Abstract
Loss-of-function mutations in the ABCC6 gene cause pseudoxanthoma elasticum (PXE) and type 2 generalized arterial calcification of infancy (GACI), heritable ectopic mineralization disorders without effective treatment. ABCC6 encodes the putative efflux transporter ABCC6 which is predominantly expressed in the liver. While the substrate of ABCC6 remains unknown, recent studies demonstrated that PXE is a metabolic disorder caused by reduced circulating levels of pyrophosphate (PPi), a potent mineralization inhibitor. Here, we hypothesized that reconstitution of ABCC6 might counteract ectopic mineralization in an Abcc6−/− mouse model of PXE. Intravenous administration of a recombinant adenovirus expressing wild type human ABCC6 in Abcc6−/− mice showed sustained high-level expression of human ABCC6 in the liver for up to 4 weeks, increasing PPi levels in plasma. In addition, adenovirus injection every 4 weeks restored plasma PPi levels, and consequently, significantly reduced ectopic mineralization in the skin of young mice. By contrast, the same treatment in old mice with already established mineral deposits failed to reduce mineralization. These results suggest that adenovirus-mediated ABCC6 gene delivery, when initiated early, is a promising prevention therapy for PXE and GACI, diseases that currently lack preventive or therapeutic options.
Keywords: Pseudoxanthoma elasticum, ectopic mineralization, mouse model, ABC transporter, adenovirus, gene therapy
INTRODUCTION
Ectopic mineralization, aberrant deposition of calcium hydroxyapatite in soft connective tissues, is a leading cause of morbidity and mortality (Giachelli, 1999). Pseudoxanthoma elasticum (PXE) is one example of heritable multisystem ectopic mineralization disorders with protean manifestations in the skin, eyes, and cardiovascular system (Neldner, 1988; Uitto et al., 2017). Early cutaneous findings are yellowish papules on the predilection sites which coalesce into lax and inelastic skin. The skin findings signify the development of serious, debilitating and occasionally life-threatening complications in the eyes and cardiovascular system caused by ectopic mineralization in these tissues. PXE, affecting 1 in every 50,000 person worldwide, is a late-onset and slowly-progressive disease for which there is presently no specific treatment.
In most cases, PXE is caused by the loss-of-function mutations in the ABCC6 gene (Bergen et al., 2000; Le Saux et al., 2000; Ringpfeil et al., 2000). ABCC6 mutations can also cause type 2 generalized arterial calcification of infancy (GACI) (Nitschke et al., 2012; Li et al., 2014), a severe early-onset vascular mineralization disorder that affects the fetus in utero or in infancy. The ABCC6 gene encodes ABCC6, a member in the ATP-binding cassette (ABC) transporter superfamily. Based on sequence homology with ABCC1, ABCC6 is thought to be a transmembrane efflux transporter, however, its substrate remains unknown. ABCC6 is predominantly expressed in hepatocytes and, to a lesser extent, in proximal tubule cells in the kidneys, but not in cells at sites of mineralization in the skin, eyes and vasculature (Belinsky and Kruh, 1999; Scheffer et al., 2002). This observation favors the metabolic hypothesis of PXE which postulates that absence of ABCC6 transporter activity in the liver, the primary site of ABCC6 expression, results in deficiency of circulating factor(s) that are physiologically required to prevent ectopic mineralization in remote tissues (Uitto et al., 2017).
It was recently shown that ABCC6 mediates ATP release from hepatocytes to extracellular space where ATP is converted by pyrophosphatase/phosphodiesterase ENPP1 to AMP and inorganic pyrophosphate (PPi), the latter being a principal inhibitor of ectopic mineralization (Jansen et al., 2013; Jansen et al., 2014). The role of ABCC6-mediated ATP release was supported by reduced levels of PPi in plasma of Abcc6 knockout mice and rats as well as in patients with PXE (Jansen et al., 2014; Li et al., 2018; Li et al., 2017). The results suggested that absence of ATP release in an ABCC6-dependent manner and reduced plasma PPi levels are critical pathogenic features of PXE and type 2 GACI as a result of defective ABCC6 transporter activity in the liver (Uitto et al., 2017; Favre et al., 2017). These findings form the conceptual basis to develop therapeutics capable of counteracting ectopic mineralization by liver-specific ABCC6 gene therapy targeting PPi deficiency for treatment of PXE.
Human species C adenovirus serotype 5 (Ad5) is among the most frequently used gene delivery systems in animal and clinical studies which aim to correct genetic and acquired diseases (Lee et al., 2017). The extreme propensity of the virus for hepatocyte transduction after its intravascular delivery has made Ad5 the vector of choice for applications requiring high level and long-term transgene expression in hepatocytes in vivo (Thomas et al., 2003). To express human ABCC6 protein in mouse, a recombinant adenovirus was generated carrying human ABCC6 cDNA under the transcriptional control of a CMV promoter. The efficacy of adenovirus-mediated ABCC6 gene therapy to inhibit ectopic mineralization was tested in the Abcc6−/− mouse model of PXE in which mouse ABCC6 protein is not expressed (Klement et al., 2005). In this proof-of-concept preclinical study, two complementary approaches, prevention and reversal, were performed to determine whether the adenovirus vector provides sustained and titratable ABCC6 expression, and consequently, might counteract ectopic mineralization in the dermal sheath of vibrissae in muzzle skin, an early and reliable biomarker in Abcc6−/− mice (Klement et al., 2005). Our results demonstrated that adenovirus-mediated ABCC6 reconstitution prevented, but did not reverse, ectopic mineralization in the Abcc6−/− mice, suggesting that gene therapy should be considered in PXE and GACI patients with ABCC6 mutations at the early stage of diagnosis.
RESULTS
Adenoviral vector carrying human ABCC6 transgene resulted in dose-dependent expression in vitro
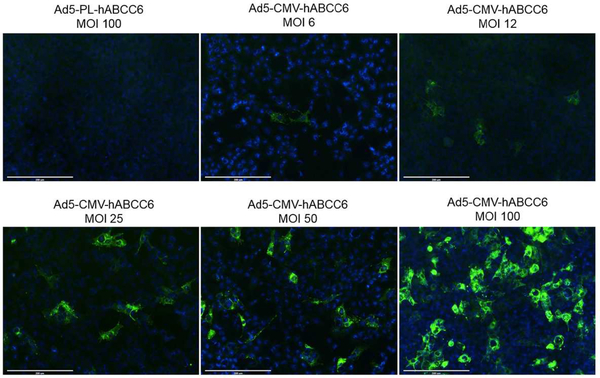
An adenovirus vector was generated carrying the wild type human ABCC6 cDNA under the control of a CMV promoter (Ad5-CMV-hABCC6). As a control, an adenovirus vector was generated carrying promoterless human ABCC6 cDNA (Ad5-PL-hABCC6). Mouse liver epithelial cells (HEPA) were transduced at different multiplicity of infections (MOI). Cells at 36 hours post transduction were immunostained with a human ABCC6-specific antibody. HEPA cells transduced with Ad5-CMV-hABCC6 vector resulted in dose-dependent expression of human ABCC6 while cells transduced with Ad5-PL-hABCC6 resulted in no expression of ABCC6 (Figure 1).
