Abstract
Background:
Individuals with HIV have ~ 2-fold increased risk of developing pulmonary fibrosis. The mechanism(s) by which this occurs has yet to be determined. HIV-1 protein gp120 activates CXCR4 in the lymphocyte, promoting a variety of intracellular signaling pathways including those common to TGFβ1 associated with lung fibroblast-to-myofibroblast transdifferentiation. We hypothesized that gp120 promotes pulmonary fibrotic changes via activation of CXCR4 in the lung fibroblast.
Methods:
Mouse primary lung fibroblasts (PLFs) were cultured ± gp120, then analyzed for α-SMA expression and stress fiber formation. In parallel, PLFs were cultured ± gp120 ± AMD3100 (a CXCR4 antagonist), and α-SMA, pan and phospho-Akt, and total and phospho-MAPK (or ERK1/2) protein expression was quantified. Finally, lungs and PLFs from wild-type and HIV-1 transgenic mice were analyzed for hydroxyproline and α-SMA content.
Results:
gp120 treatment increased α-SMA expression and myofibroblast differentiation in PLFs. gp120 treatment activated phosphorylation of ERK1/2, but not PI3K-Akt. Pre-treatment with AMD3100 inhibited gp120-induced ERK1/2 phosphorylation and gp120-induced α-SMA expression. In parallel, there was a significant increase in hydroxyproline content in lungs from older HIV-1 transgenic mice and a > 3-fold increase in α-SMA expression in PLFs isolated from HIV-1 transgenic mice.
Conclusions:
gp120 induces α-SMA expression and fibroblast-to-myofibroblast transdifferentiation by activating the CXCR4-ERK1/2 signaling pathway in mouse PLFs. Lungs of older HIV-1 transgenic mice contain higher hydroxyproline content and their PLFs have a striking increase in α-SMA expression. These results suggest a mechanism by which individuals with HIV are at increased risk of developing pulmonary fibrotic changes as they age.
Keywords: gp120, fibroblast, myofibroblast, CXCR4
INTRODUCTION
HIV infection is associated with increased risk for infectious and non-infectious chronic pulmonary complications including tuberculosis, emphysema, venous thromboembolism, pulmonary hypertension, and lung cancer1, 2. In a large cohort of VA patients, HIV-positive status conferred a nearly two-fold increased risk of pulmonary fibrotic changes in older patients independent of multiple covariates1. Additionally, in a cross-sectional multicenter cohort, fibrosis-like changes were present on computed tomography in approximately one third of HIV-positive individuals3. Despite these clinical and radiographic findings, the underlying mechanism(s) by which HIV predisposes to fibrotic changes in the lung has yet to be determined. In particular, it is unclear whether changes are a consequence of the increased frequency of pulmonary infections and post-infectious lung remodeling, or whether they are directly attributed to HIV-related proteins and their effects on cell signaling and function.
Pulmonary fibrosis, or lung tissue scarring, is due to excessive deposition of collagen and extracellular matrix in the lung, which may lead to reduction in pulmonary function, impairment of gas exchange, and resultant increase in respiratory symptoms such as dyspnea and cough4. While fibrosis can be initiated by a variety of insults, the key effector cell is the myofibroblast, which is believed to originate from at least three sources: native lung fibroblasts undergoing fibroblast-to-myofibroblast transdifferentiation, pulmonary epithelial cells undergoing epithelial-to-mesenchymal transition, and circulating fibrocytes5. Regardless of their origin, myofibroblasts are characterized by production of α-smooth muscle actin (α-SMA) stress fibers, and are believed to be responsible for the majority of excess collagen and extracellular matrix production seen in pulmonary fibrosis5.
The HIV-1 genome encodes for a variety of viral proteins necessary for target cell entry and viral replication6. gp120 is an HIV-1 envelope glycoprotein that mediates viral particle entry by binding to cell surface receptor CD4 and co-receptors CXCR4 and/or CCR5 depending on viral tropism6. While CXCR4 is a chemokine receptor classically associated with leukocytes, its presence has also been noted on a variety of other cell types, including hepatic stellate cells (HSCs) and circulating fibrocytes7–9. In addition to aiding viral cell entry, gp120 can serve as an agonist of CXCR4 in human CD4+ T-lymphocytes, promoting a variety of potentially pro-fibrotic intracellular signaling pathways, which in the lung fibroblast have been associated with fibroblast-to-myofibroblast transdifferentiation10–12. Finally, CXCR4-tropic (X4-tropic) gp120 has been shown to activate hepatic stellate cells with resultant increased expression of α-SMA and collagen type I13. However, no study to date has evaluated if gp120 has any effect on the lung fibroblast.
While the possibility of CXCR4 blockade has been extensively studied, there are no FDA-approved anti-CXCR4 medications indicated for HIV management; however, AMD3100, one of the early CXCR4 antagonists, is approved in individuals with multiple myeloma and non-Hodgkin lymphoma to aid in mobilization of hematopoietic stem cells to the peripheral blood14, 15. AMD3100 is a bicyclam that is a specific CXCR4 antagonist and does not affect any other chemokine receptor, nor does it act as a CXCR4 agonist16. Its ability to block HIV-1 viral entry via prevention of gp120 binding to CXCR4 prompted a phase I trial with promising results; however, further studies regarding its therapeutic use in HIV have been limited due to inability to block CCR5-tropic (R5) viral entry and limited oral bioavailability14, 17, 18.
In the present study, we hypothesized that HIV-1 directly mediates an increase in pulmonary fibrotic changes, at least in part, through gp120-mediated activation of CXCR4 on the lung fibroblast, with resultant increase in fibroblast-to-myofibroblast transdifferentiation and increased deposition of matrix proteins, such as collagen, over time. Further, we asked whether or not gp120-mediated fibroblast-to-myofibroblast transdifferentiation could be inhibited by treatment with the CXCR4 antagonist AMD3100. To test our hypothesis, we used the well-characterized HIV-1 transgenic mouse model that allowed us to examine the effect of HIV-1 infection in primary lung fibroblasts ex vivo in addition to the in vivo effect of HIV-1 on collagen deposition in lung tissue.
