Abstract
Objective:
Evidence supports an inverse association between vitamin D and bacterial vaginosis (BV) during pregnancy. Furthermore, both the vaginal microbiome and vitamin D status correlate with pregnancy outcome. Women of African ancestry are more likely to experience BV, to be vitamin D deficient, and to have certain pregnancy complications. We investigated the association between vitamin D status and the vaginal microbiome.
Study Design:
Subjects were assigned to a treatment (4 400 IU), or a control group (400 IU vitamin D daily), sampled 3 times during pregnancy, and vaginal 16S rRNA profiles and plasma 25-hydroxyvitamin D [25(OH)D] concentrations were examined.
Result:
Gestational age and ethnicity were significantly associated with the microbiome. Megasphaera correlated negatively (p = 0.0187) with 25(OH)D among women of African ancestry. Among controls, women of European ancestry exhibited a positive correlation between plasma 25(OH)D and L. crispatus abundance.
Conclusion:
Certain vaginal bacteria are associated with plasma 25(OH)D concentration.
INTRODUCTION
The composition of the vaginal microflora can significantly impact both reproductive and neonatal health. Vaginal lactobacilli, through the production of lactic acid, create a vaginal environment characterized by a low pH, and this, in combination with bacteriocins and possibly other components, inhibits the colonization and growth of potentially pathogenic microorganisms and reduces the phylogenetic diversity of the vaginal microbiome. In a state of dysbiosis termed bacterial vaginosis (BV), the lactobacilli are depleted and replaced with a polymicrobial, anaerobic microflora that includes Gardnerella vaginalis, Atopobium vaginae, Sneathia, Prevotella spp, Megasphaera, and others. Although BV is frequently asymptomatic, this dysbiotic state is significantly associated with other clinical complications including pelvic inflammatory disease, infertility, and spontaneous abortion. When BV occurs during pregnancy, it is associated with a more than 2-fold increased risk for preterm birth. This is important because preterm birth accounts for as much as 70% of neonatal mortality, 75% of neonatal morbidity, and nearly 50% of long-term neurologic sequelae. A significant portion of moderate and late preterm births and the majority of very preterm births (<32 weeks) may be attributable to infection and subsequent inflammation and recent microbiome analyses reveal an association between term birth and abundance of healthy lactobacilli and an association between preterm birth and BV-associated bacterial taxa1,2
Numerous studies support the important role for sufficient serum or plasma concentrations of 25-hydroxy vitamin D (25(OH)D) during pregnancy in preventing negative outcomes3–10. Although some studies failed to detect an association, possibly because vitamin D deficiency is low among certain populations, many others and a recent meta-analysis, suggest that vitamin D deficiency increases the rate of preterm birth, particularly early preterm birth3. It plays roles in maintenance of maternal and fetal calcium homeostasis, which is linked to skeletal integrity, in general fetal growth and development, and in the regulation of immune function11,12. There also appears to be a relationship between vitamin D and BV status during pregnancy. Multiple reports describe higher rates of BV among women with insufficient 25(OH)D concentrations (often defined as <20 nmol/L or <30 nmol/L)13–15. However, studies in non-pregnant women have yielded conflicting results. One randomized controlled trial that investigated the effectiveness of vitamin D supplementation in conjunction with metronidazole in eliminating BV in non-pregnant women did not detect a positive effect of vitamin D16 while another study found that vitamin D supplementation was effective in eliminating BV17.
Race/ethnicity has significant population-level impacts on vitamin D status, BV status, and pregnancy outcomes. Vitamin D deficiency is more prevalent among people with limited sun exposure and among people with dark skin. Children of African ancestry are more likely than those of European ancestry to experience hypocalcaemic seizures and to develop rickets18. Women of African ancestry are also twice as likely to receive a clinical diagnosis of BV, and analyses of the vaginal microbiota reveal that they are more likely to be colonized by certain BV-associated bacteria19. Furthermore, African American women (though not African-born women) are nearly twice as likely to give birth preterm (<37 weeks gestation) and more than twice as likely to give birth early preterm (<34 weeks)20,21 and vitamin D deficiency has been linked to preterm birth and small-for-gestational-age infants born to women of African ancestry22,23. There is an urgent need to understand and address these important health disparities and an important first step in understanding whether or not the three parameters, vitamin D status, vaginal microbiome, and preterm birth, are linked. As a component of a prospective vitamin D supplementation trial in pregnant women funded by the W. F. F. Kellogg Foundation, referred to as the Kellogg Study, we analyzed a cohort of 230 healthy women and investigated the relationship between plasma 25(OH)D concentrations, the vaginal microbiome, and preterm birth.
MATERIALS AND METHODS
Participant recruitment
This study was approved by the Medical University of South Carolina Institutional Review Board for Human Research (PRO 00020570), registered at clinicaltrials.gov as NCT 01932788, and conducted from 1 January 2013 to 30 April 2018 at the Medical University of South Carolina (Charleston, South Carolina). All subjects gave written, informed consent. Women of diverse racial/ethnic backgrounds presenting to their obstetrician or midwife at the Medical University of SC (MUSC), Charleston, SC obstetrical facilities within the first 14 weeks after last menstrual period (LMP) with confirmation of a singleton pregnancy were eligible for enrollment in the Kellogg Study. Obstetrical dating was confirmed by early ultrasound by an MUSC obstetrician at the first prenatal visit. In those women with greater than a 2-week discrepancy between dating by LMP and ultrasound, the ultrasound dating was used. Mothers with pre-existing calcium, uncontrolled thyroid disease, parathyroid conditions, or who required chronic diuretic or cardiac medication therapy including calcium channel blockers were excluded. Mothers with pre-existing sickle cell disease (not trait only), sarcoidosis, Crohn’s disease, or ulcerative colitis were also ineligible to participate in the study. In addition, because of the potentially confounding effect of multiple fetuses, mothers with multiple gestations as determined by obstetrical ultrasound, were not eligible for participation in the study. Women who met the entry criteria were approached by the Kellogg Study team for enrollment in the study following their written, informed consent. A sub-group of approximately 100 subjects with known diabetes, hypertension, HIV, or morbid obesity (body mass index > 49) did participate but were subsequently excluded from analysis in the present study as the present study’s focus was on healthy women at the start of pregnancy.
The women were randomized into 1 of 2 groups: (1) Group A: 400 IU vitamin D3/day—Standard dose treatment of placebo (0 IU vitamin D3 gummy) plus prenatal vitamin (400 IU/day); or (2) Group B: 4 400 IU/day (4 000 IU/2 gummies/day + 400 IU/day in prenatal). A sample size of 240 subjects, 120 in each arm, was calculated to be sufficient to detect a clinically important difference of 0.3 between groups in achieving a total circulating 25(OH)D concentration of at least 40 ng/mL assuming a standard deviation of 10 using a two-tailed t-test of difference between means with 80% power and a 5% level of significance. Considering a dropout rate of 10% the sample size required is 264 (132 per group). To attain equal group numbers and balanced numbers by racial/ethnic group (African-American, Caucasian and Hispanic, which represent the predominant groups delivering at MUSC), a stratified block randomization was used. Adherence to treatment was assessed by monthly pill count and women with an adherence rate of <60% predicted pill intake for two consecutive months exited the study (n= 60: 7 white/Caucasian; 18 Hispanic; and 35 black/African American women). Because of the effect of adherence to treatment on vitamin D status24, the biomarker—25-hydroxy-vitamin D (25(OH)D was used as an additional measure of maternal vitamin D status.