Figure 1. Recombinant adenovirus transduction demonstrated dose-dependent human ABCC6 expression in vitro.
Mouse liver epithelial cells were transduced with Ad5-CMV-hABCC6 or Ad5-PL-hABCC6 adenoviruses. Cells were stained with human ABCC6-specific antibody 36 hours after transduction (Green). Staining of cells transduced with Ad5-PL-hABCC6 virus did not reveal ABCC6 expression. In contrast, dose-dependent human ABCC6 expression was detected in cells transduced with Ad5-CMV-hABCC6 virus at 6 to 100 multiplicity of infection (MOI). Scale bar, 0.2 mm. Blue = DAPI.
Administration of adenovirus resulted in long-term expression in the liver of Abcc6−/−Rag1−/− mice
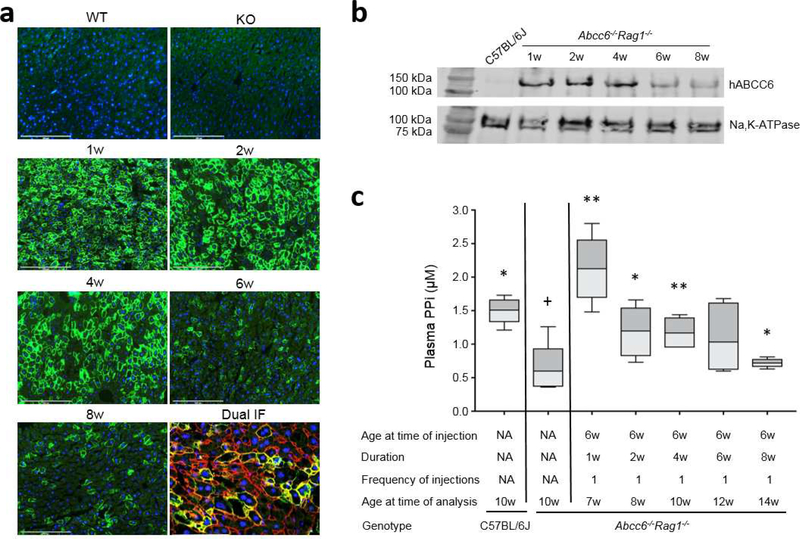
To prevent adaptive (antibody and T-cell) immune responses against the xenogeneic human ABCC6 protein, the Ad5-CMV-hABCC6 adenovirus was administered by tail vein injection to 6-week-old Abcc6−/− mice on Rag1−/− immunodeficient background (Jiang et al., 2009; Jiang et al., 2010). These mice were analyzed at 1, 2, 4, 6, and 8 weeks after a single injection of adenovirus at a dose of 4×108 IFU per mouse. Human ABCC6 expression in mouse liver was analyzed by immunostaining with an antibody recognizing human ABCC6. The results showed a temporal ABCC6 expression that peaked at 1 week and was sustained at peak levels for 4 weeks. The expression declined at 6 and 8 weeks after injection (Figure 2a). Dual immunostaining with antibodies against ABCC6 and Na, K-ATPase, a plasma membrane marker at the basolateral side of hepatocytes (Wolters et al., 1991), demonstrated that human ABCC6 was expressed in the basolateral plasma membrane of hepatocytes, the physiologic location of ABCC6 expression and function (Figure 2a). ABCC6 was expressed selectively in the liver, whereas no expression was observed in the kidneys and other tissues (data not shown).
Figure 2. Administration of recombinant adenovirus revealed temporal ABCC6 expression and increase of plasma PPi in Abcc6−/−Rag1−/− mice.
A total of 25 Abcc6−/−Rag1−/− mice were administered a single injection of 4×108 IFU of Ad5-CMV-hABCC6 at 6 weeks of age. The mice were analyzed at 1, 2, 4, 6, and 8 weeks after injection. (a) Immunofluorescent labeling of human ABCC6 revealed peak response at 1 week and sustained expression up to 4 weeks (Green). Dual immunofluorescent labeling with human ABCC6 (Green) and Na,K-ATPase (Red) revealed co-localization in the basolateral plasma membrane of hepatocytes. Expression was not noted in the wild type C57BL/6J (WT) and Abcc6−/−Rag1−/− (KO) mice for human ABCC6 protein. Scale bar, 0.2 mm. Blue = DAPI. (b) Quantitation of ABCC6 protein in mouse liver by Western blot. A plasma member marker, Na,K-ATPase, served as internal control. Note negative expression of human ABCC6 in the liver of wild type C57BL/6J mice. (c) Administration of Ad5-CMV-hABCC6 resulted in increased plasma PPi at 1 week which persisted up to 6 weeks. n = 4 – 6 mice per group. *p < 0.05, **p < 0.01, compared to Abcc6−/−Rag1−/− control mice. +p < 0.05, ++p < 0.01, compared to C57BL/6J control mice.
To further quantify the expression of human ABCC6 at the protein level, Western blot was performed with proteins extracted from the liver of these mice. The Abcc6−/−Rag1−/− mice receiving Ad5-CMV-hABCC6 demonstrated high levels of ABCC6 expression that persisted up to 4 weeks then reduced at 6 and 8 weeks after administration which correlated with immunofluorescence of liver tissues from these mice (Figure 2a, b).
To explore the potency of reconstituted ABCC6, plasma levels of PPi, an indicator of ABCC6 function and biomarker of PXE, were quantified in these mice. Consistent with previous results (Jansen et al., 2014; Li et al., 2018), the Abcc6−/−Rag1−/− mice had approximately 30% plasma PPi levels of that in wild type mice (Figure 2c). Plasma PPi levels at 1 week after Ad5-CMV-hABCC6 administration exceeded that of wild type mice, and remained at high levels, similar to that in wild type mice, up to 6 weeks after administration. Plasma PPi levels at 8 weeks were still significantly higher than that of the Abcc6−/−Rag1−/− mice (Figure 2c).