METHODS
HIV-1 transgenic mouse model
Three to six and eight to nine-month old wild-type (WT) or HIV-1 transgenic (TG) mice were utilized. HIV-1 TG mice in the FVB background were originally obtained from Dr. Paul Klotman19 and were then backcrossed into the C57BL/6 background for 10 generations by our colleague Dr. Roy Sutliff (Atlanta VA Medical Center, Decatur, GA). We then developed a colony in our laboratory that provides WT and TG litter-mate mice in a 1:1 ratio by breeding heterozygous TG male mice with WT female mice. All studies were approved by the Institutional Animal Care and Use Committee (IACUC) at Emory University and conformed to institutional standards for the humane treatment of laboratory animals.
Cell culture and treatment
Primary lung fibroblasts (PLFs) were isolated from the lungs of three to six-month old WT or HIV-1 TG mice as we previously described20. PLFs (passage 2–7) were cultured in DMEM with 4.5 g/L glucose supplemented with 20% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 U/ml streptomycin. Cells were cultured in complete culture medium containing 5% FBS 24 hours prior to treatment initiation. Treatments included oligomeric X4-tropic recombinant gp120, cloned from HIV-1 IIIB and expressed in the Baculovirus Expression System at >98% purity (ImmunoDX, 10ng/mL) ± AMD3100 (Sigma, St. Louis, MO, USA, 5μM and 10μM) in complete culture medium containing 2% FBS for 30 minutes, 1 hour, 2 hours, 24 hours, and 72 hours. In cells treated with both AMD3100 and gp120, cells were pretreated with AMD3100 for 15 minutes prior to gp120 treatment.
Messenger RNA expression analyses
Messenger RNA (mRNA) was isolated from whole lung or PLFs as previously described using RNeasy kit (Qiagen, Germantown, MD, USA)20. First-strand cDNA was synthesized and quantitative PCR was performed with primers set for 18s and α-SMA using iQ SYBR Green Supermix (Bio-Rad); the real-time iCycler sequence detection system (Bio-Rad) was used for the real-time PCR analysis. The level of target mRNA expression was normalized to 18s housekeeping gene levels and relative target mRNA levels were determined according to the comparative cycle threshold method (Applied Biosystems 7900HT Sequence Detection System, User Bulletin No. 2; Applied Biosystems, Foster City, CA, USA) and relative expression values were calculated as previously described21.
Protein isolation and analyses
Total protein from whole lung or PLFs was isolated as previously described20. Equal amounts of protein samples were separated on 4–20% gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel, and transferred to nitrocellulose membranes. The blots were blocked in blocking buffer (5% non-fat dry milk, 0.1% Tween 20 in TBS, pH 7.4) for 1 hour at room temperature, then incubated with rabbit anti-mouse α-SMA (Abcam, Cambridge, UK, 1:2,000), goat anti-mouse CXCR4 (Abcam 1:500), rabbit anti-mouse phosphor-Akt and pan Akt (Ser473) (Cell Signaling, Danvers, MA, 1:1,000), rabbit anti-mouse phospho-p44/42-MAPK (ERK1/2) and total p44/42-MAPK (ERK1/2) (Thr202/Tyr204) (Cell Signaling, Danvers, MA, 1:2,000), and rabbit anti-mouse GAPDH (Sigma, 1:50,000) at 4°C overnight in 5% bovine serum albumin in 0.1% Tween 20 in TBS. They were then washed and incubated for 1 hour at room temperature with an appropriate horseradish peroxidase–conjugated secondary antibody (Amersham Biosciences, Pittsburgh, PA, USA), washed, and visualized via enzyme-linked chemiluminescence using the SuperSignal West Pico kit (Pierce Biotechnology, Rockford, IL, USA).
Immunofluorescent staining
Mouse PLFs were cultured in chamber glass slide (Lab-Tek, Rochester, NY, USA) in the presence or absence of gp120 (10ng/mL), heat-inactivated gp120 (10ng/mL), and TGFβ1 (R&D Systems, Minneapolis, MN, USA, 5ng/mL) for 96 hours. Cells were fixed in 4% paraformaldehyde in PBS for 10 minutes at 37°C, washed with PBS, then permeabilized in 0.5% Triton X-100 (Sigma) in PBS and stained for α-SMA overnight using rabbit anti-mouse α-SMA antibody at a 1:200 dilution at 4°C (Abcam). Slides were incubated with goat anti-rabbit 488 secondary antibody (Invitrogen, Carlsbad, CA, USA, 1:500); DAPI nuclear stain was applied, and slides were examined using an Olympus BX-41 fluorescence microscope (Olympus, Center Valley, PA, USA) at 20× magnification. Cell count was performed from 3–5 random fields per sample (20× magnification) from triplicate experiments. Data were expressed as percent of cells with stress fiber formation compared to number of total cells.
Hydroxyproline assay
Frozen right lung of WT and TG mice were analyzed for hydroxyproline content using the manufacturer’s protocol from a commercially available kit (BioVision, San Francisco, CA, USA). A hydroxyproline standard was used to generate a standard curve, and hydroxyproline content was calculated using this curve.
Statistical analyses
Unpaired two tailed t-tests or one-way ANOVA were used for comparisons between groups using GraphPad Prism and GraphPad InStat version 4. Post-test analysis following one-way ANOVA using Dunnett’s correction method was performed if statistical significance was reached. GraphPad Prism and GraphPad InStat version 5 were used to calculate statistics. Significant differences were accepted at a P level of < 0.05.