Sampling and sample processing
Samples were self-obtained using CultureSwab EZ (Becton Dickinson, Franklin Lakes, NJ) from the mid-vaginal wall at visit 1 (baseline; 7.6-15.1 weeks), visit 4 (21.5-28.3 weeks gestation), and visit 7 (31.6-40.4 weeks). The swab handles were broken off and swabs were stored in microtubes at −80°C until they were transported from MUSC to VCU. DNA was extracted from the swabs using the Powersoil® kit (MoBio). The swabs were swirled directly in the Powerbead tubes supplied with the kit and processing was according to manufacturer’s instructions. Nugent score was calculated and assigned by a single, blinded obstetrician by assessment of presence of Gram-positive rods, small Gram-variable rods, and curved Gram-variable rods per high power field as described25.
Measurement of total circulating 25(OH)D
Whole blood samples were obtained, placed in EDTA-treated vacutainers, and centrifuged to achieve plasma separation. The plasma was stored at −80°C until measurement for total circulating 25(OH)D, which was measured using a commercially available radioimmunoassay (Diasorin, Stillwater, MN).
Maternal sociodemographic and health characteristics
Baseline maternal sociodemographic and health data were obtained at the first study visit. During each subsequent study visit, maternal health status was obtained by utilizing a validated pregnancy health status questionnaire used in two prior studies by this group. Pregnancy complications were obtained by electronic medical record and patient records after obtaining written informed consent, and utilized definitions of preterm labor, preterm birth (delivery <37 weeks) and significant preterm birth (<34 weeks at delivery), preeclampsia as defined by the clinical team using ACOG guidelines, bacterial vaginosis, abnormal glucose screening, and gestational diabetes, as previously defined by this group26. Sexually transmitted diseases were categorized as trichomoniasis, chlamydia, HSV, gonorrhea, and HPV. Bacterial vaginosis was diagnosed by a single obstetrician who was blinded to treatment using ACOG guidelines27.
16S rRNA gene survey
The V1-V3 hypervariable regions of the bacterial 16S rRNA gene was PCR amplified using barcoded primers 28–30. Briefly, for each reaction, 2μL of extracted DNA was combined with 12.5μL 2× Phusion Hot Start II High-Fidelity PCR Master Mix (Thermo Fisher Scientific, Waltham, MA), 3% DMSO and 100nM each of forward and reverse primers. The primers included an Illumina linker adaptor (used for binding and sequencing), a unique index sequence followed immediately by a variable sequence spacer (0-6 bases) and 16S rRNA gene primers (see S1 Appendix). The forward primer was a mixture (4 : 1) of primers Fwd-P1 (5’ - CCATCTCATCCCTGCGTGTCTCCGACTCAG BBBBBB AGAGTTYGATYMTGGCTYAG) and Fwd-P2 (5’ - CCATCTCATCCCTGCGTGTCTCCGACTCAG BBBBBB AGARTTTGATCYTGGTTCAG). The reverse primer was Rev1B (5’ - CCTATCCCCTGTGTGCCTTGGCAGTCTCAG ATTACCGCGGCTGCTGG). The PCR was carried out in a 25µl reaction in a Thermal Cycler (Applied BioSystem GeneAmp PCR system 9700) with the following parameters: initial denaturation at 98°C for 30 s, followed by 30 cycles of 98°C for 15 s, 58°C for 15 s, and 72°C for 15 s with a final extension at 72°C for 1 min. The PCR was performed in 96-well format with two PCR controls, a negative water control and a positive MOMS-PI Mock Community control. All amplicons were quantified using Picogreen (Invitrogen/Thermo Scientific) and a spectrofluorimeter (Biotek). Equal amounts of amplicon were combined followed by removal of unincorporated primers, salts and enzymes using Agencourt AMpure XP beads. The DNA concentration of this concentrated pool was verified by qPCR using the KAPA Library Quantification Kit for Illumina platform using Thermo Fisher Scientific ViiA™ 7 Real-Time PCR System. The library pool was diluted and denatured according to the Illumina MiSeq library preparation guide. The sequencing run was conducted on the Illumina MiSeq sequencer using the 2 × 300 PE reagent kit 3.
Bioinformatics Analysis
Sequence reads were de-multiplexed using an in-house python script. The raw overlapping paired-end sequence data was merged and quality-filtered using MeFiT31 with meep (maximum expected error rate) cutoff of 1.0. Non-overlapping high-quality paired end reads were retained for downstream analysis by linking them with 15 N’s. Samples with less than 1 000 high-quality reads were removed. Species-level taxonomic assignments were performed using STIRRUPS32, employing the USEARCH algorithm33 combined with a curated vaginal 16S rRNA gene database. All below-threshold (< 97% identity) assignments were removed. Relative abundance of each species was calculated as the proportion of total classified reads at > 97% identity.
Spurious taxonomic assignments, i.e. relative abundance < 0.01%, were removed from each sample’s microbial profile and relative abundances were re-normalized. For downstream statistical analysis (diversity measures and LEfSe analysis), species present in less than 5% samples were not taken into consideration.
Statistical Analysis
The predominant taxon in a sample refers to the taxon for which the largest number of reads were assigned taxonomic classification with confidence (i.e. the highest percentage of reads in the sample). Vaginal 16S rRNA profiles were assigned to community state types (CSTs)/vagitypes based on the taxon with the largest proportion of reads. Samples where the largest proportion was less than 30% were not assigned a vagitype. This “predominant taxon” rule has been shown to exhibit over 90% agreement with clustering-based methods across a variety of vaginal microbiome datasets34, yet is not population or dataset dependent and is therefore more conducive to use in a clinical setting.
Within-sample alpha diversity was measured using the Shannon and inverse Simpson’s index. Pairwise bray-curtis dissimilarity distances were calculated for between-sample beta diversity comparisons. Diversity measures were calculated using the vegan R package. Principal Coordinates Analysis on dissimilarity distances was performed using the phyloseq R package.
For each race/ethnicity, a linear mixed effect model with random subject effect (to account for the longitudinal nature of the data) was fit to relate 25(OH)D concentration as a function of treatment group, gestational age, bacteria relative abundances from a subject’s microbiome profile (A. vaginae, G. vaginalis, BVAB1, L. crispatus, L. gasseri, L. iners, Megasphaera), as well as the interaction of treatment with gestational age and relative abundances. Interaction terms were included to determine if the effect of gestational age and/or taxa relative abundances on 25(OH)D depend on treatment group. Given the significant association between education level and treatment/control group (see Table 1), education level was also included as a covariate. All linear mixed effect models were fit using JMP software (version 14).
