Abstract
Niemann Pick disease, type C (NPC) is a neurodegenerative lysosomal storage disease affecting the visceral organs and the central nervous system. The age of initial presentation varies from fetal to adult onset, although childhood onset is most common. The life expectancy for the full spectrum of NPC patients is not well defined, and it is unknown if current supportive care impacts the natural history. In order to assess age of death for a large cohort of NPC patients, we “crowd-sourced” age and year of death from information posted on disease support group website memorial walls. We analyzed data from 338 individuals who died between 1968 and 2018. In addition to age of death, gender can be inferred from given names and photographs. The median age of death was 13 years with a range from 0.1-69 years. Although sex significantly affects survival of NPC1 mutant mice, we did not observe a gender dependent survival difference in NPC patients. Median age of survival across time increased between the earliest patients and the most recently deceased patient; however, we found no significant change in survival over the last 20 years. These data suggest that supportive medical care has not impacted survival in the recent past and provides support for the use of historic controls in evaluating therapeutic interventions.
Keywords: Niemann-Pick disease, type C, NPC, Lysosomal Storage Disease
1. Introduction
Niemann-Pick disease, type C (NPC) is an autosomal recessive, neurodegenerative lysosomal storage disorder that is caused by mutations in either NPC1 or NPC2. The proteins encoded by NPC1 and NPC2 are involved in intracellular cholesterol trafficking. Impaired function of either NPC1 or NPC2 leads to an endolysosomal accumulation of cholesterol and glycosphingolipids [1]. NPC1 is an integral membrane protein that facilitates the transport of cholesterol across the endolysosomal membrane, and NPC2 is a non-enzymatic soluble lysosomal protein that likely transports cholesterol from the lysosomal lumen to NPC1 [2]. NPC1 mutations are more common with more than 95% of diagnosed individuals having NPC disease due to NPC1 mutations [3]. NPC is a rare disease with an estimated incidence of approximately 1 in 100,000 live births for the classical disease and potentially higher frequency for late-onset presentations [3, 4]. Over 250 pathogenic mutations have been documented in NPC1 with the most common encoding p.I1061T which has been identified in approximately 15% of disease-associated alleles [5].
The NPC1 phenotype is very heterogeneous both with respect to age of onset and the specific sign/symptom complex present in each patient [3]. Initial presentation ranges from infantile cholestatic liver disease to progressive neurological disease later in life. Neurological symptoms are progressive and can include progressive developmental delay/school difficulties, cerebellar ataxia, seizures, gelastic cataplexy, vertical supranuclear gaze palsy and dementia. Although age of disease onset is a continuum, NPC patients have been categorized as early-infantile (<2 years), late-infantile (2-<6 years), or juvenile (6-15 years) depending on the age of first neurological symptom [6]. Neurological disease progression has been characterized in several cohorts [5-7]. In contrast, available information on lifespan of NPC patients is limited. Given that a number of potential therapeutic agents are being developed [8-11], information on lifespan will be critical to understanding the ultimate efficacy of therapeutic interventions.
We were able to obtain survival data on a large cohort of NPC patients by crowd sourcing public information posted on patient support group websites. Both Fight NPC (www.fightnpc.com) and The National Niemann Pick Disease Foundation (NNPDF; https://nnpdf.org) have incorporated a memorial webpage on their site. We were able to use these sites to obtain information on date of birth, gender and age of death for 338 individuals with NPC. These data can be used to define lifespan, elucidate effect of gender, and determine if changes in supportive medical care have altered the natural history of NPC. Although there are limitations, crowd-sourced information has the potential of providing critical data regarding the natural history of rare disorders.