RESULTS
gp120 increases α-SMA expression in mouse primary lung fibroblasts
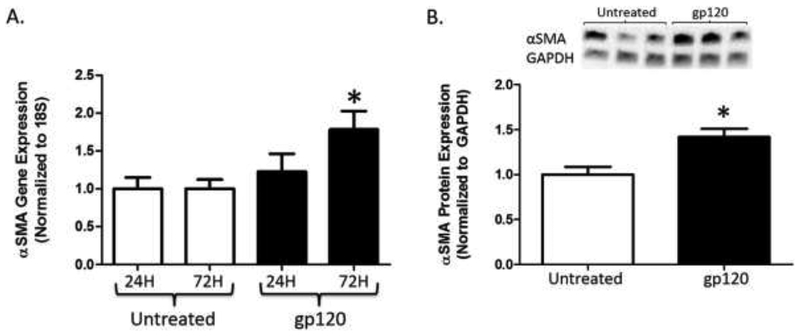
To determine if gp120 had a direct effect on myofibroblastic differentiation, we first measured α-SMA gene and protein expression in mouse PLFs treated with gp120 for 24 and 72 hours. Figure 1A highlights that PLFs treated with gp120 for 24 hours had no significant change in α-SMA gene expression; however, gene expression was significantly increased by almost 2-fold after treatment with gp120 for 72 hours as compared to untreated cells. In a similar fashion, α-SMA protein expression (Figure 1B) was almost 50 percent higher in cells treated with gp120 as compared to untreated cells after 72 hours. These results imply that gp120 directly affects the lung fibroblast and induces α-SMA protein expression, suggestive of fibroblast-to-myofibroblast transdifferentiation.
Figure 1. gp120 augments α-SMA expression in mouse primary lung fibroblasts.
Mouse primary lung fibroblasts (PLFs) were isolated from wild-type mice, and after passages 3–7, cells were treated with or without gp120 (10ng/mL). (A) At 24 and 72 hours, cells were harvested for α-SMA gene expression analysis by quantitative PCR. (B) At 72 hours, cells were harvested for α-SMA protein expression analysis by Western immunoblot. (A) Untreated PLFs at 24 and 72 hours N = 12 and 11, and gp120-treated PLFs at 24 and 72 hours N = 12 and 10. (B) Untreated and gp120-treated PLFs N = 12. *P<0.05 increased compared to untreated cells.
gp120 augments the myofibroblast phenotype in mouse primary lung fibroblasts
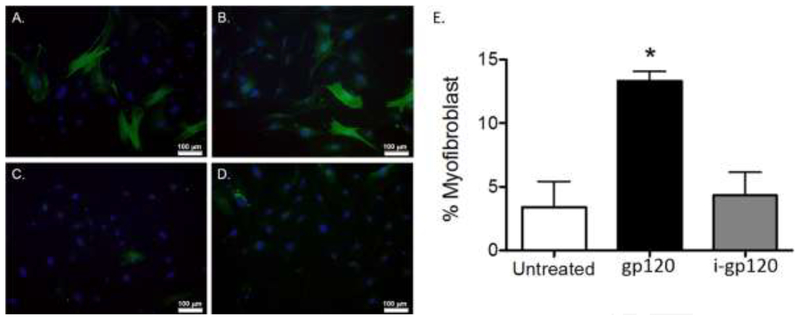
To confirm that increased α-SMA gene and protein expression was associated with increased myofibroblastic transdifferentiation, we treated cells with gp120 (10ng/mL), TGFβ1 (as a positive control; 5ng/mL), or heat-inactivated gp120 (as a negative control; 10ng/mL), for 96 hours, and evaluated cells for the presence of α-SMA stress fibers via fluorescent microscopy. In Figure 2A, gp120 treatment induced cells with high α-SMA expression and stress fiber formation (shown in green), consistent with the myofibroblast morphology, in a fashion similar to TGFβ1-treated cells (Figure 2B). As expected, untreated cells (Figure 2C) and cells treated with heat-inactivated gp120 (Figure 2D) expressed low levels of α-SMA without stress fiber formation. Consistent with these fluorescent images, quantifying myofibroblasts in each condition identified that gp120 treatment increased the percentage of cells bearing a myofibroblast phenotype greater than 3-fold compared to untreated and heat-inactivated gp120-treated cells (Figure 2E). gp120-treated cells had a similar percentage of cells with a myofibroblast phenotype compared to cells treated with TGFβ1 (data not shown). These results confirm that gp120 directly augments lung fibroblast-to-myofibroblast transdifferentiation.
Figure 2. gp120 induces the myofibroblast phenotype in mouse primary lung fibroblasts.
Mouse primary lung fibroblasts (PLFs) were isolated from wild-type mice, and after passages 3–7, PLFs were treated with (A) gp120 (10ng/mL), (B) TGFβ1 (5ng/mL), (C) no treatment, and (D) heat-inactivated gp120 (10ng/mL). At 96 hours, cells were fixed and stained for α-SMA and DAPI. Cells were analyzed for morphology by immunofluorescence microscopy. (E) Graph depicts quantification of myofibroblasts by cell count. Total PLFs counted for untreated group = 222, gp120-treated group = 371, and heat-inactivated gp120-treated group = 328. Green = α-SMA, blue = DAPI nuclear stain. All images were captured at 20× magnification. *P<0.05 increased compared with untreated and heat-inactivated gp120-treated groups.
gp120 induces the ERK1/2 but not PI3K-Akt signaling pathway.
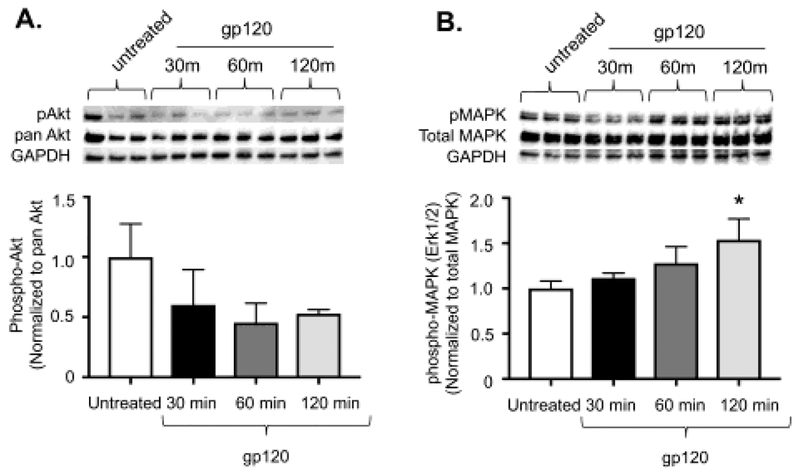
Next, we attempted to identify the intracellular signaling pathway responsible for fibroblast-to-myofibroblast transdifferentiation following gp120-mediated activation of CXCR4. Activation of CXCR4 can result in several biological responses including activation of pro-fibrotic intracellular signaling pathways important for myofibroblat development; e.g. phosphatidylinositol 3-kinase (PI3K)-Akt and mitogen-activated protein kinase (MAPK, also known as ERK1/2)12, 22, 23. Therefore, we examined whether gp120 induces phosphorylation of PI3K/Akt or ERK1/2 in PLFs. As shown in Figure 3A, treatment with gp120 did not induce phosphorylation of Akt at 30, 60, or 120 minutes. However, treatment with gp120 significantly induced phosphorylation of ERK1/2 at 120 minutes as shown in Figure 3B.