Table 1:
Baseline demographics
| Baseline Maternal Characteristic | All subjects | Controls (400 IU) | Treated (4000 IU) | p-value | |
|---|---|---|---|---|---|
| Subjects | 236 | 112 | 124 | ||
| Age in Years, Mean (Range) | 29 (18-42) | 29 (18-42) | 29 (20-41) | 0.6421 | |
| BMI at baseline visit 1, Mean | 33.19 | 30.33 | 35.79 | 0.4941 | |
| Race/Ethnicity, N (%) | American Indian | 2 (1%) | 1 (1%) | 1 (1%) | 0.3902 |
| African American | 83 (35%) | 39 (35%) | 44 (35%) | ||
| Hispanic | 61 (26%) | 34 (30%) | 27 (22%) | ||
| White/Caucasian | 90 (38%) | 38 (34%) | 52 (42%) | ||
| Gravidity, Median (Range) | 2 (1-14) | 2 (1-14) | 2 (1-6) | ||
| Parity, Median (Range) | 1 (0-4) | 1 (0-4) | 1 (0-3) | ||
| Education, N (%) | < High School | 25 (10%) | 17 (15%) | 8 (6%) | 0.0182 |
| High School | 56 (24%) | 31 (28%) | 25 (20%) | ||
| College | 155 (66%) | 64 (57%) | 91 (74%) | ||
| Insurance Status, N (%) | Private insurance | 105 (44%) | 44 (39%) | 61 (49%) | 0.3122 |
| Medicaid | 84 (36%) | 44 (39%) | 40 (32%) | ||
| Self-Pay | 47 (20%) | 24 (22%) | 23 (19%) | ||
| Mean Gestational Age at Delivery (weeks. days) | 38.90 | 39.0 | 38.81 | 0.4101 | |
| Number term (>=37 weeks) | 217 | 107 | 111 | 0.0912 | |
| Number preterm (<37, >=34 weeks) | 18 | 4 | 12 | ||
| Number early preterm (<34 weeks) | 2 | 1 | 1 | ||
P values calculated using 1Welch’s t-test or 2Fisher’s exact test, as appropriate.
Linear discriminant analysis effect size (LEfSe) applies a Kruskal-Wallis rank sum test for each bacterium, then uses linear discriminant analysis to estimate effect size. The effect size is the contribution of a variable to the ability to distinguish two different groups.
RESULTS
Subject characteristics
402 women were consented and enrolled in the Kellogg Study. Of these, 387 were randomized and received supplement: 191 received 400 IU (control group), of whom, 142 were followed through to delivery; 196 received 4400 IU (treatment group), of whom, 155 were followed through to delivery. There were 19 miscarriages (10 in black/African American women and 9 in Hispanic women). The concentration of 25(OH)D was determined from blood samples collected monthly starting at 10-14 weeks of gestation through delivery. Women (n=108) exited the study due to loss of interest, moving away and nonadherence to protocol (n=60). Three women were enrolled in the study for 2 separate pregnancies; the results from the second pregnancy was not included in the analyses, but all three women delivered at term gestation. Women who exited the Kellogg Study were more likely to be black/African American (p<0.0001), had less than college education (p=0.005), were less likely to be employed full-time (p=0.03); more likely to have Medicaid/self-pay insurance (p<0.0001), higher gravidity (p=0.008) and parity (0.0015); and lower 25(OH)D concentration at baseline (p=0.0003), but did not differ by body mass index (BMI), season, dietary vitamin D intake, or serum calcium or phosphorus concentrations.
While a circulating 25(OH)D concentration was obtained for each participant on a monthly basis, vaginal swab samples were obtained in women who chose to provide a sample, and in some cases, women were late to be seen for their appointments, and chose not to provide a vaginal swab. This resulted in fewer vaginal swabs than blood samples, and hence the discrepancy in numbers. For the purpose of this nested study, we analyzed circulating 25(OH)D concentrations and vaginal swab samples that were obtained at visit 1 (baseline; 7.6-15.1 weeks gestation; n=177), visit 4 (21.5-28.3 weeks gestation; n=220), and visit 7 (31.6-40.4 weeks gestation; n=200) from 112 women in the control group and 124 women in the treatment group. Baseline demographics for the cohort are summarized in Table 1. Subjects were randomly stratified into control and treatment groups in blocks of 10 and, by chance, there was a significant difference in education between the groups. This was accounted for in analyses as appropriate.
Effect of treatment on Total Circulating 25(OH)D Concentration
The treatment group demonstrated a greater increase in 25(OH)D concentrations over the 3 time-points; however, even the control group exhibited a significant increase over the time-course of the study (Fig. 1a). Ethnicity was a significant covariate, so the effect of treatment on 25(OH)D concentration was also examined within each ethnic group (Fig 1b, 1c, 1d). Women of European ancestry and Hispanic women in the control group exhibited a significant increase in 25(OH)D but women of African ancestry in the control group did not. All ethnic groups in the treatment group displayed a significant increase in 25(OH)D over the 3 time-points. Gestational age was a significant covariate; however, a linear mixed effect model indicated that the effect of treatment on 25(OH)D concentrations remained significant for each of the 3 groups (p < 0.0001 for African ancestry, p = 0.0127 for European ancestry, and p < 0.0001 for Hispanic).
Figure 1. Total circulating 25(OH)D concentrations increase over pregnancy.
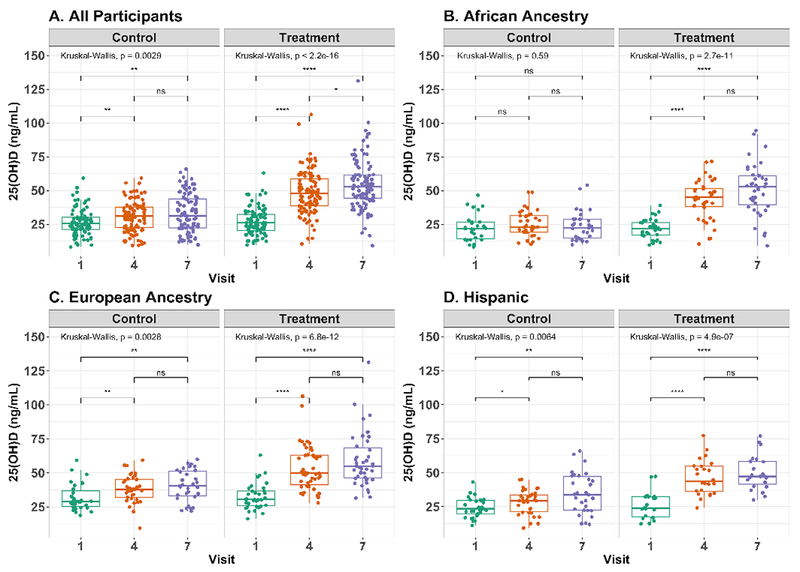
Boxplots of 25(OH)D concentration in plasma collected longitudinally over pregnancy. Whiskers extend to the highest/lowest value within 1.5 times the interquartile range and outliers beyond the whiskers are plotted as points. Significant differences in 25(OH)D between visits are indicated for A. all participants, B. women of African ancestry, C. women of European ancestry, and D. Hispanic women. Symbols indicating statistical significance - ns: p>0.05; *: p<=0.05; **: p<=0.01; ***: p<=0.001; ****: p<=0.0001. Figure was prepared using ggpubr61 R package.