2. Materials and Methods
Because all individuals are deceased, this work did not require Institutional Review Board oversight. All information is publicly available. On September 28, 2018, we accessed the Fight NPC (http://fightnpc.com/en/memorial_wall) and NNPDF ((https://nnpdf.org/family-services/memorials/) websites to obtain data on deceased individuals. Duplicate entries were tabulated only once. For each individual we extracted birth date, date of death and inferred gender using photographs and given names. Gender was inferred by three assessors based on agreement of at least two. Individuals were excluded from analysis if the information posted on the website was insufficient to calculate the age at death. Where possible, we cross-checked and confirmed the extracted data with our natural history study database (NCT00344331). Mouse data are from homozygous mutant Npc1m1N mice on a BalbC background [12]. Descriptive statistics, median and range, are used in this report. Survival curves are compared using either the log-rank test or log-rank test for trend. The data were graphed and analyzed utilizing Graph Pad Prism 6.0.
3. Results
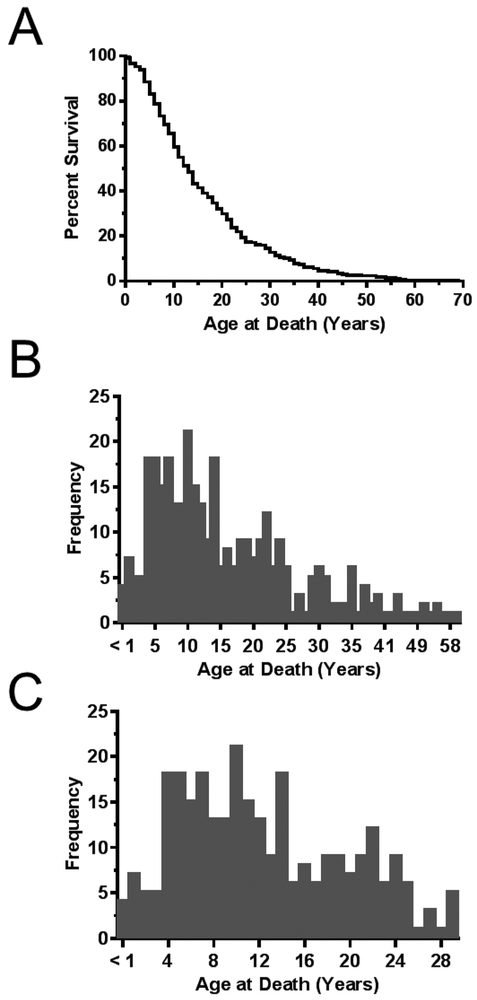
From the Fight NPC and the NNPDF websites we were able to obtain data corresponding to 338 individuals with NPC. Age at death was calculated based on dates of birth and death. Figure 1A provides a survival curve for these individuals. Median age of death for this cohort was 13 years. Age of death ranged from 0.1 to 69 years. These data are presented as histograms in figures 1B and 1C. The histograms clearly show a marked increase in deaths starting at 4 years of age. Although the distribution is continuous, the under 30-year-old histogram (Fig. 1C) potentially suggests a bimodal distribution with one group of patients dying in the first decade of life and a second group dying early in the third decade of life.
Figure 1. Age of death.
A) Kaplan-Meier survival curve of 338 individuals diagnosed with NPC between 1968 and 2018. Median age of death was 13 years with a range from 0.1-69 years. B) Frequency distribution of age of death for the 338 individuals with NPC. C) Frequency distribution of the 289 individuals who died prior to 30 years of age.