Administration of adenovirus optimized dose for raising plasma PPi levels in Abcc6−/−Rag1−/− mice
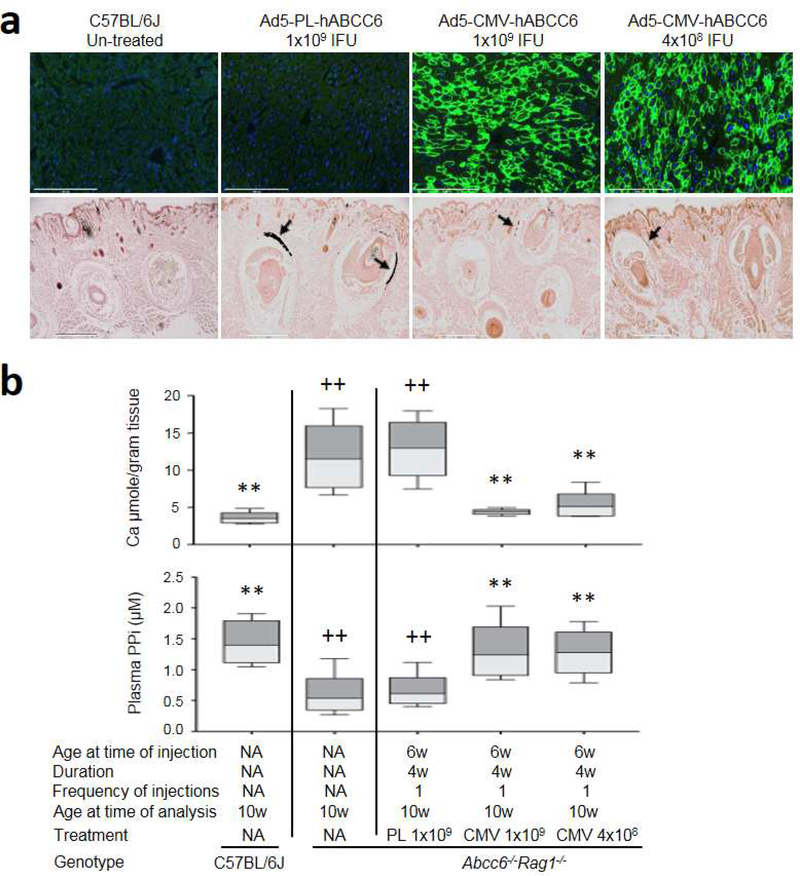
Two different dose levels of adenovirus, 4×108 and 1×109 IFU per mouse, were administered intravenously to Abcc6−/−Rag1−/− mice, initiated at 6 weeks of age, a time point at the earliest stages of ectopic mineralization in the dermal sheath of vibrissae. Mice were analyzed 4 weeks after injection. The level of human ABCC6 expression determined by immunofluorescent staining remained high and indistinguishable between the two doses of Ad5-CMV-hABCC6 adenovirus (Figure 3a, upper panel). As expected, ABCC6 expression was not found in mice injected with Ad5-PL-hABCC6 (Figure 3a, upper panel). As a result of adenovirus treatment, plasma PPi levels in the Abcc6−/−Rag1−/− mice increased significantly, similar to those of the wild type mice (Figure 3b, lower panel).
Figure 3. Administration of recombinant adenovirus optimized dose for therapy in Abcc6−/−Rag1−/− mice.
Abcc6−/−Rag1−/− mice at 6 weeks of age received Ad5-CMV-hABCC6 or Ad5-PL-hABCC6 at 4×108 or 1×109 IFU. 8 mice per group. The mice were analyzed 4 weeks after injection, at 10 weeks of age. (a) Upper panel: Administration of 4×108 and 1×109 IFU Ad5-CMV-hABCC6 per mouse did not reveal significant differences in ABCC6 expression (Green). Note negative expression in C57BL/6J un-treated mice and Abcc6−/−Rag1−/− mice receiving Ad5-PL-hABCC6. Scale bar, 0.2 mm. Blue = DAPI. Lower panel: Ectopic mineralization in the dermal sheath of vibrissae (arrows) was analyzed by histopathology with von Kossa stains. Abcc6−/− Rag1−/− mice treated with Ad5-PL-hABCC6 had similar levels of mineralization in the muzzle skin to that of Abcc6−/−Rag1−/− control mice. In contrast, administration of Ad5-CMV-hABCC6 at 4×108 or 1×109 IFU per mouse revealed only residual mineralization; Scale bar, 0.4 mm. (b) Treatment outcome measures. Upper panel: The chemical assay of calcium demonstrated significant reduction in the amount of calcium in the muzzle skin in the Abcc6−/−Rag1−/− mice treated with Ad5-CMV-hABCC6 at both doses; Lower panel: Increased plasma PPi levels in Abcc6−/−Rag1−/− mice treated with recombinant adenovirus. n = 8 mice per group. *p < 0.05, **p < 0.01, compared to Abcc6−/−Rag1−/− mice. +p < 0.05, ++p < 0.01, compared to C57BL/6J mice.
Single administration of recombinant adenovirus for 4 weeks of follow-up prevented ectopic mineralization in young Abcc6−/−Rag1−/− mice
To further examine whether restored plasma PPi levels after recombinant adenovirus therapy would prevent ectopic mineralization, the degree of mineralization of the dermal sheath of vibrissae in the muzzle skin, an early and reliable biomarker in the overall mineralization process in the Abcc6−/−Rag1−/− mice, was examined by two independent assays. One piece of muzzle skin was processed for histopathologic examinations. A mineralization-specific stain, von Kossa, revealed robust mineralization in the dermal sheath of vibrissae in the Abcc6−/−Rag1−/− mice administered with Ad5-PL-hABCC6. The Abcc6−/−Rag1−/− mice receiving Ad5-CMV-hABCC6, either at 4×108 or 1×109 IFU per mouse, showed significantly reduced mineralization in the muzzle skin 4 weeks after a single administration (Figure 3a, lower panel). Another piece of muzzle skin was biopsied and calcium levels were quantified. The results showed a significant reduction of more than 62% in the amount of calcium in the muzzle skin in the Abcc6−/−Rag1−/− mice with levels indistinguishable from those in wild type C57BL/6J mice negative for ectopic mineralization (Figure 3b, upper panel).
