Abstract
Background
Cardiovascular disease (CVD) is more frequent among people with HIV (PWH) and may relate to traditional and nontraditional factors, including inflammation and immune activation. A critical need exists to develop effective strategies to prevent CVD in this population.
Methods
The Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE) (A5332) is a prospective, randomized, placebo-controlled trial of a statin strategy for the primary prevention of major adverse cardiovascular events (MACE) in PWH with low to moderate traditional risk. At least 7500 PWH, 40-75yrs of age, on stable antiretroviral therapy (ART), will be randomized to pitavastatin calcium (4 mg/day) or identical placebo and followed for up to 7 years. Participants are enrolled based on the 2013 ACC/AHA ASCVD risk score and LDL cholesterol (LDL-C) level with a goal to identify a low to moderate risk population who might benefit from a pharmacologic CVD prevention strategy. Potential participants with a risk score ≤ 15% were eligible, based on decreasing LDL-C thresholds for increasing risk score > 7.5% [LDL-C<190 mg/dL for risk score <7.5%; LDL-C<160 mg/dL for risk score 7.6-10% and LDL-C<130 mg/dL for risk score 10.1-15%]. The primary objective is to determine effects on a composite endpoint of MACE. Formal and independent adjudication of clinical events will occur utilizing standardized criteria. Key secondary endpoints include effects on MACE components, all-cause mortality, specified non-CVD events, AIDS and non-AIDS events, and safety.
Results
To date, REPRIEVE has enrolled over 7300 participants, at approximately 120 sites across 11 countries, generating a diverse and representative population of PWH, to investigate the primary objective of the trial.
Conclusion
REPRIEVE is the first trial investigating a primary CVD prevention strategy in PWH. REPRIEVE will inform the field of the efficacy and safety of a statin strategy among HIV-infected participants on ART and provide critical information on CVD mechanisms and non-CVD events in PWH.
Keywords: Major adverse cardiovascular events (MACE), HIV, myocardial infarction, statin, inflammation, lipids, antiretroviral therapy
Introduction
Approximately 37 million people worldwide are infected with HIV [1]. Although people with HIV (PWH) are surviving longer [2] due to the success of antiretroviral therapy (ART), cardiovascular disease (CVD) is increasing. Large studies comparing PWH and people without HIV, suggest increased rates of myocardial infarction (MI) and stroke, with varying hazard ratio (HR) estimates, of up to 50% or more [3-9]. In addition, CVD mortality has increased in HIV and CVD mortality is now one of the leading non-AIDS causes of death [10], at a time when CVD mortality rates are dropping in the population without HIV [11].
Traditional CVD risk factors, such as dyslipidemia, smoking, fat redistribution, including excess visceral adiposity, and diabetes are increased among PWH, but explain only some of the excess risk of CVD events in HIV [5, 9]. Increased CVD events occur even among PWH with low to moderate traditional risk factors, suggesting that non-traditional risk factors contribute to increased CVD in this population. HIV results in increased inflammation, immune activation, arterial inflammation, and a unique atherosclerotic plaque phenotype, with noncalcified, vulnerable plaque [12–16]. In addition, thrombosis may be increased, as suggested by increased levels of D-Dimer and tissue factor-expressing monocytes [17], contributing to increased cardiovascular events [18]. A prevailing hypothesis is that immune activation, endothelial factors, thrombogenic mechanisms, and an atherogenic lipid phenotype characterized by increased oxidized LDL (oxLDL) may contribute to accelerated atherogenesis in HIV. Use of ART may improve CVD rates [19], but is unlikely to be sufficient to fully prevent CVD in HIV, as residual inflammation, immune activation and arterial inflammation, persist after effective therapy [20, 21].
An effective primary CVD prevention strategy that addresses both traditional and HIV-specific mediators of CVD is critical for PWH. Statin therapy uniquely meets these criteria. In the general population, statins reduce LDL-C and prevent CVD events [22-25]. In addition, statins are known to have anti-inflammatory and immunomodulatory characteristics, which may also contribute to cardio-protective effects [26]. The JUPITER trial suggested an effect of statins to prevent CVD in HIV-uninfected persons with increased C-reactive protein (CRP) but nonelevated LDL-C [27]. Of note, the hazard ratio for ASCVD reduction in JUPITER, 0.56, was greater than that anticipated based on LDL-C lowering alone, suggesting a potential mechanistic effect beyond LDL-C lowering [27]. Studies evaluating statins in PWH have demonstrated the significant potential to decrease LDL-C, reduce inflammatory, immune activation and arterial inflammation indices; reduce proatherogenic oxLDL; and improve coronary plaque characteristics, including noncalcified plaque [28-31]. Statins may also reduce activation of atypical monocytes, thereby decreasing tissue factor activation [32]. Statins may therefore be useful as a primary prevention strategy for CVD in the setting of HIV.
Despite the evidence that statins may be an effective strategy to reduce traditional and nontraditional risk factors in HIV, statin use in the HIV-infected population continues to be relatively low [33]. Specific statins may interact with ART via metabolism by the CYP3A system and other mechanisms [34, 35]. Although short-term studies suggest that specific statins, including pitavastatin calcium (hereafter referred to as pitavastatin), can be safely administered to PWH [28, 36], the low use of statins may reflect the uncertain efficacy for CVD prevention, potential side effects, and ART interactions during long-term use. This is particularly true for those at low to moderate risk for CVD, in whom LDL-C levels are not substantially increased. These factors suggest equipoise for a large randomized trial to assess efficacy and safety of a statin therapy strategy for primary CVD prevention in HIV.
We designed the Randomized Controlled Trial to Prevent Vascular Events in HIV (REPRIEVE) (A5332) as the first large-scale, long-term, randomized trial to assess a primary CVD prevention strategy among PWH, using pitavastatin therapy. REPRIEVE will assess potential statin mechanisms and determine effects on key comorbidities and long-term safety. REPRIEVE addresses an urgent national healthcare priority to prevent CVD among PWH.
METHODS
Major Objectives
The ongoing REPRIEVE trial is assessing the efficacy of a statin to reduce the risk of CVD in PWH. The primary objective of REPRIEVE is to determine the effects of pitavastatin as a primary prevention strategy for major adverse cardiovascular events (MACE) in HIV (Figure 1). The primary outcome measure of MACE is a composite of MI (classified as type 1, type 2 or other), CVD death, hospitalization for unstable angina, revascularization (coronary, carotid or peripheral arterial), transient ischemic attack, stroke or hospitalization for peripheral arterial ischemia (Table I). While events included the primary endpoint are largely atherosclerotic in nature it is also inclusive of nonatherosclerotic events such as CVD death, type 2 MI and heart failure, therefore the broader term CVD is utilized to describe the endpoint and endpoints of interest.
