Abstract
The Ca2+-sensing receptor (CaSR) is a dimeric family C G-protein-coupled receptor that is expressed in calcitropic tissues such as the parathyroid glands and kidneys, and signals via G-proteins and beta-arrestin. The CaSR plays a pivotal role in bone and mineral metabolism by regulating parathyroid hormone secretion, urinary Ca2+ excretion, skeletal development and lactation. The importance of the CaSR for these calcitropic processes is highlighted by loss- and gain-of-function CaSR mutations, which cause familial hypocalciuric hypercalcaemia and autosomal dominant hypocalcaemia, respectively, and also by alterations in parathyroid CaSR expression, which contribute to the pathogenesis of primary and secondary hyperparathyroidism. Moreover, the CaSR is an established therapeutic target for hyperparathyroid disorders. The CaSR is also expressed in organs not involved in Ca2+ homeostasis, where it has non-calcitropic roles that include lung and neuronal development, vascular tone, gastro-intestinal nutrient sensing, secretion of insulin and entero-endocrine hormones, and wound healing. Furthermore, abnormal expression or function of the CaSR is implicated in cardiovascular and neurological diseases, as well as in asthma, and the CaSR is reported to protect against colorectal cancer and neuroblastoma, but increase the malignant potential of prostate and breast cancers. This review will discuss these physiological and pathophysiological roles of the CaSR.
Introduction
The extracellular calcium (Ca2+)-sensing receptor (CaSR) is an ~120-160 kDa G-protein-coupled receptor (GPCR) that is most highly expressed in the parathyroid glands and kidneys1,2, where it influences systemic Ca2+ homeostasis by detecting increases in the prevailing circulating Ca2+ concentration, which lead to intracellular signalling events that mediate a decrease in parathyroid hormone (PTH) secretion and reduction in renal tubular Ca2+ reabsorption (FIG. 1)3. The importance of the CaSR, which is a family C GPCR, for the regulation of circulating Ca2+ concentrations, i.e. its calcitropic actions, has been demonstrated by the identification of germline loss- and gain-of-function mutations affecting this GPCR and its intracellular partner proteins that result in inherited hypercalcaemic and hypocalcaemic disorders such as familial hypocalciuric hypercalcaemia (FHH) and autosomal dominant hypocalcaemia (ADH), respectively4. Furthermore, the CaSR, which is present as a dimer of ~240-310 kDa5 has been shown to represent a therapeutic target for such calcitropic disorders, and cinacalcet, a CaSR positive allosteric modulator (PAM), is used in clinical practice to treat hyperparathyroid disorders, and calcilytic drugs that are CaSR negative allosteric modulators (NAMs) are being investigated as a targeted therapy for symptomatic forms of ADH6. The CaSR is also expressed in other tissues, such as the intestine, pancreatic islets, lungs, brain, skin and vasculature, where it has been shown to be involved in non-calcitropic actions that include regulation of molecular and cellular processes such as gene expression, proliferation, differentiation and apoptosis, as well as influencing the physiological regulation of entero-endocrine hormone secretion, cardiac function, vascular tone, and also lung and neuronal development (TABLE 1)7–14. Furthermore, abnormal expression or function of the CaSR in these non-calcitropic tissues has been reported to contribute to the pathogenesis of cardiovascular diseases, asthma, Alzheimer’s disease, and breast and colon cancer9,14–16. This review focuses on the evolutionary origins, structure and signalling pathways of the CaSR, together with the roles of the CaSR in calcitropic and non-calcitropic diseases. Many of these aspects were discussed at the Third International Symposium on the Ca2+-Sensing Receptor (Florence, May 2017), which brought together researchers who are studying these basic, translational and clinical aspects of CaSR physiology and pathophysiology.
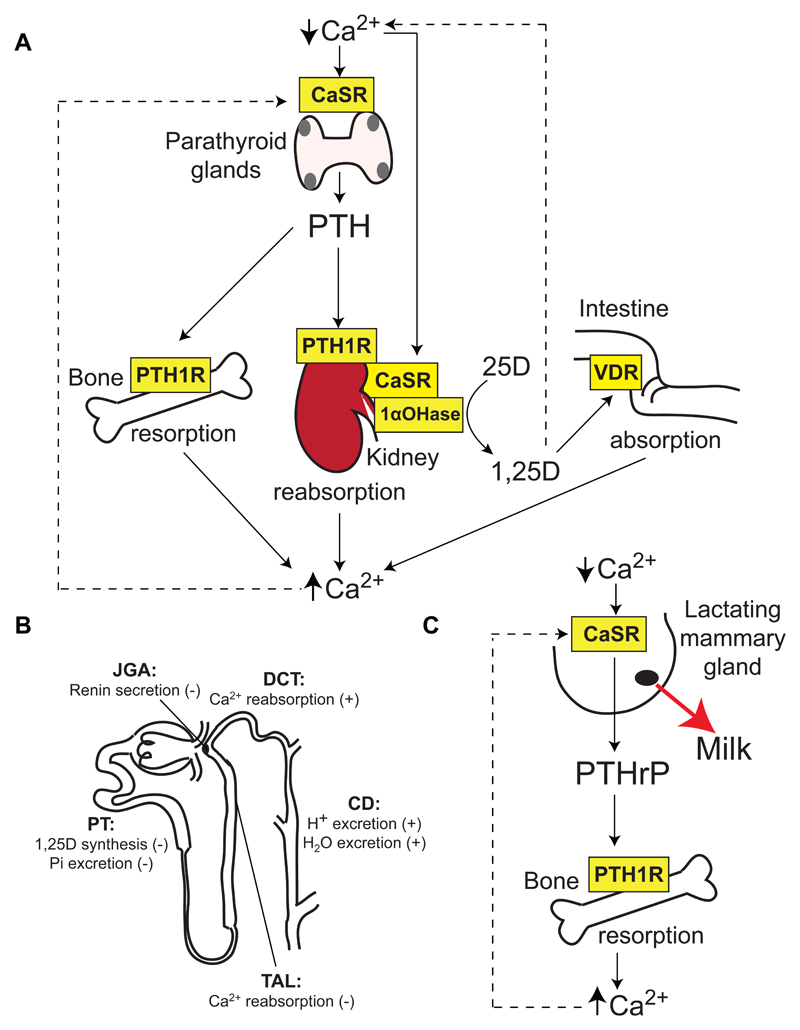
Figure 1. Role of the CaSR in Ca2+o homeostasis.
A. The CaSR is highly expressed in the parathyroid glands (grey), which are located adjacent and posterior to the thyroid gland (pink). The parathyroid CaSR detects reductions in Ca2+o, which leads to the release of PTH. PTH acts on the PTH1 receptor (PTH1R) to increase resorption of Ca2+ from bone, promote urinary Ca2+ reabsorption, and enhance expression of the renal 1-α-hydroxylase (1αOHase) enzyme, which converts the 25-hydroxyvitamin D (25D) precursor metabolite to biologically active 1,25-dihydroxyvitamin D (1,25D). The elevated 1,25D increases absorption of dietary calcium by acting on the intestinal vitamin D receptor (VDR)3. The kidney CaSR acts independently of PTH to regulate urinary Ca2+ reabsorption60,61. Increases in Ca2+o and 1,25D concentrations lead to negative feedback on the parathyroid glands, thereby inhibiting further PTH release. B. Nephron segment-specific roles of the CaSR. The CaSR is expressed in the: apical membrane of the proximal tubule (PT), where it regulates 1,25D synthesis and phosphate (Pi) excretion; basolateral membrane of the cortical thick ascending limb (TAL) of the Loop of Henle, and apical and basolateral membranes of the distal convoluted tubule (DCT), where it regulates Ca2+ reabsorption; apical and basolateral membranes of the collecting duct (CD), where it regulates H+ and water excretion; and juxtaglomerular apparatus (JGA), where it regulates renin secretion58,64. (+), stimulatory action of CaSR; (-), inhibitory action of CaSR. C. During lactation, the mammary gland CaSR detects reductions in Ca2+o, which leads to increased PTHrP secretion from mammary epithelial cells into the circulation9. PTHrP acts on the PTH1R to increase bone resorption, which in turn releases Ca2+o for milk production9. Stimulatory and inhibitory actions are indicated by solid lines and dashed lines, respectively.