Association between vitamin D sufficiency and the vaginal microbiome
DNA from vaginal swab samples was analyzed by 16S rRNA gene survey. 16S amplicon sequences were classified against a custom database of vaginally-relevant species, using STIRRUPS32, and relative abundance were computed as a percentage of reads classified. Taxonomic assignments below 0.01% relative abundance were removed and the proportions were re-normalized. Furthermore, bacterial species not present in at least 5% of samples were removed. This protects against features with small mean and trivially large coefficient of variance. Vaginal 16S rRNA gene profiles from all samples were grouped based on total circulating plasma 25(OH)D concentrations of either < 30 ng/mL or > 40 ng/mL. There was no significant association between 25(OH)D concentration and alpha diversity as measured by Shannon index or Inverse Simpson Index (supplementary figure S1a). Principal Coordinates Analysis performed on the bray-curtis dissimilarity distances between samples did not reveal any patterns between plasma 25(OH)D concentration and bacterial species (supplementary figure S1b). When the samples were grouped by dominant taxon, it was apparent, as expected, that women with vaginal microbiome dominated by lactobacilli had lower Nugent scores, overall (Fig 2). Furthermore, it appeared that women with > 40 ng/mL 25(OH)D at the time of sampling had vaginal microbiomes with greater abundance of lactobacilli relative to women with < 30 ng/mL 25(OH)D (Fig 2a, 2b). The microbiomes in this group were 1.6 times, 2.3 times, and 1.5 times as likely to be dominated by the healthy Lactobacillus species L. crispatus, L. jensenii, and L. gasseri (respectively), and 0.2 times as likely to be dominated by the unhealthy BV-associated species G. vaginalis or BVAB1 (Table 2). When separated by ethnicity, the effect appeared to be more pronounced among women of African ancestry (Fig 3).
Figure 2. Vaginal microbial taxa differ between women with plasma 25(OH)D <30 ng/mL or >40 ng/mL.
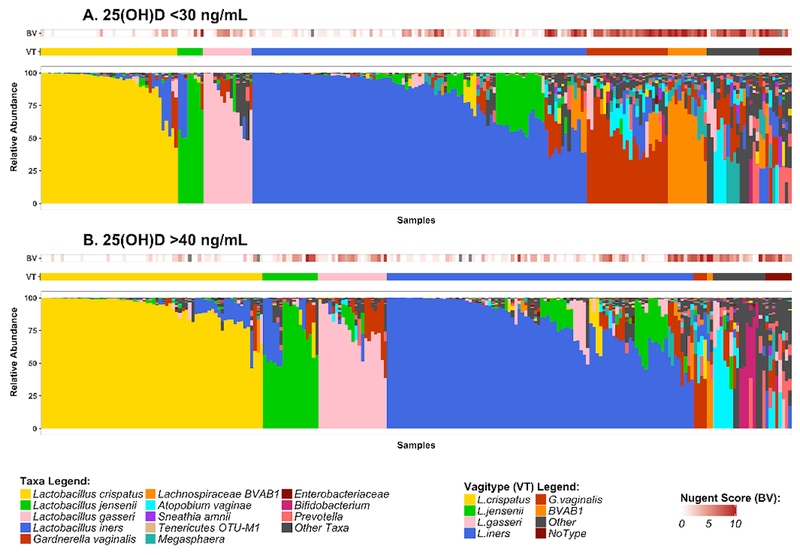
A. and B. stacked bar plots showing vaginal microbial community profiles from 3 visits from each of the 236 women in the cohort. The profiles are grouped by the most abundant species and samples within each community group are clustered on bray distances using ward method. The microbial profiles are annotated by Nugent score. The distances were calculated using vegan R package and the figure was prepared using ggplot2.
Table 2.
Prevalence of communities in 25(OH)D deficient and sufficient subjects
| L. crispatus | L. jensenii | L. gasseri | L. iners | G. vaginalis | BVAB1 | Other | NoType | Total | |
|---|---|---|---|---|---|---|---|---|---|
| <30 ng/mL | 43 (19% ) | 8 (3%) | 15 (6%) | 103 (44%) | 25 (11%) | 12 (5%) | 16 (7%) | 10 (4%) | 232 (100%) |
| >40ng/mL | 68 (30%) | 17 (7%) | 21 (9%) | 94 (41%) | 4 (2%) | 2 (1%) | 16 (7%) | 8 (3%) | 230 (100%) |
Number of samples and (percent of total) dominated by one of the named bacterial taxa, a rare taxon “Other”, or not dominated by a single taxon “No Type”.
Figure 3. The association between 25(OH)D and microbiome differs among ethnic groups.
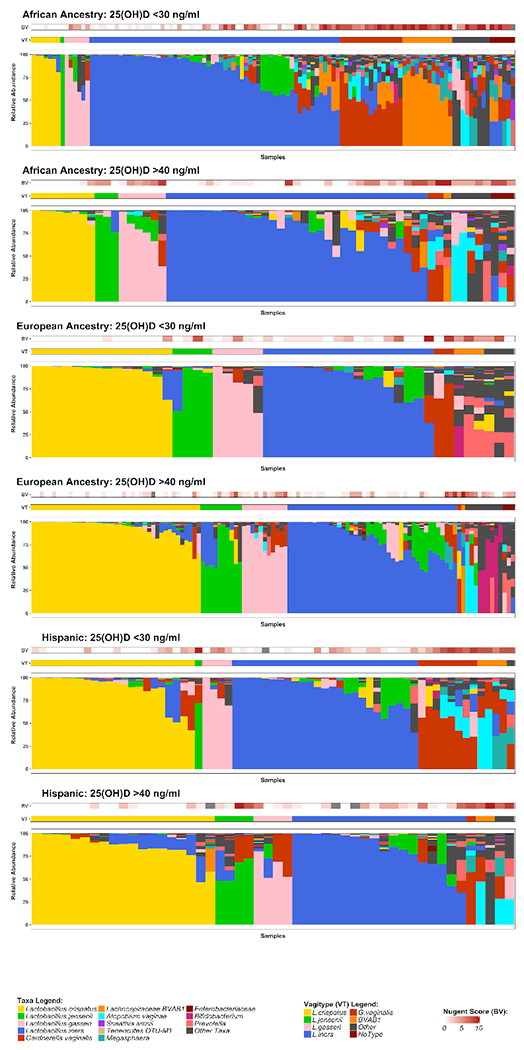
Stacked bar plots showing vaginal microbial community profiles from 3 visits from each of the 236 women in the cohort grouped by ethnicity and 25(OH)D status.