Single and multiple administrations of recombinant adenovirus demonstrated long-term prevention of ectopic mineralization in young Abcc6−/−Rag1−/− mice
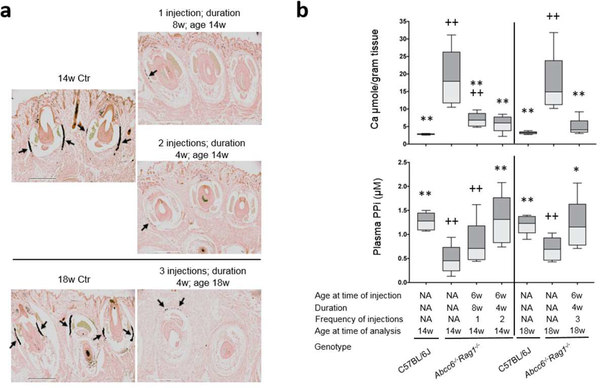
Based on the results that ABCC6 expression persisted up to 4 weeks after a single injection, restored plasma PPi levels, and attenuated ectopic mineralization in Abcc6−/−Rag1−/− mice (Figure 3), a prevention trial was conducted in 6-week-old Abcc6−/−Rag1−/− mice and these mice were analyzed 8 weeks after a single injection of adenovirus at 4×108 IFU per mouse. In addition, multiple injections of adenovirus 4 weeks apart were performed and these mice were analyzed 4 weeks after the last injection. All mice, irrespective of the frequency of injections and duration of treatment, demonstrated by histologic examination significant reduction in the amount of mineralization, as compared to their respective age-matched Abcc6−/−Rag1−/− control mice (Figure 4a). The therapeutic effect of adenovirus gene therapy was substantiated by the calcium content in muzzle skin biopsies measured using a chemical assay. The results confirmed a significant, over 62%, reduction in the amount of calcium in the Abcc6−/−Rag1−/− mice treated with adenovirus, as compared to the age-matched untreated Abcc6−/−Rag1−/− mice (Figure 4b, upper panel). The mice administered one injection but euthanized 8 weeks later still revealed significantly less mineralization than the age-matched Abcc6−/−Rag1−/− controls (Figure 4b, upper panel).
Figure 4. Single and multiple administrations of recombinant adenovirus prevented ectopic mineralization in young Abcc6−/−Rag1−/− mice.
A total of 32 Abcc6−/−Rag1−/− mice, 6 weeks of age, received Ad5-CMV-hABCC6 at a dose of 4×108 IFU per mouse. Ten mice were analyzed 8 weeks after injection, at 14 weeks of age. Twenty-two mice received 2 or 3 injections 4 weeks apart and analyzed at 14 and 18 weeks of age, respectively. (a) Muzzle skin biopsies were collected from Abcc6−/−Rag1−/− mice at 14 and 18 weeks of age and ectopic mineralization in the dermal sheath of vibrissae (arrows) was analyzed by histopathology with von Kossa stains. Regardless of the frequency of injection and duration of treatment, mice treated with Ad5-CMV-hABCC6 had only residual mineralization foci in the dermal sheath of vibrissae in the muzzle skin. Scale bar, 0.4 mm. (b) Treatment outcome measures. Upper panel: The chemical assay of calcium demonstrated significantly reduced calcium in the muzzle skin in the Abcc6−/−Rag1−/− mice treated with Ad5-CMV-hABCC6; Lower panel: Plasma PPi levels in Abcc6−/−Rag1−/− mice treated with Ad5-CMV-hABCC6 adenovirus 4 weeks after the last injection were significantly increased, with levels similar to that in age-matched wild type mice. n = 9 – 14 mice per group. *p < 0.05, **p < 0.01, compared to age-matched Abcc6−/−Rag1−/− mice. +p < 0.05, ++p < 0.01, compared to age-matched C57BL/6J control mice.
The plasma PPi levels in mice administered adenovirus every 4 weeks remained high similar to that of the age-matched wild type mice (Figure 4b, lower panel). The mice administered one injection but euthanized 8 weeks later revealed decreased levels of plasma PPi reflecting reduced ABCC6 expression in these mice. Of note, the slowly decrease of plasma PPi levels from 4 to 8 weeks after injection was still accompanied by significantly reduced mineralization in these mice, suggesting that ABCC6 expression had long-lasting effect on plasma PPi levels to prevent mineralization.
Single administration of recombinant adenovirus for 4 weeks of follow-up did not reverse ectopic mineralization in old Abcc6−/−Rag1−/− mice
Given the fact that PXE is a late-onset and slowly progressive disease, the fundamental question remains whether established mineralization in old mice could be reversed after ABCC6 reconstitution. In this regard, Abcc6−/−Rag1−/− mice were aged to 43 weeks, the time at which ectopic mineralization is extensive in the muzzle skin containing vibrissae. Ad5-CMV-hABCC6 and Ad5-PL-hABCC6 were administered at 4×108 IFU per mouse and all mice were euthanized 4 weeks later. Histopathology did not reveal differences in the amount of mineralization in the muzzle skin containing vibrissae in mice administered Ad5-CMV-hABCC6 or Ad5-PL-hABCC6 (Figure 5a). The histology observation was confirmed by the amount of calcium in the muzzle skin despite restoration of plasma PPi levels to those of age-matched wild type mice (Figure 5b). These results demonstrated that recombinant adenovirus therapy, for a total of 4 weeks of follow-up, did not reverse existing mineralization in old Abcc6−/−Rag1−/− mice.
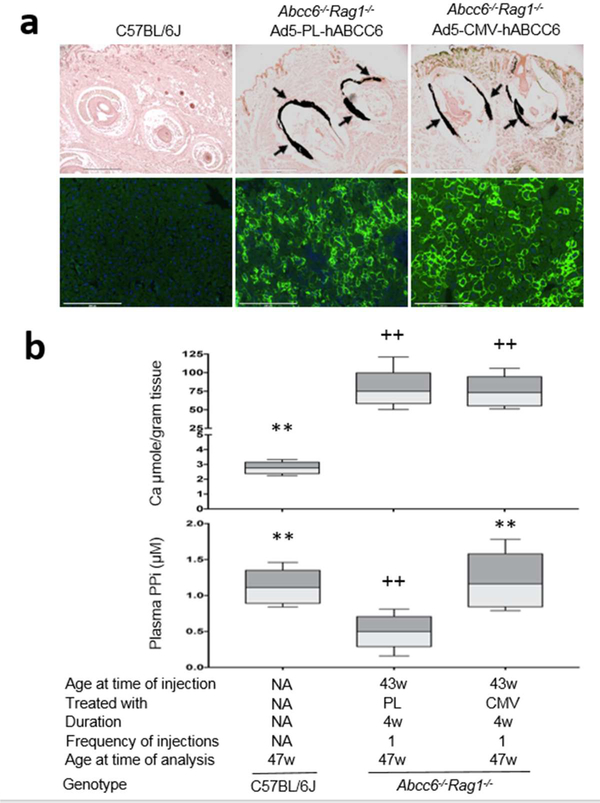
Figure 5. Administration of recombinant adenovirus did not reverse ectopic mineralization in old Abcc6−/−Rag1−/− mice.