Figure 1.
REPRIEVE Trial Schema
Table I.
General Principles Guiding Definitions of MACE Components1
| CVD Death | • Includes death resulting from an acute MI, sudden cardiac death, death due to HF, death due to stroke, death due to CV procedures, death due to CV hemorrhage, and death due to other CV causes. |
| Myocardial Infarction | • The diagnosisof Ml requires the combination of: evidence of myocardial necrosis (either changes in cardiac biomarkers or post mortem pathological findings); and supporting information derived from the clinical presentation, electrocardiographic changes, or the results of myocardial or coronary artery imaging suggesting an event consistent with coronary ischemia. |
| • The totality of the clinical, electrocardiographic, and cardiac biomarker information will be considered to determine whether or not an MI has occurred. Specifically, timing and trends in cardiac biomarkers and electrocardiographic information will be included whenever possible, but the diagnosis can still be determined if these results are not available. | |
| Unstable angina hospitalization | • Ischemic discomfort or equivalent requiring hospitalization within 24 hours with objective signs of coronary ischemia in absence of MI. ECG, angiographic and imaging criteria will be considered. |
| Coronary, carotid, or peripheral arterial revascularization | • Invasive percutaneous or surgical procedure intended to restore or improve blood flow in a coronary or peripheral artery including but not limited to angioplasty, stent, stent graft, or bypass graft. |
| Stroke | • An acute episode of focal or global neurological dysfunction caused by brain, spinal cord, or retinal vascular injury as a result of hemorrhage or infarction. |
| Transient lschemic Attack | • A transient episode of focal neurological dysfunction caused by brain, spinal cord, or retinal ischemia, without stroke. |
| Peripheral Arterial Ischemia | • Urgent hospitalization for insufficiency of the peripheral arterial circulation, including but not limited to, acute limb ischemia, chronic limb ischemia, amputation, or other vascular abnormality of an ischemic and noninfectious nature. |
| Additional Endpoints of Interest | |
| Non-CVD Death | • In addition to CVD death which is a component of the primary endpoint, all deaths will be adjudicated by the CEC to determine likely cause. |
| Hospitalization for Heart Failure | • Defined as an event that meets 5 specific criteria including a hospital admission with primary diagnosis of HF, length of hospitalization must extend for at least 24 hours, the participant exhibits new or worsening symptoms of HF and finally the participant received initiation or intensification of treatment for HF. |
The primary endpoint of Major Adverse Cardiovascular Events (MACE) includes the composite of the CVD events listed below. Formal definitions for each component are contained within the Clinical Events (CEC) charters and are based on the Standardized Defini tions for Cardiovascular and Stroke Endpoint Events in Clinical Trials.
Abbreviations: MACE, major adverse cardiovascular event; MI, myocardial infarction; HF, heart failure; CV, cardiovascular; CVD, cardiovascular disease
The key secondary objectives are: to evaluate the effects of pitavastatin on each of the components of the primary composite MACE endpoint and all-cause mortality; determine the effects of pitavastatin on LDL-C and non-HDL cholesterol (non-HDL-C) in relationship to MACE; evaluate whether baseline traditional risk factors and time updated HIV-specific risk factors are predictive of MACE and pitavastatin effects on MACE; evaluate the effects of pitavastatin on the incidence of serious AIDS and non-AIDS non-CVD events; and determine the safety of pitavastatin in the HIV population, including the development of diabetes mellitus (DM), liver dysfunction, and myopathy.
Substudies and Ancillary Objectives
The Mechanistic Substudy of REPRIEVE (A5333s) aims to determine the effects of pitavastatin on the morphology and composition of non-calcified coronary atherosclerotic plaque, including the progression of plaque volume and whether these effects are modulated by markers of inflammation and immune activation, including activated T- cell and monocyte subsets determined by flow cytometry, sCD14, sCD163, IL-6, LP-PLA2, and oxLDL. Additional ancillary objectives of REPRIEVE include assessment of sex-based differences in statin-induced immunomodulation and CVD risk reduction, the relationship of ovarian reserve to CVD among women with HIV, and statin effects on renal function. To ensure increased participation in REPRIEVE by women living with HIV, a unique recruitment campaign called Follow YOUR Heart [37] has been developed.
Stand-alone ancillary studies leveraging REPRIEVE include the Pitavastatin to Reduce Physical Function Impairment and Frailty in HIV (PREPARE) substudy, to evaluate the effects of pitavastatin on muscle strength and function (ClinicalTrials.gov Identifier: NCT03070223) and a study to evaluate the effects of pitavastatin on myocardial fibrosis, myocardial steatosis and cardiac function utilizing MRI (ClinicalTrials.gov Identifier: NCT03070223).
Funding
Funding for this project comes from U01HL123336 awarded to the Clinical Coordinating Center (CCC) and U01HL123339 awarded to the Data Coordinating Center (DCC) from the National Heart, Lung, and Blood Institute (NHLBI), by the Office of AIDS Research (OAR), and by the National Institute of Allergy and Infectious Diseases (NIAID) Division of AIDS (DAIDS) and the AIDS Clinical Trials Group (ACTG). Additional funding comes from Kowa Pharmaceuticals America, Inc., including for study product and matching placebo, and Gilead Sciences, Inc. The authors are solely responsible for the design and conduct of this study including, all study analyses, the drafting and editing of this manuscript, and its final contents. The views expressed are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; National Institute of Allergy and Infectious Diseases; National Institutes of Health; or the United States of Department of Health and Human Services.
Overall Study Design
REPRIEVE is a prospective, double-blind, randomized, placebo-controlled, multi-center, phase III efficacy study with two arms (4mg daily pitavastatin vs. matching placebo for pitavastatin) among HIV-1 infected adults currently on ART, followed for approximately 36 to 96 months, depending on date of enrollment in the trial.
Choice of Statin Therapy
Pitavastatin was chosen as it interacts minimally with ART and effectively decreases LDL-C cholesterol among PWH [36], while simultaneously reducing markers of immune activation, arterial inflammation and oxLDL [38], without increasing glucose levels.