Figure 3. gp120 induces phosphorylation of ERK1/2 but not PI3K-Akt signaling.
Mouse primary lung fibroblasts (PLFs) were isolated from wild-type mice, and after passages 2–7, cells were treated with or without gp120 (10ng/mL). Cells were harvested at 30, 60, or 120 minutes after treatment with gp120 and analyzed for (A) phosphorylated Akt, pan Akt, and GAPDH protein expression. Graph depicts phosphorylated Akt normalized to pan Akt. Upper panel shows representative blots. (B) phosphorylated mitogen-activated protein kinase 1/2 (ERK 1/2), total ERK 1/2, and GAPDH protein expression. Graph depicts phosphorylated ERK1/2 normalized to total ERK1/2. Upper panel shows representative blots. N = 6 per group. *P<0.05 increased compared to untreated cells.
The pro-fibrotic effect of gp120 can be inhibited by treatment with the CXCR4 antagonist, AMD3100
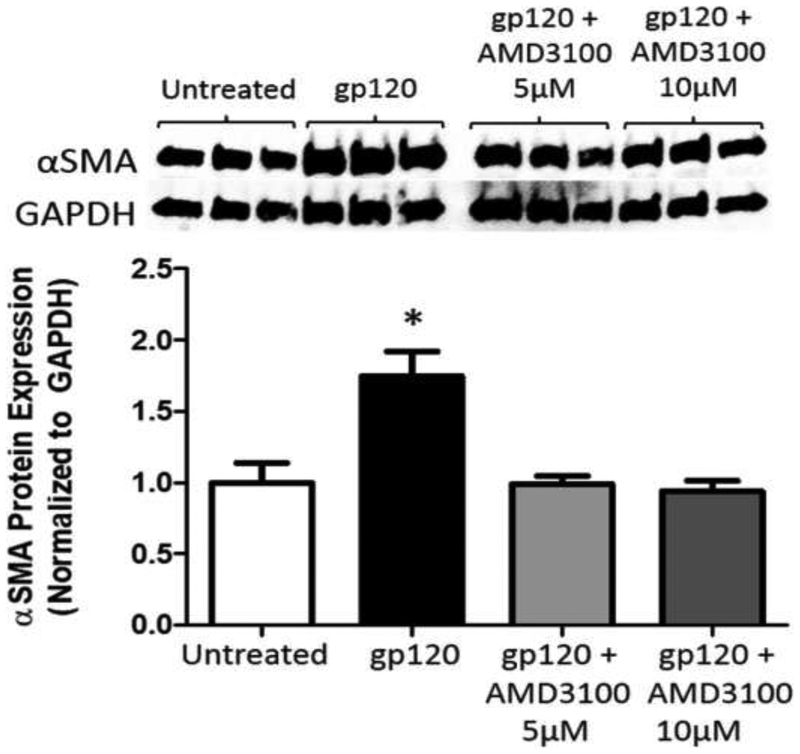
To determine the role of CXCR4 in gp120-mediated fibroblast-to-myofibroblast transdifferentiation, we treated primary lung fibroblasts with gp120 ± AMD3100, a CXCR4-specific antagonist. We first ensured that CXCR4 was produced by mouse primary lung fibroblasts, and that gp120 had no effect on protein expression (data not shown). As shown in Figure 4, we again demonstrated that gp120 treatment for 72 hours induced α-SMA protein expression. Notably, the effect of gp120 on α-SMA protein expression was entirely inhibited by co-treatment with AMD3100 (5μM and 10μM, Figure 4). These data confirm that gp120 directly induces fibroblast-to-myofibroblast differentiation through its ability to bind to and activate CXCR4.
Figure 4. Inhibition of CXCR4 signaling with AMD3100 attenuates gp120-induced α-SMA expression in mouse primary lung fibroblasts.
Mouse primary lung fibroblasts (PLFs) were isolated from wild-type mice, and after passages 2–7, cells were treated with or without gp120 (10ng/mL). In a separate group, cells were pre-treated with AMD3100 (5μM and 10μM) for 15 minutes prior to addition of gp120 (10ng/mL). Cells were then maintained in their respective treatment for the duration of the experiment. At 72 hours, cells were harvested for α-SMA protein expression analysis by Western immunoblot. Upper panel shows representative blot. Graph depicts relative α-SMA protein expression analyzed by densitometry. N = 3 per group. *P<0.05 increased compared to untreated and AMD3100 pretreated cells.
