Abstract
Rationale & Objective:
Hepatitis C virus (HCV) infection is common among maintenance dialysis patients. Few studies have examined both dialysis survival as well as transplantation outcomes for HCV-seropositive patients because registry datasets lack information on HCV serostatus.
Study design:
Retrospective cohort study.
Setting and Participants:
Adult long-term dialysis patients treated by a US national dialysis provider between 1/1/2004 – 12/31/2014.
Exposure:
HCV antibody serostatus obtained as part of clinical data from a national dialysis provider.
Outcomes:
Mortality on dialysis, entry onto the kidney transplant waiting list, kidney transplantation, and estimated survival benefit from kidney transplantation versus remaining on the waitlist.
Analytical approach:
After linking clinical data with data from the Organ Procurement and Transplantation Network, Cox and cause-specific hazards regression were implemented to estimate the associations between HCV seropositivity and mortality as well as entry onto the kidney transplant waitlist. Cox regression was also used to estimate the survival benefit from transplantation versus dialysis among HCV-seropositive individuals.
Results:
Among 442,171 dialysis patients, 31,624 (7.2%) were HCV seropositive. HCV seropositivity was associated with a small elevation in the rate of death (adjusted hazard ratio [aHR], 1.09; 95% CI, 1.07–1.11) and a substantially lower rate of entry onto the kidney transplant waitlist (sub-distribution hazard ratio [sHR], 0.67; 95% CI, 0.61 – 0.74). Once wait-listed, the kidney transplant rate was not different for HCV-seropositive (sHR 1.10; 95% CI, 0.96–1.27) versus HCV-seronegative patients. HCV-seropositive patients lived longer with transplantation (aHR at 3 years, 0.42; 95% CI, 0.27–0.63). Receiving an HCV-seropositive donor kidney provided a survival advantage at the 2-year post-transplantation timepoint compared to remaining on dialysis waiting for an HCV-negative kidney.
Limitations:
No data on HCV viral load or liver biopsy.
Conclusions:
HCV-seropositive patients experience reduced access to the kidney transplantation waitlist despite deriving a substantial survival benefit from transplantation. HCV-seropositive patients should consider foregoing HCV treatment while accepting kidneys from HCV-infected donors to facilitate transplant and prolong survival.
Keywords: dialysis, kidney transplantation, hepatitis C virus (HCV), chronic kidney disease (CKD), waitlisting, survival benefit, end-stage renal disease (ESRD), ESRD modality, HCV seropositive, delisting, barriers to transplantation
Introduction
Hepatitis C virus (HCV) prevalence in the general US population is approximately 1%,1 while prevalence among hemodialysis patients is substantially higher, ranging between 3 – 14%.2–4 In contemporary US practice, all dialysis patients are screened for HCV infection by testing for anti-HCV antibody and, more recently, HCV RNA, to confirm chronic infection. Historically, HCV infection has been associated with higher mortality rates in the dialysis population,5,6 primarily due to an increased risk of hepatic decompensation7 and cardiovascular disease.8 Previous studies of dialysis patients with HCV infection have been limited by small sample sizes, limited comorbidity data, and/or an inability to account for transplantation events.
Transplantation is the ideal therapy for many patients with kidney failure but characterization of HCV-seropositive dialysis patients’ outcomes on the kidney waitlist has been impeded by the fact that national registry data – including the Organ Procurement and Transplantation Network (OPTN) and the United States Renal Data Systems - do not include HCV serostatus for wait-listed patients. HCV-seropositive individuals undergo a more complex medical assessment that includes hepatology evaluation to be wait-listed; these additional steps may create barriers to transplantation.9 If waiting-list registration challenges can be overcome, HCV-infected patients can reduce their time to transplantation by accepting kidneys from donors with HCV infection.10 However, access to kidneys from HCV-infected donors is inconsistent because of geographic variations in HCV prevalence and center willingness to transplant these kidneys; in 2017, <50% of centers utilized HCV-infected kidneys.11,12
Irrespective of donor type, HCV-seropositive transplant recipients have diminished graft and post-transplantation survival compared to HCV-seronegative patients,13 with higher rates of complications, including post-transplantation diabetes, glomerulonephritis, cirrhosis, and hepatocellular carcinoma. 14–17 While the availability of direct-acting antivirals (DAAs) has been hailed as a great advance for patients with HCV and emerging data18 suggest a positive impact of DAAs on post-transplantation outcomes, clinical adoption has been slow; only 12.9% of kidney transplant recipients and 1.5% of dialysis patients have been treated for HCV, leaving most still at risk of infectious complications. Furthermore, economic decision analyses indicate that HCV treatment post-transplantation is preferred if HCV-infected kidneys are readily available.19 Taken together, these data suggest that the majority of HCV-infected transplant candidates may remain untreated; understanding their barriers to transplantation and outcomes after wait-listing remain an important priority for the nephrology community.
We assembled a large, national cohort of maintenance dialysis patients with information about HCV serostatus, wait-listing, and kidney transplantation. We aimed to determine the association between HCV serostatus, dialysis survival, and transplant access. Among HCV-seropositive patients, we aimed to determine whether kidney transplantation offered a survival benefit versus remaining on the waitlist. We examined the impact of transplantation with an HCV-seropositive donor kidney on survival compared to waiting for a kidney from an HCV-negative donor.
Methods
Study Design and Data Sources
We performed a retrospective cohort study of adult (≥18 years) incident and prevalent patients receiving outpatient maintenance dialysis between January 1, 2004 and December 31, 2014 (the last date of follow-up) at US facilities managed by a large national dialysis provider (DaVita). DaVita operates more than 2500 dialysis units in 47 states, providing a broad national sample of dialysis patients. DaVita data include detailed information about treatment modality, dialysis access, administrative claims related to comorbidities (ICD-9 codes), and laboratory measurements, including HCV serostatus.20,21 We excluded patients with indeterminate HCV serostatus and/or with HIV infection (which adversely affects transplant access).
DaVita clinical data were linked to data from the OPTN22 using five identifiers: Social Security Number, date of birth, first name, last name, and sex. The OPTN data system includes data on all donors, wait-listed candidates, and transplant recipients in the US, submitted by the members of the OPTN, The Health Resources and Services Administration (HRSA), US Department of Health and Human Services, provides oversight to the activities of the OPTN contractor. Analyses were performed using a de-identified dataset. The protocol was approved by the University of Pennsylvania Institutional Review Board (#822070) and informed consent was not required due to use of de-identified data.
Primary Exposure
The primary exposure was HCV antibody serostatus, which was assessed by dialysis provider protocol by either enzyme immunoassay or recombinant immunoblot assay. Results were reported as “positive,” “negative,” or “indeterminate.
Outcomes Assessment
The primary outcomes were: a) mortality on dialysis, b) wait-listing for kidney transplant, c) kidney transplantation, and d) estimated survival benefit from kidney transplantation versus remaining on the waitlist. Secondary outcomes included delisting (waitlist removal). Mortality was ascertained by either the dialysis provider or the OPTN, through linkage to the Social Security Master Death file (Item S1).21 Wait-listing and kidney transplantation events were ascertained from the OPTN.