A total of 10 Abcc6−/−Rag1−/− mice, 43 weeks of age, received Ad5-CMV-hABCC6 or Ad5-PL-hABCC6 at a dose of 4×108 IFU per mouse. The mice were analyzed at 47 weeks of age. (a) Upper panel: Administration of Ad5-CMV-hABCC6 revealed significant ABCC6 expression in the liver (Green). Scale bar, 0.2 mm. Blue = DAPI. Lower panel: Ectopic mineralization in the dermal sheath of vibrissae (arrows) was analyzed by histopathology with von Kossa stains. Abcc6−/−Rag1−/− mice receiving Ad5-PL-hABCC6 revealed robust mineralization in the muzzle skin. The Ad5-CMV-hABCC6-treated mice had similar levels of mineralization in the muzzle skin to those receiving Ad5-PL-hABCC6. Scale bar, 0.4 mm. (b) Treatment outcome measures. Upper panel: The chemical assay of calcium demonstrated similar calcium levels in the muzzle skin in Abcc6−/−Rag1−/− mice treated with Ad5-CMV-hABCC6 and Ad5-PL-hABCC6; Lower panel: Increased plasma PPi levels in Abcc6−/−Rag1−/− mice treated with Ad5-CMV-hABCC6. n = 8 mice per group. **p < 0.01, compared to Abcc6−/−Rag1−/− mice treated with Ad5-PL-hABCC6. ++p < 0.01, compared to age-matched C57BL/6J control mice.
DISCUSSION
The pathologic feature in PXE and type 2 GACI is reduced plasma PPi levels as a result of defective ABCC6 transporter activity in the liver (Uitto et al., 2017). These discoveries have now provided a platform to develop liver-targeted therapy for PXE using plasma PPi as a biomarker (Dedinszki et al., 2017). Previously, transduction of hepatocytes by hydrodynamic tail vein injection with plasmid DNA provided transient, up to several days only, and low level expression of ABCC6 in mouse liver (Jin et al., 2015; Pomozi et al., 2014). To achieve long-term human ABCC6 expression and stable increase of PPi levels in plasma, we took advantage of adenovirus as highly efficient delivery and expression vehicle. Compared with other viral mediated gene therapy systems, adenoviral vector attracts tremendous attention due to its well-defined biology, genetic stability, high transduction efficiency, ability to accommodate large transgenes, ease of large-scale production, and its supreme biosafety (Lee et al., 2017). Adenovirus is the vector of choice for the relative large size of human ABCC6 cDNA which exceeds the packaging limit of adeno-associated virus. In addition, the liver tropism of adenovirus enables transgene expression almost exclusively in the liver where ABCC6 is predominantly expressed. Adenovirus, first described in 1984, has been utilized extensively as a gene transfer platform in multiple pre-clinical and clinical applications for both gene therapy and vaccine based therapy (Amalfitano, 2004; Ghosh et al., 2006; Crystal, 2014). Adenovirus vectors are the most common vectors used in clinical trials worldwide and account for >20% of all gene therapy trials with the recognition that the immune reaction against the adenovirus capsid is the major hurdle for gene therapy (Ginn et al., 2018).
While the precise level of expression of ABCC6 needed in individual patients to prevent ectopic mineralization is currently unknown, an expression level of 50% is sufficient as attested to by the fact that heterozygous carriers of a mutation do not develop mineralization phenotypes. Plasma PPi are reduced to approximately 30–40% of normal levels in patients with PXE and in Abcc6−/− murine models (Jansen et al., 2014; Li et al., 2017; Dedinszki et al., 2017). Several variables, including virus dose levels and injection schedules, were carefully examined to optimize expression of ABCC6 and restoration of plasma PPi levels. Our results demonstrated that adenovirus-mediated ABCC6 expression remained high up to 4 weeks while plasma PPi levels were sustained up to 6 weeks after a single administration. The decline of ABCC6 expression could be due to the nature of non-integrating adenoviral vector and the renewal of liver cells during growth and development.
Upon optimization of dose and temporal expression, two sets of experiments were designed to test the efficacy of adenovirus-mediated human ABCC6 substitution in amelioration of the ectopic mineralization phenotype in the mouse model of PXE. The Prevention Study tested young Abcc6−/−Rag1−/− mice with administration of adenovirus initiated early at 6 weeks of age, a time point when mineralization just starts to develop. These mice were analyzed at 10, 14 and 18 weeks of age after different injection frequency and duration regimens. It is obvious that treatments with a prolonged therapeutic effect are desired. In this regard, the long-lasting effects were observed in mice 8 weeks after a single administration. The Reversal Study tested 43-week-old mice with established mineralization in the muzzle skin. Adenovirus therapy, for a duration of 4 weeks, stabilized if not reversed existing mineralization despite high levels of ABCC6 expression and increased plasma PPi levels, suggesting that therapy should be initiated in PXE and GACI patients as soon as diagnosis is made. Collectively, the results support a proof-of-concept therapeutic approach employing adenovirus to restore plasma PPi levels from expression of ABCC6, the gene at default in PXE. This is in contrast to previous findings that restoration of plasma PPi levels by ENPP1 overexpression did not completely prevent ectopic mineralization in Abcc6−/− mice (Zhao et al., 2017), suggesting distinct biologic functions of ABCC6 and ENPP1.
Unlike the transgenic mouse model expressing low levels of wild type human ABCC6 in Abcc6−/− mice (Pomozi et al., 2017b), our approach is adenovirus-mediated, and the effects of single administration on plasma PPi levels and ectopic mineralization are unlikely to last a lifetime. Because PPi is relatively unstable in circulation and has a short half-life, adenovirus-mediated ABCC6 gene therapy might require repeated injections. While this approach may not provide permanent cure of PXE, sustained maintenance of patients on the ABCC6 gene therapy treatment, analogous to patients on maintenance therapy for hemophilia (Baruteau et al., 2017), would prevent long-term complications. Despite the promising efficacy profile demonstrated in clinical trials, a major hurdle to adenovirus-mediated gene therapy is the anti-viral immune response. The studies performed in the immunodeficient Abcc6−/− mice provide proof-of-principle of ABCC6 gene therapy. As such, further studies to monitor and minimize immune reactions to recombinant adenovirus therapy are necessary in patients with PXE and GACI.