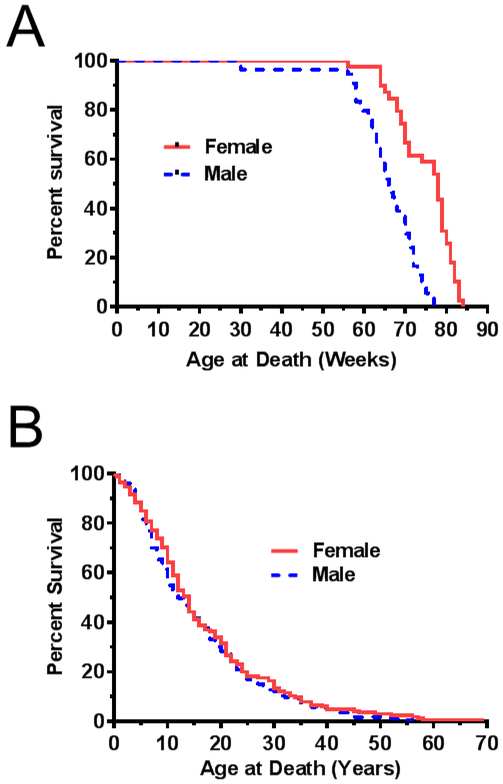
Multiple research groups have recognized a sex difference in survival between male and female Npc1 mutant mice as a potential confounder in preclinical evaluation of therapies. Figure 2A provides data on survival of male and female Npc1 mutant mice from our research group. Female Npc1 mutant mice live significantly longer (p<0.0001) than male Npc1 mice with a median survival of 66 and 78 days for male and female NPC1 mutant mice, respectively. We used our current survival dataset to determine if a similar survival difference was present in male and female NPC1 patients. The male and female survival difference observed in mice was not observed in NPC1 humans (Fig. 2B). Median survival for males and females were 12 and 14 years respectively. The survival curves were not significantly different (p=0.31).
Figure 2. Effect of gender/sex on the age of death.
A) Kaplan-Meier survival curves corresponding to female (n=78, solid line) and male (n=66, dashed line) Npc1 mutant mice. B) Kaplan-Meier survival curves corresponding to female (n=165, solid line) and male (n=173, dashed line) individuals with NPC.
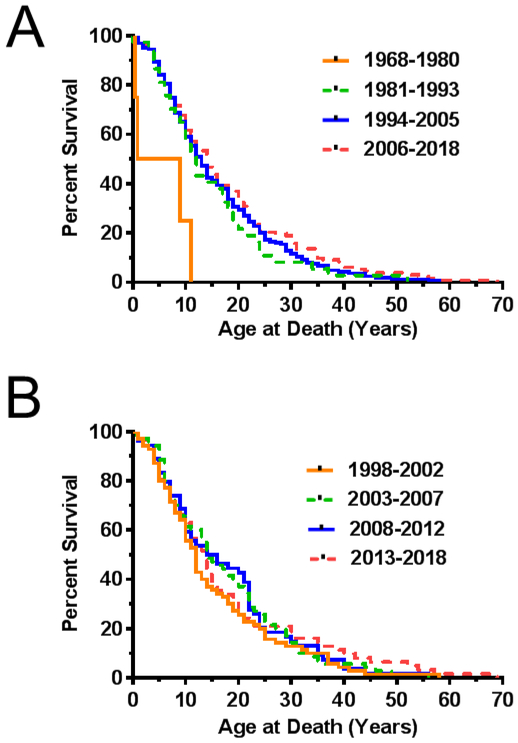
We were also interested in determining if the age of death has changed over time due to advances in available diagnostic methods and medical care. This is critical to know if one is ultimately interested in comparing survival of treated individuals to historical controls. We also evaluated these data by segmenting the dataset into four equal time intervals based on the year of death (Fig. 3A). The first cohort had 4 subjects (1968-1980), second cohort had 37 subjects (1981-1993), third cohort had 163 subjects (1994-2005) and the fourth cohort had 133 subjects (2006-2018). Median ages of death for these four-time intervals were 5, 12, 13, 14 years, respectively. Except for the first time period, where the number of subjects was very low, there did not appear to be a significant increase in survival from 1998 to 2018. The 1968-1980-time interval was excluded from analysis due to the minimal data available. Consistent with our impression, there was no significant trend (p=0.12) for the remaining three survival curves.
Figure 3. Effect of year of death on survival curves for individuals with NPC.