We analyzed the abundance of bacterial taxa by linear determinant analysis effect size (LEfSe) to determine whether any were associated with 25(OH)D status. Among samples from women with >40 ng/mL 25(OH)D, L. crispatus, L. gasseri and Bifidobacterium species were more abundant (Fig 4). In addition to these lactic acid-producing taxa, additional taxa that are not associated with vaginal health, including A. vaginae, were more abundant in this group. Among samples from women with <30 ng/mL 25(OH)D, G. vaginalis, BVAB1, TM7_OTU_H1, Prevotella, BVAB1, and Sneathia were more abundant.
Figure 4. Statistical association analysis using LEfSe.
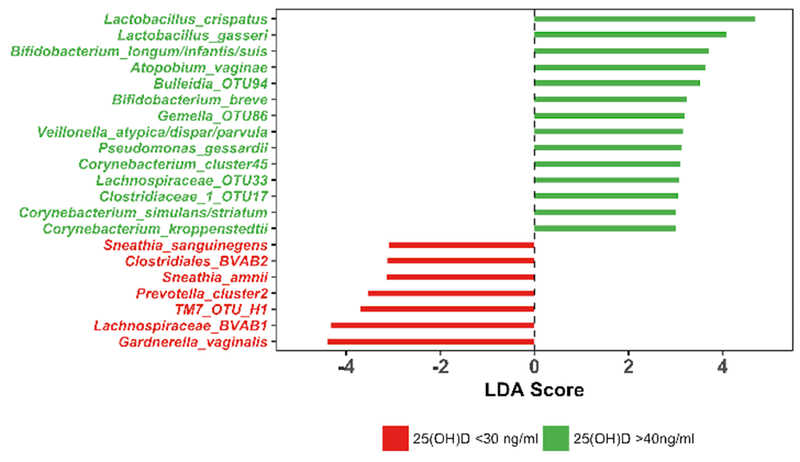
Bacterial species with significant differential abundance between women with plasma 25(OH)D concentrations >40 ng/mL or <30 ng/mL were identified using LEfSe. Features with LDA score greater than 3.0 are shown in the figure, with bacterial species associated with plasma 25(OH)D concentrations >40 ng/mL shown in green, while those associated with <30 ng/mL are shown in red.
Relationship between G. vaginalis, plasma 25(OH)D, ethnicity, and gestational age
Because of its strong association with BV and because it appeared to be negatively associated with vitamin D sufficiency, we examined the abundance of G. vaginalis over the course of pregnancy more carefully. We examined a subset of the women of African ancestry who had a relative abundance of at least 1% G. vaginalis at their first visit. Among the controls within this subset, there was not a significant increase in total circulating 25(OH)D between visit 1 and visit 7 (Wilcoxon, p = 0.14) but there was a significant increase in the treated subjects (Wilcoxon p < 0.0001) (Fig 5a). There was a decrease in G. vaginalis abundance between visits 1 and 7 in both the control and treatment group (p = 0.0029 and 0.0153, respectively) (Fig 5b).
Figure 5. G. vaginalis abundance decreases over pregnancy irrespective of 25(OH)D concentration.
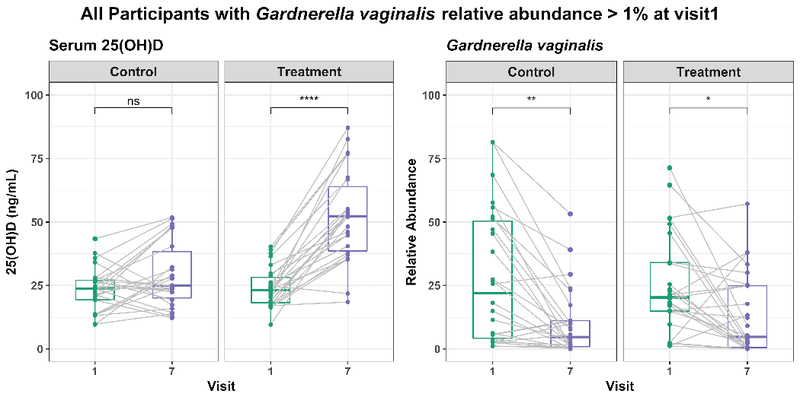
Box-plots showing plasma 25(OH)D concentration (left-panel) and G. vaginalis abundance (right-panel) for all subjects with G. vaginalis abundance >1% at visit 1. Data points from same subject at visit 1 and visit 7 are connected by gray line. There is a significant increase in 25(OH)D among the treatment group and significant decrease in G. vaginalis abundance in both control and treatment group. The figure was prepared using ggpubr R package.
Collinearity between plasma 25(OH)D and gestational age
Because of the observed decrease in the BV-associated taxon G. vaginalis over pregnancy, even among the control group, we examined baseline (before treatment) 25(OH)D concentrations and Nugent scores and found that women of African ancestry with 25(OH)D < 20 ng/mL at baseline had significantly higher Nugent score than women with 25(OH)D > 40 ng/mL. We also developed a model to incorporate the mixed effects of treatment group and gestational age, and we analyzed the different ethnic groups separately. Because education differed between the control and treatment groups, education was accounted for in the model. When treatment group and gestational age were fixed, both treatment and gestational age significantly correlated with 25(OH)D concentrations, but the associations between many of the taxa and 25(OH)D were no longer significant, suggesting that covariation of abundance levels with gestational age, treatment group, and/or ethnicity exerted an effect. Despite the covariation, there remained a significant correlation (p = 0.0088) between Megasphaera species and 25(OH)D concentrations among women of African ancestry insofar as women with higher 25(OH)D had less Megasphaera. Among women of European ancestry, a significant interaction between L. crispatus and treatment (p=0.0123) was observed and suggests that the association of L. crispatus with 25(OH)D depends on treatment/control. In particular, among women of European ancestry in the control group, but not the treatment group, total circulating 25(OH)D concentrations correlated positively with abundance of L. crispatus.
DISCUSSION
The hypothesis driving this study was that vitamin D status is associated with the vaginal microbiome. Vitamin D could affect the vaginal microbiome through a number of mechanisms. First, it could promote the barrier integrity of the vaginal epithelium. Vitamin D was shown to upregulate genes encoding epithelial cell junction proteins and stimulated proliferation of the vaginal epithelium35,36. Furthermore, two studies found that postmenopausal women receiving vitamin D treatment had increased numbers of superficial vaginal epithelial cells compared to women not receiving treatment, and that this increase was accompanied by decreases in vaginal pH37,38. Together these studies suggest that vitamin D may promote vaginal epithelial cell growth, differentiation, and function, which may increase vaginal thickness to prevent atrophy and improve barrier function. Second, adequate vitamin D during pregnancy may impact the vaginal environment so that it is more supportive of beneficial flora. It is well established that insulin stimulates glycogen synthesis39, and more recently it has been proposed that vitamin D induces insulin synthesis40,41. Vitamin D was also shown to increase the phosphorylation and inactivation of glycogen synthase kinase, an inhibitor of glycogen synthesis, in adipose tissue42. Therefore there is also a possibility that vitamin D sufficiency alters glucose homeostasis in the vagina to promote increased glycogen deposition. Increased vaginal levels of free glycogen positively correlate with Lactobacillus relative abundance43, and so if vitamin D is indeed promoting vaginal glycogen production, the accompanying increase in lactobacilli and decrease in pH may result in the decreased abundance of pathogenic and BV-associated organisms. A third possibility is that vitamin D influences abundance of BV-associated bacteria through its effects on immune function. Previous studies have shown that vitamin D treatment induces the expression of the LL-37 antimicrobial peptide in keratinocytes, neutrophils, monocytes, and bladder and gingival epithelial cells as well as β2 defensin in keratinocytes, neutrophils, and monocytes44–46, as well as potentiates the secretion of IL-8 and CXCL10 from airway epithelial cells47. These effects could promote leukocyte recruitment to the vaginal epithelium and modulate the antimicrobial response of leukocytes upon arrival to the tissue48.