Site Selection and Qualification
There are approximately 120 sites in 11 countries in the REPRIEVE trial (see site map: http://reprievetrial.org/collaborating-sites/). These sites represent the majority of ACTG sites and select non-ACTG sites. The Site Selection and Performance Committee selected the sites, in collaboration with the DAIDS Office of Clinical Site Oversight (OCSO) and the ACTG Network Coordinating Center, for their demonstrated experience enrolling in HIV trials, ability to collaborate with a cardiologist as well as additional expectations of the clinical site based on a site survey. The role of the cardiologist was not specified, but sites were encouraged to work with a local cardiologist to assist in questions regarding overall education about CVD in HIV, eligibility, adjudication triggers, and safety management of statin therapy. Specific involvement at individual visits was left up to each site. In addition, all sites have access to a cardiologist (PD) on the primary team, for any such questions throughout the trial. Before beginning enrollment, each site was required to undergo DAIDS OCSO approval that included completion of activities such as lab certification, Good Clinical Practice training, training on reporting expedited adverse events, etc. In parallel, all sites were required to be protocol activated by completing requirements such as IRB approval, contract execution, DAIDS protocol registration of the protocol, protocol training, and submission of completed Protocol Activation Checklist. These processes ensured that all sites were qualified and prepared to enroll and follow participants for the duration of REPRIEVE.
Study Population
At least 7500 HIV-infected men and women ≥40 and ≤75 years of age, on any ART regimen for at least 6 months prior to study entry, with CD4+T-cell count >100 cells/mm3 are enrolling into REPRIEVE. Participants with a 10-year ASCVD risk score estimated by the 2013 ACC/AHA Pooled Cohort Equations of ≤15% and with fasting LDL-C meeting specific thresholds depending on the ASCVD risk score are eligible for enrollment in REPRIEVE (Table II). Because of the requirement to meet specific fasting LDL-C thresholds combined with specific ASCVD risk score thresholds, REPRIEVE participants are considered to be at relatively low to moderate risk for the development of CVD using conventional classification tools.
Participant Eligibility
Eligibility for REPRIEVE is determined at a screening visit at which the 10-year ASCVD risk score (http://tools.acc.org/ASCVD-Risk-Estimator/) is calculated. In addition, sites collect information on medical history, including cardiovascular risk history, physical exam, and medication history. Laboratory values are drawn or obtained from clinical care, for the estimation of glomerular function, and calculation of FIB-4 for persons with HCV and/or HBV. See Table II for the full list of inclusion and exclusion criteria.
Randomization
Eligible participants provide written informed consent and are randomly assigned in a ratio of 1:1 to either 4mg pitavastatin or identical placebo for pitavastatin, 1 pill daily. A computer-generated permuted block randomization scheme is used with stratification by sex at birth, CD4+T-cell count (≤500 vs. >500 cells/mm), and participation in the Mechanistic Substudy of REPRIEVE. All entry evaluations occur after randomization but before initiation of study treatment. Participants initiate study treatment within 72 hours of randomization unless they are co-enrolled to the MRI or Mechanistic Substudy which allow initiation of study treatment up to 14 days after randomization. Randomization is blinded to the study investigators and participants.
Intervention and Subsequent Study Visits
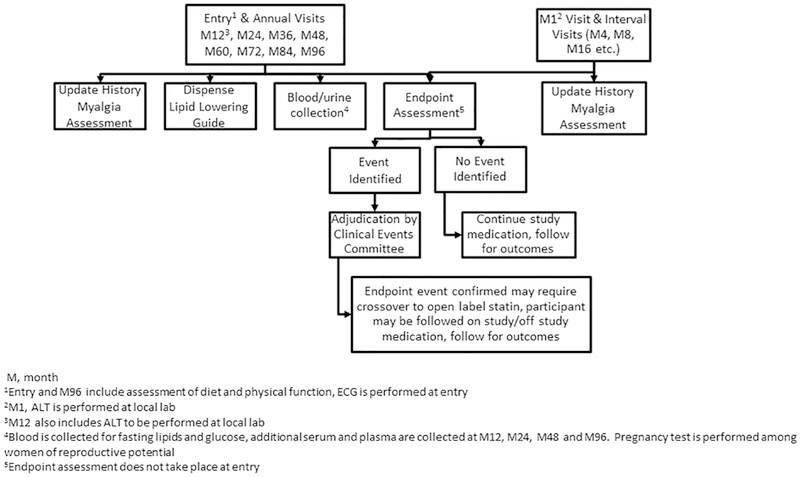
After completion of the entry visit, participants begin treatment with blinded study medication and return for study visits every 4 months and a safety visit 1 month after the entry visit, see Figure 2 for details of each visit type. Participants and investigators are blinded to centrally tested lab results collected during the study (lipids, glucose, and inflammatory markers). The Clinical Coordinating Center had no access to prespecified lipid, glucose and inflammatory data. Sites were neither encouraged nor prohibited from assessing lipid levels for clinical care. Given the relatively low levels of LDL-C in the study population, a relatively low cross over rate was anticipated and this is monitored throughout the trial by the DSMB (see Sample Size and Power Calculations). All participants receive information on healthy lifestyle activities annually in the Lipid-Lowering Diet, Activity, and Smoking Cessation Guide.
Figure 2.
Flow Diagram of REPRIEVE Study Visits
Outcome Measures
Primary Outcome Measures
The primary outcome measure of REPRIEVE is time to first MACE. Primary events are prospectively determined and adjudicated by an independent clinical events review process, conducted by the Thrombolysis in Myocardial Infarction (TIMI) group at the Brigham and Women’s Hospital, Boston, MA, USA based on standardized criteria used in prior cardiovascular trials and developed by consensus groups and the FDA [39]. The REPRIEVE DCC staff arranges for the necessary materials to be provided to the TIMI group’s Clinical Events Committee (CEC). Study and site investigators do not receive decisions on event adjudications. The measurement of the time to event is from the date of randomization to the date of the event onset.