A) Kaplan-Meier survival curves corresponding to individuals who died in the 11-12 year intervals of 1968-1980 (n=4 gray solid line), 1981-1993 (n=37, gray dashed line), 1994-2005 (n=163, black solid line), 2006-2018 (n=133, black dashed line). B) Kaplan-Meier survival curves corresponding to 256 individuals with NPC who have died in the last 20 years. Each curve represents a 5-year interval. Intervals are 1998-2002 (n=70, gray solid line), 2003-2007 (n=70, gray dashed line), 2008-2012 (n=54, black solid line) and 2013-2018 (n=62, black dashed line).
NPC1 and NPC2 were initially identified in 1997 and 2000, respectively [13, 14]. The availability of molecular confirmation of an NPC diagnosis may have expanded the phenotypic spectrum of NPC patients. In addition, miglustat was approved by the EMA in 2002 for the treatment of NPC. Although miglustat was not approved by the FDA for NPC, off-label use approaches 40% in our NIH cohort of NPC1 natural history trial participants. To gain insight into whether these two factors impacted NPC lifespan, we analyzed age of death in 5-year intervals over the last two decades (Fig. 3B). The number of subjects in the four groups was 70 (1998-2002), 70 (2003-2007), 54 (2008-2012) and 62 (2013-2018). Median age of death for these time periods were 12, 14, 15 and 14 years, respectively. No significant trend (p=0.67) was observed for the four survival curves.
4. Discussion
Niemann-Pick disease, type C is a lethal neurodegenerative disease resulting in premature death. A number of studies have looked at survival as a function of age of neurological disease onset. Imrie, et al. [6] published a report of 74 patients in a United Kingdom cohort. In the neonatal onset group the mean age of death (± SD) was 0.19 ± 0.22 years, in the early-infantile group mean age of death was 5.6 ± 2 years, in the late infantile group the mean age of death was 13.4 ± 6.7, and in the juvenile onset group the mean age of death was noted to be 25.9 ± 8.9 years. The adolescent /adult group had a mean age of death of 33.7 ± 6.2 years. In a review by Vanier and Millat [1], the typical age of death is reported as a function of the age of onset of neurological symptom onset. Individuals presenting with neurological symptoms evident by the age of 18 months typically died before the age of 5; whereas, individuals with later neurological onset in childhood and adolescence typically died as teenagers with some individuals surviving into their twenties or thirties. Walterfang et al. [15] provided data on the survival of 97 NPC patients stratified by age of neurological onset. Fifty percent of NPC patients with neurological onset either prior to 2 years or between 2-11 years of age died within approximately 3 or 7 years of diagnosis. In contrast, 64% of individuals with neurological disease onset after 11 years of age were still alive after 10 years of follow-up. Their data, although limited, also suggest that miglustat might alter the survival time of NPC patients. Jahnova et al. also showed a stability of diagnostic rates and disease manifestions over a 40 year span, with only the adolescent/ adult phenotype being ascertained more often in more recent decades [16].
This study reports on the age of death of a cohort of 338 individuals with NPC who died between 1968 and 2018. The median age of death was 13 years with a range from 0.1-69 years. The histograms show an increase in rate of death starting at 4 years of age. This observation argues for early presymptomatic diagnosis by newborn screen [17] and early intervention as potential therapies become available. From this data set, in contrast to what has been observed in mice, we are to conclude that there does not appear to be a significant difference in survival of males and females with NPC. In addition, there does not appear to be a significant difference in lifespan in individuals that have died more recently. This suggests that to date, changes in diagnostic testing and clinical management have not significantly impacted the overall survival of patients with NPC disease. These factors are frequently cited as concerns when historical control groups are used to evaluate therapeutic efficacy.