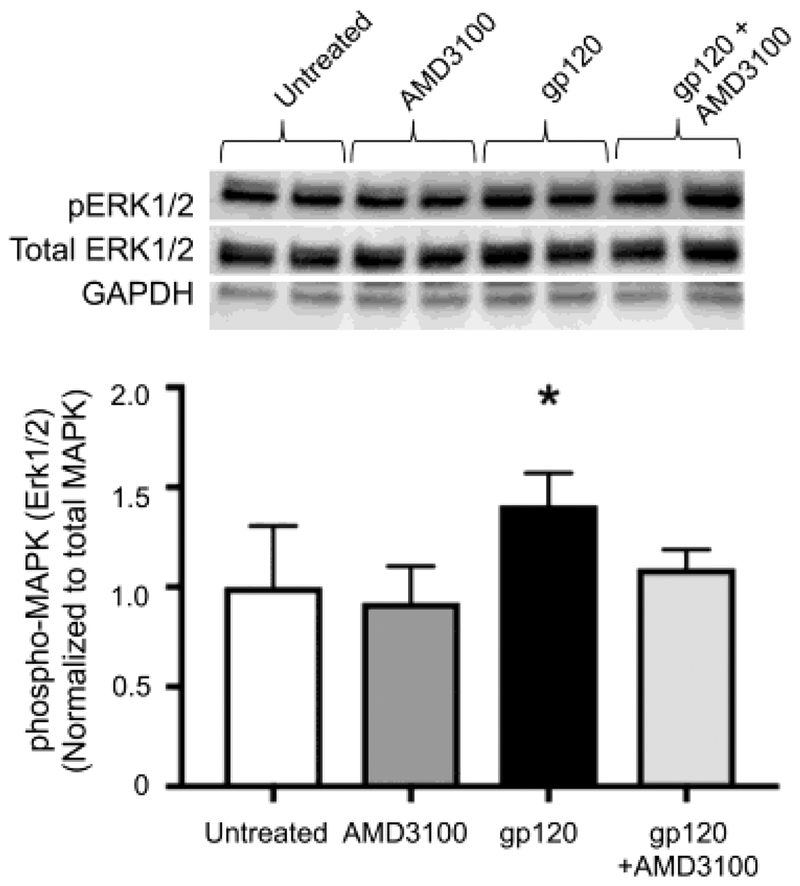
Inhibition of CXCR4 with AMD3100 prevented phosphorylation of ERK1/2 in primary lung fibroblasts.
To confirm the effect of gp120 on ERK1/2 phosphorylation was mediated via activation of CXCR4, we pre-treated PLFs with AMD3100 prior to treatment with gp120. Figure 5 showed that AMD3100 alone did not induce ERK1/2 phosphorylation, and consistent with data shown in Figure 4, gp120 alone induced ERK1/2 phosphorylation. Pretreatment with AMD3100 attenuated the effect of gp120 on ERK1/2 phosphorylation. These data suggest that gp120 activates fibroblast-to-myofibroblast transdifferentiation via the CXCR4-ERK1/2 intracellular signaling pathway, which can be effectively prevented by CXCR4 antagonism.
Figure 5. Inhibition of CXCR4 signaling with AMD3100 attenuates gp120-mediated phosphorylation of ERK1/2.
Mouse primary lung fibroblasts (PLFs) were isolated from wild-type mice, and after passages 2–7, cells were treated with or without gp120 (10ng/mL). In a separate group, cells were pre-treated with AMD3100 (5μM) for 15 minutes prior to addition of gp120 (10ng/mL). Cells were then maintained in their respective treatment for the duration of the experiment. Cells were harvested at 120 minutes after treatment and analyzed for phosphorylated mitogen-activated protein kinase 1/2 (ERK1/2), total ERK1/2, and GAPDH protein expression. Graph depicts phosphorylation ERK1/2 normalized to total ERK1/2. Upper panel shows representative blots. N = 6 per group. *P<0.05 increased compared to untreated cells.
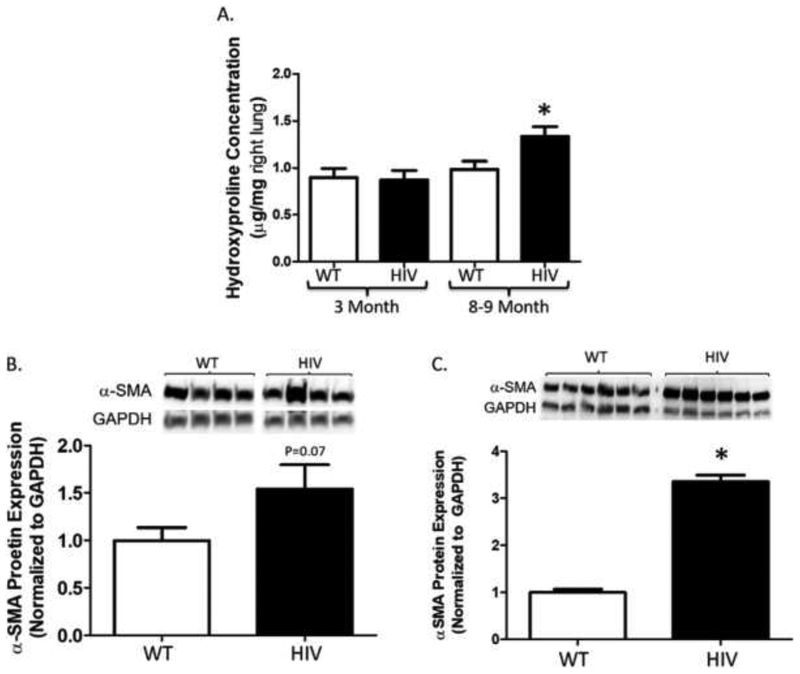
HIV-1 transgenic mice express greater hydroxyproline content in lung tissue as they age, and have a marked increase in α-SMA expression in their primary lung fibroblasts
To correlate these findings to the clinical observation that individuals with HIV infection at the age of 50 years and older have an increased risk of pulmonary fibrotic changes1, we next determined if the lungs of HIV-1 TG mice develop any potential evidence of fibrotic changes as they age. We speculated that older HIV-1 TG mice (age 8–9-month old) might have increased collagen deposition in their lungs compared to younger mice (age 3-month old). To evaluate this, we assessed hydroxyproline content in the right lung as a surrogate for pulmonary collagen deposition. We found that hydroxyproline protein concentration (Figure 6A) was significantly higher in lungs from older (8–9 month) HIV-1 TG as compared to younger (3 month) HIV-1 TG and older and younger WT mice. Next, we assessed α-SMA protein expression in whole lungs from older HIV-1 TG and WT mice in order to determine if the increase in hydroxyproline content seen in the lungs of older HIV-1 TG mice was due to an increase in the myofibroblast pool. There was a trend toward increased α-SMA protein expression (Figure 6B) in lungs from older HIV-1 TG mice compared to older WT mice, though these results were just below statistical significance (P = 0.07). Finally, we quantified α-SMA in PLFs from four to six month-old HIV-1 TG and WT mice given our concern that this marker of the myofibroblast may have been diluted by other cell types present in whole lung. We identified that PLFs from HIV-1 TG mice expressed more than three times as much α-SMA as PLFs from WT mice (Figure 6C). Taken together, these findings suggest that chronic expression of HIV-related proteins, as occurs in individuals living with HIV, promotes pro-fibrotic changes in the lung that correlate with the increased risk of pulmonary fibrosis in this vulnerable population.