Table 1. Major calcitropic and non-calcitropic cellular roles of the CaSR.
| Process | Cell type | Role | Reference |
|---|---|---|---|
| Calcitropic | |||
| Parathyroid | PTH secretion (-) | 1,56 | |
| Proliferation (-) | 1,133 | ||
| Kidney | |||
| Proximal tubule | Phosphate excretion (-) | 65 | |
| 1,25(OH)2D synthesis (-) | 62 | ||
| TAL | Ca2+ reabsorption (-) | 60,61 | |
| Distal convoluted tubule | Ca2+ reabsorption (+) | 68 | |
| Collecting duct | H+ excretion (+) | 69 | |
| Water excretion (+) | 69 | ||
| Juxtaglomerular apparatus | Renin secretion (-) | 70 | |
| Bone | |||
| Osteoblast | Differentiation and mineralization (+) | 55,71 | |
| Growth plate chondrocyte | Differentiation (+) | 55 | |
| Breast epithelium | Ca2+ transport into milk (+) | 9,76 | |
| PTHrP production (-) | 9,75 | ||
| Non-calcitropic | |||
| Cardiovascular | |||
| Vascular smooth muscle cell | Proliferation (+) | 200 | |
| Arterial contractility (+) | 13 | ||
| Endothelial cell | Nitric oxide production (+) | 201 | |
| Ventricular cardiomyocyte | Apoptosis (+) | 203 | |
| Foetal lung epithelium | Fluid secretion (+) | 206,207 | |
| Proliferation and lung branching morphogenesis (-) | 207 | ||
| Gastrointestinal | |||
| Gastric parietal cell | H+ excretion (+) | 216,217 | |
| Gastric mucosal cell | Ghrelin secretion (-) | 210,212 | |
| Gastric G cell | Gastrin secretion (+) | 215 | |
| Duodenal I cell | Cholecystokinin secretion (+) | 220 | |
| Intestinal L cell | Glucagon-like peptide-1 (+) | 210 | |
| Peptide YY (+) | 210,221 | ||
| Colon | Barrier function (+) | 229 | |
| Enteric nervous system (-) | 224 | ||
| Fluid secretion (-) | 223,224 | ||
| Proliferation (-) | 10 | ||
| Pancreatic islet | |||
| α-cell | Glucagon secretion (+) | 7,232 | |
| β-cell | Insulin secretion (+) | 232,233 | |
| Adipocyte | Proliferation (+) | 162 | |
| Differentiation (+) | 162 | ||
| Inflammatory cytokine expression (+) | 162 | ||
| Skin keratinocyte | Proliferation (-) | 236 | |
| Differentiation (+) | 236 | ||
| Apoptosis (-) | 236 | ||
| Central nervous system | |||
| Cerebellar granule cell | Differentiation and migration (+) | 190 | |
| Oligodendrocyte | Differentiation and myelinogenesis (+) | 8 | |
| GNRH neuron | Chemotaxis (+) | 12 | |
| Sub-fornical organ | Neuronal excitability and blood pressure regulation (+) | 12 |
GNRH, gonadotrophin-releasing hormone; PTH, parathyroid hormone: PTHrP, parathyroid hormone related protein; TAL, thick ascending limb of Loop of Henle; (+), stimulatory action of CaSR; (-), inhibitory action of CaSR.
Structure of the CaSR
The CaSR belongs to the family C GPCRs, which play pivotal roles in neurotransmission, nutrient-sensing and Ca2+ homeostasis17. These receptors are functionally active as homodimers or heterodimers18–20, and are characterised by the presence of a large extracellular domain (ECD), which contains a bilobed venus flytrap (VFT) module (FIG. 2) that closes upon ligand binding, and has structural similarity to nutrient-scavenging bacterial periplasmic proteins21. The CaSR is considered to have arisen during vertebrate evolution22, and has been shown to be functionally active in vertebrates ranging from cartilaginous fishes to terrestrial mammals22. Recent X-ray crystallography studies have demonstrated the human CaSR to have a glycosylated ECD, which binds Ca2+o at three distinct sites within the VFT, and also at a site located between the VFT and cysteine-rich domain (CRD) (FIG. 2)23. The CaSR also binds amino acids within the cleft of the VFT (FIG. 2)23,24, and the binding of both Ca2+ and amino acids may be required to fully activate the CaSR23. The CaSR VFT is predicted to contain additional binding sites for anions such as phosphate and sulphate, and these anions likely stabilise the CaSR in an inactive conformation23. The CaSR is maintained as a dimer (FIG. 2) by covalent and non-covalent interactions that involve the N-terminal VFT lobe of each monomer, and ligand-binding has been shown to induce closure of the VFT, as well as extending the dimer interface to include the C-terminal VFT lobe and the CRD23. These agonist-induced conformational changes of the CaSR ECD may lead to rearrangements of the helices within the transmembrane domain (TMD) that facilitate G-protein binding and intracellular signal transduction3,25. The CaSR TMD consists of seven hydrophobic helical domains connected by three extracellular loops and three intracellular loops (FIG. 2). The TMD anchors the CaSR in the plasma membrane, and also plays a critical role in signal transduction. Indeed, mutational analysis has identified residues within intracellular loops 2 and 3, which are essential for the activation of downstream effector proteins26. The CaSR TMD has not yet been crystallised, however, homology modelling studies using the related metabotropic glutamate receptor crystal structure indicates the presence of a cavity located between the mid-portion and extracellular aspect of the CaSR TMD, which is the site of binding of allosteric modulators such as the phenylalkylamine calcimimetic drugs and amino-alcohol calcilytic drugs (FIG. 2)27. The intracellular domain (ICD) of the CaSR regulates downstream signalling events27, and also undergoes phosphorylation and ubiquitination, which facilitates desensitisation and degradation or recycling of the CaSR28,29. Moreover, the ICD is considered to be the site of binding for the intracellular adaptor-related protein complex 2 (AP2) (FIG. 2), which facilitates clathrin-mediated endocytosis of GPCRs such as the CaSR30.
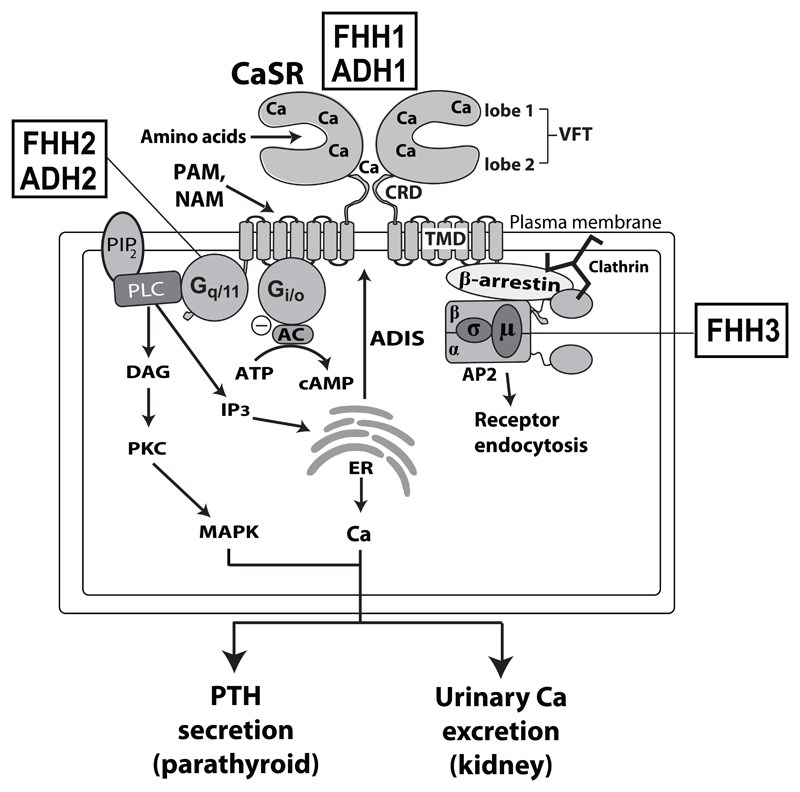
Figure 2. CaSR signalling and trafficking.