There are some treatment approaches in development for different types of mutations in ABCC6. Premature termination codon (PTC) read through utilizing PTC124 as a prototypic drug has been tested for therapy for PXE carrying ABCC6 nonsense mutations (Zhou et al., 2013). Another example of the mutation-based application of precision medicine for PXE revolves around chaperone-based correction of improper targeting of mutant ABCC6 proteins. Amino acid substitutions in ABC transporter proteins, such as ABCC6, can result in loss-of-function either through changes in the catalytic/transport activity, in the intracellular trafficking, or the conformational stability, or any combination thereof (Pomozi et al., 2014). A prototypic molecule, 4-phenylbutyrate, has been shown in in vitro culture systems and in mice to be capable of correcting the trafficking defect, allowing the mutant ABCC6 protein to be targeted to the proper localization at the basolateral surface of hepatocytes (Pomozi et al., 2014; Pomozi et al., 2017a). In contrast to these therapies, adenovirus-mediated ABCC6 gene therapy might have the potential to treat PXE patients regardless of the types of mutations in the ABCC6 gene. Future clinical trials are warranted to validate this approach.
MATERIALS AND METHODS
Vector design and manufacturing
Human ABCC6 coding sequence (NP_001162.4) was cloned into pENTR™/D-TOPO vector (Life Technologies, Grand Island, NY) followed by recombination into the E1 region of pAd/CMV/V5-DEST and pAd/PL-DEST vectors (Thermo Fisher, Grand Island, NY) containing E1- and E3-deleted human adenovirus serotype 5 (Ad5). The replication-deficient recombinant Ad5-CMV-hABCC6 and Ad5-PL-hABCC6 adenoviruses were produced in HEK293A cells and purified using Adeno-X Maxi Purification Kit (Clontech, Mountain View, CA). The viruses were titered using Adeno-X rapid Titer Kit (Clontech).
In vitro transduction study
The dose-response and time-course experiments were carried out in mouse liver hepatoma cells (CRL1830™, ATCC, Manassas, VA). Recombinant Ad5-CMV-hABCC6 and Ad5-PL-hABCC6 viruses were added to the cultures at different doses and human ABCC6 expression were detected 36 hours post transduction by immunostaining using human ABCC6-specific antibody that does not cross react mouse ABCC6 protein (Cell Sciences, Newburyport, MA).
Mice and treatments
The Abcc6tm1JfK mouse was descripted previously and referred to as Abcc6−/− (Klement et al., 2005). The Abcc6−/− mice on immunodeficient Rag1tm1Mom/J mouse background (this mouse is referred to as Rag1−/−) (The Jackson Laboratory, Bar Harbor, ME) were divided into four groups: Time-course expression (Set 1), Dose-response (Set 2), Prevention Study (Set 3), and Reversal Study (Set 4), with appropriate age-matched controls (Table 1).
Table 1.
Experimental groups of mice by genotype and treatment*
| Genotype | No. of mice examined (M+F) | Injected with | Dose per mouse (IFU) | Frequency of injections | Age at time of injection | Duration | Age at time of analysis |
|---|---|---|---|---|---|---|---|
| Set 1 (Time course) | |||||||
| C57BL/6J | 6 (3+3) | NA | NA | NA | NA | NA | 10w |
| Abcc6−/−Rag1−/− | 4 (2+2) | NA | NA | NA | NA | NA | 10w |
| Abcc6−/−Rag1−/− | 6 (2+4) | Ad5-CMV-hABCC6 | 4×108 | 1 | 6w | 1w | 7w |
| Abcc6−/−Rag1−/− | 6 (1+5) | Ad5-CMV-hABCC6 | 4×108 | 1 | 6w | 2w | 8w |
| Abcc6−/−Rag1−/− | 4 (2+2) | Ad5-CMV-hABCC6 | 4×108 | 1 | 6w | 4w | 10w |
| Abcc6−/−Rag1−/− | 5 (1+4) | Ad5-CMV-hABCC6 | 4×108 | 1 | 6w | 6w | 12w |
| Abcc6−/−Rag1−/− | 4 (2+2) | Ad5-CMV-hABCC6 | 4×108 | 1 | 6w | 8w | 14w |
| Set 2 (Dose response) | |||||||
| C57BL/6J | 6 (3+3) | NA | NA | NA | NA | NA | 10w |
| Abcc6−/−Rag1−/− | 4 (2+2) | NA | NA | NA | NA | NA | 10w |
| Abcc6−/−Rag1−/− | 8 (4+4) | Ad5-PL-hABCC6 | 1×109 | 1 | 6w | 4w | 10w |
| Abcc6−/−Rag1−/− | 8 (5+3) | Ad5-CMV-hABCC6 | 1×109 | 1 | 6w | 4w | 10w |
| Abcc6−/−Rag1−/− | 8 (5+3) | Ad5-CMV-hABCC6 | 4×108 | 1 | 6w | 4w | 10w |
| Set 3 (Prevention study) | |||||||
| C57BL/6J | 7 (2+5) | NA | NA | NA | NA | NA | 14w |
| C57BL/6J | 9 (4+5) | NA | NA | NA | NA | NA | 18w |
| Abcc6−/−Rag1−/− | 14 (9+5) | NA | NA | NA | NA | NA | 14w |
| Abcc6−/−Rag1−/− | 13 (9+4) | NA | NA | NA | NA | NA | 18w |
| Abcc6−/−Rag1−/− | 10 (6+4) | Ad5-CMV-hABCC6 | 4×108 | 1 | 6w | 8w | 14w |
| Abcc6−/−Rag1−/− | 9 (5+4) | Ad5-CMV-hABCC6 | 4×108 | 2 | 6w, 10w | 4w, 4w | 14w |
| Abcc6−/−Rag1−/− | 13 (9+4) | Ad5-CMV-hABCC6 | 4×108 | 3 | 6w, 10w, 14w | 4w, 4w, 4w | 18w |
| Set 4 (Reversal study) | |||||||
| C57BL/6J | 6 (3+3) | NA | NA | NA | NA | NA | 47w |
| Abcc6−/−Rag1−/− | 8 (3+5) | Ad5-PL-hABCC6 | 4×108 | 1 | 43w | 4w | 47w |
| Abcc6−/−Rag1−/− | 8 (3+5) | Ad5-CMV-hABCC6 | 4×108 | 1 | 43w | 4w | 47w |
Mice with desired genotypes were injected with either Ad5-PL-hABCC6 or Ad5-CMV-hABCC6 adenovirus at designated frequency and duration as indicated in the table. The mice were sacrificed for determination of human ABCC6 expression in mouse liver, PPi levels in plasma and the degree of ectopic mineralization in the muzzle skin. M, male; F, female; w, weeks.