Secondary Outcome Measures
Secondary outcome measures include time to the first of each individual component of the primary endpoint; time to death (all-cause mortality); time to death and/or MACE; time to any or each of the following clinical diagnoses including non-AIDS-defining cancers; AIDS-defining events; initiation of dialysis or renal transplantation, cirrhosis, or hepatic decompensation requiring hospitalization; and calculated fasting LDL-C and non-HDL-C cholesterol levels at study entry and annually thereafter, as well as change from baseline. Safety outcome measures include time to a serious adverse event, incident diabetes, grade 3 or 4 ALT), and grade 3 or 4 myopathy, which were all defined utilizing the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events (https://rsc.niaid.nih.gov/sites/default/files/daidsgradingcorrectedv21.pdf). Exploratory outcome measures include fasting total and HDL cholesterol (HDL-C), LDL-C/HDL-C ratio, and time to heart failure. Stored plasma and serum at baseline, yearly study visits, and end of study will be available, for secondary analyses, to address safety and mechanistic questions raised by the study.
Statistical Methods
Sample Size and Power Considerations
The initial design of REPRIEVE was to enroll 6500 participants over 2.5 years, with median follow-up of 4.75 years. This design was chosen to achieve 90% power to detect a 30% reduction in MACE and assumed an underlying control event rate of 15/1000PY, 10% cross-over rate and 5% annual loss to follow up. The control rate was determined from data from the Partners Research Patient Data Registry (RPDR) for individuals ≥ 40 years of age in conjunction with published data from the JUPITER trial (a primary CVD prevention trial in HIV-uninfected persons) [27]. Specifically, among 3,213 PWH followed over 10 years between 2000-2009 at Partners Health Care in Boston, MA, USA a MACE rate of 13/1000 PY that excluded CVD death was estimated (unpublished data). In JUPITER, CVD death represented 20% of all MACE. Applying this adjustment to the Partners RPDR estimate gave an estimated MACE rate in HIV to 16.2/1000 PY. The MACE rate for the placebo group among HIV-uninfected participants in JUPITER was 13.6/1000 PY. A final MACE rate of 15/1000 PY was chosen for REPRIEVE as a reasonable compromise and in line with these rates.
Under its current design (see section below on Design Changes), REPRIEVE will enroll at least 7500 participants over 4 years with median follow up of 6 years. Under this design (with the original assumptions for cross-over and loss to follow up rates maintained), the study has 85% power to detect the target HR of 0.7 in the setting of a MACE rate in the control group as low as 8.4 events/1000 PY.
Interim Monitoring Plan and Benchmarks
REPRIEVE undergoes review by an NHLBI-appointed Data Safety Monitoring Board (DSMB) for study conduct, continued feasibility, safety, and efficacy. The Board convenes to review accumulated data at six-month intervals and provides recommendations regarding termination, continuing or modifying the study protocol as necessary. A summary of the monitoring schedule is provided in Table III and Figure 3.
Table III.
Overview of DSMB Monitoring Focus and frequency of Review
| Primary Focus | Frequency of Review (unless otherwise requested by the DSMB) |
|---|---|
|
Feasibility and Conduct: Site activation, enrollment, data and visit completeness, rates of loss to follow-up and cross-over. |
Approximately every 6 months |
|
Safety: Rates of adverse events by treatment group |
Approximately every 6 months |
|
Event Rate Evaluation (adequacy of sample size): Pooled rates of events observed to date. |
Approximately every 6 months, starting 1 year after enrollment of first participant |
| If the pooled rate falls below a specified target, predicted confidence interval analysis of the pooled event rate under a range of realistic scenarios | |
|
Efficacy and Futility: Treatment group comparison for the primary endpoint utilizing group sequential methods |
Annually starting once the adequacy of the sample size has been established. A total of 3 interim looks for efficacy are planned, at 40%, 60%, and 80% of total information (i.e., expected number of endpoints) |
Accrual targets for the entire enrollment course of REPRIEVE were mutually agreed upon between the REPRIEVE Principal Investigators and NHLBI to allow the study to be fully enrolled over 4 years.
Figure 3. REPRIEVE Trial Accrual Figure.
Dotted line shows actual accrual progress. Colored bands denote predefined NIH target enrollment zones: yellow/light gray (100% to 75%), orange/medium gray (75% to 25%), red/dark gray (<25%).
Statistical Analyses
All major treatment comparisons between the randomized groups will be performed according to the principle of “intention-to-treat” and statistical comparisons using two-sided significance tests with a 5% type I error. Time to event outcomes will be defined from time of randomization; treatment comparison of these outcomes will use methods for competing risks, notably Cox proportional hazards models for estimation of cause-specific hazard ratios and Gray’s test for comparison of cumulative incidence curves; all testing will be stratified by sex and CD4 cell count per randomization. For the primary outcome of time to first MACE, deaths from non-CVD causes will be treated as competing risk events. In supportive analyses, Poisson regression with robust variance estimates will also be used to incorporate multiple and repeated events in evaluation of event incidence rates by treatment group and rate ratios. To complement the primary analyses, the same analytic approach will be used for evaluation of individual components of the primary MACE endpoint as well as other pre-specified secondary endpoints. In the absence of a competing risk event, treatment comparisons of all-cause mortality and a composite of MACE and all-cause mortality will use a stratified log-rank test.
Design Changes
After early recruitment demonstrated a lower than anticipated median 10-year predicted ASCVD risk in the study population as a whole, a number of design changes have been made to ensure that the REPRIEVE population represents the intended broad crosssection of individuals with low to moderate cardiovascular risk: the upper threshold of risk score for eligibility has been increased and an enrollment limit for participants with the lowest risk estimates has been set. See Figure 4 for timeline of design changes.
Figure 4.
Timeline of REPRIEVE Design Changes and Amendments
As previously described, the target sample size and follow-up duration have also been increased. These changes were implemented following DSMB review of pooled event rate data. A timeline of relevant amendments is shown in Figure 4. All changes were made before the first DSMB interim look at the outcome data by treatment arm.
Organization and Human Studies Approval
Conduct of the trial is in partnership with the ACTG under the sponsorship of DAIDS. DAIDS holds the study IND, and provides regulatory support, clinical monitoring and oversight of sites (ACTG and non-ACTG). Coordination of clinical activities and execution of the protocol is overseen by the CCC at MGH while coordination of data flow and storage is overseen by the Data Coordinating Center (DCC) with responsibilities shared between MGH (imaging and administration), Center for Biostatistics and AIDS Research (CBAR) at Harvard School of Public Health (biostatistics) and Frontier Science Foundation (data management, including specimen handling and shipping).