The CaSR is functionally active as a constitutive homodimer, and may also form heterodimers with other family C GPCRs such as the metabotropic glutamate receptors and gamma-amino butryric acid (GABA) type B receptors in growth plate chondrocytes and the central nervous system18–20. The CaSR ECD binds calcium (Ca2+) at multiple sites within the lobes of the venus flytrap (VFT) and cysteine-rich domain (CRD). The CaSR also binds amino acids within the VFT cleft. Synthetic positive allosteric modulators (PAMs) and negative allosteric modulators (NAMs) bind to the CaSR transmembrane domain (TMD). The binding of Ca2+ leads to Gq/11-dependent activation of phospholipase C (PLC) and the production of diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3) from membrane bound phosphatidylinositol 4,5-bisphosphate (PIP2). The increase in intracellular IP3 levels facilitates the release of Ca2+ from intracellular stores such as the endoplasmic reticulum (ER). DAG activates protein kinase C (PKC) and the mitogen-activated protein kinase (MAPK) pathway. The CaSR also activates the Gi/o protein, which leads to inhibition of adenylate cyclase (AC)-mediated production of cAMP. These signalling events cause a decrease in parathyroid hormone (PTH) secretion and reduction in renal tubular Ca2+ reabsorption. CaSR cell-surface expression is regulated by agonist-driven insertional signalling (ADIS), which mediates anterograde receptor trafficking39; and also by an endocytic complex comprising clathrin, β-arrestin and the heterotetrameric adaptor-related protein complex 2 (AP2) complex, which mediates retrograde receptor trafficking40. Loss- and gain-of function mutations of the CaSR lead to FHH1 and ADH1, respectively; loss- and gain-of function mutations of the Gα11 subunit are associated with FHH2 and ADH2, respectively; and loss-of-function mutations of the AP2σ subunit are associated with FHH3.
CaSR signalling
The CaSR activates downstream signalling cascades by coupling to heterotrimeric guanine-nucleotide binding proteins (G-proteins) such as the Gq/11, Gi/o and G12/13 family of proteins31,32. The Gq/11 proteins are critical for CaSR signal transduction, and germline mutations of G-protein subunit α11 (Gα11) have been shown to cause hypercalcaemic and hypocalcaemic disorders (TABLE 2)33. Through Gq/11, the CaSR activates phospholipase C (PLC) to convert phosphatidylinositol 4,5-bisphosphate (PIP2) into diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3), which in turn releases Ca2+ from intracellular stores by binding to IP3 receptors on endoplasmic reticulum and activates Ca2+ influx via store-operated channels located in the plasma membrane (FIG. 2)31. The lipid derived second messenger, DAG, activates protein kinase C (PKC), which regulates protein phosphorylation cascades involved in cell survival, differentiation and proliferation such as the mitogen activated protein kinases (MAPKs) (FIG. 2)31,34. Key components of the MAPK cascade activated by the CaSR include the extracellular-signal regulated kinases 1/2 (ERK1/2), p38 kinase (p38K) and c-Jun N-terminal kinase (JNK)35,36. By coupling to the pertussis toxin-sensitive Gi/o proteins, the CaSR suppresses adenylyl cyclase activation and cyclic AMP (cAMP) production (FIG. 2), and also stimulates MAPKs such as ERK1/231,34,35. The CaSR may also stimulate the MAPK pathway via a G-protein independent mechanism, which involves the beta-arrestin proteins37. The CaSR additionally has been reported to couple to the G12/13 proteins to activate RhoA, which is a small GTPase protein that plays a role in the Wnt3a-beta-catenin signalling cascade38 and also facilitates cadherin-mediated intercellular adhesion11. CaSR signal transduction also determines the level of cell-surface expression of this GPCR via a process known as agonist-driven insertional signalling (ADIS), which increases anterograde trafficking of newly synthesised CaSRs to the plasma membrane (FIG. 2), and likely prevents the CaSR from undergoing functional desensitization in response to continual exposure to Ca2+o39,40. CaSR signalling is additionally mediated by the σ-subunit of the heterotetrameric AP2 complex, and germline loss-of-function AP2σ mutations have been shown to impair intracellular Ca2+ and MAPK signalling responses in CaSR-expressing cells, and to cause hypercalcaemia (TABLE 2)30,41–43. These CaSR-mediated signalling cascades modulate diverse physiological functions in calcitropic and non-calcitropic tissues, including hormone secretion, growth, survival, migration, adhesion, and differentiation (see sections below). In calcitropic tissues, loss- and gain of CaSR expression and/or signalling lead to hypercalcaemic and hypocalcaemic disorders, respectively. However, in non-calcitropic tissues reduced CaSR activity/signalling has been associated with disorders ranging from impaired wound healing to vascular calcification and colorectal carcinoma11,16,44, and upregulation of CaSR expression in cells and tissues with a low basal level of CaSR expression and activity, such as neurons and airway smooth muscle cells, has been reported to occur in brain injury or asthma14,45, with the resulting increase in CaSR activity and signalling further contributing to disease progression.
Table 2. Calcitropic disorders associated with CaSR, Gα11 and AP2σ mutations or autoantibodies.
| Disorder | OMIMa | Inheritance | Geneb | Chromosomal localisation | Clinical features |
|---|---|---|---|---|---|
| Hypercalcaemic disorders | |||||
| Genetic causes | |||||
| Familial hypocalciuric hypercalcaemia type 1 (FHH1) | 145980 | Autosomal dominant | CASR | 3q21.1 | Asymptomatic in majority of patients |
| Familial hypocalciuric hypercalcaemia type 2 (FHH2) | 145981 | Autosomal dominant | GNA11 | 19p13.3 | Asymptomatic in majority of patients |
| Familial hypocalciuric hypercalcaemia type 3 (FHH3) | 600740 | Autosomal dominant | AP2S1 | 19q13.3 | Hypercalcaemic symptoms in >20% of patients Low bone mineral density in >50% of patients Childhood cognitive deficits in >75% of patients |
| Neonatal severe hyperparathyroidism (NSHPT) | 239200 | Autosomal recessive or dominant | CASR | 3q21.1 | Severe hypercalcaemia Hyperparathyroid bone disease |
| Adult-onset primary hyperparathyroidism (PHPT) | - | Autosomal recessive or dominant | CASR | 3q21.1 | Nephrolithiasis in >40% of patients Low bone mineral density in >25% of patients |
| Acquired causes due to CaSR autoantibodies | |||||
| Autoimmune hypocalciuric hypercalcaemia (AHH) | - | - | - | - | Asymptomatic or symptomatic hypercalcaemia, which may be responsive to glucocorticoids |
| Hypocalcaemic disorders | |||||
| Genetic causes | |||||
| Autosomal dominant hypocalcaemia type 1 (ADH1) | 601198 | Autosomal dominant | CASR | 3q21.1 | Hypocalcaemic symptoms in ~50% of patients Ectopic calcifications in ~35% of patients |
| Autosomal dominant hypocalcaemia type 2 (ADH2) | 615361 | Autosomal dominant | GNA11 | 19p13.3 | Hypocalcaemic symptoms in >75% of patients Short stature reported in two kindreds |
| Bartter syndrome type V | 601198 | Autosomal dominant | CASR | 3q21.1 | Renal salt wasting and hypokalaemia Hypocalcaemic symptoms in >75% of patients |
| Acquired causes due to CaSR autoantibodies | |||||
| Autoimmune hypoparathyroidism | - | - | - | - | Asymptomatic or symptomatic hypocalcaemia |
Online Mendelian Inheritance in Man.
CASR encodes the CaSR; GNA11 encodes Gα11; and AP2S1 encodes AP2σ.
Adapted from Hannan FM, Babinsky VN, Thakker RV. Disorders of the calcium-sensing receptor and partner proteins: insights into the molecular basis of calcium homeostasis. J Mol Endocrinol. 2016; 57(3): R127-42.
