastatic breast cancer in 2016. Therapeutic Advances in Medical Oncology. 2015;7(6):304–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolff A CDK4 and CDK6 Inhibition in Breast Cancer — A New Standard. N Engl J Med. 2016;375:1993–1994. [DOI] [PubMed] [Google Scholar]
- 10.Fry DW, Harvey PJ, Keller PR, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004;3:1427–1438. [PubMed] [Google Scholar]
- 11.Finn RS, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11:R77–R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16:25–35. [DOI] [PubMed] [Google Scholar]
- 13.Finn RS, Martin M, Rugo HS, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med. 2016;375:1925–1936. [DOI] [PubMed] [Google Scholar]
- 14.Turner NC, Ro J, André F, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373:209–219. [DOI] [PubMed] [Google Scholar]
- 15.Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425–439. [DOI] [PubMed] [Google Scholar]
- 16.Verma S, Bartlett CH, Schnell P, et al. Palbociclib in Combination With Fulvestrant in Women With Hormone Receptor-Positive/HER2-Negative Advanced Metastatic Breast Cancer: Detailed Safety Analysis From a Multicenter, Randomized, Placebo-Controlled, Phase III Study (PALOMA-3). The Oncologist. 2016;21(10):1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh H A U.S. food and drug administration pooled analysis of outcomes of older women with hormone-receptor positive metastatic breast cancer treated with a CDK4/6 inhibitor as initial endocrine based therapy. Presented at 2017 San Antonio Breast Cancer Symposium; Dec 2017; San Antonio, TX. [Google Scholar]
- 18.Rugo HS, Turner NC, Finn RS et al. Palbociclib plus endocrine therapy in older women with HR+/HER2- advanced breast cancer: a pooled analysis of randomised PALOMA clinical studies. Eur J Cancer. 2018;101:123–133. [DOI] [PubMed] [Google Scholar]
- 19.Yates JW. Comorbidity considerations in geriatric oncology research. CA Cancer J Clin. 2001;51(6):329–36. [DOI] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, MacKenzie R A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases. 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 21.Finn RS, Crown JP, Ettl J, et al. Efficacy and safety of palbociclib in combination with letrozole as first-line treatment of ER-positive, HER2-negative, advanced breast cancer: expanded analyses of subgroups from the randomized pivotal trial PALOMA-1/TRIO-18. Breast Cancer Research. 2016;18(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu W, Sung T, Jessen BA, et al. Mechanistic Investigation of Bone Marrow Suppression Associated with Palbociclib and its Differentiation from Cytotoxic Chemotherapies. Clin Cancer Res. 2016;22(8):2000–8. [DOI] [PubMed] [Google Scholar]
- 23.Johnson SM, Torrice CD, Bell JF, et al. Mitigation of hematologic radiation toxicity in mice through pharmacological quiescence induced by CDK4/6 inhibition. J Clin Invest. 2010;120(7):2528–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sammons SL, Topping DL, Blackwell KL. HR+, HER2− Advanced Breast Cancer and CDK4/6 Inhibitors: Mode of Action, Clinical Activity, and Safety Profiles. Current Cancer Drug Targets. 2017;17(7):637–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palbociclib (Ibrance) [package insert]. Pfizer, New York, New York; 2017. [Google Scholar]