Covariate Assessment
For analyses of dialysis mortality and access to the waitlist, covariates were obtained from the DaVita dataset during the 6 months before the index date: HCV serostatus, age, sex, kidney failure cause, initial dialysis modality, pre-observation dialysis exposure (any/none), primary insurance type, body mass index (kg/m2), and median household income (estimated using zip codes and US census data; additional methods description in Item S1). Cardiovascular disease, diabetes mellitus, severe liver disease, and chronic obstructive pulmonary disease (COPD) were determined with ICD-9 codes (Item S2).23–25 We also collected laboratory data, including serum hemoglobin, platelets, and albumin.
For time to transplantation and survival benefit analyses, OPTN covariates were primarily used and supplemented with DaVita data (e.g. HCV serostatus, laboratory values, several comorbidities) only when not available in the OPTN dataset. OPTN covariates included blood group, panel-reactive antibody, donor HCV status, and donor service area (DSA).
Statistical Analyses
Descriptive statistics.
Analyses were performed using Stata 14.0 (StataCorp LP, College Station, TX). Descriptive statistics were generated to compare baseline clinical and demographic characteristics between patients with and without HCV seropositivity. Nonparametric continuous variables were compared between groups using Wilcoxon rank-sum. Categorical and binary variables were compared using the chi-square test.
Dialysis mortality by HCV serostatus.
The index date was the first day of dialysis in a DaVita facility. We used multivariable Cox regression to determine adjusted hazard ratios (aHRs) for mortality, with censoring on the date of transfer to a non-DaVita dialysis unit, kidney transplantation, or end of follow-up.
Time to wait-listing by HCV serostatus.
The index date was the first day of dialysis in a DaVita facility. We fit multivariable Cox regression models for the outcome of wait-listing for kidney transplantation. We used the Fine-Gray method26 to estimate cause-specific sub-distribution hazard ratios (sHRs) and treated death as a competing risk. Both models clustered on DSA. These analyses excluded individuals listed for multi-organ transplant or wait-listed before their first DaVita dialysis treatment. When an individual had multiple waitlist registrations during the observation period, we selected the earliest episode.
Time to kidney transplantation by HCV serostatus.
The index date was the day of addition to the transplant waitlist. We fit multivariable Cox models for time to transplantation, censoring for death, de-listing, or living donor transplantation. Additionally, we fit models using the Fine-Gray method26 that treated death as well as de-listing as competing risks for transplantation. Both models clustered on DSA.
Survival benefit from kidney transplantation versus dialysis among HCV-seropositive patients.
The index date was the day of addition to the transplant waitlist. We examined survival benefit from kidney transplantation only among HCV-seropositive patients using Cox regression with kidney transplantation as a time-updated covariate; we included an indicator variable for time post-transplantation.27 The hazard ratio for each time indicator variable represents the hazard for death during that period post-transplantaion versus remaining on the waiting list. We also examined whether the strategy of accepting a kidney from an HCV-seropositive donor provided a survival benefit compared to the strategy of remaining wait-listed and/or accepting a kidney from a donor without HCV infection.28,29 Patients were censored for de-listing.
For all multivariable models, variables were selected a priori for inclusion based on clinical relevance and prior studies.30–34 We confirmed the proportional hazards assumption by visual inspection of log-log plots and examination of Schoenfeld residuals. Variables not meeting the proportional hazards assumption were refit as categorical variables (hemoglobin, albumin, platelets) or with an interaction with time (waitlist year in survival benefit analyses). We generated Lowess plots for non-normally distributed continuous covariates (such as age) to identify appropriate cut-points; these variables were categorized based on identified cut-points.
Missing data.
For all models, we conducted a complete case analysis. All covariates were <5% missing, with the exception of BMI at the start of dialysis (14.2% missing); this covariate was only included in supplemental models restricted to hemodialysis patients. We used multiple imputation to address missing data in supplemental analyses (Item S1).
Sensitivity analyses.
In recognition of the fact that patients can spontaneously clear HCV infection, yet remain antibody-positive,35–37 we performed secondary analyses in which we randomly reassigned 30% of the HCV-seropositive cohort as HCV-seronegative. When sufficient laboratory data were available, we calculated the Fibrosis-4 (FIB-4) index, which is highly associated with severity of liver fibrosis (Item S1).38–40 We also fit models that accounted for patient acceptance of HCV-seropositive organs.
Results
Patient Characteristics
We identified 442,171 adult maintenance dialysis patients with a defined HCV serostatus (Figure S1), among whom 410,547 were HCV-seronegative and 31,624 were HCV-seropositive (Table 1). HCV-seropositive dialysis patients were younger (median 56 versus 64 years, P<0.001) and more likely to be male (65.9% versus 54.4%, P<0.001) and African American (54% versus 29.1%, <0.001) than HCV-seronegative patients. HCV-seropositive patients were twice as likely to have Medicaid as their primary insurance (7.4% versus 3.3%, P<0.001). The majority of the cohort received in-center hemodialysis, with central venous catheters most often used for vascular access. Median serum albumin and platelet counts were lower among HCV-seropositive versus HCV-negative patients, but differences were not clinically meaningful.