CaSR ligands and biased signalling
The CaSR binds to an array of physiological ligands such as cations (e.g. H+, Na+, Ca2+, Mg2+), L-amino acids and polyamines, and crystal structures indicate that the CaSR may also bind anions such as PO43- and SO42- 23,31. Low molecular weight molecules which allosterically enhance or suppress CaSR activity and are referred to as calcimimetic or calcilytic compounds, respectively, have also been identified6. Such calcimimetic and calcilytic compounds are either in clinical use or under investigation for the management of calcitropic and non-calcitropic disorders, which are associated with activating or inactivating mutations, aberrant expression of the CaSR, or its downstream signaling molecules3,14,42,46–48.
Several CaSR ligands, including L-amino acids, polyamines, Mg2+, calcimimetics, and calcilytics, have been shown to induce biased signalling (also referred to as functional selectivity), thereby leading to preferential activation of distinct intracellular signalling responses37,49. CaSR-mediated biased signalling may provide an explanation for the CaSR’s ability to have differing responsiveness to extracellular Ca2+ and mediating diverse physiological functions in different cell systems in response to locally available allosteric factors. This biased signalling may also explain the differences in the efficacies of calcimimetics or calcilytics in different target tissues. For example, it has been shown that the calcimimetic compound R-568 is 40-fold more potent in suppressing PTH secretion in parathyroid cells than in stimulating calcitonin secretion from thyroid C-cells50.
The mechanisms underlying CaSR-mediated biased signalling remain to be elucidated, and the recent characterization of a novel ADH-causing mutation, Arg680Gly, has led to the identification of a transmembrane salt-bridge (Arg680-Glu767) (FIG. 3), which regulates signalling via a G-protein independent mechanism51. Indeed, mutations disrupting this salt bridge, which is located at the entrance to the TMD allosteric modulator binding pocket, were shown to upregulate beta-arrestin-mediated signalling without altering G-protein-dependent signalling (FIG. 3)51. The Arg680Gly CaSR mutation also abrogated the effect of NPS 2143 (FIG. 3)51, which is an amino-alcohol calcilytic compound52, most likely by disrupting a hydrogen bond interaction between the wild-type Arg680 residue and NPS 214327. These findings indicate that a structurally distinct class of CaSR NAMs, such as the quinazolinone calcilytic compounds52, may be required to treat patients with symptomatic hypocalcemia caused by the gain-of-function Arg680Gly CaSR mutation51.
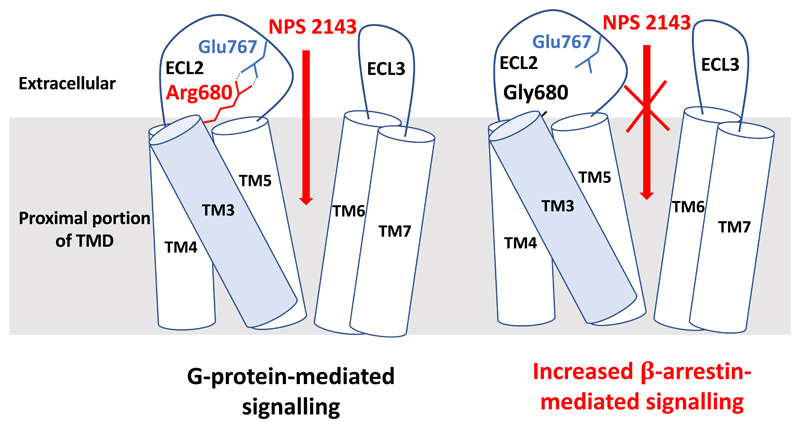
Figure 3. Disruption of a salt-bridge within the CaSR TMD causes biased signalling.
The salt-bridge is formed by the Arg680 residue located in the proximal portion of transmembrane helix 3 (TM3, shaded blue) and the Glu767 residue located in extracellular loop 2 (ECL2). The Arg680-Glu767 salt-bridge is situated at the entrance of the allosteric modulator binding pocket, which is formed by residues from TM3 and TM5-TM727. The Arg680 residue mediates the binding of the NPS 2143 calcilytic compound27. The Arg680-Glu767 salt-bridge is associated with G-protein-mediated signalling, whereas disruption of the salt-bridge by the ADH-causing CaSR mutation, Arg680Gly, selectively increases β-arrestin-mediated signalling, as well as abrogating the inhibitory effect of the NPS 2143 compound51.
Role of the CaSR in Ca2+ homeostasis
Extracellular Ca2+ (Ca2+o) is critical for a diverse range of biological functions that include mineralisation of bone matrix, neuronal and neuromuscular function, blood coagulation, and provision of a constant supply of Ca2+ for intracellular processes that range from signal transduction to the synthesis and secretion of hormones53. The concentration of Ca2+o is tightly regulated by a homeostatic system consisting of the following components: 1) the parathyroid glands, which sense Ca2+o and secrete PTH; 2) the kidneys and small intestine, which shift Ca2+ between the extracellular fluid and external environment; 3) the skeleton, which is the main Ca2+ reservoir and buffers acute fluxes in Ca2+o concentrations; and 4) calcitropic hormones such as PTH and 1,25-dihydroxyvitamin D3, which mediate interactions between the parathyroid glands and target calcitropic organs such as the bone, kidney and intestine (FIG. 1)3. Moreover, during lactation the mammary glands act as a calcitropic endocrine organ, which secretes PTH-related peptide (PTHrP) to mobilise Ca2+ from bone. The CaSR is expressed in the parathyroid glands, kidneys, bone and breast, and its calcitropic actions in these tissues are outlined below.
Parathyroid gland
The CaSR is most highly expressed in the parathyroid glands2, where it regulates PTH synthesis and secretion in an inverse manner54. Thus, increases in Ca2+o lead to CaSR-mediated suppression of PTH release, whereas a decrease in Ca2+o releases this suppression to promote tonic PTH release54. In keeping with this, mice with a parathyroid-specific CaSR ablation have been shown to develop severe hyperparathyroidism and hypercalcemia55. In the parathyroid glands, the CaSR has been shown to interact with Klotho, which is a transmembrane protein, to regulate PTH secretion56. Moreover, expression of the parathyroid CaSR is upregulated by 1,25-dihydroxyvitamin D (1,25(OH)2D), which acts on vitamin D response elements within the CASR gene promoter57. CaSR-mediated PTH secretion leads to activation of the PTH1 receptor (PTH1R) in bone and the kidneys to increase: osteoclast-mediated bone resorption; Ca2+ reabsorption by the renal thick ascending limb (TAL) of the loop of Henle and distal renal tubule; phosphate excretion by the proximal renal tubule; and synthesis of 1,25(OH)2D by the proximal renal tubule, which increases intestinal Ca2+ absorption (FIG. 1)3,58,59. These calcitropic effects lead to an increase in Ca2+o concentrations, which in combination with 1,25(OH)2D, causes feedback inhibition of PTH release (FIG. 1)3.
Kidney
The CaSR is highly expressed in the basolateral membrane of the renal TAL (FIG. 1), where it regulates Ca2+ reabsorption independently of the actions of PTH60,61. The effect of the TAL CaSR on renal Ca2+ excretion is likely mediated by the claudin-14 tight junction protein, which regulates the paracellular reabsorption of divalent cations59,62,63. The CaSR is also expressed at a lower level in the proximal renal tubule64 where it regulates expression of the 1-α-hydroxylase enzyme, thereby influencing 1,25(OH)2D synthesis62. The proximal renal tubular CaSR has also been shown to inhibit PTH-mediated phosphate excretion65. The kidney CaSR is considered to play a key physiological role in the defense against hypercalcaemia, by promoting renal Ca2 excretion independently of the actions of PTH60,66, and also by potentially inhibiting 1,25(OH)2D synthesis62,67. The CaSR is also expressed in the distal convoluted tubule64, where it may promote Ca2+ reabsorption via the transient receptor potential vanilloid member 5 (TRPV5) channel68. Furthermore, the CaSR is expressed in the renal collecting ducts64, where it prevents the development of hypercalciuria-mediated nephrocalcinosis by enhancing urinary acidification and water excretion69. Finally, the CaSR has been reported to be expressed in the juxtaglomerular apparatus, where it inhibits renin secretion70, although the physiological consequence of this finding remains to be established.