In Set 1, 6-week-old Abcc6−/−Rag1−/− mice were injected through tail vein with Ad5-CMV-hABCC6 at 4×108 IFU per mouse. At time points indicated in Table 1, mice were euthanized to analyze human ABCC6 expression in the liver and PPi levels in plasma. In Set 2, 6-week-old Abcc6−/−Rag1−/− mice were injected with Ad5-CMV-hABCC6 or Ad5-PL-hABCC6 at 4×108 or 1×109 IFU per mouse. Mice were euthanized 4 weeks afterwards, at 10 weeks of age. In Set 3, 6-week-old Abcc6−/−Rag1−/− mice were injected with Ad5-CMV-hABCC6 virus at 4×108 IFU per mouse. Some mice received one injection and euthanized 8 weeks afterwards, at 14 weeks of age. Other mice received 2 or 3 injections 4 weeks apart and euthanized 4 weeks after the last injection, at 14 and 18 weeks of age, respectively. Set 4 consists of 43-week-old Abcc6−/−Rag1−/− mice injected with Ad5-CMV-hABCC6 or Ad5-PL-hABCC6 at 4×108 IFU per mouse. Mice were euthanized 4 weeks after injection, at 47 weeks of age.
All mice, including the wild type C57BL/6J mice and the Abcc6−/−Rag1−/−mice, were maintained on a standard rodent diet. All protocols were approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University.
Immunostaining of the ABCC6 protein
Immunostaining of liver samples was performed on 6 μm frozen sections. The rat monoclonal anti-human ABCC6 antibody M6II-7 (Cell Sciences) was used to identify human ABCC6 protein. A mouse monoclonal antibody was used to label the basolateral plasma membrane marker Na,K-ATPase (Abcam, Cambridge, MA). The Alexa Fluor 488 donkey anti-rat and Alexa Fluor 594 goat anti-mouse secondary antibodies (Invitrogen) were used. Images were acquired with EVOS FL Auto Imaging Microscopy (Thermo Fisher).
Western blot
Plasma membrane proteins from mouse liver were prepared and separated on 8% SDS-PAGE. An anti-ABCC6 antibody M6II-7 (Cell Sciences) was used to detect the human ABCC6 protein. A mouse monoclonal anti Na,K-ATPase antibody was used as a plasma membrane marker for equal protein loading. To visualize the signal, the membrane was incubated with secondary antibodies (LI-COR, Lincoln, NE) and scanned with an Odyssey Infrared Imager (LI-COR).
Histopathological analysis
Left muzzle skin biopsies from euthanized mice were collected and processed for histology. Tissue sections were stained with von Kossa using standard procedures.
Chemical quantitation of calcium
Right muzzle skin biopsies were harvested and decalcified with 1.0 mol/L HCl for 48 hours at room temperature. Solubilized calcium was then determined by colorimetric analysis using the ơ-cresolphthalein complexone method (calcium (CPC) LiquiColor; Stanbio Laboratory, Boerne, TX). The values were normalized to tissue weight.
Plasma collection and PPi assay
Whole blood was collected by cardiac puncture using CTAD (citrate, theophylline, adenosine, and dipyridamole) and EDTA as anti-coagulants (Becton, Dickinson and Company, Franklin Lakes, NJ), followed by depletion of platelets by filtration through a Centrisart I 300-kDa mass cutoff filter (Sartorius, New York, NY, USA). Determination of PPi concentration in plasma was performed using a methodology adopted world-wide (Jansen et al., 2014; Li et al., 2017; Dedinszki et al., 2017; Pomozi et al., 2017b; Bauer et al., 2018).
Statistical analysis
The data were analyzed using multivariable linear regression with the predictor of sex and treatment and their interactions for each group. Statistical significance was reached with p < 0.05. All statistical computations were completed using R version 3.5.0 software.
ACKNOWLEDGMENTS
This study was supported by NIH/NIAMS grants R01AR055225 (JU), K01AR064766 (QL), and R01AR072695 (JU and QL). The authors thank Dian Wang, Joshua Kingman, Jingyi Zhao, Sarah Siu, Diana Li, Ida Joely Jacobs, and Tingting Zhan for assistance. Carol Kelly helped in manuscript preparation.