Table 1.
Baseline characteristics of the cohort
| All Dialysis Patients | All Wait-listed Patients | |||||
|---|---|---|---|---|---|---|
| HCV− (n=410,547) |
HCV+ (n=31,624) |
P value |
HCV− (n=31,944) |
HCV+ (n=2074) |
P value |
|
| Age, y | 64 (52–74) | 56 (50–63) | <0.001 | 53 (42–62) | 55 (49–60) | <0.001 |
| Male sex | 22,862 (54.4) | 20,847 (65.9) | <0.001 | 19,492 (61) | 1444 (69.6) | <0.001 |
| Race | <0.001 | <0.001 | ||||
| Caucasian | 195,687 (47.7) | 8732 (27.6) | 11,471 (35.9) | 517 (24.9) | ||
| African-American | 119,325 (29.1) | 17,092 (54) | 11,627 (36.4) | 1171 (56.5) | ||
| Asian | 14,548 (3.5) | 629 (1.9) | 2183 (6.8) | 59 (2.8) | ||
| Latino | 60,002 (14.6) | 3853 (12.2) | 5942 (18.6) | 290 (14) | ||
| Other | 18,547 (4.5) | 1161 (3.6) | 721 (2.3) | 37 (1.8) | ||
| Missing | 2438 (0.6) | 157 (0.7) | 0 | 0 | ||
| Cause of ESRD | <0.001 | <0.001 | ||||
| DM | 179,190 (43.6) | 12,601 (39.9) | 13,751 (43) | 803 (38.7) | ||
| HTN | 111,782 (27.2) | 10,175 (32.3) | 8509 (26.6) | 674 (32.5) | ||
| GN | 39,687 (9.7) | 3307 (10.4) | 4489 (14.1) | 262 (12.6) | ||
| PKD | 7215 (1.7) | 310 (0.9) | 909 (2.8) | 27 (1.3) | ||
| Other | 40,379 (9.8) | 2837 (8.9) | 2722 (8.5) | 185 (8.9) | ||
| Missing | 32,294 (8) | 2394 (7.6) | 1564 (4.9) | 123 (5.9) | ||
| Any preobservation dialysis | 348,637 (84.9) | 27,913 (88.3) | <0.001 | - | - | |
| Preobservation dialysis days | 11 (5–173) | 15 (6–465) | <0.001 | - | - | |
| CVD | 69,560 (16.9) | 4580 (14.5) | <0.001 | 3863 (12.1) | 238 (11.5) | 0.4 |
| DM | 262,680 (64) | 19,684 (62.2) | <0.001 | 19,617 (61.4) | 1255 (60.5) | 0.4 |
| Liver disease | 5003 (1.2) | 2440 (7.7) | <0.001 | 351 (1.1) | 150 (7.2) | <0.001 |
| COPD | 11,575 (2.8) | 817 (2.6) | <0.001 | 604 (1.9) | 35 (1.7) | 0.5 |
| BMI (kg/m2) | 27.2 (23.3–32.6) | 25.5 (22.2–30.1) | <0.001 | 28.3 924.4–32.8) | 26.9 (23.6–30.8) | <0.001 |
| Insurance | ||||||
| Medicare | 245,292 (59.7) | 18,093 (57.2) | <0.001 | 14,938 (46.7) | 1104 (53.2) | <0.001 |
| Medicaid | 13,378 (3.3) | 2326 (7.4) | 1051 (3.3) | 96 (4.6) | ||
| Private | 151,143 (36.8) | 11,145 (35.2) | 15,917 (49.9) | 874 (42.1) | ||
| Missing | 734 (0.2) | 60 (0.2) | 50 (0.1) | 1 (0.1) | ||
| Initial access type | ||||||
| AVF/AVG | 174,492 (42.5) | 14,684 (46.4) | <0.001 | 13,580 (42.5) | 1021 (49.2) | <0.001 |
| CVC | 197,872 (48.2) | 14,872 (47) | 13,507 (42.3) | 825 (39.8) | ||
| PD catheter | 31,366 (7.6) | 1543 (4.9) | 4367 (13.7) | 194 (9.4) | ||
| Other | 4539 (1.2) | 348 (1.1) | 0 | 0 | ||
| Missing | 2278 (0.5) | 177 (0.6) | 490 (1.5) | 34 (1.6) | ||
| Initial modality | ||||||
| HD | 381,142 (92.8) | 30,159 (95.3) | <0.001 | 28,056 (87.8) | 1878 (90.5) | 0.002 |
| Home HD | 1306 (0.3) | 86 (0.2) | 132 (0.4) | 10 (0.5) | ||
| Nocturnal | 575 (0.1) | 42 (0.1) | 90 (0.3) | 5 (0.2) | ||
| PD | 27,421 (6.7) | 1331 (4.3) | 3663 (11.5) | 181 (8.7) | ||
| Missing | 103 (0.1) | 6 (0.1) | ||||
| annual income (US$) | 47,625 (37,529–63,065) | 45,835 (35,717–60,157) | <0.001 | 48,697 (38,052–64,234) | 46,059 (35,134–60,230) | <0.001 |
| FIB-4 Index | ||||||
| Low | 136,378 (33.2) | 6650 (21) | <0.001 | 11,515 (36) | 225 (10.8) | <0.001 |
| Intermediate | 74,968 (18.2) | 5445 (17.2) | 2680 (8.3) | 128 (6.2) | ||
| High | 16,238 (3.9) | 2253 (7.1) | 213 (0.6) | 30 (1.4) | ||
| Missing | 182,963 (44.7) | 17,276 (54.7) | 17,536 (55.1) | 1691 (81.6) | ||
| Albumin (g/dL) | 3.6 (3.2–3.9) | 3.5 (3–3.9) | <0.001 | 4 (3.7–4.2) | 3.9 (3.6–4.1) | <0.001 |
| Hb (g/dL) | 10.4 (9.4–11.5) | 10.5 (9.4–11.7) | <0.001 | 11.3 (10.5–12.2) | 11.7 (10.8–12.5) | <0.001 |
| Platelets x103/mm3 | 233 (180–298) | 218 (162–286) | <0.001 | 232 (188–287) | 214 (168–269) | <0.001 |
| PRA>80% | - | - | - | 4669 (15) | 293 (14.5) | 0.8 |
| Blood group | 0.006 | |||||
| O | - | - | - | 16,760 (52.5) | 1076 (51.9) | |
| A | - | - | - | 9164 (28.7) | 538 (25.9) | |
| B | - | - | - | 5117 (16) | 385 (18.6) | |
| AB | - | - | - | 903 (2.8) | 75 (3.6) | |
Continuous data given as median [interquartile range]; categorical data as count (percentage).
Abbreviations: HCV−, Hepatitis C virus seronegative; HCV+, HCV seropositive; IQR – Interquartile range; ESRD - end-stage renal disease; DM – Diabetes mellitus; HTN – hypertension; GN – glomerulonephritis; PKD – polycystic kidney disease; CVD – cardiovascular disease; COPD – chronic obstructive pulmonary disease; BMI – body mass index; AVF – arteriovenous fistula; AVG – arteriovenous graft; CVC – central venous catheter; PD – peritoneal dialysis; FIB-4 – Fibrosis-4 Index; PRA – panel reactive antibody; Hb, hemoglobin
Mortality
HCV-seropositivity was associated with an increased risk of death on dialysis (aHR, 1.09; 95% CI, 1.07–1.11; Tables S1 and S2). Median follow-up was 652 (IQR, 232–1339) days. Results were similar in secondary analyses limited to hemodialysis patients or including the FIB-4 index (Tables S3 – S5). The most commonly reported causes of death were cardiovascular disease (33% HCV-seropositive vs. 35.8% HCV-seronegative) and infection (8.5% HCV-seropositive vs. 7.7% HCV-seronegative); patient death was attributed to liver disease in 3.3% of the HCV seropositive cohort, compared to 0.6% in seronegative patients (P<0.001).
Wait-listing
After exclusions, we analyzed 410,804 patients (29,263 HCV-seropositive; 381,541 HCV-negative). Median time to wait-listing was 354 (IQR, 173–716) days in the HCV-seronegative cohort and 473 (IQR, 222–970) days in the HCV-seropositive cohort (P<0.001).
HCV-seropositivity was associated with a lower likelihood of wait-listing for kidney transplant (aHR, 0.67; 95% CI, 0.61–0.74; Tables S6 and S2), which remained consistent in models that accounted for death as a competing risk for wait-listing (sHR 0.67; 95% CI, 0.61–0.74; Table 2 and Figure 1a) and in secondary analyses (Tables S7 – S9).
Table 2.