Figure 6. HIV-1 transgenic mice have greater hydroxyproline concentration in the lung and α-SMA expression in primary lung fibroblasts.
Lungs and primary lung fibroblasts were harvested from HIV-1 transgenic mice and littermate wild-type controls, and analyzed for hydroxyproline and α-SMA content. (A) Graph depicts hydroxyproline concentration (μg per mg right lung) in younger (3 month) and older (8–9 month) HIV-1 transgenic compared to wild-type mice. (B) Graph depicts the summary data for α-SMA protein expression relative to GAPDH in right whole lung from older HIV-1 transgenic compared to wild-type mice. Top panel shows a representative blot. (C) Graph depicts the summary data for α-SMA protein expression relative to GAPDH in primary lung fibroblasts from four to six-month old HIV-1 transgenic compared to wild-type mice. Top panel shows a representative blot. (A) 3-month old wild-type and HIV N = 5, and 8–9-month old wild-type N = 10 and HIV N = 9. *P<0.05 increased compared to 3-month old HIV and wild-type and 8–9-month old wild-type groups. (B) Wild-type N = 10 and HIV N = 9. P = 0.07 increased compared to wild-type group. (C) Wild-type N = 6 and HIV N = 6. *P<0.05 increased compared to wild-type group.
DISCUSSION
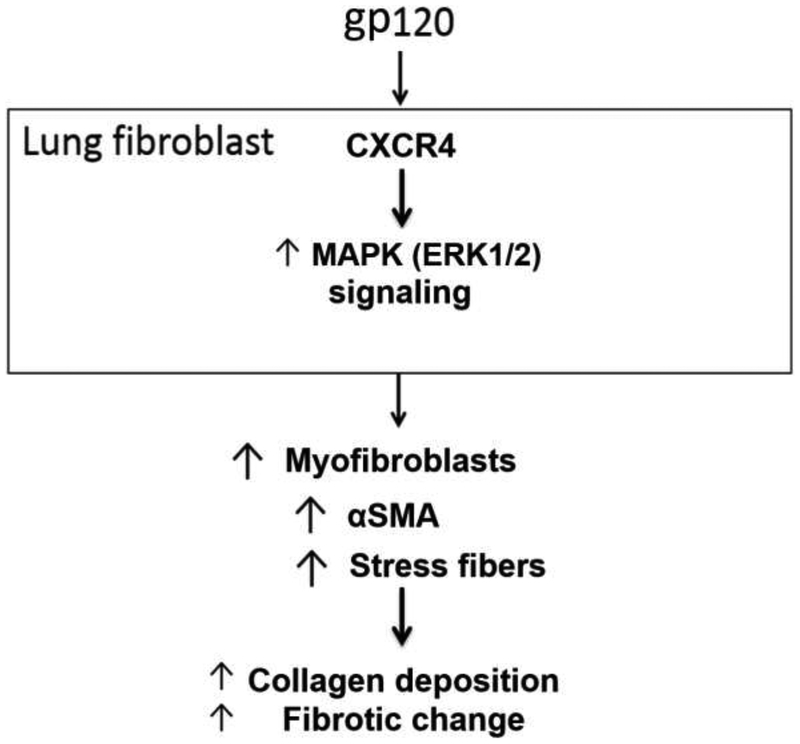
In the present study, mouse PLFs treated with gp120 had increased α-SMA expression. In parallel, direct visualization of gp120-treated PLFs confirmed there was a significantly higher percentage undergoing myofibroblast transdifferentiation. We further showed that gp120 induced activation of ERK1/2 but not PI3K-Akt intracellular signaling pathway. Additionally, the CXCR4 antagonist AMD3100 inhibited gp120-mediated augmentation of α-SMA and gp120-induced ERK1/2 phosphorylation. Finally, HIV-1 TG mice had age-dependent increased hydroxyproline concentration in their lungs and greater α-SMA expression in their PLFs. Taken together, these results suggest that activation of the chemokine receptor CXCR4 by the HIV-1-related protein gp120 mediates lung fibroblast-to-myofibroblast transdifferentiation with resultant increased pulmonary collagen deposition and potentially reveal a novel mechanism by which older individuals living with HIV are susceptible to pulmonary fibrotic changes1, 3; this pathophysiological pathway is shown schematically in Figure 7. Further, this study raises the provocative possibility that antagonizing CXCR4, such as with the FDA-approved AMD3100, could prevent and/or limit the development of fibrotic changes in these vulnerable individuals.
Figure 7. Schematic representation of hypothesized effect of gp120 on mouse PLFs.
The data present in this manuscript suggests that HIV-1 envelope protein gp120 binds to the cell surface receptor CXCR4 in the mouse primary lung fibroblast, resulting in activation of the CXCR4-ERK1/2 intracellular signaling cascade, and promoting fibroblast-to-myofibroblast transdifferentiation. This mechanism could explain the increase in fibrotic change seen in the lung of individuals infected with HIV as previously described by Crothers et al1.
We discovered that gp120 directly induces lung fibroblast-to-myofibroblast differentiation by activating CXCR4. This pathway was discerned in vitro by demonstrating that PLFs treated with gp120 had greater α-SMA expression and myofibroblastic phenotype as compared to untreated cells and the effect of gp120 on α-SMA could be inhibited by a CXCR4-specific antagonist. While speculative, a potential in vivo scenario by which this mechanism occurs in individuals infected with HIV is via direct contact of either free of virion-bound gp120 with circulating fibrocytes recruited to injured lung24,6. We were drawn to study the association of gp120 and CXCR4 in the lung fibroblast because activation of CXCR4 by stromal cell-derived factor 1 (SDF-1) can induce PI3K-Akt and ERK1/2 signaling pathways, both of which have been independently associated with increased fibroblast-to-myofibroblast transdifferentiation via non-canonical TGFβ1 signaling12, 25, 26. In our current study, we identified that gp120 activates the ERK1/2 but not the PI3K-Akt signaling pathway. These results are consistent with previous findings which showed that gp120 activates CXCR4 in the human hepatic stellate cell (HSC) with resultant increase in α-SMA and collagen type I via increase in ERK signaling13. In a similar fashion, our results are compatible with prior studies which showed that gp120 is a partial agonist of chemokine receptors CXCR4 and CCR5 and activates intracellular signaling comparable to the receptors’ natural ligands in multiple cell types outside of the lung fibroblast10, 13, 27.