In this study, we analyzed a sub-cohort from a randomized, placebo-controlled clinical trial of vitamin D supplementation of pregnant women who were enrolled during the first trimester of pregnancy and followed until delivery. We analyzed 16S rRNA gene survey data to determine whether plasma 25(OH)D concentrations were associated with the vaginal microbiome. Women in both the control (400 IU vitamin D daily) and treatment groups (4 400 IU vitamin D daily) displayed higher circulating 25(OH)D concentrations with increasing gestational age. While initial analyses suggested a substantive association between 25(OH)D and the vaginal microbiome, we found that gestational age was a strong covariate. While multiple studies have shown that the abundances of certain BV-associated bacterial taxa decrease during pregnancy49,50, a correlation with gestational age has not been demonstrated and a report of a longitudinal study suggested that the vaginal microbiome is remarkably constant over the course of pregnancy51. In our study, however, G. vaginalis decreased significantly in abundance between visit 1 (7.6-15.1 weeks), and visit 7 (31.6-40.4 weeks) (Fig 5).
A model that took into account the covariation between the vaginal microbiome and gestational age, found only three taxa that were associated with 25(OH)D concentrations, and these associations were dependent upon ethnicity. Specifically, among women of African ancestry, there was a negative correlation between 25(OH)D and abundance of Megasphaera spp.. The majority of reads that were categorized with the genus Megasphaera were Megasphaera Type 1, which we have found in another study (Glascock, Fettweis, manuscript in preparation), to be the most common vaginal type. Detection of Megasphaera by PCR has been shown to accurately predict of BV52 and Megasphaera is associated with bacteria vaginosis and spontaneous preterm delivery53–55. Megasphaera has also been linked to genital tract inflammation56 and HIV acquisition among African women57. Among women of European ancestry in the control group, women with higher 25(OH)D concentrations were more likely to have higher abundance of L. crispatus. L. crispatus is a healthy H2O2-producing lactobacillus species that is associated with decreased risk for developing BV and decreased risk for HIV acquisition58,59. Thus, although only two taxa were significantly associated with 25(OH)D, these results suggest that vitamin D could have a positive impact on the vaginal microbiome.
A limitation of this study is that the cohort contained few women who had profound vitamin D deficiency (defined as 25(OH)D <2 ng/mL). In our prior NICHD vitamin D pregnancy study involving 350 women randomized to three treatment groups (400, 2000 and 4000 IU vitamin D3/day)60, even the control group showed an increase in 25(OH)D over the course of pregnancy, especially in white/Caucasian women. Most women are not taking a prenatal vitamin or a multivitamin at the start of pregnancy and since most women have limited sunlight exposure or use sunscreen limiting their endogenous synthesis of vitamin D, and because the average American woman is provided ~200 IU vitamin D/day from her diet60, the addition of 400 IU/day results in a slight but measurable increase in 25(OH)D over time.
Another potential limitation of the study is the loss of subjects during the study period. There were 108 women who exited the study, 60 due to nonadherence to protocol. The women who exited the study tended to be of lower socioeconomic status, were black/African American, less educated, more likely to be of higher gravidity and parity, and with a lower baseline 25(OH)D concentration than those women who completed the study. The loss of these subjects in the study could have created potential bias in the results.
It is possible that within the range of total circulating 25(OH)D concentration among women in the cohort, there is little impact on the vaginal microbiome, but that severe vitamin D deficiency has a greater effect. In this study, plasma 25(OH)D was the only marker of vitamin D status that was measured; however, maternal 1,25D and other vitamin D metabolites such as 24,25(OH)2D and the 3-epi-25(OH)D could also be important factors. Because the subjects in this cohort were pregnant, plasma 1,25D concentration would be expected to be higher and could influence immune function and the vaginal microbiota. Another limitation was that the relatively small cohort size may not have provided the study with adequate power to detect significant associations between 25(OH)D concentrations and bacterial taxa, particularly in light of other covariates (gestational age, ethnicity, and random treatment group assignment). While all participants in the study provided a blood sample to measure total circulating 25(OH)D, only a subset of those women provided a vaginal sample at the 3 timepoints, which further decreased the sample size. The vaginal microbiome is a complex parameter and consequently, microbiome studies often require large cohorts to detect biologically relevant results. An additional potential confounder is that women who were diagnosed with BV by Nugent score were prescribed treatment, and while treatment occurred after vaginal swab samples were obtained, it could have influenced the composition of the microbiome at subsequent visits.
Supplementary Material
ACKNOWLEDGEMENTS
This work was funded in part from a grant from the W. K. Kellogg Foundation and by the South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina, NIH/NCAT Grant number UL1 TR000062. This work was also supported by National Institutes of Health [grant U54 DE023786 “A Multi-’omic Analysis of the Vaginal Microbiome during Pregnancy”]. All sequencing and analysis of sequence data was performed in the Genomics Core of the Nucleic Acids Research Facilities at VCU.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
SUPPLEMENTARY DATA
Supplementary information is available at Journal of Perinatology’s website.