REPRIEVE is conducted under the supervision of an Executive Committee (EC) which includes NIH (NHLBI and NIAID representation). The EC developed the protocol and oversees trial execution. The EC supervises the Publications Committee, the Operational Leadership Committee, the Protocol Committee, and the Site Selection and Performance Committee, which perform all aspects of protocol monitoring, protocol development and site selection, evaluation and performance monitoring through prespecified metrics. The DAIDS Medical Officer is responsible for safety oversight. See Figure 5 for the REPRIEVE trial Organization.
Figure 5.
REPRIEVE Trial Organization
The Partners Health Care Institutional Review Board (IRB) approved the protocol and provides oversight of central activities of the Coordinating Centers, including implementation of design changes and overall trial structure. Each clinical research site has obtained local IRB/ethics committee approval and any other applicable regulatory entity (RE) approval. Each participant has been provided with study information and signed a declaration of informed consent.
A parallel but identical study is being planned in Europe under the supervision of the NEAT-ID trial network and is co-sponsored by the Massachusetts General Hospital, Boston, MA, USA and NEAT-ID, London, UK, to facilitate additional enrollment of approximately 250 participants. Data from this parallel trial will be will be analyzed in combination with the main data.
Results
Enrollment
As of December 3rd, 2018, REPRIEVE has enrolled approximately 7300 participants since the first enrollment in March of 2015. Currently enrolling sites include 51 ACTG and 66 non-ACTG sites, located in the US (85), Canada (6), Brazil (10), Thailand (2), South Africa (5), Haiti (2), Peru (2), Botswana (1), Uganda (1), Zimbabwe (1), and India (2).
Discussion
The REPRIEVE trial is a landmark outcome trial designed to determine the efficacy of a statin strategy to prevent primary CVD, defined using a standard MACE definition, among PWH, receiving ART, with low to moderate CVD risk. The study began in 2015 and has nearly completed enrollment, utilizing almost 120 sites across the globe in 11 different countries. The trial has enrolled a highly representative population, reflecting the diversity of sex, race, ethnicity and demographics of a large at-risk HIV population, for whom little information and no proven primary CVD prevention exists. This significant achievement is based on a successful and novel collaboration between multiple public and private stakeholders, including NHLBI, NIAID, OAR, ACTG, Kowa Pharmaceuticals America, Inc. and Gilead Sciences, Inc. Unique strategies to recruit this population were developed and employed by the REPRIEVE investigators. Importantly, REPRIEVE is a public resource to be leveraged to understand the mechanism of CVD in HIV. Numerous ancillary studies have already been funded in this regard, including studies on the sex specific CVD mechanisms and statin effects in HIV, and statin effects on kidney and muscle function, and cardiac fibrosis, steatosis and function.
This trial was designed to address the increased incidence of CVD among the HIV population, occurring often in relatively young PWH, without significant traditional CVD risk or abnormal LDL-C. Risk stratification strategies have not yet been fully developed that accurately predict CVD events in HIV, and accurately identifying those most at risk is difficult. REPRIEVE relied on a recently developed algorithm, the 2013 ACC/AHA risk calculator based on the Pooled Cohort Equation with modification to allow in higher risk participants with lower LDL-C levels. Data on the utility of this score are retrospective, suggesting potentially that the score may under-predict events [40, 41]. The ACC/AHA risk prediction algorithm was not specifically designed for use in the HIV population and did not include significant numbers of PWH in the cohorts. As such, REPRIEVE is the first large study to utilize this algorithm to recruit participants. Over the course of recruitment for the trial, adjustments were made in the ACC/AHA thresholds, and the size of the low risk groups with less than 2.5% and 5% predicted 10-year risk were limited to generate the most highly representative, and scientifically and clinically valid population, with a high enough risk score to potentially benefit from the treatment strategy, but not too high, to maintain equipoise.
To help ensure equipoise, REPRIEVE requires progressively lower LDL-C thresholds for entry among participants with > 7.5% ASCVD risk, a level often recommended for statin use in the general population. These entry criteria aim to identify a low to moderate risk group for whom the efficacy of primary prevention remains unknown and thus the need for statin prescribing unclear among PWH. Limiting enrollment to only lower risk individuals (< 7.5%) has the risk of including individuals at too low a risk to benefit from statin therapy, whereas including those with a higher risk score, risks enrolling indiviudals for whom statins have proven beneficial in the general population, albeit not in any studies of the HIV population. The REPRIEVE trial has made a significant effort to strike a balance in this regard, enrolling across a range of relevant ASCVD risk scores and using LDL-C as an additional variable to help ensure equipoise. Initial consideration was given to enrolling strictly based on risk score < 7.5%, but with ongoing monitoring of the trial, the DSMB recommended that this threshold be increased to capture the intended population, with a caveat that specific LDL-C thresholds be used for higher risk scores (see Design Changes). Prior to enrollment, all potential participants are encouraged to have an informed discussion with the investigator and relevant clinical caregivers to discuss options, including potential clinical use of statins as recommended by current guidelines in lieu of study participation. An important outcome of the REPRIEVE trial will be to prospectively assess the accuracy of this prediction algorithm in a highly representative HIV population and to determine how best to identify those with increased risk for CVD events.
REPRIEVE utilizes a newer statin, pitavastatin, which minimally interacts with ART, as a strategy to prevent CVD. Statin therapy reduces CVD in primary and secondary prevention strategies in HIV-uninfected individuals, including among those with normal LDL-C and increased evidence of inflammation. Statins have been used, albeit in a limited fashion, among PWH, and have been shown to reduce LDL-C effectively, improve key inflammatory and immune indices [29, 32, 38] thought to drive accelerated atherogenesis in HIV, and have been shown to reduce vulnerable non-calcified coronary atherosclerotic plaque in small studies [29]. Nonetheless, the long-term efficacy as a primary prevention strategy; and the safety of these agents remains unknown. Efficacy in terms of LDL-C lowering and safety were key considerations for the choice of the statin utilized in REPRIEVE. Compared to other statins, pitavastatin at 4 mg/day, a moderate intensity regimen, reduces LDL-C more than 40 mg/day of pravastatin in PWH [27], but may reduce LDL-C less than high dose atorvastatin or rosuvastatin, depending on doses used. Both atorvastatin and rosuvastatin have known interactions with ART. Moreover, certain statins have been shown to increase the incidence of diabetes in large studies of HIV-uninfected persons, including rosuvastatin in JUPITER [36], though the effect was small in comparison to the overall benefit. In addition, rosuvastatin increased insulin and glucose parameters in the SATURN trial among PWH [42]. This is an important issue in the HIV population, in whom insulin resistance is common. We chose pitavastatin, which was shown to be neutral to glucose in a large study of PWH, INTREPID [43], but it will be important to ascertain long-term effects in this regard, as well as effects on muscle and liver, and overall tolerability, and balance these in the context of degree of efficacy. Depending on the results, safety and determination of the relationship of LDL-C lowering to event reduction in the HIV-infected population studied in REPRIEVE, even more potent strategies to lower LDL-C, including higher intensity statins, could be considered for future trials. In addition, other strategies may be useful for the HIV population, including other anti-inflammatory strategies, but these may be limited by side effects and lack of efficacy. Canakinumab was shown to be effective in HIV-uninfected persons, but increased the incidence of fatal infections, so may not be optimal for PWH [27]. Methotrexate has not been proven to prevent CVD events in HIV-uninfected persons and was not shown to effectively lower markers of immune activation and inflammation, nor significantly reduce arterial inflammation in PWH [43]. In contrast, statin therapy may have beneficial pleotropic and effects on lipid and inflammatory indices and thus may be useful for the HIV population. REPRIEVE will answer this hypothesis.