gp120-mediated fibroblast-to-myofibroblast transdifferentiation could be inhibited by pre-treatment with the CXCR4 antagonist AMD3100. In addition, inhibition of CXCR4 signaling with AMD3100 also attenuated activation of ERK1/2 by gp120. To confirm there was no dose-dependent response we performed a dose titration at concentrations of 5μM and 10μM; Figure 3 showed no difference between the two doses examined in our study. Additionally, we chose to treat cells with AMD3100 at 10μM because this concentration was previously shown to limit SDF-1-induced stress fiber formation in human lung fibroblasts28. Our results not only confirm that the effect of gp120 on the lung fibroblast was due to activation of CXCR4, but this effect could be entirely inhibited by a medication shown to be safe in humans18, 29. AMD3100 is a bicylcam that was first studied as a potential inhibitor of HIV-1 target cell fusion and entry via CXCR4-selective antagonism, limiting binding of gp120 to the co-receptor16, 30. In fact, there was a phase 1 trial evaluating AMD3100 as a potential treatment for HIV-1 with a promising side effect profile and intravenous pharmacokinetics; however, further studies of the drug for HIV-1 treatment were not pursued primarily due to its inability to inhibit R5-tropic HIV-1 fusion and poor oral bioavailability14, 18. Despite limitations to its use, we believe the results of our current study argue for further analysis of AMD3100 treatment in individuals infected with HIV-1 in order to attenuate or prevent HIV-associated pulmonary fibrotic changes.
To effectively evaluate prospective mechanisms by which chronic HIV infection induces pulmonary fibrotic changes in humans, we confirmed that predisposition to fibrotic change in older individuals could be mimicked in a mouse model1. Hydroxyproline is a non-essential amino acid required for the synthesis of collagen, which in turn, is abundant in pulmonary fibrosis31. We chose to quantify hydroxyproline as a surrogate of collagen and predisposition to fibrotic-like change in order to detect any difference in collagen content that may predate true fibrosis and could be potentially missed on histology. Additionally, we chose to measure α-SMA expression in whole lung in order to determine if any difference in hydroxyproline content could be attributed to increased myofibroblast activity5. Our results confirm that in the lungs of older HIV-1 TG as compared to WT mice, there is higher collagen content as assessed by a hydroxyproline assay. This finding is likely due to an increase in the myofibroblast pool in the HIV-1 TG lung as evidenced by increased α-SMA expression in HIV-1 TG PLFs. gp120-mediated activation of CXCR4 could potentially explain these in vivo results; however, this is purely speculative as HIV-TG mice express multiple other viral genes, including tat, nef, rev, vif, vpr, and vpu, all of which may contribute19. Within this limitation, these results are the first of their kind and provide a potential explanation as to why chronic infection with HIV may lead to pulmonary fibrotic changes in humans.
There are a number of limitations to this study. We chose a mouse model rather than human cell line or human PLFs in order to better examine the putative mechanistic pathway by which HIV-1 predisposes to pulmonary fibrotic changes, and to readily discern the relevance of this pathway in vivo; as such, the applicability of our findings in humans is speculative and these experimental findings need to be extended to the clinical setting with the obvious caveat that it is not feasible to isolate primary lung fibroblasts and lung tissue from individuals living with HIV. In addition, although we determined by PCR and Western immunoblot that CXCR4 was expressed in mouse primary lung fibroblasts, cell-surface presence of CXCR4 was not confirmed. We did not feel the need to confirm this because prior studies have demonstrated that CXCR4 is expressed on the surface of both mouse and human lung fibroblasts8, 28. AMD3100 limits fibroblast-to-myofibroblast differentiation and could potentially limit fibrotic changes attributed to X4-tropic HIV-1; however, it is unlikely to have any effect on R5 or dual-tropic strains of the virus30. The significance of this limitation is currently unknown, as prior studies evaluating the risk of pulmonary fibrosis in patients with HIV-1 did not account for viral tropism, and it is not clear what proportion of patients infected with X4-tropic, R5-tropic, and dual-tropic HIV-1 are at risk for fibrosis1. Finally, while we demonstrated that direct activation of CXCR4 by gp120 in the lung fibroblast may provide at least one explanation for why individuals infected with HIV are prone to pulmonary fibrotic changes, we have not excluded other potential mechanisms; in particular, it remains unknown if an indirect effect from recurrent pulmonary infections secondary to immunosuppression may also be a risk factor.
In conclusion, we determined that gp120-mediated activation of the CXCR4-ERK1/2 signaling pathway, with resultant fibroblast-to-myofibroblast differentiation, is a previously unrecognized mechanism by which HIV-1 might promote pulmonary fibrotic changes in older individuals living with HIV. Further, we identified that this effect can be inhibited by treatment with the CXCR4-specific antagonist AMD3100. Finally, we correlated our in vitro findings with increased markers of pro-fibrotic changes in the lungs of HIV-1 TG mice in which gp120 and other HIV-1-related proteins are chronically expressed in vivo, consistent with the pulmonary fibrotic changes associated with chronic HIV infection in older humans. Although these findings in a relevant pre-clinical mouse model need to be confirmed in human lung fibroblasts and, eventually, in individuals living with HIV, our experimental findings raise the intriguing possibility that treatment of HIV-infected individuals with a CXCR4-specific antagonist such as AMD3100 may be efficacious in preventing and/or limiting pulmonary fibrosis.
ACKNOWLEDGEMENTS
The authors wish to thank Xian Fan, Bashar Staitieh, and Wendy Neveu for their scientific discussions and helpful suggestions with this manuscript. We also wish to thank Todd Mills for general technical support.
Research support: NIH T32 HL116271 for LTM, NIH K08 AA021404-01 for VS, NIH R01 HL125042 for DMG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: No conflicts of interest are declared by the authors.