This study has several limitations. The most significant limitation of this study is that we do not have access to correlating clinical data. Thus, we are unable to stratify this cohort based on age of neurological onset, age of diagnosis, presence or absence of visceral disease, specific neurological manifestations such as seizures, cause of death or miglustat treatment status. Another limitation is that we cannot independently confirm the diagnosis of NPC or separate case due to mutation of NPC1 versus NPC2. A third limitation is that ascertainment may be biased. We are not able ascertain the completeness of the dataset and the dataset is clearly biased toward individuals whose guardians are comfortable with the English language and the use of social media. It would be interesting to know if similar datasets are available in other countries and interesting to compare if they show a similar distribution. The dataset is also biased toward individuals who have died more recently. However, even with these limitations, this study still informs our understanding of the natural history of NPC.
To our knowledge, this is the first example of extraction of crowd-sourced mortality data that is not part of a curated patient registry. Other rare diseases such as Pompe disease (http://www.amda-pompe.org/index.php/main/patients/memorial/), Batten Disease (https://bdsra.org/family-profiles/), leukodystrophies (https://www.huntershope.org/family-care/wall-of-fame/), chronic intestinal pseudoobstruction (https://www.g-pact.org/memorials/memorial-wall), or Krabbe disease (https://krabbeconnect.org/our-heroes/) have similar memorial walls or member walls in honor of affected children. The available information will vary from site to site; however, as long as the limitations are recognized, the information collected by these sites may complement more traditional sources of natural history data such as formal observational trials, review of case reports and case series, surveys and patient registries.
Acknowledgements
We would like to acknowledge both Fight NPC and the National Niemann-Pick Disease Foundation for their sustained efforts to support NPC patients and families and their dedication to memorialize the lives of individuals whose lives have been cut short by NPC disease. We appreciate the statistical assistance of Dr. Ninet Sinaii. Finally, we would like to dedicate this paper to the parents and guardians who have suffered the loss of a child due to NPC.
Funding
This work was supported by the intramural research program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- [1].Vanier MT, Millat G, Niemann-Pick disease type C Clinical genetics 64 (2003) 269–281. [DOI] [PubMed] [Google Scholar]
- [2].Kwon HJ, Abi-Mosleh L, Wang ML, Deisenhofer J, Goldstein JL, Brown MS, Infante RE, Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol Cell 137 (2009) 1213–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Vanier MT, Niemann-Pick disease type C Orphanet journal of rare diseases 5 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wassif CA, Cross JL, Iben J, Sanchez-Pulido L, Cougnoux A, Platt FM, Ory DS, Ponting CP, Bailey-Wilson JE, Biesecker LG, Porter FD, High incidence of unrecognized visceral/neurological late-onset Niemann-Pick disease, type C1, predicted by analysis of massively parallel sequencing data sets Genet Med 18 (2016) 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Runz H, Dolle D, Schlitter AM, Zschocke J, NPC-db, a Niemann-Pick type C disease gene variation database Human mutation 29 (2008) 345–350. [DOI] [PubMed] [Google Scholar]