REPRIEVE will assess effects of statin therapy on MACE. The effect size chosen for REPRIEVE, 30%, is more than the 22% based on LDL-C lowering alone, seen among HIV-uninfected persons in the CTT collaboration meta-analysis [44]. This is consistent with the hypothesis of REPRIEVE that statin therapy will have an effect beyond LDL-C lowering. In contrast, 30% is more conservative than the observed effect size of 44% seen in JUPITER, given the uncertainty of statin effects in PWH. The effect size of 30% was deemed clinically relevant for the HIV population and consistent with prior data and the hypotheses of the study.
Beyond the primary endpoint of MACE, the study will assess effects on MACE plus allcause mortality, as a broad index of benefit. Effects on individual components of MACE and all cause mortality will also be assessed. Importantly, REPRIEVE will assess whether MIs in PWH are predominantly Type 1 or 2. In addition, REPRIEVE will assess effects on AIDS endpoints and non-AIDS endpoints including cancer, renal and liver disease. Statin therapy may have potential benefits on these endpoints through anti-inflammatory or other mechanisms. Critically REPRIEVE will assess time-updated immunological indices and determine effects of statins on these endpoints, and whether effects on primary endpoints are modified or predicted by baseline or change in immune function. Effects on glucose will be determined as will incident diabetes to further assess metabolic and safety parameters. Effects of statin therapy on coronary plaque and detailed immune and inflammatory indices will be addressed in a large 800-person Mechanistic Substudy of REPRIEVE, in which participants are followed after randomization, with coronary computed tomography angiography at baseline and 2 years. The design of this embedded substudy is described in a separate manuscript.
Table II.
Eligibility Criteria in REPRIEVE (A5332)
| Inclusion Criteria |
|---|
| Men and women age ≥40 and ≤75 years of age |
| Documentation of HIV-1 infection |
| Combination ART for at least 180 days prior to study entry |
| CD4+ cell count >100 cells/mm3 |
| Fasting LDL-C as follows: |
| ○ If ASCVD riskscore <7.5%, LDL-C must be <190 mg/dL |
| ○ If ASCVD riskscore ≥7.5% and ≤10%, LDL-C must be <160 mg/dL |
| ○ If ASCVD riskscore >10% and ≤15%, LDL-C must be <130 mg/dL |
| Fasting triglycerides <500 mg/dL |
| Hemoglobin ≥8 g/dL for female participants and ≥9 g/dL for male participants |
| GFR ≥60 mL/min/1.73m2or CrCl ≥60 mL/min |
| ALT ≤2.5 × ULN |
| For persons with known chronic active HBV or HCV, calculated FIB-4 score must be ≤3.25 |
| Female participants of reproductive potential must have a negative serum or urine pregnancy test |
| For women of reproductive potential, willingness to use contraceptives as described in the product information for pitavastatin |
| Ability and willingness of participant or legal representative to provide written informed consent |
| Exclusion Criteria |
| Clinical ASCVD, as defined by 2013 ACC/AHA guidelines |
| Current diabetes mellitus if LDL-C ≥70 mg/dL |
| 10-year ASCVD risk score estimated by Pooled Cohort Equations >15% |
| Active cancer within 12 months prior to study entry |
| Known decompensated cirrhosis |
| History of myositis or myopathy with active disease in the 180 days prior to study entry |
| Known untreated symptomatic thyroid disease |
| History of allergy or severe adversereactionto statins |
| Use of specific immunosuppressants or immunomodulatory agents in the 30 days prior to study entry |
| Current use of erythromycin, colchicine, or rifampin |
| Use of any statin drugs, gemfibrozil, or PCSK9 inhibitors in the 90 days prior to study entry |
| Current use of an investigational new drug that would be contraindicated |
| Serious illness or trauma requiring systemic treatment or hospitalization in the 30 days prior to study entry |
| Known active or recent (not fully resolved within 30 days prior to study entry) systemic bacterial, fungal, parasitic, or viral infections (except HIV, HBV, HPV, or HCV) |
| Current breastfeeding |
| Alcohol or drug use that, in the opinion of the site investigator, would interfere with completion of study procedures |
| Other medical, psychiatric, or psychological condition that, in the opinion of the site investigator, would interfere with completion of study procedures and or adherence to study drug |
Abbreviations: ART, antiretroviral therapy; ASCVD, atherosclerotic cardiovascular disease; GFR, glomerular filtration rate; CrCI, creatinine clearance; ALT, alanine aminotransferase; HBV, hepatitis B virus;HCV, hepatitis C virus; FIB-4, fibrosis-4; HPV, human papillomavirus
Summary and Significance.
REPRIEVE is a landmark trial and the first large, randomized trial of a primary prevention strategy for CVD in HIV. Data from REPRIEVE will inform clinicians on the efficacy and safety of statins in PWH. The trial will provide critical information on the utility of CVD risk prediction algorithms, mechanisms of CVD, including sex-related factors, plaque biology, and statin effects on other comorbidities in a diverse, global population of PWH receiving ART.
Acknowledgements:
The study investigators would like to thank the study participants, site staff and study-associated personnel, REPRIEVE Community Advisory Board members and Clinical Trials Specialists (Barbara Bastow, Laura Moran, and Jhoanna Roa) for their efforts to make this study possible.