Abbreviations:
- Ad5
adenovirus serotype 5
- PXE
pseudoxanthoma elasticum
- GACI
generalized arterial calcification of infancy
- PPi
inorganic pyrophosphate
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCS
- Amalfitano A Utilization of adenovirus vectors for multiple gene transfer applications. Methods 2004;33:173–8. [DOI] [PubMed] [Google Scholar]
- Baruteau J, Waddington SN, Alexander IE, Gissen P. Gene therapy for monogenic liver diseases: clinical successes, current challenges and future prospects. J Inherit Metab Dis 2017;40:497–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer C, le Saux O, Pomozi V, Aherrahrou R, Kriesen R, Stolting S, et al. Etidronate prevents dystrophic cardiac calcification by inhibiting macrophage aggregation. Sci Rep 2018;8:5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belinsky MG, Kruh GD. MOAT-E (ARA) is a full-length MRP/cMOAT subfamily transporter expressed in kidney and liver. Br J Cancer 1999;80:1342–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen AA, Plomp AS, Schuurman EJ, Terry S, Breuning M, Dauwerse H, et al. Mutations in ABCC6 cause pseudoxanthoma elasticum. Nat Genet 2000;25:228–31. [DOI] [PubMed] [Google Scholar]
- Crystal RG. Adenovirus: the first effective in vivo gene delivery vector. Hum Gene Ther 2014;25:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedinszki D, Szeri F, Kozak E, Pomozi V, Tokesi N, Mezei TR, et al. Oral administration of pyrophosphate inhibits connective tissue calcification. EMBO Mol Med 2017;9:1463–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre G, Laurain A, Aranyi T, Szeri F, Fulop K, Le Saux O, et al. The ABCC6 transporter: A new player in biomineralization. Int J Mol Sci 2017;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh SS, Gopinath P, Ramesh A. Adenoviral vectors: a promising tool for gene therapy. Appl Biochem Biotechnol 2006;133:9–29. [DOI] [PubMed] [Google Scholar]
- Giachelli CM. Ectopic calcification: gathering hard facts about soft tissue mineralization. Am J Pathol 1999;154:671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginn SL, Amaya AK, Alexander IE, Edelstein M, Abedi MR. Gene therapy clinical trials worldwide to 2017: An update. J Gene Med 2018;20:e3015. [DOI] [PubMed] [Google Scholar]
- Jansen RS, Kucukosmanoglu A, de Haas M, Sapthu S, Otero JA, Hegman IE, et al. ABCC6 prevents ectopic mineralization seen in pseudoxanthoma elasticum by inducing cellular nucleotide release. Proc Nat Acad Sci USA 2013;110:20206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen RS, Duijst S, Mahakena S, Sommer D, Szeri F, Varadi A, et al. ABCC6-mediated ATP secretion by the liver is the main source of the mineralization inhibitor inorganic pyrophosphate in the systemic circulation-brief report. Arterioscler Thromb Vasc Biol 2014;34:1985–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Endo M, Dibra F, Wang K, Uitto J. Pseudoxanthoma elasticum is a metabolic disease. J Invest Dermatol 2009;129:348–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Oldenburg R, Otsuru S, Grand-Pierre AE, Horwitz EM, Uitto J. Parabiotic heterogenetic pairing of Abcc0−/−/Rag1−/− mice and their wild-type counterparts halts ectopic mineralization in a murine model of pseudoxanthoma elasticum. Am J Pathol 2010;176:1855–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Jiang Q, Wu Z, Shao C, Zhou Y, Yang L, et al. Genetic heterogeneity of pseudoxanthoma elasticum: The Chinese signature profile of ABCC6 and ENPP1 mutations. J Invest Dermatol 2015;135:1294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klement JF, Matsuzaki Y, Jiang QJ, Terlizzi J, Choi HY, Fujimoto N, et al. Targeted ablation of the Abcc6 gene results in ectopic mineralization of connective tissues. Mol Cell Biol 2005;25:8299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Saux O, Urban Z, Tschuch C, Csiszar K, Bacchelli B, Quaglino D, et al. Mutations in a gene encoding an ABC transporter cause pseudoxanthoma elasticum. Nat Genet 2000;25:223–7. [DOI] [PubMed] [Google Scholar]
- Lee CS, Bishop ES, Zhang R, Yu X, Farina EM, Yan S, et al. Adenovirus-mediated gene delivery: Potential applications for gene and cell-based therapies in the new era of personalized medicine. Genes Dis 2017;4:43–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Brodsky JL, Conlin L, Pawel B, Glatz A, Gafni RI, et al. Mutations in the ABCC6 gene as a cause of generalized arterial calcification of infancy: Genotypic overlap with pseudoxanthoma elasticum. J Invest Dermatol 2014;134:658–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Kingman J, van de Wetering K, Tannouri S, Sundberg JP, Uitto J. Abcc6 knockout rat model highlights the role of liver in PPi homeostasis in pseudoxanthoma elasticum. J Invest Dermatol 2017;137:1025–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Huang J, Pinkerton AB, Millian JL, van Zelst BD, Levine MA, et al. Inhibition of tissue-nonspecific alkaline phosphatase attenuates ectopic mineraliation in the Abcc6−/− mouse model of PXE but not in the Enpp1 mutant mouse models of GACI. J Invest Dermatol 2018;(in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neldner KH. Pseudoxanthoma elasticum. Clin Dermatol 1988;6:1–159. [DOI] [PubMed] [Google Scholar]
- Nitschke Y, Baujat G, Botschen U, Wittkampf T, du Moulin M, Stella J, et al. Generalized arterial calcification of infancy and pseudoxanthoma elasticum can be caused by mutations in either ENPP1 or ABCC6. Am J Hum Genet 2012;90:25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomozi V, Brampton C, Fulop K, Chen LH, Apana A, Li Q, et al. Analysis of pseudoxanthoma elasticum-causing missense mutants of ABCC6 in vivo; pharmacological correction of the mislocalized proteins. J Invest Dermatol 2014;134:946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomozi V, Brampton C, Szeri F, Dedinszki D, Kozak E, van de Wetering K, et al. Functional rescue of ABCC6 deficiency by 4-phenylbutyrate therapy reduces dystrophic calcification in Abcc6(−/−) mice. J Invest Dermatol 2017a;137:595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomozi V, Brampton C, van de Wetering K, Zoll J, Calio B, Pham K, et al. Pyrophosphate supplementation prevents chronic and acute calcification in ABCC6-deficient mice. Am J Pathol 2017b;187:1258–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringpfeil F, Lebwohl MG, Christiano AM, Uitto J. Pseudoxanthoma elasticum: mutations in the MRP6 gene encoding a transmembrane ATP-binding cassette (ABC) transporter. Proc Natl Acad Sci U S A 2000;97:6001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer GL, Hu X, Pijnenborg AC, Wijnholds J, Bergen AA, Scheper RJ. MRP6 (ABCC6) detection in normal human tissues and tumors. Lab Invest 2002;82:515–8. [DOI] [PubMed] [Google Scholar]
- Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet 2003;4:346–58. [DOI] [PubMed] [Google Scholar]
- Uitto J, Van de Wetering K, Varadi A, Terry SF. Novel insights into pathomechanisms and treatment development in heritable ectopic mineralization disorders. Summary of the PXE International Biennial Research Symposium. J Invest Dermatol 2017;137:790–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters H, Spiering M, Gerding A, Slooff MJ, Kuipers F, Hardonk MJ, et al. Isolation and characterization of canalicular and basolateral plasma membrane fractions from human liver. Biochim Biophys Acta 1991;1069:61–9. [DOI] [PubMed] [Google Scholar]
- Zhao J, Kingman J, Sundberg JP, Uitto J, Li Q. Plasma PPi deficiency is the major, but not the exclusive, cause of ectopic mineralization in an Abcc6−/− mouse model of PXE. J Invest Dermatol 2017;137:2336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Jiang Q, Takahagi S, Shao C, Uitto J. Premature termination codon read-through in the ABCC6 gene: Potential treatment for pseudoxanthoma elasticum. J Invest Dermatol 2013;133:2672–7. [DOI] [PMC free article] [PubMed] [Google Scholar]