Skeleton
The CaSR is expressed within bone and the growth plate, and its roles in these tissues have been characterized by using mice with osteoblast- and chondrocyte-specific ablations of the CaSR55,71,72. These studies have demonstrated that the chondrocyte CaSR has a critical role in growth plate chondrogenesis, longitudinal bone growth and skeletal mineralization, by counteracting PTH-related protein (PTHrP)/PTH1R signalling73. Indeed, mice with chondrocyte-specific ablation of the CaSR have shortened long bones, and also undermineralisation of the ribs, long bones and calvariae55. Mice with an osteoblast-specific CaSR ablation also showed reductions in bone mineralisation and long bone size, as well as developing spontaneous long bone fractures55,71. These findings occurred in association with impaired osteoblast differentiation and increased osteoclast activation, and highlight a role for the osteoblast CaSR in skeletal development, mineralisation and remodelling71.
Breast
The CaSR has been shown to be expressed in human and mouse breast epithelial cells, and its expression is increased during lactation74,75. The CaSR promotes lactation by increasing the supply of Ca2+ for milk production through effects on the plasma membrane Ca2+-ATPase 2 (PMCA2), which transports Ca2+ from the mammary cell into milk76. Studies in mice have shown that during lactation, the breast CaSR also influences Ca2+o homeostasis in order to regulate the mobilisation of skeletal Ca2+ for milk production9,75. Thus, in low Ca2+o states, reduced activation of the breast CaSR leads to increased PTHrP production from breast epithelial cells, which in turn stimulates osteoclastic resorption of Ca2+ from bone (FIG. 1)9. These systemic effects of the breast CaSR ensure a steady supply of Ca2+ for milk production, and also protect against maternal hypocalcaemia during lactation9.
Role of CaSR signalling pathways in inherited calcitropic diseases
Loss-of-function of the CaSR or the downstream Gα11 and AP2σ signalling proteins gives rise to hypercalcaemic disorders, such as FHH types 1-3 (FHH1, FHH2 and FHH3), respectively, as well as primary hyperparathyroidism (PHPT) and neonatal severe hyperparathyroidism (NSHPT); whereas gain-of-function mutations of the CaSR or Gα11 cause ADH types 1 (ADH1) and 2 (ADH2), respectively (TABLE 2).
Hypercalcaemic disorders
FHH is considered to be a rare disorder, with one study involving FHH patients in the west of Scotland reporting an estimated minimum prevalence of 1:78,00077. FHH is characterised by mild-to-moderate elevations of serum Ca2+ concentrations, mild hypermagnesaemia, and normal or elevated circulating PTH concentrations4. FHH has a similar serum biochemical phenotype to PHPT, and these conditions are distinguished in clinical practice by assessment of urinary calcium excretion, as ~80% of FHH patients are hypocalciuric with a calcium-to-creatinine clearance ratio (CCCR) <0.0178,79. However, a low CCCR is also observed in ~10% of PHPT patients, and thus mutational analysis of the known FHH-causing genes may be required to differentiate FHH from PHPT80. FHH comprises a genetically heterogeneous group of autosomal dominant disorders, designated as FHH types 1-3 (TABLE 2)3, and these will be briefly discussed.
FHH1
FHH1 (OMIM #145980) occurs most commonly and accounts for ~65% of all FHH cases4. This condition is usually benign and non-progressive, however, a higher prevalence of chondrocalcinosis with increasing age has been noted81, and some patients may develop recurrent pancreatitis82. FHH1 is caused by germline heterozygous loss-of-function mutations of the CASR gene, which is located on chromosome 3q21.1 (TABLE 2)83,84, and to-date >150 different CASR mutations have been reported in FHH patients (http://www.casrdb.mcgill.ca)85, and the majority (>85%) of these are missense substitutions and the remaining (<15%) are nonsense, deletion, insertion and splice-site mutations that result in truncated CaSR proteins4,83. The offspring of FHH1 parents may harbour homozygous or compound heterozygous CASR mutations that lead to NSHPT (OMIM #239200), which is a potentially life-threatening disorder characterized by severe hypercalcaemia (serum calcium concentrations typically 3.5-5.0 mmol/L (normal range 2.10-2.55 mmol/L)), bone demineralization leading to fractures, and respiratory distress (TABLE 2)4. Hypercalcaemia in some patients with NSHPT may respond to treatment with cinacalcet, a calcimimetic (see below), but for long-term treatment parathyroidectomy is usually required86. Some patients who have the clinical features of FHH, but do not harbour CASR mutations, may have autoimmune hypocalciuric hypercalcaemia (AHH), which is associated with the presence of autoantibodies against the CaSR and also lymphocytic infiltrates within the parathyroid gland87,88. The hypercalcaemia caused by AHH may be responsive to glucocorticoid therapy87,88.
Loss-of-function mutations affect all regions of the CaSR protein, however, mutations commonly occur within the first 350 amino acid residues of the ECD, which contains the four Ca2+ binding sites and the VFT cleft amino acid binding site23,24. Indeed, this region is a hotspot for recurrently occurring FHH1 mutations, and some amino acid residues are also affected by multiple different loss-of-function mutations3,83. Approximately 50% of the FHH1 mutations are predicted to impair the biosynthesis and post-translational processing of CaSR proteins within the endoplasmic reticulum or Golgi apparatus, thus leading to enhanced proteasomal degradation of these nascent receptors, with reduced anterograde trafficking and cell-surface expression89,90. Some other FHH1 mutations, which are located in the ECD and TMD, have instead been demonstrated to induce biased signalling responses by switching the wild-type CaSR from preferentially coupling with intracellular Ca2+ (Ca2+i) signalling to a mutant receptor that signals equally via the Ca2+i and MAPK pathways, or which acts mainly through MAPK91,92. Biased signalling has also been observed with an AHH-causing autoantibody, which enhanced CaSR-mediated accumulation of inositol phosphates but impaired ERK1/2 phosphorylation93.
FHH2
FHH2 (OMIM#145981) is caused by germline heterozygous loss-of-function mutations of the GNA11 gene on chromosome 19p13.3, which encodes the Gα11 protein (TABLE 2)33. To date, FHH2-causing mutations have been reported in four probands, and these affected individuals exhibit mild hypercalcaemia (serum adjusted-calcium concentrations <2.80 mmol/L (normal range 2.10-2.55 mmol/L)), normal serum PTH concentrations, and normal or low urinary Ca2+ excretion33,47,94. The GNA11 mutations identified in FHH2 patients comprise three missense substitutions, Thr54Met, Leu135Gln, Phe220Ser, and an in-frame isoleucine deletion at residue 200 (Ile200del)33,47,94. All of these mutations have been demonstrated to impair CaSR-mediated signalling responses, and are predicted to affect key domains of the Gα11 subunit33,47,94. Thus, the Ile200del and Phe220Ser mutations are located within the Gα11 GTPase domain and predicted to impair the coupling of Gα11 with the upstream GPCR (i.e. CaSR) or downstream effector protein (i.e. PLC), respectively33,47. However, the Leu135Gln mutation is located within the portion of the Gα11 helical domain that interacts with downstream effectors, and the Thr54Met mutation is situated at the interface between GTPase and helical domains, and predicted to impair guanine-nucleotide binding at this interface33,94. These studies of FHH2 mutations have highlighted residues critical for Gα11 function.
FHH3
FHH3 (OMIM#600740) represents a more severe form of FHH, and is characterized by significantly higher serum calcium and magnesium concentrations and a significantly reduced FECa, when compared to FHH141,95. Moreover, symptomatic hypercalcaemia, reduced bone mineral density, recurrent pancreatitis and cognitive dysfunction have been reported in some FHH3 patients41,96. This disorder is caused by germline heterozygous loss-of-function mutations of the AP2S1 gene on chromosome 19q13.3, which encodes the AP2σ protein (TABLE 2)30. AP2S1 mutations have been reported in >60 FHH probands to date, and >99% of affected individuals harbour a mutation affecting the AP2σ Arg15 residue, which may give rise to an Arg15Cys, Arg15His or Arg15Leu missense substitution30,41,42,95,97–99. To date, only one FHH patient has a mutation not involving the Arg15 residue, but the Met117 residue100. The prevalence of FHH3 has been reported to be ~7.8 per 100:000100. Patients harbouring the Arg15Leu AP2σ mutation have been shown to be more hypercalcaemic with an earlier age of presentation compared to probands with Arg15Cys or Arg15His AP2σ mutations41,99. Crystal structure analyses have demonstrated the AP2σ Arg15 residue to bind to cell-surface cargo proteins101, and it is predicted that these FHH3-causing Arg15 mutations disrupt an interaction between the AP2 complex and the CaSR ICD, thereby diminishing endocytosis of this GPCR30. Indeed, in vitro studies have shown FHH3-causing AP2σ mutations to alter CaSR cell-surface expression and impair signal transduction in a dominant-negative manner30,41. Thus, these studies highlight a role for the AP2 complex in Ca2+o homeostasis.