Grants and Funding Support U01 HL123336 (to SKG and PD); U01 HL123339 (to UH and HJR); 5UM1AI068636 (to JSC); Investigator initiated grant, study drug and blinded matching placebo from Kowa Pharmaceuticals America, Inc. (to SKG); Investigator initiated grant from Gilead Sciences (to SKG and MVZ).
Footnotes
Disclaimer: The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; National Institute of Allergy and Infectious Diseases; National Institutes of Health; or the United States Department of Health and Human Services.
ClinicalTrials.gov Identifier: NCT02344290
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Steven K. Grinspoon, M.D. has received grant support through his institution from Kowa Pharmaceuticals America, Inc. and Gilead Sciences, Inc. for the conduct of the study; from Theratechnologies, and Navidea unrelated to this project and received consulting fees from Theratechnologies, Navidea, Gilead, Merck, and Bristol Myers Squibb, all unrelated to this Project.
Kathleen V. Fitch, M.S.N. has no disclosures to report.
Pharmaceuticals America, Inc. and Gilead Sciences, Inc. for the conduct of the study.
Edgar Turner Overton, M.D. has received grant support through his institution from Gilead Sciences and ViiV unrelated to this project and received consulting fees from ViiV and Merck. Markella V. Zanni, M.D. has participated in a scientific advisory board meeting for Roche Diagnostics and has received grant support through her institution from Gilead Sciences, unrelated to this project. She has received grant support through her institution from Gilead Sciences for the conduct of the study.
Carl J. Fichtenbaum, M.D., has received grant support through his institution from Gilead Sciences, ViiV Healthcare, Janssen, Pfizer, Merck, Amgen and Cytodyn unrelated to this project. He also receives consulting fees for advisory board from Janssen.
Judith A. Aberg, M.D. has received research grants from Bristol-Myers Squibb, Gilead Sciences and Viiv Healthcare, and received scientific advisory board personal fees from Gilead, Janssen, Merck, and ViiV Healthcare, all unrelated to the present study
Carlos Malvestutto, M.D. received consulting fees from ViiV Healthcare unrelated to this project.
Michael T. Lu, M.D. has received grant support through his institution from Kowa.
Judith S. Currier, M.D. has no disclosures to report
Craig A. Sponseller, M.D. is an employee of Kowa Pharmaceuticals America, Inc.
Myron Waclawiw, Ph.D. is an employee of the National Institutes of Health.
Beverly Alston-Smith, M.D. is an employee of the National Institutes of Health.
Katharine Cooper-Arnold, M.D. is an employee of the National Institutes of Health.
Karin L. Klingman, M.D. is an employee of the National Institutes of Health.
Patrice Desvigne-Nickens, M.D. is an employee of the National Institutes of Health.
Udo Hoffmann M.D. has received grant support through his institution from Kowa. Pharmaceuticals America, Inc. for the conduct of the study and has received grant support from Siemens Healthcare, the American College of Radiology Imaging Network and HeartFlow Inc. unrelated to this project.
Heather J. Ribaudo, Ph.D. has no disclosures to report.
Pamela Douglas M.D. has no disclosures to report.
References
- 1.UNAIDS. Fact Sheet - Latest Statistics on the status of the AIDS epidemic. In: UNAIDS; 2017. pp. Fact Sheet. [Google Scholar]
- 2.UNAIDS/WHO 2010 AIDS Epidemic Update. In UNAIDS Report on the Global AIDS Epidemic; 2010. [Google Scholar]
- 3.Klein D, Hurley LB, Quesenberry CP Jr., Sidney S Do protease inhibitors increase the risk for coronary heart disease in patients with HIV-1 infection? J Acquir Immune Defic Syndr 2002,30:471–477. [DOI] [PubMed] [Google Scholar]
- 4.Currier JS, Taylor A, Boyd F, Dezii CM, Kawabata H, Burtcel B, et al. Coronary Heart Disease in HIV-Infected Individuals. J Acquir Immune Defic Syndr 2003,33:506–512. [DOI] [PubMed] [Google Scholar]
- 5.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007,92:2506–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Obel N, Thomsen HF, Kronborg G, Larsen CS, Hildebrandt PR, Sorensen HT, et al. Ischemic heart disease in HIV-infected and HIV-uninfected individuals: a population-based cohort study. Clin Infect Dis 2007,44:1625–1631. [DOI] [PubMed] [Google Scholar]
- 7.Lang S, Mary-Krause M, Cotte L, Gilquin J, Partisani M, Simon A, et al. Increased risk of myocardial infarction in HIV-infected patients in France, relative to the general population. AIDS 2010,24:1228–1230. [DOI] [PubMed] [Google Scholar]
- 8.Durand M, Sheehy O, Baril JG, Lelorier J, Tremblay CL. Association between HIV infection, antiretroviral therapy, and risk of acute myocardial infarction: a cohort and nested case-control study using Quebec's public health insurance database. J Acquir Immune Defic Syndr 2011,57:245–253. [DOI] [PubMed] [Google Scholar]
- 9.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV Infection and the Risk of Acute Myocardial Infarction. JAMA Intern Med 2013:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith CJ, Ryom L, Weber R, Morlat P, Pradier C, Reiss P, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet 2014,384:241–248. [DOI] [PubMed] [Google Scholar]
- 11.Feinstein MJ, Bahiru E, Achenbach C, Longenecker CT, Hsue P, So-Armah K, et al. Patterns of Cardiovascular Mortality for HIV-Infected Adults in the United States: 1999 to 2013. Am J Cardiol 2016,117:214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burdo TH, Lentz MR, Autissier P, Krishnan A, Halpern E, Letendre S, et al. Soluble CD163 Made by Monocyte/Macrophages Is a Novel Marker of HIV Activity in Early and Chronic Infection Prior to and After Anti-retroviral Therapy. J Infect Dis 2011,204:154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitch KV, Srinivasa S, Abbara S, Burdo TH, Williams KC, Eneh P, et al. Noncalcified Coronary Atherosclerotic Plaque and Immune Activation in HIV-Infected Women. J Infect Dis 2013,208:1737–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zanni MV, Abbara S, Lo J, Wai B, Hark D, Marmarelis E, et al. Increased Coronary Atherosclerotic Plaque Vulnerability by Coronary Computed Tomography Angiography in HIV-Infected Men. AIDS 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanna DB, Lin J, Post WS, Hodis HN, Xue X, Anastos K, et al. Association of Macrophage Inflammation Biomarkers With Progression of Subclinical Carotid Artery Atherosclerosis in HIV- Infected Women and Men. J Infect Dis 2017,215:1352–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, et al. Arterial inflammation in patients with HIV. JAMA 2012,308:379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Funderburg NT, Mayne E, Sieg SF, Asaad R, Jiang W, Kalinowska M, et al. Increased tissue factor expression on circulating monocytes in chronic HIV infection: relationship to in vivo coagulation and immune activation. Blood 2010,115:161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nordell AD, McKenna M, Borges AH, Duprez D, Neuhaus J, Neaton JD. Severity of cardiovascular disease outcomes among patients with HIV is related to markers of inflammation and coagulation. J Am Heart Assoc 2014,3:e000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Sadr WM, Lundgren J, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 2006,355:2283–2296. [DOI] [PubMed] [Google Scholar]