REFERENCES
- 1.Crothers K, Huang L, Goulet JL, et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era Am J Respir Crit Care Med 2011;183:388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bibas M, Biava G, Antinori A HIV-Associated Venous Thromboembolism Mediterranean journal of hematology and infectious diseases 2011;3:e2011030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leader JK, Crothers K, Huang L, et al. Risk Factors Associated With Quantitative Evidence of Lung Emphysema and Fibrosis in an HIV-Infected Cohort Journal of acquired immune deficiency syndromes 2016;71:420–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Thoracic Society ERS American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001 Am J Respir Crit Care Med 2002;165:277–304. [DOI] [PubMed] [Google Scholar]
- 5.Scotton CJ, Chambers RC Molecular targets in pulmonary fibrosis: the myofibroblast in focus Chest 2007;132:1311–21. [DOI] [PubMed] [Google Scholar]
- 6.Abbas W, Herbein G T-Cell Signaling in HIV-1 Infection Open Virol J 2013;7:57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busillo JM, Benovic JL Regulation of CXCR4 signaling Biochim Biophys Acta 2007;1768:952–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehrad B, Burdick MD, Strieter RM Fibrocyte CXCR4 regulation as a therapeutic target in pulmonary fibrosis Int J Biochem Cell Biol 2009;41:1708–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong F, Tuyama A, Lee TF, et al. Hepatic stellate cells express functional CXCR4: role in stromal cell-derived factor-1alpha-mediated stellate cell activation Hepatology 2009;49:2055–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balabanian K, Harriague J, Decrion C, et al. CXCR4-tropic HIV-1 envelope glycoprotein functions as a viral chemokine in unstimulated primary CD4+ T lymphocytes J Immunol 2004;173:7150–60. [DOI] [PubMed] [Google Scholar]
- 11.Webber J, Jenkins RH, Meran S, et al. Modulation of TGFbeta1-dependent myofibroblast differentiation by hyaluronan Am J Pathol 2009;175:148–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caraci F, Gili E, Calafiore M, et al. TGF-beta1 targets the GSK-3beta/beta-catenin pathway via ERK activation in the transition of human lung fibroblasts into myofibroblasts Pharmacol Res 2008;57:274–82. [DOI] [PubMed] [Google Scholar]
- 13.Hong F, Saiman Y, Si C, et al. X4 Human immunodeficiency virus type 1 gp120 promotes human hepatic stellate cell activation and collagen I expression through interactions with CXCR4 PloS one 2012;7:e33659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Clercq E AMD3100/CXCR4 Inhibitor Front Immunol 2015;6:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Hout A, D’Huys T, Oeyen M, et al. Comparison of cell-based assays for the identification and evaluation of competitive CXCR4 inhibitors PloS one 2017;12:e0176057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatse S, Princen K, Bridger G, et al. Chemokine receptor inhibition by AMD3100 is strictly confined to CXCR4 FEBS Lett 2002;527:255–62. [DOI] [PubMed] [Google Scholar]
- 17.Donzella GA, Schols D, Lin SW, et al. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor Nat Med 1998;4:72–7. [DOI] [PubMed] [Google Scholar]
- 18.Hendrix CW, Flexner C, MacFarland RT, et al. Pharmacokinetics and safety of AMD-3100, a novel antagonist of the CXCR-4 chemokine receptor, in human volunteers Antimicrob Agents Chemother 2000;44:1667–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kopp JB, Klotman ME, Adler SH, et al. Progressive glomerulosclerosis and enhanced renal accumulation of basement membrane components in mice transgenic for human immunodeficiency virus type 1 genes Proceedings of the National Academy of Sciences of the United States of America 1992;89:1577–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sueblinvong V, Neujahr DC, Mills ST, et al. Predisposition for disrepair in the aged lung Am J Med Sci 2012;344:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sueblinvong V, Loi R, Eisenhauer PL, et al. Derivation of lung epithelium from human cord blood-derived mesenchymal stem cells Am J Respir Crit Care Med 2008;177:701–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conte E, Fruciano M, Fagone E, et al. Inhibition of PI3K prevents the proliferation and differentiation of human lung fibroblasts into myofibroblasts: the role of class I P110 isoforms PLoS One 2011;6:e24663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Midgley AC, Rogers M, Hallett MB, et al. Transforming growth factor-beta1 (TGF-beta1)-stimulated fibroblast to myofibroblast differentiation is mediated by hyaluronan (HA)-facilitated epidermal growth factor receptor (EGFR) and CD44 co-localization in lipid rafts J Biol Chem 2013;288:14824–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oh SK, Cruikshank WW, Raina J, et al. Identification of HIV-1 envelope glycoprotein in the serum of AIDS and ARC patients Journal of acquired immune deficiency syndromes 1992;5:251–6. [PubMed] [Google Scholar]
- 25.Nishimura Y, Ii M, Qin G, et al. CXCR4 antagonist AMD3100 accelerates impaired wound healing in diabetic mice J Invest Dermatol 2012;132:711–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kulkarni AA, Thatcher TH, Olsen KC, et al. PPAR-gamma ligands repress TGFbeta-induced myofibroblast differentiation by targeting the PI3K/Akt pathway: implications for therapy of fibrosis PloS one 2011;6:e15909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Y, Yoder A Chemokine coreceptor signaling in HIV-1 infection and pathogenesis PLoS Pathog 2009;5:e1000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin CH, Shih CH, Tseng CC, et al. CXCL12 induces connective tissue growth factor expression in human lung fibroblasts through the Rac1/ERK, JNK, and AP-1 pathways PloS one 2014;9:e104746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DiPersio JF, Micallef IN, Stiff PJ, et al. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin’s lymphoma J Clin Oncol 2009;27:4767–73. [DOI] [PubMed] [Google Scholar]
- 30.De Clercq E The AMD3100 story: the path to the discovery of a stem cell mobilizer (Mozobil) Biochem Pharmacol 2009;77:1655–64. [DOI] [PubMed] [Google Scholar]
- 31.Srivastava AK, Khare P, Nagar HK, et al. Hydroxyproline: A Potential Biochemical Marker and Its Role in the Pathogenesis of Different Diseases Curr Protein Pept Sci 2016;17:596–602. [DOI] [PubMed] [Google Scholar]