Hypocalcaemic disorders including ADH
ADH is caused by increased sensitivity of the CaSR signalling pathway to Ca2+o concentrations 33,102 and has the opposite biochemical phenotype to FHH. Thus, ADH patients have low serum calcium concentrations, mild hypomagnesaemia, normal or low PTH concentrations, and a relative or absolute hypercalciuria3. Approximately 50% of ADH patients have symptomatic hypocalcaemia and >30% of patients have renal and/or intracerebral calcifications (TABLE 2)3. Some ADH patients with marked CaSR activation may additionally have Bartter syndrome type V, which is characterised by hypokalaemic alkalosis, renal salt wasting and hyperreninaemic hyperaldosteronism (TABLE 2)103,104. Active vitamin D metabolites, combined with adequate dietary calcium intake and/or use of calcium supplements, are currently used to treat symptomatic ADH patients, although their use may predispose patients to the development of marked hypercalciuria, nephrocalcinosis, nephrolithiasis and renal impairment102,105.
ADH is a genetically heterogeneous disorder with two types (ADH type 1 (ADH1; OMIM #601198) and ADH type 2 (ADH2; OMIM #615361) described3. ADH1 and ADH2 are caused by germline heterozygous gain-of-function CASR and GNA11 mutations, respectively (TABLE 2)83,102 33,106–108. To date, >70 different ADH1-causing CaSR mutations have been identified (http://www.casrdb.mcgill.ca)85, and ~95% of these are missense substitutions, whereas ~5% are inframe or frameshift insertion/deletion mutations83. Structure-function analyses have shown ADH1 mutations to cluster within the second peptide loop of the CaSR ECD (residues 116-136)109, which contributes to the CaSR dimer interface23,24,110, and also within the distal portion of the TMD (residues 819-837)111, which is involved in G-protein binding112. Moreover, the Leu173 and Pro221 ECD residues have been shown to be the site of both ADH and FHH mutations, and structure-function analysis indicates that these residues may act as intra-molecular switches to regulate ligand binding or potentially influence the receptor conformational changes that occur upon agonist binding83,113. Some patients with hypoparathyroidism, which may be isolated or due to autoimmune polyglandular syndrome type 1 (APS1) have been found to harbour activating autoantibodies to the CaSR114–116. These patients may have mild and asymptomatic hypocalcemia, or develop hypocalcaemic seizures that require treatment with calcium and vitamin D preparations115.
Six different gain-of-function missense GNA11 mutations (Arg60Cys, Arg60Leu, Arg181Gln, Ser211Trp, Val340Met and Phe341Leu) have been reported to cause ADH233,106–108, and affected individuals have a similar phenotype to ADH1 patients. However, ADH2 patients may have milder alterations in urinary Ca2+ excretion compared to ADH1106, and short stature has also been reported in two ADH2 kindreds106,117. Structural modelling studies have shown ADH2 mutations to cluster at the interface between the helical and GTPase domains of the Gα11 protein, which mediates GDP/GTP exchange; and also to be located at the Gα11 C-terminus, which is involved in GPCR-G-protein coupling3,33,108.
Role of the CaSR in common calcitropic diseases
Altered CaSR expression is implicated in the pathogenesis of common parathyroid disorders such as primary and secondary hyperparathyroidism. Moreover, CASR SNPs have been reported to be associated with hypercalciuria and nephrolithiasis.
Primary hyperparathyroidism
PHPT is associated with an increased set-point for Ca2+o-mediated PTH release, which indicates a role for altered CaSR function in the pathogenesis of this disorder. In support of this, germline heterozygous or homozygous loss-of-function CASR mutations have occasionally been reported in patients with adult-onset PHPT118. However, somatic CASR mutations have not been identified in parathyroid tumours from patients with sporadic PHPT119–121. Instead, the parathyroid set-point abnormalities associated with PHPT may be partly attributed to alterations in CaSR expression, which is reduced in the majority of hyperplastic and adenomatous parathyroid tumours122,123. In keeping with this, ex-vivo studies have shown the set-point for Ca2+o-mediated PTH release to be inversely related to the amount of CaSR expression in parathyroid adenomas124. This reported decreased expression of CaSR in some parathyroid tumors may be associated with downregulation of filamin A125, which is scaffolding protein that interacts with the CaSR in parathyroid cells126, and it is reported that there are no alterations in CASR gene promoter methylation127. The mechanisms causing this reduced CaSR expression in parathyroid tumours remain to be fully elucidated. Common CASR polymorphisms have been reported to influence the clinical severity of PHPT. In particular, the Arg990Gly SNP, which is located in the CaSR cytoplasmic domain, has been reported to be associated with lower serum PTH concentrations, increased urinary excretion, and also increased occurrence of nephrocalcinosis in PHPT patients128,129.
Chronic kidney disease and secondary hyperparathyroidism
Secondary hyperparathyroidism (SHPT) is a common complication of chronic kidney disease (CKD), and may arise early in the pathogenesis of this disorder130. Altered CaSR function is involved in the progression of SHPT in CKD patients130,131. Thus, hypocalcaemia, which occurs as a consequence of hyperphosphataemia and reduced 1,25(OH)2D synthesis in CKD, inactivates the parathyroid CaSR, thereby increasing PTH secretion130,131. Prolonged PTH secretion in CKD is associated with increased parathyroid cell proliferation and gland hyperplasia, and studies in uraemic rats have shown that this is accompanied by reduced CaSR expression, which can be upregulated following calcimimetic treatment132,133. The reduction in CaSR expression is most marked in nodular parathyroid gland hyperplasia caused by advanced SHPT130,134. Furthermore, ex-vivo studies involving freshly excised parathyroid glands from haemodialysis patients with SHPT, have shown CaSR expression to inversely correlate with parathyroid gland weight and to be associated with an increase in the set-point for Ca2+o-mediated PTH release135. Studies in rats have shown that renal CaSR expression is also reduced in CKD and may contribute to the hypocalciuria associated with this disorder136.
Idiopathic hypercalciuria and nephrolithiasis
Idiopathic hypercalciuria (IH) is associated with nephrolithiasis, and may be familial137, although germline CASR mutations have not been identified in families with autosomal dominant inheritance of IH138. However, common SNPs located within the coding and regulatory regions of the CASR gene have been implicated in nephrolithiasis in patient-based studies. Thus, the common Arg990Gly CASR SNP has been reported in association with an increased risk of hypercalciuric nephrolithiasis139, and a common CASR promoter-region SNP (rs6776158 (G>A)), which has been shown to impair CASR transcriptional activity in vitro and decrease CaSR expression within the renal medulla, is reported to be associated with an increased risk of Ca2+-containing renal calculi140. The mechanisms underlying the development of nephrolithiasis in patients harbouring the rs6776158 CASR promoter-region SNP remain to be established as these patients did not have alterations in the urinary concentrations of calcium or phosphate140.
Drugs for calcitropic diseases
CaSR allosteric modulators, which comprise calcimimetic and calcilytic compounds, are being used as a targeted therapy for parathyroid disorders and also for symptomatic hypercalcaemia and hypocalcaemia caused by germline loss- and gain-of-function CaSR mutations, respectively52,141.