REFERENCES
- 1.Tabatabaei N, Eren AM, Barreiro LB, Yotova V, Dumaine A, Allard C et al. Vaginal microbiome in early pregnancy and subsequent risk of spontaneous preterm birth: a case-control study. BJOG Int J Obstet Gynaecol 2018. doi: 10.1111/1471-0528.15299. [DOI] [PubMed] [Google Scholar]
- 2.Callahan BJ, DiGiulio DB, Goltsman DSA, Sun CL, Costello EK, Jeganathan P et al. Replication and refinement of a vaginal microbial signature of preterm birth in two racially distinct cohorts of US women. Proc Natl Acad Sci U S A 2017; 114: 9966–9971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amegah AK, Klevor MK, Wagner CL. Maternal vitamin D insufficiency and risk of adverse pregnancy and birth outcomes: A systematic review and meta-analysis of longitudinal studies. PloS One 2017; 12: e0173605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tabatabaei N, Auger N, Herba CM, Wei S, Allard C, Fink GD et al. Maternal Vitamin D Insufficiency Early in Pregnancy Is Associated with Increased Risk of Preterm Birth in Ethnic Minority Women in Canada. J Nutr 2017; 147: 1145–1151. [DOI] [PubMed] [Google Scholar]
- 5.Zhao X, Fang R, Yu R, Chen D, Zhao J, Xiao J. Maternal Vitamin D Status in the Late Second Trimester and the Risk of Severe Preeclampsia in Southeastern China. Nutrients 2017; 9. doi: 10.3390/nu9020138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wen J, Hong Q, Zhu L, Xu P, Fu Z, Cui X et al. Association of maternal serum 25-hydroxyvitamin D concentrations in second and third trimester with risk of gestational diabetes and other pregnancy outcomes. Int J Obes 2005 2017; 41: 489–496. [DOI] [PubMed] [Google Scholar]
- 7.Toko EN, Sumba OP, Daud II, Ogolla S, Majiwa M, Krisher JT et al. Maternal Vitamin D Status and Adverse Birth Outcomes in Children from Rural Western Kenya. Nutrients 2016; 8. doi: 10.3390/nu8120794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiely ME, Zhang JY, Kinsella M, Khashan AS, Kenny LC. Vitamin D status is associated with uteroplacental dysfunction indicated by pre-eclampsia and small-for-gestational-age birth in a large prospective pregnancy cohort in Ireland with low vitamin D status. Am J Clin Nutr 2016; 104: 354–361. [DOI] [PubMed] [Google Scholar]
- 9.Miliku K, Vinkhuyzen A, Blanken LM, McGrath JJ, Eyles DW, Burne TH et al. Maternal vitamin D concentrations during pregnancy, fetal growth patterns, and risks of adverse birth outcomes. Am J Clin Nutr 2016; 103: 1514–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner CL, Hollis BW, Kotsa K, Fakhoury H, Karras SN. Vitamin D administration during pregnancy as prevention for pregnancy, neonatal and postnatal complications. Rev Endocr Metab Disord 2017; 18: 307–322. [DOI] [PubMed] [Google Scholar]
- 11.Weinert LS, Silveiro SP. Maternal-fetal impact of vitamin D deficiency: a critical review. Matern Child Health J 2015; 19: 94–101. [DOI] [PubMed] [Google Scholar]
- 12.Tamblyn JA, Hewison M, Wagner CL, Bulmer JN, Kilby MD. Immunological role of vitamin D at the maternal-fetal interface. J Endocrinol 2015; 224: R107–121. [DOI] [PubMed] [Google Scholar]
- 13.Bodnar LM, Krohn MA, Simhan HN. Maternal vitamin D deficiency is associated with bacterial vaginosis in the first trimester of pregnancy. J Nutr 2009; 139: 1157–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunlop AL, Taylor RN, Tangpricha V, Fortunato S, Menon R. Maternal vitamin D, folate, and polyunsaturated fatty acid status and bacterial vaginosis during pregnancy. Infect Dis Obstet Gynecol 2011; 2011: 216217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hensel KJ, Randis TM, Gelber SE, Ratner AJ. Pregnancy-specific association of vitamin D deficiency and bacterial vaginosis. Am J Obstet Gynecol 2011; 204: 41.e1–9. [DOI] [PubMed] [Google Scholar]
- 16.Turner AN, Carr Reese P, Fields KS, Anderson J, Ervin M, Davis JA et al. A blinded, randomized controlled trial of high-dose vitamin D supplementation to reduce recurrence of bacterial vaginosis. Am J Obstet Gynecol 2014; 211: 479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taheri M, Baheiraei A, Foroushani AR, Nikmanesh B, Modarres M. Treatment of vitamin D deficiency is an effective method in the elimination of asymptomatic bacterial vaginosis: A placebo-controlled randomized clinical trial. Indian J Med Res 2015; 141: 799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Callaghan KM, Kiely ME. Ethnic disparities in the dietary requirement for vitamin D during pregnancy: considerations for nutrition policy and research. Proc Nutr Soc 2018; 77: 164–173. [DOI] [PubMed] [Google Scholar]
- 19.Fettweis JM, Brooks JP, Serrano MG, Sheth NU, Girerd PH, Edwards DJ et al. Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiol Read Engl 2014; 160: 2272–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oliver EA, Klebanoff M, Yossef-Salameh L, Oza-Frank R, Moosavinasab S, Reagan P et al. Preterm Birth and Gestational Length in Four Race-Nativity Groups, Including Somali Americans. Obstet Gynecol 2018; 131: 281–289. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton BE, Martin JA, Osterman MJK, Curtin SC, Matthews TJ. Births: Final Data for 2014. Natl Vital Stat Rep Cent Dis Control Prev Natl Cent Health Stat Natl Vital Stat Syst 2015; 64: 1–64. [PubMed] [Google Scholar]
- 22.Seto TL, Tabangin ME, Langdon G, Mangeot C, Dawodu A, Steinhoff M et al. Racial disparities in cord blood vitamin D levels and its association with small-for-gestational-age infants. J Perinatol Off J Calif Perinat Assoc 2016; 36: 623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagner CL, Baggerly C, McDonnell S, Baggerly KA, French CB, Baggerly L et al. Post-hoc analysis of vitamin D status and reduced risk of preterm birth in two vitamin D pregnancy cohorts compared with South Carolina March of Dimes 2009–2011 rates. J Steroid Biochem Mol Biol 2016; 155: 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abercrombie M, Shary J, Ebeling M, Hollis B, Wagner C. Analysis of the NICHD Vitamin D Pregnancy Cohort on a Per-Protocol vs. Intent-to-Treat Basis: The Effect of Adherence on Trial Results. J Nutr Food Sci 2018; 8: 696. [Google Scholar]
- 25.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol 1991; 29: 297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hollis BW, Wagner CL. Vitamin D supplementation during pregnancy: Improvements in birth outcomes and complications through direct genomic alteration. Mol Cell Endocrinol 2017; 453: 113–130. [DOI] [PubMed] [Google Scholar]
- 27.ACOG Committee on Practice Bulletins--Gynecology. ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists, Number 72, May 2006: Vaginitis. Obstet Gynecol 2006; 107: 1195–1206. [DOI] [PubMed] [Google Scholar]