- 20.Zanni MV, Toribio M, Robbins GK, Burdo TH, Lu MT, Ishai AE, et al. Effects of Antiretroviral Therapy on Immune Function and Arterial Inflammation in Treatment-Naive Patients With Human Immunodeficiency Virus Infection. JAMA Cardiol 2016,1:474–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim CJ, Rousseau R, Huibner S, Kovacs C, Benko E, Shahabi K, et al. Impact of intensified antiretroviral therapy during early HIV infection on gut immunology and inflammatory blood biomarkers. AIDS 2017,31:1529–1534. [DOI] [PubMed] [Google Scholar]
- 22.Pedersen TR, Kjekshus J, Berg K, Haghfelt T, Faergeman O, Faergeman G, et al. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). 1994. Atheroscler Suppl 2004,5:81–87. [DOI] [PubMed] [Google Scholar]
- 23.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med 1996,335:1001–1009. [DOI] [PubMed] [Google Scholar]
- 24.Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med 1995,333:1301–1307. [DOI] [PubMed] [Google Scholar]
- 25.Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998,279:1615–1622. [DOI] [PubMed] [Google Scholar]
- 26.Greenwood J, Mason JC. Statins and the vascular endothelial inflammatory response. Trends Immunol 2007,28:88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr., Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N EnglJ Med 2008,359:2195–2207. [DOI] [PubMed] [Google Scholar]
- 28.Silverberg MJ, Leyden W, Hurley L, Go AS, Quesenberry CP Jr., Klein D, et al. Response to newly prescribed lipid-lowering therapy in patients with and without HIV infection. Ann Intern Med 2009,150:301–313. [DOI] [PubMed] [Google Scholar]
- 29.Lo J, Lu MT, Ihenachor EJ, Wei J, Looby SE, Fitch KV, et al. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. The Lancet HIV 2015,2:e52–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Funderburg NT, Jiang Y, Debanne SM, Storer N, Labbato D, Clagett B, et al. Rosuvastatin treatment reduces markers of monocyte activation in HIV-infected subjects on antiretroviral therapy. Clin Infect Dis 2014,58:588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Longenecker CT, Sattar A, Gilkeson R, McComsey GA. Rosuvastatin slows progression of subclinical atherosclerosis in patients with treated HIV infection. AIDS 2016,30:2195–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Funderburg NT, Jiang Y, Debanne SM, Labbato D, Juchnowski S, Ferrari B, et al. Rosuvastatin reduces vascular inflammation and T-cell and monocyte activation in HIV-infected subjects on antiretroviral therapy. J Acquir Immune Defic Syndr 2015,68:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clement ME, Park LP, Navar AM, Okeke NL, Pencina MJ, Douglas PS, et al. Statin Utilization and Recommendations Among HIV- and HCV-infected Veterans: A Cohort Study. Clin Infect Dis 2016,63:407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aberg JA, Kaplan JE, Libman H, Emmanuel P, Anderson JR, Stone VE, et al. Primary care guidelines for the management of persons infected with human immunodeficiency virus: 2009 update by the HIV medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2009,49:651–681. [DOI] [PubMed] [Google Scholar]
- 35.Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA, et al. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014,58:1–10. [DOI] [PubMed] [Google Scholar]
- 36.Aberg JA, Sponseller CA, Ward DJ, Kryzhanovski VA, Campbell SE, Thompson MA. Pitavastatin versus pravastatin in adults with HIV-1 infection and dyslipidaemia (INTREPID): 12 week and 52 week results of a phase 4, multicentre, randomised, double-blind, superiority trial. Lancet HIV 2017,4:e284–e294. [DOI] [PubMed] [Google Scholar]
- 37.Zanni MV, Fitch K, Rivard C, Sanchez L, Douglas PS, Grinspoon S, et al. Follow YOUR Heart: development of an evidence-based campaign empowering older women with HIV to participate in a large-scale cardiovascular disease prevention trial. HIV Clin Trials 2017,18:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toribio M, Fitch KV, Sanchez L, Burdo TH, Williams KC, Sponseller CA, et al. Effects of pitavastatin and pravastatin on markers of immune activation and arterial inflammation in HIV. AIDS 2017,31:797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hicks KA, James Hung K, Mahaffey KW, Mehran R, Nissen SE, Stockbridge NL, et al. Taskforce Recommendations for Standardized Definitions for Cardiovascular and Stroke End Point Events in Clinical Trials. In; 2012. pp. 1–31. [Google Scholar]
- 40.Feinstein MJ, Nance RM, Drozd DR, Ning H, Delaney JA, Heckbert SR, et al. Assessing and Refining Myocardial Infarction Risk Estimation Among Patients With Human Immunodeficiency Virus: A Study by the Centers for AIDS Research Network of Integrated Clinical Systems. JAMA Cardiol 2017,2:155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Triant VA, Perez J, Regan S, Massaro JM, Meigs JB, Grinspoon SK, et al. Cardiovascular Risk Prediction Functions Underestimate Risk in HIV Infection. Circulation 2018,137:2203–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Benedetti F, Gattorno M, Anton J, Ben-Chetrit E, Frenkel J, Hoffman HM, et al. Canakinumab for the Treatment of Autoinflammatory Recurrent Fever Syndromes. N Engl J Med 2018,378:1908–1919. [DOI] [PubMed] [Google Scholar]
- 43.Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010,376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A, et al. 2014 ACC/AHA Key Data Elements and Definitions for Cardiovascular Endpoint Events in Clinical Trials: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). J Am Coll Cardiol 2015,66:403–469. [DOI] [PubMed] [Google Scholar]