Calcimimetics
Calcimimetics are ligands that mimic the effects of Ca2+o at the CaSR, and are divided into two types: type I calcimimetics are agonists, and include naturally occurring ligands; and type II calcimimetics are PAMs that increase the sensitivity of the CaSR to Ca2+o3,142. Calcimimetic compounds, which decrease PTH secretion and parathyroid gland proliferation, are used for the treatment of hyperparathyroid disorders143,144. Cinacalcet, which is a phenylalkylamine compound that binds to the CaSR TMD, was the first calcimimetic to be licensed as a therapy for hyperparathyroid conditions such as secondary hyperparathyroidism due to end-stage renal failure; inoperable forms of primary hyperparathyroidism; and parathyroid carcinoma6. More recently, etelcalcetide, which is an intravenously administered synthetic polycationic peptide that acts as a type II calcimimetic and agonist of the CaSR145, has been approved for the management of secondary hyperparathyroidism in adult patients on haemodialysis146. Etelecalcetide has been shown to bind to the Cys482 residue within the CaSR VFT147.
Calcimimetics also represent a targeted therapy for symptomatic forms of FHH52, and have been shown to improve the signalling responses of cells expressing loss-of-function CaSR, Gα11 or AP2σ mutant proteins in vitro42,46,47,92,148, and to significantly lower serum calcium concentrations in patients with symptomatic hypercalcaemia caused by FHH types 1-342,48,149,150. Cinacalcet has also been successfully used to manage life-threatening hypercalcaemia in NSHPT patients harbouring a heterozygous CaSR mutation, Arg185Gln151, but is ineffective for NSHPT caused by biallelic deletional CaSR mutations152.
Calcilytics
Calcilytics are synthetic NAMs that bind to the CaSR TMD27, and comprise two main classes of compounds, which are the amino alcohols (e.g. NPS-2143, ronacaleret, NPSP795 and JTT-305/MK-5442) and quinazolinones (e.g. ATF936 and AXT914)6. These compounds were originally investigated as potential therapies for osteoporosis, as they induced a transient rise in PTH secretion, which had the potential to induce anabolic effects on bone mass153. However, clinical trials have shown that calcilytics such as ronacaleret and JTT-305/MK-5442 are not effective for treating postmenopausal osteoporosis154,155, and these compounds have instead been investigated as a potential targeted therapy for ADH52. In vitro studies have shown the NPS-2143 calcilytic to rectify the increased signalling responses associated with gain-of-function CaSR and Gα11 mutations, which cause ADH1 and ADH2, respectively46,92,156,157. However, NPS-2143 has limited efficacy for severe gain-of-function mutations, which cause Bartter syndrome type V157; whereas the quinazolinone-derived calcilytic drugs have successfully rectified constitutive activation caused by Bartter syndrome-associated CaSR mutations158. Calcilytics have also been assessed in vivo, and single dose studies have shown NPS-2143 to significantly increase plasma calcium and PTH concentrations in mouse models for ADH1 and ADH248,156,159. Moreover, longer-term studies involving ADH1 mouse models have shown the JTT-305/MK-5442 calcilytic to prevent the development of nephrocalcinosis, which was observed in mice treated with the drug vehicle or recombinant PTH159. Intravenous administration of the NPSP795 calcilytic compound in a phase IIa clinical trial involving five ADH1 patients, significantly increased plasma PTH concentrations and reduced urinary calcium excretion160.
Non-calcitropic roles of the CaSR
The CaSR is widely expressed in tissues not involved in Ca2+o homeostasis, thereby indicating that it likely has non-calcitropic roles. These non-calcitropic roles of the CaSR involve regulation of physiological and pathophysiological processes in a tissue-dependent manner, and these include: proliferation, differentiation and apoptosis; vascular tone; renal and intestinal water transport; inflammation; maintenance of the integrity of epithelial layers in the intestine and skin; neuronal development and function; and entero-endocrine hormone secretion (TABLE 1)7,9–11,13,14,161,162. Thus, abnormal CaSR function or expression in non-calcitropic organs may be associated with cancer, cardiovascular diseases, asthma, gastro-intestinal disorders, Alzheimer’s disease, pancreatic insulin secretion and poor wound healing (FIG. 4)163,164. Some of these physiological and pathophysiological non-calcitropic roles of the CaSR are reviewed briefly, below.
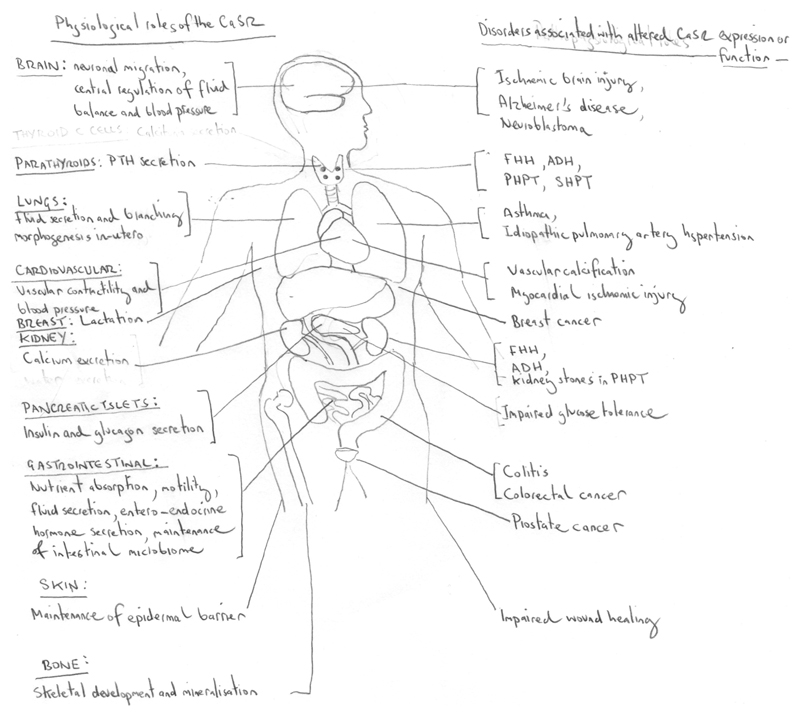
Figure 4. Physiological roles and disease associations of the CaSR.
The CaSR has key physiological roles in calcitropic tissues (e.g. parathyroid glands, kidneys and bone) and in non-calcitropic tissues (e.g. brain, cardiovascular system, lungs, breast, intestine and pancreas, and skin). Altered CaSR activity in calcitropic tissues causes inherited and sporadic diseases of Ca2+ homeostasis3, whereas alterations in CaSR function or expression in non-calcitropic tissues is associated with cardiovascular disease, asthma and malignancy. ADH, autosomal dominant hypocalcaemia; FHH, familial hypocalciuric hypercalcaemia; PHPT, primary hyperparathyroidism; SHPT, secondary hyperparathyroidism.
Tumourigenesis
The CaSR regulates cell fate and its expression has been reported to be increased or decreased in different cancers, thereby suggesting that the CaSR may have possible roles as an oncogene or tumour suppressor in different organs. For example, increased CaSR expression is found in metastatic breast and prostate cancers16,165,166, whereas loss of CaSR expression is found in colorectal cancer and some neuroblastic tumours16,167. In breast cancer cells, the CaSR has been reported to act as an oncogene and increase proliferation and inhibit apoptosis168 by likely switching G-protein coupling from Gαi, to Gαs, which leads to an upregulation of PTHrP expression169. This increase in PTHrP results in the inhibition of the apoptosis inducing factor (AIF) and cell cycle inhibitor p27kip1, thereby reducing apoptosis and promoting cell proliferation, respectively168. Moreover, in vitro studies involving the stimulation of malignant breast carcinoma cells with Ca2+o or the R-568 calcimimetic have shown the CaSR to increase the migratory potential of these tumour cells, and also promote the secretion of pro-angiogenic and chemotactic factors that are implicated in breast cancer metastases170,171. Furthermore, metastatic breast cancer cells in bone that express the CaSR may exacerbate PTHrP-mediated osteolysis by sensing high concentrations of Ca2+ released during bone remodelling172, and thereby promoting further PTHrP secretion. Thus, the CaSR has been hypothesised to drive a vicious cycle of skeletal metastasis173. In support of this, PTHrP secretion from breast cancer cells has been shown to be stimulated by high (7.5-10 mM) Ca2+o concentrations173, which are present in the bone microenvironment during remodelling172, and breast cancer cells overexpressing the CaSR have been demonstrated to cause an increase in osteolytic lesions when injected intratibially into Balb/c-Nude mice174. Thus, these findings indicate that the CaSR plays a role in the pathogenesis of breast cancer skeletal metastases.