Association of HCV serostatus with the outcome of wait-listing for kidney transplantation, in a multivariable model with clustering on donor service area
| Characteristic | Death Censored (n=388,437) |
Death as a Competing Risk (n=388,437) |
||
|---|---|---|---|---|
| HCV-seropositive | 0.67 (0.61–0.74) | 0.67 | 0.61–0.74 | |
| Age | ||||
| <40 y | 1.00 (reference) | 1.00 (reference) | ||
| 40–70 y | 0.56 | 0.53–0.59 | 0.51 | 0.48–0.54 |
| >70 y | 0.06 | 0.05–0.07 | 0.05 | 0.04–0.06 |
| Male sex | 1.33 | 1.30–1.37 | 1.33 | 1.30–1.37 |
| Race | ||||
| Caucasian | 1.00 (reference) | ref | ref | |
| African American | 0.85 | 0.79–0.92 | 0.96 | 0.89–1.03 |
| Asian | 1.30 | 1.06–1.59 | 1.49 | 1.25–1.78 |
| Latino | 0.80 | 0.62–1.02 | 0.92 | 0.73–1.16 |
| Other | 1.01 | 0.90–1.13 | 1.13 | 1.01–1.26 |
| Dialysis Modality | ||||
| Hemodialysis | ref | ref | ref | ref |
| Home Hemodialysis | 1.35 | 1.16–1.56 | 1.39 | 1.17–1.64 |
| Nocturnal | 1.45 | 1.22–1.72 | 1.45 | 1.25–1.69 |
| Peritoneal dialysis | 1.71 | 1.59–1.84 | 1.78 | 1.66–1.92 |
| Insurance | ||||
| Medicare | ref | ref | ref | ref |
| Medicaid | 0.75 | 0.69–0.82 | 0.73 | 0.67–0.80 |
| Private | 1.49 | 1.32–1.70 | 1.49 | 1.32–1.68 |
| Cardiovascular disease | 0.79 | 0.75–0.83 | 0.78 | 0.74–0.82 |
| Diabetes mellitus | 0.78 | 0.74–0.82 | 0.77 | 0.73–0.81 |
| Liver disease | 1.00 | 0.90–1.11 | 0.94 | 0.84–1.05 |
| COPD | 0.46 | 0.42–0.52 | 0.43 | 0.38–0.48 |
| Income quintile | ||||
| $8,993–35,363 | ref | ref | ref | ref |
| $35,370–43,265 | 1.09 | 1.01–1.17 | 1.08 | 0.99–1.16 |
| $43,331–52,143 | 1.18 | 1.04–1.33 | 1.16 | 1.04–1.31 |
| $52,173–67,316 | 1.31 | 1.18–1.45 | 1.31 | 1.19–1.44 |
| $67,331–180,120 | 1.45 | 1.27–1.66 | 1.45 | 1.27–1.66 |
| Albumin < 3.5 g/dl | 0.86 | 0.84–0.89 | 0.75 | 0.73–0.78 |
| Hemoglobin | ||||
| <10 g/dL | 1.35 | 1.29–1.41 | 1.27 | 1.22–1.33 |
| 10–12 g/dL | 1.16 | 1.13–1.20 | 1.11 | 1.08–1.14 |
| >12 g/dl | ref | ref | ref | ref |
| Platelets <150 × 103/mm3 | 0.79 | 0.77–0.83 | 0.69 | 0.66–0.72 |
Values shown are adjusted hazard ratio (95% confidence interval).
Abbreviations: COPD – chronic obstructive pulmonary disease; BMI – body mass index; AVF – arteriovenous fistula; AVG – arteriovenous graft; CVC – central venous catheter; PD – peritoneal dialysis; PRA – panel reactive antibody
Figure 1a and 1b.
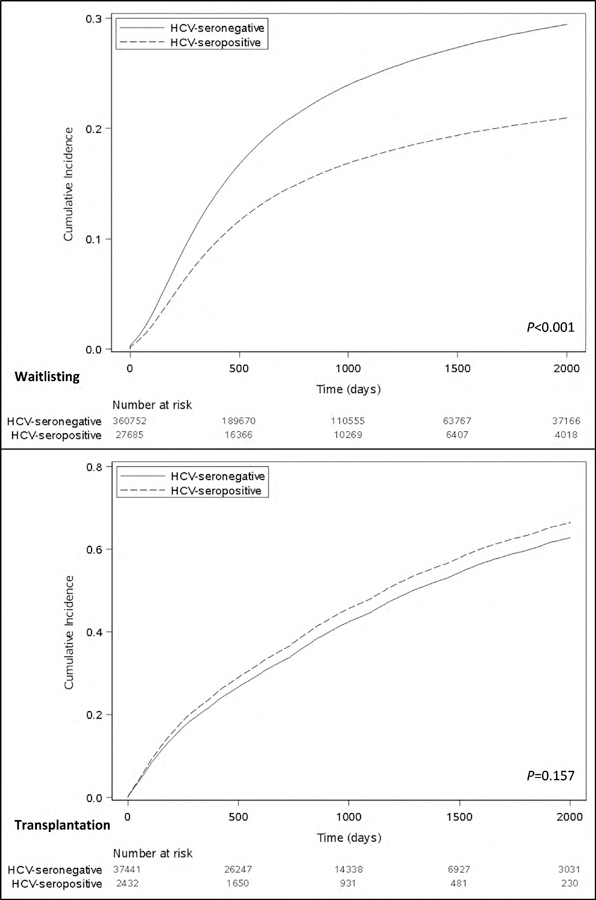
Cumulative incidence of a) waitlisting and b) transplantation, treating death as a competing risk in each case.
Kidney transplantation
Of the 51,625 patients wait-listed, 16,490 HCV-seronegative and 1117 HCV-seropositive patients underwent kidney transplantation. Overall time to transplantation was not significantly different between the groups (507 days [IQR, 178–1010] for HCV-seronegative; 433 days [IQR, 134–920] for HCV-seropositive; p=0.629), but was significantly shorter for recipients of HCV-seropositive kidneys (251 [IQR, 82–604] days, P<0.001). In unadjusted and adjusted analyses, patient HCV-seropositivity was not significantly associated with the rate of transplantation (aHR, 1.11; 95% CI, 0.97–1.28; Tables S10 and S3). Results were consistent in secondary analyses (Tables S11 – S14) and when death or delisting were considered as competing risks. The most common reported causes of death among wait-listed and transplanted patients, independent of HCV serostatus, were cardiovascular disease, infection, and malignancy. Depleting induction was more common in the HCV-seronegative group (77% vs. 73.4%). The majority of patients overall were discharged on a tacrolimus-based immunosuppression.
Transplantation survival benefit
Among HCV-seropositive patients, kidney transplantation was associated with a decreased risk of death, compared to remaining on the waitlist, and this benefit was achieved by 9 months after transplantation (Figure 2a). By 3 years, the adjusted hazard of death associated with transplantation compared to remaining on the waiting list was 0.42 (95% CI 0.27–0.63; Table S15). Of the 1117 HCV-seropositive recipients, 394 received kidneys from HCV-seropositive donors and survival benefit with transplantation was independent of donor HCV serostatus (aHR for death at 3 years, 0.42 [95% CI 0.25–0.72] for kidneys from HCV-seronegative donors vs 0.52 [95% CI 0.30–0.93] for kidneys from HCV-seropositive donors; Figure 2b; Table S16).
Figure 2a and 2b.
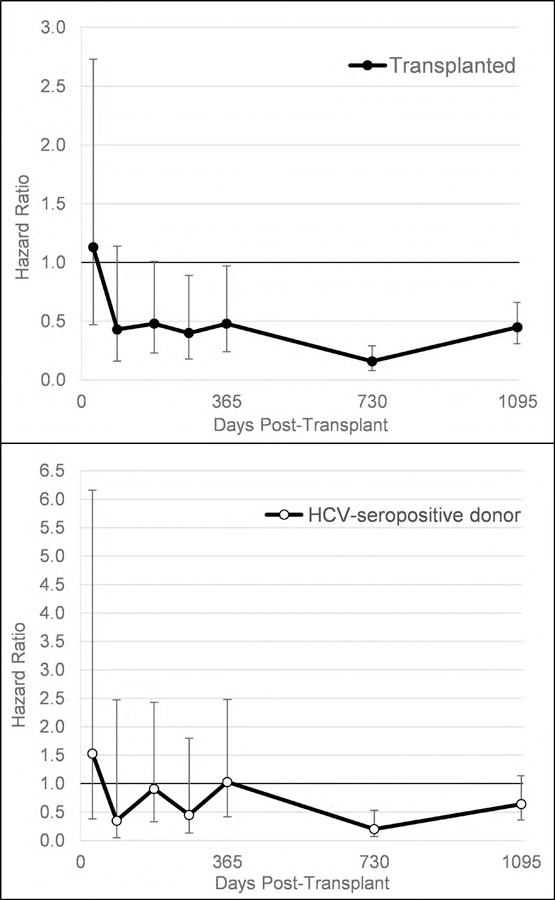
Hazard ratio for death among HCV-seropositive candidates associated with a) kidney transplantation compared to remaining on the waiting list, b) kidney transplantation with a HCV-seropositive kidney compared to the strategy of remaining on the waitlist and/or accepting a HCV-negative kidney*
* Models were time updated for transplantation and included age, gender, race, diabetes, cardiovascular disease, severe liver disease, insurance, income, panel reactive antibody, dialysis vintage, prior kidney transplant, body mass index, dialysis modality, year of waitlisting interacted with time, hemoglobin, platelets, and albumin.