- [6].Imrie J, Heptinstall L, Knight S, Strong K, Observational cohort study of the natural history of Niemann-Pick disease type C in the UK: a 5-year update from the UK clinical database BMC Neurol 15 (2015) 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yanjanin NM, Velez JI, Gropman A, King K, Bianconi SE, Conley SK, Brewer CC, Solomon B, Pavan WJ, Arcos-Burgos M, Patterson MC, Porter FD, Linear clinical progression, independent of age of onset, in Niemann-Pick disease, type C Am J Med Genet B Neuropsychiatr Genet 153B (2010) 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Patterson MC, Vecchio D, Prady H, Abel L, Wraith JE, Miglustat for treatment of Niemann-Pick C disease: a randomised controlled study The Lancet. Neurology 6 (2007) 765–772. [DOI] [PubMed] [Google Scholar]
- [9].Ory DS, Ottinger EA, Farhat NY, King KA, Jiang X, Weissfeld L, Berry-Kravis E, Davidson CD, Bianconi S, Keener LA, Rao R, Soldatos A, Sidhu R, Walters KA, Xu X, Thurm A, Solomon B, Pavan WJ, Machielse BN, Kao M, Silber SA, McKew JC, Brewer CC, Vite CH, Walkley SU, Austin CP, Porter FD, Intrathecal 2-hydroxypropyl-beta-cyclodextrin decreases neurological disease progression in Niemann-Pick disease, type C1: a non-randomised, open-label, phase 1-2 trial Lancet (London, England) 390 (2017) 1758–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chandler RJ, Williams IM, Gibson AL, Davidson CD, Incao AA, Hubbard BT, Porter FD, Pavan WJ, Venditti CP, Systemic AAV9 gene therapy improves the lifespan of mice with Niemann-Pick disease, type C1 Human molecular genetics 26 (2017) 52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kirkegaard T, Gray J, Priestman DA, Wallom KL, Atkins J, Olsen OD, Klein A, Drndarski S, Petersen NH, Ingemann L, Smith DA, Morris L, Bornaes C, Jorgensen SH, Williams I, Hinsby A, Arenz C, Begley D, Jaattela M, Platt FM, Heat shock protein-based therapy as a potential candidate for treating the sphingolipidoses Science translational medicine 8 (2016) 355ra118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pentchev PG, Comly ME, Kruth HS, Patel S, Proestel M, Weintroub H, The cholesterol storage disorder of the mutant BALB/c mouse. A primary genetic lesion closely linked to defective esterification of exogenously derived cholesterol and its relationship to human type C Niemann-Pick disease The Journal of biological chemistry 261 (1986) 2772–2777. [PubMed] [Google Scholar]
- [13].Carstea ED, Morris JA, Coleman KG, Loftus SK, Zhang D, Cummings C, Gu J, Rosenfeld MA, Pavan WJ, Krizman DB, Nagle J, Polymeropoulos MH, Sturley SL, Ioannou YA, Higgins ME, Comly M, Cooney A, Brown A, Kaneski CR, Blanchette-Mackie EJ, Dwyer NK, Neufeld EB, Chang TY, Liscum L, Strauss JF 3rd, Ohno K, Zeigler M, Carmi R, Sokol J, Markie D, O'Neill RR, van Diggelen OP, Elleder M, Patterson MC, Brady RO, Vanier MT, Pentchev PG, Tagle DA, Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis Science (New York, N.Y.) 277 (1997) 228–231. [DOI] [PubMed] [Google Scholar]
- [14].Naureckiene S, Sleat DE, Lackland H, Fensom A, Vanier MT, Wattiaux R, Jadot M, Lobel P, Identification of HE1 as the second gene of Niemann-Pick C disease Science (New York, N.Y.) 290 (2000) 2298–2301. [DOI] [PubMed] [Google Scholar]
- [15].Walterfang M, Chien YH, Imrie J, Rushton D, Schubiger D, Patterson MC, Dysphagia as a risk factor for mortality in Niemann-Pick disease type C: systematic literature review and evidence from studies with miglustat Orphanet journal of rare diseases 7 (2012) 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jahnova H, Dvorakova L, Vlaskova H, Hulkova H, Poupetova H, Hrebicek M, Jesina P, Observational, retrospective study of a large cohort of patients with Niemann-Pick disease type C in the Czech Republic: a surprisingly stable diagnostic rate spanning almost 40 years Orphanet journal of rare diseases 9 (2014) 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jiang X, Sidhu R, Mydock-McGrane L, Hsu FF, Covey DF, Scherrer DE, Earley B, Gale SE, Farhat NY, Porter FD, Dietzen DJ, Orsini JJ, Berry-Kravis E, Zhang X, Reunert J, Marquardt T, Runz H, Giugliani R, Schaffer JE, Ory DS, Development of a bile acid-based newborn screen for Niemann-Pick disease type C Science translational medicine 8 (2016) 337ra363. [DOI] [PMC free article] [PubMed] [Google Scholar]