- 28.Fadrosh DW, Ma B, Gajer P, Sengamalay N, Ott S, Brotman RM et al. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2014; 2: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a Dual-Index Sequencing Strategy and Curation Pipeline for Analyzing Amplicon Sequence Data on the MiSeq Illumina Sequencing Platform. Appl Environ Microbiol 2013; 79: 5112–5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bartram AK, Lynch MDJ, Stearns JC, Moreno-Hagelsieb G, Neufeld JD. Generation of Multimillion-Sequence 16S rRNA Gene Libraries from Complex Microbial Communities by Assembling Paired-End Illumina Reads. Appl Environ Microbiol 2011; 77: 3846–3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parikh HI, Koparde VN, Bradley SP, Buck GA, Sheth NU. MeFiT: merging and filtering tool for illumina paired-end reads for 16S rRNA amplicon sequencing. BMC Bioinformatics 2016; 17: 491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fettweis JM, Serrano MG, Sheth NU, Mayer CM, Glascock AL, Brooks JP et al. Species-level classification of the vaginal microbiome. BMC Genomics 2012; 13 Suppl 8: S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinforma Oxf Engl 2010; 26: 2460–2461. [DOI] [PubMed] [Google Scholar]
- 34.Brooks JP, Buck GA, Chen G, Diao L, Edwards DJ, Fettweis JM et al. Changes in vaginal community state types reflect major shifts in the microbiome. Microb Ecol Health Dis 2017; 28: 1303265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gniadecki R, Gajkowska B, Hansen M. 1,25-dihydroxyvitamin D3 stimulates the assembly of adherens junctions in keratinocytes: involvement of protein kinase C. Endocrinology 1997; 138: 2241–2248. [DOI] [PubMed] [Google Scholar]
- 36.Lee A, Lee MR, Lee H-H, Kim Y-S, Kim J-M, Enkhbold T et al. Vitamin D Proliferates Vaginal Epithelium through RhoA Expression in Postmenopausal Atrophic Vagina tissue. Mol Cells 2017; 40: 677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rad P, Tadayon M, Abbaspour M, Latifi SM, Rashidi I, Delaviz H. The effect of vitamin D on vaginal atrophy in postmenopausal women. Iran J Nurs Midwifery Res 2015; 20: 211–215. [PMC free article] [PubMed] [Google Scholar]
- 38.Yildirim B, Kaleli B, Düzcan E, Topuz O. The effects of postmenopausal Vitamin D treatment on vaginal atrophy. Maturitas 2004; 49: 334–337. [DOI] [PubMed] [Google Scholar]
- 39.Forde JE, Dale TC. Glycogen synthase kinase 3: a key regulator of cellular fate. Cell Mol Life Sci CMLS 2007; 64: 1930–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maestro B, Campión J, Dávila N, Calle C. Stimulation by 1,25-dihydroxyvitamin D3 of insulin receptor expression and insulin responsiveness for glucose transport in U-937 human promonocytic cells. Endocr J 2000; 47: 383–391. [DOI] [PubMed] [Google Scholar]
- 41.Maestro B, Molero S, Bajo S, Dávila N, Calle C. Transcriptional activation of the human insulin receptor gene by 1,25-dihydroxyvitamin D(3). Cell Biochem Funct 2002; 20: 227–232. [DOI] [PubMed] [Google Scholar]
- 42.Parker L, Levinger I, Mousa A, Howlett K, de Courten B. Plasma 25-Hydroxyvitamin D Is Related to Protein Signaling Involved in Glucose Homeostasis in a Tissue-Specific Manner. Nutrients 2016; 8. doi: 10.3390/nu8100631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mirmonsef P, Hotton AL, Gilbert D, Burgad D, Landay A, Weber KM et al. Free glycogen in vaginal fluids is associated with Lactobacillus colonization and low vaginal pH. PloS One 2014; 9: e102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang T-T, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol Baltim Md 1950 2004; 173: 2909–2912. [DOI] [PubMed] [Google Scholar]
- 45.Hertting O, Holm Å, Lüthje P, Brauner H, Dyrdak R, Jonasson AF et al. Vitamin D induction of the human antimicrobial Peptide cathelicidin in the urinary bladder. PloS One 2010; 5: e15580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McMahon L, Schwartz K, Yilmaz O, Brown E, Ryan LK, Diamond G. Vitamin D-mediated induction of innate immunity in gingival epithelial cells. Infect Immun 2011; 79: 2250–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brockman-Schneider RA, Pickles RJ, Gern JE. Effects of vitamin D on airway epithelial cell morphology and rhinovirus replication. PloS One 2014; 9: e86755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng Y, Niyonsaba F, Ushio H, Nagaoka I, Ikeda S, Okumura K et al. Cathelicidin LL-37 induces the generation of reactive oxygen species and release of human alpha-defensins from neutrophils. Br J Dermatol 2007; 157: 1124–1131. [DOI] [PubMed] [Google Scholar]
- 49.Romero R, Hassan SS, Gajer P, Tarca AL, Fadrosh DW, Nikita L et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome 2014; 2: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stout MJ, Zhou Y, Wylie KM, Tarr PI, Macones GA, Tuuli MG. Early pregnancy vaginal microbiome trends and preterm birth. Am J Obstet Gynecol 2017; 217: 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DiGiulio DB, Callahan BJ, McMurdie PJ, Costello EK, Lyell DJ, Robaczewska A et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc Natl Acad Sci U S A 2015; 112: 11060–11065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fredricks DN, Fiedler TL, Thomas KK, Oakley BB, Marrazzo JM. Targeted PCR for detection of vaginal bacteria associated with bacterial vaginosis. J Clin Microbiol 2007; 45: 3270–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kusters JG, Reuland EA, Bouter S, Koenig P, Dorigo-Zetsma JW. A multiplex real-time PCR assay for routine diagnosis of bacterial vaginosis. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol 2015; 34: 1779–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nelson DB, Hanlon A, Nachamkin I, Haggerty C, Mastrogiannis DS, Liu C et al. Early pregnancy changes in bacterial vaginosis-associated bacteria and preterm delivery. Paediatr Perinat Epidemiol 2014; 28: 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zozaya-Hinchliffe M, Martin DH, Ferris MJ. Prevalence and abundance of uncultivated Megasphaera-like bacteria in the human vaginal environment. Appl Environ Microbiol 2008; 74: 1656–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lennard K, Dabee S, Barnabas SL, Havyarimana E, Blakney A, Jaumdally SZ et al. Microbial Composition Predicts Genital Tract Inflammation and Persistent Bacterial Vaginosis in South African Adolescent Females. Infect Immun 2018; 86. doi: 10.1128/IAI.00410-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McClelland RS, Lingappa JR, Srinivasan S, Kinuthia J, John-Stewart GC, Jaoko W et al. Evaluation of the association between the concentrations of key vaginal bacteria and the increased risk of HIV acquisition in African women from five cohorts: a nested case-control study. Lancet Infect Dis 2018; 18: 554–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gajer P, Brotman RM, Bai G, Sakamoto J, Schütte UME, Zhong X et al. Temporal dynamics of the human vaginal microbiota. Sci Transl Med 2012; 4: 132ra52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Verstraelen H, Verhelst R, Claeys G, De Backer E, Temmerman M, Vaneechoutte M. Longitudinal analysis of the vaginal microflora in pregnancy suggests that L. crispatus promotes the stability of the normal vaginal microflora and that L. gasseri and/or L. iners are more conducive to the occurrence of abnormal vaginal microflora. BMC Microbiol 2009; 9: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res Off J Am Soc Bone Miner Res 2011; 26: 2341–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.ggplot2 - Elegant Graphics for Data Analysis | Hadley Wickham | Springer; http://www.springer.com/us/book/9780387981413 (accessed 13 Jul2016). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