To assess the strategy of accepting a HCV-seropositive kidney versus waiting for a HCV-seronegative kidney, we compared the survival benefit associated with accepting an HCV-seropositive donor versus outcomes for individuals who remained wait-listed and/or received an HCV-seronegative kidney. Accepting an HCV-seropositive donor organ provided a survival advantage at the 2-year post-transplantation compared to waiting for a kidney offer from a donor without HCV (Figure 2b).
Secondary analyses
Our results were confirmed in models using multiple imputation to account for missing data (Tables S17 – S19).
Discussion
In this national cohort, HCV-seropositive patients experienced a modest elevation in their risk of death on dialysis, but were 33% less likely to gain access to the kidney transplantation waiting list. However, among HCV-seropositive patients who were wait-listed, transplantation rates were similar to HCV-seronegative individuals, and kidney transplantation was associated with a lower risk of death compared to remaining wait-listed. For HCV-seropositive transplant candidates, accepting a kidney from an HCV-seropositive donor provided a survival advantage versus waiting for an uninfected organ.
HCV was only associated with a 9% increase in dialysis mortality. This finding is consistent with a contemporary report from the international Dialysis Outcomes and Practice Patterns Study (DOPPS) cohort,7 but lower in magnitude than the hazard of death reported in older studies of US dialysis populations;5,41 this difference may be due in part to improvements in dialysis care over time. In the DOPPS cohort, HCV-seropositive patients had only a modestly higher adjusted risk of hospitalization (HR, 1.09; 95% CI, 1.04–1.13) versus seronegative patients. However, this persistently elevated mortality for HCV-seropositive patients in our study and others should concern nephrologists, as these patients were younger and had fewer comorbid conditions than their HCV-seronegative counterparts, and would have been expected to have equivalent or better outcomes. Taken together, these findings highlight the need for interventions to improve the health of patients with HCV.
The fate of HCV-seropositive patients on the waitlist has been previously impossible to study on a national level due to limitations inherent in registry data. Our finding that HCV-seropositive patients in the US were much less likely to be wait-listed is a novel and troubling insight, especially since these patients were almost a decade younger than their HCV-seronegative counterparts. This finding is consistent with a study from France42 that demonstrated that patients with “hepatic disease” were less likely to be wait-listed for transplantation (aHR, 0.34), but the authors did not specify if this population was limited to patients with HCV or included others with Hepatitis B virus. The reasons for this disparity are unclear. It is possible that HCV-seropositive patients are less likely to be referred for transplantation or wait-listed because providers overestimate the deleterious health effects of HCV, assuming it will undermine the usual benefits of transplantation. Rigorous performance oversight for transplant centers may also cause physicians to be reluctant to waitlist patients considered “high risk,”43,44 such as those with HCV. At the patient level, the medical evaluation for transplant is complex. Patients with HCV have to contend with additional steps, including an assessment of liver fibrosis and potentially consideration of simultaneous liver-kidney transplantation.9 Importantly, HCV-seropositive patients in our cohort were more likely to be African-American and had lower median household income; thus, merely curing their HCV infection may be insufficient to reverse their diminished access to transplantation. Candidates from racial/ethnic minorities or who have limited financial resources face challenges completing the transplant evaluation process, and HCV infection may exacerbate this disparity.45,46 These findings should motivate efforts to identify and overcome the sources of these disparities in transplant access. HCV-seropositive patients may benefit from enhanced support in navigating the medical workup. Providers may also need education about post-transplantation outcomes for HCV-seropositive patients.
Despite the challenges in getting wait-listed, our study provides evidence for the survival benefit associated with kidney transplantation for HCV-seropositive individuals. Studies from the US and Canada47–50 have reported a survival benefit beyond 6 months post-transplantation for HCV-seropositive patients, but these were limited by the relatively small number of patients, dependence on single center/organ procurement organization data and an inability to address the impact of HCV-infected donors. Our study extends their work by assessing outcomes on a national level and specifically addressing the contribution of donor HCV serology to outcomes. Demonstration of a survival benefit with transplantation for HCV-seropositive recipients is important in light of the decreased access to transplantation that they currently face; these results should motivate efforts to facilitate their listing.
Previous studies of the impact of donor HCV status on transplant outcomes have yielded conflicting results; registry data51–54 suggest a negative impact on patient and allograft survival, while single and multi-center reports55,9 found no differences. This study provides new evidence that transplantation with kidneys from HCV-infected donors is advantageous to HCV-seropositive patients.51 Recipients of HCV-seropositive donor kidneys experienced shorter waiting times, and use of these kidneys was associated with better survival than remaining on dialysis to wait for a seronegative kidney. While this finding is similar to what has been shown with expanded criteria, high Kidney Donor Profile Index, and kidneys from donors with diabetes, the decision that we studied -- acceptance of HCV-infected kidneys -- is crucial for patients with HCV.28,56,57 The opioid abuse epidemic has led to a dramatic increase in HCV-infected donors, and recent data suggest that outcomes using HCV-infected organs are excellent, yet not all centers transplant these organs.12,58,59 Our findings should encourage all centers to utilize organs from HCV-infected donors to maximize the benefit for their listed HCV-seropositive patients. Furthermore, in light of the availability of HCV treatment regimens that can be administered to patients with ESRD, these data provide a clear rationale for providers and patients to forego HCV treatment while on dialysis in order to preserve the option of accepting an HCV-infected organ and accelerating transplantation (provided that HCV-infected organs are readily available in that area).19,60
This study advances the understanding of outcomes for HCV-seropositive patients with ESRD by leveraging a dataset from a large dialysis provider providing care in all 50 states and with linkage to the transplant registry, permitting for an analysis of their outcomes from dialysis through waitlisting and transplantation. These data demonstrate a clear disparity in access to the waiting list and provide a compelling clinical rationale, rather than an economic argument, for patients to postpone HCV therapy in the hopes of sooner transplantation with an HCV-infected organ.19
Our study has limitations. We only had HCV-antibody data and lacked information on HCV viral loads; therefore, we were unable to assess which patients spontaneously cleared their HCV infection or were treated. We cannot assess if any HCV infections were nosocomially acquired. However, because interferon-based therapy for HCV has limited effectiveness in this population and direct-acting antivirals were only approved in the year 2013 and not widely adopted in ESRD populations by 2014, it is very unlikely many patients were treated.7 Sensitivity analyses that randomly reassigned 30% of the HCV-seropositive cohort to HCV-seronegative and revealed similar outcomes to the main analysis. We were also unable to assess which patients may have had HBV co-infection, but our dialysis mortality estimates were similar to those reported in studies adjusting for HBV.7 Another limitation is incomplete information regarding liver disease severity. Among individuals with sufficient data, we calculated the FIB-4 index, which predicts liver injury; results were consistent with the main analysis. Additionally, there are also center-level differences in the selection of patients for kidney-alone versus simultaneous liver-kidney transplantation; because we lack liver biopsy data we are unable to assess the specific impact of advanced liver disease on wait-listing rates, however inclusion of the FIB-4 index as a surrogate marker did not significantly alter our results. We excluded patients who were listed for simultaneous liver-kidney transplant as the selection practices for these patients are significantly different from kidney-alone transplant, which limits generalizability of our findings. We also acknowledge that decreased access to transplantation may be due to residual confounding – for example, some HCV-seropositive patients may have lower education or health literacy on average than other patients. We adjusted for socioeconomic status and insurance, but were unable to adjust for other factors such as medication nonadherence or substance abuse, which can jeopardize acceptance onto the transplant waitlist. Finally, as with any observational study, causation cannot be inferred.
In conclusion, this study provides important and comprehensive insights into outcomes for HCV-seropositive patients with ESRD on the spectrum from maintenance dialysis through kidney transplantation. HCV-seropositive patients are younger yet experience a slightly higher adjusted rate of death on dialysis. They have diminished access to the transplant waiting list, but those who are transplanted rapidly achieve a significant survival benefit. The survival benefit from kidney transplantation among HCV-seropositive patients suggests that removing barriers to wait-listing for this patient group should be a priority for providers. The benefit in accepting a HCV-infected organ over waiting for a HCV negative one should encourage patients to carefully consider post-transplantation HCV treatment.
Supplementary Material
Table 3.
Multivariable regression analysis of the association of HCV serostatus with the outcome of kidney transplantation, clustering on donor service area*
| Characteristic | Adjusted HR (95% CI) | |||||
|---|---|---|---|---|---|---|
| Death Censored N=39,873 |
Death as a Competing Risk N=39,873 |
Death or Delisting as a Competing Risk N=39,873 |
||||
| HCV-seropositive | 1.11 (0.97–1.28) | 1.10 | 0.96–1.27 | 1.12 | 0.99–1.28 | |
| Age≥40years | 0.83 | 0.78–0.88 | 0.77 | 0.73–0.82 | 0.82 | 0.77–0.87 |
| Male sex | 1.01 | 0.97–1.06 | 1.00 | 0.96–1.05 | 0.99 | 0.95–1.04 |
| Race | ||||||
| Caucasian | ref | ref | ref | |||
| African American | 0.61 | 0.55–0.66 | 0.66 | 0.61–0.72 | 0.71 | 0.66–0.77 |
| Latino | 0.66 | 0.54–0.80 | 0.73 | 0.61–0.88 | 0.80 | 0.67–0.95 |
| Asian | 0.51 | 0.38–0.68 | 0.56 | 0.43–0.75 | 0.61 | 0.46–0.81 |
| Other | 0.68 | 0.56–0.81 | 0.75 | 0.63–0.90 | 0.80 | 0.67–0.96 |
| BMI* | ||||||
| 18.5–30 kg/m2 | ref | ref | ref | |||
| <18.5 kg/m2 | 1.06 | 0.92–1.22 | 1.04 | 0.91–1.18 | 1.05 | 0.92–1.20 |
| 30–40 kg/m2 | 0.82 | 0.79–0.86 | 0.85 | 0.82–0.89 | 0.88 | 0.84–0.92 |
| >40 kg/m2 | 0.50 | 0.42–0.60 | 0.54 | 0.49–0.64 | 0.59 | 0.50–0.70 |
| PRA>80% | 0.74 | 0.66–0.83 | 0.73 | 0.65–0.82 | 0.73 | 0.65–0.82 |
| Blood group | ||||||
| O | ref | ref | ref | |||
| A | 1.69 | 1.60–1.79 | 0.64 | 1.55–1.73 | 1.57 | 1.49–0.66 |
| B | 1.07 | 1.00–1.13 | 1.05 | 1.00–1.11 | 1.04 | 0.99–1.10 |
| AB | 2.60 | 2.32–2.91 | 2.45 | 2.20–2.72 | 2.28 | 2.04–2.54 |
| Pre-WL dialysis, per 1-d longer | 1.00 | 1.00–1.00 | 1.00 | 1.00–1.00 | 1.00 | 1.00–1.00 |
| Diabetes mellitus | 0.80 | 0.76–0.84 | 0.74 | 0.71–0.78 | 0.72 | 0.68–0.76 |
| Liver disease | 0.98 | 0.84–1.14 | 0.97 | 0.83–1.14 | 0.96 | 0.83–1.12 |
| CVD | 0.91 | 0.84–0.99 | 0.89 | 0.82–0.96 | 0.88 | 0.83–1.12 |
| Income quintile ($US) | ||||||
| $8,993–35,363 | ref | ref | ref | |||
| $35,370–43,265 | 1.00 | 0.91–1.11 | 1.00 | 0.91–1.10 | 0.99 | 0.90–1.09 |
| $43,331–52,143 | 0.94 | 0.78–1.13 | 0.93 | 0.78–1.11 | 0.93 | 0.80–1.09 |
| $52,173–67,316 | 0.90 | 0.75–1.07 | 0.89 | 0.75–1.07 | 0.90 | 0.76–1.06 |
| $67,331–180,120 | 0.89 | 0.74–1.07 | 0.89 | 0.74–1.07 | 0.88 | 0.74–1.05 |
| Insurance | ||||||
| Medicare | ref | ref | ref | |||
| Medicaid | 0.99 | 0.87–1.13 | 1.02 | 0.89–1.15 | 1.04 | 0.92–1.18 |
| Private | 1.20 | 1.14–1.26 | 1.23 | 1.17–1.29 | 1.20 | 1.14–1.26 |
| Albumin <3.5 g/dL | 0.81 | 0.74–0.88 | 0.72 | 0.66–0.79 | 0.73 | 0.67–0.80 |
| Hemoglobin | ||||||
| <10 g/dL | 0.82 | 0.77–0.89 | 0.79 | 0.74–0.86 | 0.75 | 0.70–0.81 |
| 10–12 g/dL | 0.88 | 0.84–0.93 | 0.87 | 0.83–0.92 | 0.84 | 0.80–0.89 |
| >12 g/dL | ref | ref | ref | |||
| Platelets <150 × 103/mm3 | 0.99 | 0.93–1.04 | 0.94 | 0.89–1.00 | 0.95 | 0.89–1.00 |
Values shown are adjusted hazard ratio (95% confidence interval).
Body mass index from the day of wait-listing for kidney transplantation, as reported to the OPTN
Abbreviations: HR – Hazard ratio; CI – Confidence Interval; HCV – Hepatitis C virus; BMI – body mass index; PRA – panel reactive antibody; WL – Waitlist; CVD – cardiovascular disease; COPD – chronic obstructive pulmonary disease;
Acknowledgements:
We would like to thank Michael Rudnick, MD for expert advice and efforts on obtaining national dialysis data.
Support: National Institutes of Health (R21DK108045-01A1 to Reese/Sawinski; 1K23HL133843-02 to Cohen). The funders of this study had no role in the study design, data collection, data analysis, or interpretation, writing of the report or the decision to submit the report for publication.
Financial Disclosure: Dr. Sawinski has consulted for Merck related to therapies for HCV. Drs. Reese and Goldberg have received grant support from Merck to the University of Pennsylvania to study transplantation of HCV-infected kidneys to uninfected recipients, followed by antiviral therapy (the THINKER trial; Clinicaltrials.gov identifier NCT02743897). Dr. Bloom has participated on advisory boards for Merck and Abbvie, and has performed consulting work for Abbvie. The remaining authors declare that they have no relevant financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: The data reported here have been supplied by the United Network for Organ Sharing as the contractor for the Organ Procurement and Transplantation Network (OPTN). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the OPTN or the US government.
Prior Presentation: Results from this work were presented as an abstract at the 2018 American Transplant Congress in Seattle, Washington.
Peer Review: Received ________. Evaluated by 3 external peer reviewers and a statistician, with editorial input from an Acting Editor-in-Chief (Editorial Board Member Lesley A. Inker, MD, MS). Accepted in revised form November 20, 2018. The involvement of an Acting Editor-in-Chief to handle the peer-review and decision-making processes was to comply with AJKD’s procedures for potential conflicts of interest for editors, described in the Information for Authors & Journal Policies.
References
- 1.Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med 2014;160(5):293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fabrizi F, Bunnapradist S, Lunghi G, Aucella F, Martin P. Epidemiology and clinical significance of hepatotropic infections in dialysis patients. Recent evidence. Minerva urologica e nefrologica = The Italian journal of urology and nephrology 2004;56(3):249–257. [PubMed] [Google Scholar]
- 3.Schneeberger PM, Keur I, van Loon AM, et al. The prevalence and incidence of hepatitis C virus infections among dialysis patients in the Netherlands: a nationwide prospective study. The Journal of infectious diseases 2000;182(5):1291–1299. [DOI] [PubMed] [Google Scholar]
- 4.Saab S, Martin P, Brezina M, Gitnick G, Yee HF Jr., Serum alanine aminotransferase in hepatitis c screening of patients on hemodialysis. American journal of kidney diseases : the official journal of the National Kidney Foundation 2001;37(2):308–315. [DOI] [PubMed] [Google Scholar]
- 5.Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, et al. Hepatitis C virus and death risk in hemodialysis patients. J Am Soc Nephrol 2007;18(5):1584–1593. [DOI] [PubMed] [Google Scholar]
- 6.Fabrizi F, Dixit V, Messa P. Impact of hepatitis C on survival in dialysis patients: a link with cardiovascular mortality? Journal of viral hepatitis 2012;19(9):601–607. [DOI] [PubMed] [Google Scholar]
- 7.Goodkin DA, Bieber B, Jadoul M, Martin P, Kanda E, Pisoni RL. Mortality, Hospitalization, and Quality of Life among Patients with Hepatitis C Infection on Hemodialysis. Clin J Am Soc Nephrol 2017;12(2):287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espinosa M, Martin-Malo A, Alvarez de Lara MA, Aljama P. Risk of death and liver cirrhosis in anti-HCV-positive long-term haemodialysis patients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 2001;16(8):1669–1674. [DOI] [PubMed] [Google Scholar]
- 9.Sawinski D, Murphy B. End-stage renal disease and kidney transplant in HIV-infected patients. Semin Nephrol 2008;28(6):581–584. [DOI] [PubMed] [Google Scholar]
- 10.Scalea JR, Barth RN, Munivenkatappa R, et al. Shorter waitlist times and improved graft survivals are observed in patients who accept hepatitis C virus+ renal allografts. Transplantation 2015;99(6):1192–1196. [DOI] [PubMed] [Google Scholar]
- 11.Kucirka LM, Singer AL, Ros RL, Montgomery RA, Dagher NN, Segev DL. Underutilization of Hepatitis C-Positive Kidneys for Hepatitis C-Positive Recipients. American Journal of Transplantation 2010;10(5):1238–1246. [DOI] [PubMed] [Google Scholar]
- 12.Sawinski D Where have all the (HCV-positive) kidneys gone? Am J Transplant 2018. [DOI] [PubMed]
- 13.Sawinski D, Forde KA, Eddinger K, et al. Superior outcomes in HIV-positive kidney transplant patients compared with HCV-infected or HIV/HCV-coinfected recipients. Kidney Int 2015;88(2):341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fabrizi F, Martin P, Dixit V, Bunnapradist S, Dulai G. Hepatitis C virus antibody status and survival after renal transplantation: meta-analysis of observational studies. Am J Transplant 2005;5(6):1452–1461. [DOI] [PubMed] [Google Scholar]
- 15.Bloom RD, Rao V, Weng F, Grossman RA, Cohen D, Mange KC. Association of hepatitis C with posttransplant diabetes in renal transplant patients on tacrolimus. J Am Soc Nephrol 2002;13(5):1374–1380. [DOI] [PubMed] [Google Scholar]
- 16.Gentil Govantes MA, Esforzado N, Cruzado JM, et al. Harmful effects of viral replication in seropositive hepatitis C virus renal transplant recipients. Transplantation 2012;94(11):1131–1137. [DOI] [PubMed] [Google Scholar]
- 17.Cruzado JM, Carrera M, Torras J, Grinyo JM. Hepatitis C virus infection and de novo glomerular lesions in renal allografts. Am J Transplant 2001;1(2):171–178. [PubMed] [Google Scholar]
- 18.Axelrod DA, Schnitzler MA, Alhamad T, et al. The impact of direct-acting antiviral agents on liver and kidney transplant costs and outcomes. American Journal of Transplantation 2018;18(10):2473–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shelton BA, Sawinski D, Linas BP, et al. Population level outcomes and cost-effectiveness of hepatitis C treatment pre-vs postkidney transplantation. American Journal of Transplantation 2018;18(10):2483–2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathew A, Obi Y, Rhee CM, et al. Treatment frequency and mortality among incident hemodialysis patients in the United States comparing incremental with standard and more frequent dialysis. Kidney Int 2016;90(5):1071–1079. [DOI] [PubMed] [Google Scholar]
- 21.Sawinski D, Forde KA, Locke JE, et al. Race but not Hepatitis C co-infection affects survival of HIV(+) individuals on dialysis in contemporary practice. Kidney Int 2017. [DOI] [PubMed]
- 22.Massie AB, Kucirka LM, Segev DL. Big data in organ transplantation: registries and administrative claims. Am J Transplant 2014;14(8):1723–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silber JH, Romano PS, Rosen AK, Wang Y, Even-Shoshan O, Volpp KG. Failure-to-rescue: comparing definitions to measure quality of care. Med Care 2007;45(10):918–925. [DOI] [PubMed] [Google Scholar]
- 24.Reese PP, Bloom RD, Feldman HI, et al. Mortality and cardiovascular disease among older live kidney donors. Am J Transplant 2014;14(8):1853–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kramer JR, Davila JA, Miller ED, Richardson P, Giordano TP, El-Serag HB. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Alimentary pharmacology & therapeutics 2008;27(3):274–282. [DOI] [PubMed] [Google Scholar]
- 26.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association 1999;94(446):496–509. [Google Scholar]
- 27.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. The New England journal of medicine 1999;341(23):1725–1730. [DOI] [PubMed] [Google Scholar]
- 28.Merion RM, Ashby VB, Wolfe RA, et al. Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA 2005;294(21):2726–2733. [DOI] [PubMed] [Google Scholar]
- 29.Reese PP, Feldman HI, Asch DA, et al. Transplantation of kidneys from donors at increased risk for blood-borne viral infection: recipient outcomes and patterns of organ use. Am J Transplant 2009;9(10):2338–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perl JWR, McFarlane P, et al. Hemodialysis vascular access modifies the association between dialysis modality and survival. J Am Soc Nephrol 2011;22:1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abbott KCGC, Trespalacios FC, et al. Body mass index, dialysis modality, and survival: analysis of the United States Renal Data System Dialysis Morbidity and Mortality Wave II. Study. Kidney Int 2004;65:597–605. [DOI] [PubMed] [Google Scholar]
- 32.McDonald SPMM, Johnson DW, et al. Relationship between dialysis modality and mortality. J Am Soc Nephrol 2009;20:155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kucirka LMGM, Lessler J, et al. Association of race and age with survival among patients undergoing dialysis. JAMA 2011;306:620–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kimmel PL, Fwu CW, Eggers PW. Segregation, income disparities, and survival in hemodialysis patients. J Am Soc Nephrol 2013;24(2):293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gerlach JT, Diepolder HM, Zachoval R, et al. Acute hepatitis C: high rate of both spontaneous and treatment-induced viral clearance. Gastroenterology 2003;125(1):80–88. [DOI] [PubMed] [Google Scholar]
- 36.Grebely J, Raffa JD, Lai C, Krajden M, Conway B, Tyndall MW. Factors associated with spontaneous clearance of hepatitis C virus among illicit drug users. Can J Gastroenterol 2007;21(7):447–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas DL, Astemborski J, Rai RM, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA 2000;284(4):450–456. [DOI] [PubMed] [Google Scholar]
- 38.Butt AARY, Lo Re V 3rd, Taddei TH, Kaplan DE. Comparing Child-Pugh, MELD, and FIB-4 to Predict Clinical Outcomes in Hepatitis C Virus-Infected Persons: Results From ERCHIVES. Clin Infect Dis 2017;65(1):64–72. [DOI] [PubMed] [Google Scholar]
- 39.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43(6):1317–1325. [DOI] [PubMed] [Google Scholar]
- 40.Cheng B-C, Yen Y-H, Chen J-F, et al. Transient elastography as a screening tool for liver fibrosis in a large hemodialysis population. Scientific reports 2017;7:46458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stehman-Breen CO, Emerson S, Gretch D, Johnson RJ. Risk of death among chronic dialysis patients infected with hepatitis C virus. Am J Kidney Dis 1998;32(4):629–634. [DOI] [PubMed] [Google Scholar]
- 42.Bayat S, Macher MA, Couchoud C, et al. Individual and regional factors of access to the renal transplant waiting list in france in a cohort of dialyzed patients. Am J Transplant 2015;15(4):1050–1060. [DOI] [PubMed] [Google Scholar]
- 43.Schold JD, Buccini LD, Srinivas TR, et al. The association of center performance evaluations and kidney transplant volume in the United States. Am J Transplant 2013;13(1):67–75. [DOI] [PubMed] [Google Scholar]
- 44.Schold JD, Arrington CJ, Levine G. Significant alterations in reported clinical practice associated with increased oversight of organ transplant center performance. Prog Transplant 2010;20(3):279–287. [DOI] [PubMed] [Google Scholar]
- 45.Epstein AM, Ayanian JZ, Keogh JH, et al. Racial disparities in access to renal transplantation--clinically appropriate or due to underuse or overuse? The New England journal of medicine 2000;343(21):1537–1544, 1532 p preceding 1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alexander GC, Sehgal AR. Barriers to cadaveric renal transplantation among blacks, women, and the poor. JAMA 1998;280(13):1148–1152. [DOI] [PubMed] [Google Scholar]
- 47.Bloom RD, Sayer G, Fa K, Constantinescu S, Abt P, Reddy KR. Outcome of hepatitis C virus-infected kidney transplant candidates who remain on the waiting list. Am J Transplant 2005;5(1):139–144. [DOI] [PubMed] [Google Scholar]
- 48.Knoll GA, Tankersley MR, Lee JY, Julian BA, Curtis JJ. The impact of renal transplantation on survival in hepatitis C-positive end-stage renal disease patients. Am J Kidney Dis 1997;29(4):608–614. [DOI] [PubMed] [Google Scholar]
- 49.Roth D, Gaynor JJ, Reddy KR, et al. Effect of kidney transplantation on outcomes among patients with hepatitis C. J Am Soc Nephrol 2011;22(6):1152–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pereira BJG, Levey AS. Hepatitis C virus infection in dialysis and renal transplantation. Kidney international 1997;51(4):981–999. [DOI] [PubMed] [Google Scholar]
- 51.Abbott KC, Lentine KL, Bucci JR, Agodoa LY, Peters TG, Schnitzler MA. The impact of transplantation with deceased donor hepatitis c-positive kidneys on survival in wait-listed long-term dialysis patients. Am J Transplant 2004;4(12):2032–2037. [DOI] [PubMed] [Google Scholar]
- 52.Bucci JRMC, Swanson SJ et al. Donor hepatitis C seropositivity: clinical correlates and effect on early graft and patient survival in adult cadaveric kidney transplantation. J Am Soc Nephrol 2002;13:2974–2982. [DOI] [PubMed] [Google Scholar]
- 53.Maluf DGAK, Mas VR. Kidney grafts from HCVpositive donors: advantages and disadvantages. Transplant Proc 2010;42:2436–2446. [DOI] [PubMed] [Google Scholar]
- 54.Cohen JB, Eddinger KC, Shelton B, Locke JE, Forde KA, Sawinski D. Effect of kidney donor hepatitis C virus serostatus on renal transplant recipient and allograft outcomes. Clin Kidney J 2017;10(4):564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morales JMCJ, Dominguez-Gil B et al. Long-term experience with kidney transplantation from hepatitis C positive donors into hepatitis C positive recipients. Am J Transplant 2010;10:2453–2462 [DOI] [PubMed] [Google Scholar]
- 56.Massie AB, Luo X, Chow EK, Alejo JL, Desai NM, Segev DL. Survival benefit of primary deceased donor transplantation with high-KDPI kidneys. Am J Transplant 2014;14(10):2310–2316. [DOI] [PubMed] [Google Scholar]
- 57.Cohen JB, Eddinger KC, Locke JE, Forde KA, Reese PP, Sawinski DL. Survival Benefit of Transplantation with a Deceased Diabetic Donor Kidney Compared with Remaining on the Waitlist. Clin J Am Soc Nephrol 2017;12(6):974–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goldberg DS, Blumberg E, McCauley M, Abt P, Levine M. Improving Organ Utilization to Help Overcome the Tragedies of the Opioid Epidemic. Am J Transplant 2016;16(10):2836–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reese PP, Abt PL, Blumberg EA, et al. Twelve-Month Outcomes After Transplant of Hepatitis C-Infected Kidneys Into Uninfected Recipients: A Single-Group Trial. Ann Intern Med 2018. [DOI] [PubMed]
- 60.Levitsky J, Formica RN, Bloom RD, et al. The American Society of Transplantation Consensus Conference on the Use of Hepatitis C Viremic Donors in Solid Organ Transplantation. Am J Transplant 2017;17(11):2790–2802. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