The CaSR is involved in the differentiation of neural progenitor cells and has a tumour suppressor role in malignancies affecting the developing nervous system. Thus, the CaSR is expressed in neuroblastic tumours, which comprise neuroblastomas, ganlioneuroblastomas and ganglioneuromas, and its expression in these tumours is associated with favourable prognostic outcomes such as low clinical stage and age at diagnosis <1 year175. In contrast, in highly malignant and undifferentiated neuroblastomas, the CaSR has been shown to be epigenetically silenced, and overexpression of the CaSR or treatment with cinacalcet in neuroblastoma cell lines reduces tumourigenicity by decreasing proliferation and increasing apoptosis167,176.
The CaSR may also mediate the effects of dietary Ca2+ on the development of prostate and colorectal cancer. Thus, a high dietary intake of Ca2+ is reported to enhance prostate tumourigenesis by potentially acting on the CaSR and the TRPC6 channel, which is a Ca2+-permeable channel177. In contrast, dietary calcium is reported to be protective against colorectal cancer, and this effect is likely mediated by the CaSR as it was observed only in patients with CaSR-expressing tumours178. In the colon, the CaSR has tumour suppressor roles and been shown to: inhibit proliferation, induce apoptosis and differentiation; prevent epithelial-to-mesenchymal transition179–182; and suppress canonical Wnt signalling whilst activating the non-canonical Wnt pathway183. Furthermore, CaSR expression has been demonstrated to be lost during colorectal tumourigenesis, mainly through epigenetic mechanisms184–186.
Nervous system
The CaSR is widely expressed in the central and peripheral nervous system including in: nerve terminals and fibre tracts; myelin-producing oligodendrocytes; astrocytes and microglial cells187,188. Moreover, the CaSR is considered to play a role in the development of the hippocampus, granule cell layer of the cerebellum, sympathetic nervous system, and also in GnRH neuronal migration188–191. The CaSR has been shown to modulate the growth of sympathetic axons as well as promoting dendritic growth within the hippocampus191. The CaSR may also regulate neuronal excitability within the hippocampus by regulating neuronal potassium (K+) channels and the sodium (Na+) leak channel non-selective protein (NALCN)12. Furthermore, the CaSR may facilitate neuronal migration by sensing alterations in Ca2+o or polycations such as spermine, which are present within the brain, and also by enhancing the production of chemokines such as monocyte chemoattractant protein-1189,190. The CaSR is also expressed in the sub-fornical organ (SFO), which is involved in Na+ sensing and water intake192, and the SFO-expressed CaSR may potentially influence body fluid composition12.
Abnormal CaSR function has been implicated in central nervous system disorders such as Alzheimer’s disease (AD), ischaemic brain injury and epilepsy. Indeed, a patient-based study showed the presence of a polymorphic dinucleotide repeat within intron 4 of the CASR to be significantly associated with the occurrence of AD193. Moreover, in-vitro studies have shown that: CaSR function can be upregulated by proteins such as the amyloid-β 1-42 peptide fragment (Aβ(1-42)) and apolipoprotein E, which are involved in the pathogenesis of AD193; and that the calcilytic drug NPS 2143 inhibits the secretion of Aβ(1-42) from human astrocytes and cortical neurons in vitro194. The neuronal CaSR forms heteromeric complexes with the GABAB receptor 1 (GBR1), and mouse model studies have demonstrated in ischaemic brain injury that the stoichiometry of these complexes is altered with upregulation of CaSR expression and down regulation of GBR1, which potentiates ischaemic neuronal death195. These studies also revealed that calcilytic treatment in mice with brain injury was neuroprotective and improved learning and memory retention45. CaSR mutations have been reported in patients with epilepsy196, and in vitro studies of a CaSR Arg898Gln mutation, which co-segregated with epilepsy in one family, in which the proband was normocalcaemic196, revealed that this is a gain-of-function mutation that increased CaSR expression197.
Cardiovascular
The CaSR is expressed in arterial vessels and influences cardiovascular function through direct effects within the vasculature. Thus, the CaSR is expressed in human vascular smooth muscle cells (VSMCs) and endothelial cells198,199, and it has been reported to likely play a role in VSMC proliferation200, and also in the regulation of blood pressure and blood vessel tone13. Indeed, ablation of the VSMC CaSR in mice has been shown to cause endothelium-independent decreases in the contractility of the aorta and mesenteric arteries, and also to decreases in diastolic and mean arterial blood pressures13. Furthermore, in rabbit mesenteric arteries, CaSR stimulation induced endothelium-dependent vasorelaxation through two different pathways; one involving the production of nitric oxide (NO), and the other involving activation of the intermediate Ca2+-activated potassium (IKCa) channels201. The VSMC CaSR also protects against the development of vascular calcification, and histological studies of lower-limb arteries from CKD patients have demonstrated reduced expression of the CaSR within the calcified medial arterial layer44. These findings are supported by studies of primary VSMCs, in which loss of CaSR expression or overexpression of a loss-of-function mutant CaSR in these cells leads to increased mineralisation44. Furthermore, the CaSR represents a potential therapeutic target in patients with vascular calcification, and a randomised controlled trial involving CKD patients on haemodialysis showed that cinacalcet treatment resulted in beneficial effects on arterial calcification, and particularly for calcification involving the cardiac valves202.
The CaSR is also expressed in the heart and has been shown to be functionally active in rodent ventricular cardiomyocytes, where it promotes apoptosis203 and may also protect against myocardial ischaemic injury204. Moreover, patient-based studies have demonstrated that a common CaSR SNP, Ala986Ser, is associated with an increased risk of cardiovascular diseases such as myocardial infarction15,205, and it remains to be established whether this is a direct consequence of abnormal CaSR function within the heart or vasculature, or due to an indirect effect of the polymorphic CaSR on Ca2+o homeostasis15.
Pulmonary development and function
The CaSR has been reported to be to be expressed in human fetal lungs, where it promotes fluid secretion into the pulmonary lumen, which mediates lung growth and development206, and studies in mice have also indicated a role for the CaSR in regulating lung branching morphogenesis207. The CaSR is also expressed in adult human and mouse airway smooth muscle and bronchial epithelium14, and CaSR expression has been demonstrated to be increased in the airways of asthmatic patients and mice14. Moreover, over-expression of CaSR is considered to be activated by polycations in the asthmatic airway mucosa, thereby leading to bronchoconstriction14. Calcilytic treatment was shown to diminish signalling responses that caused airway contractility, and nebulised calcilytic administration significantly suppressed airway hyper-reactivity and inflammation in a mouse model of allergic asthma14. Thus, these observations highlight the potential of inhaled calcilytics as a treatment for asthma14. Pathological upregulation of CaSR expression has also been implicated in idiopathic pulmonary artery hypertension, and calcilytic treatment in rodents has been demonstrated to prevent the development of this disorder and ameliorate the secondary occurrence of right ventricular hypertrophy208.
Gastro-intestinal tract
The CaSR, which is expressed in the gastro-intestinal (GI) tract of amphibia, birds, fish and mammals209, has been reported to act as a nutrient sensor that influences gastric acid and entero-endocrine hormone secretion, as well as regulating intestinal fluid homeostasis, and intestinal barrier function and inflammation (TABLE 1)10,209–211.
Nutrient sensing and entero-endocrine hormone secretion
The CaSR senses nutrients such as Ca2+ and aromatic amino acids within the GI lumen and responds to alterations in these nutrients by regulating hormone secretion from entero-endocrine cells, which are located throughout the GI tract and include: gastrin-secreting G-cells; ghrelin-secreting gastric cells; cholecystokinin (CCK)-secreting I-cells; and glucagon-like peptide-1 (GLP-1) and peptide YY (PYY)-secreting L-cells212–214. The CaSR is expressed in gastric G-cells, and studies involving CaSR null mice have shown the CaSR to mediate Ca2+- and aromatic amino acid-induced gastrin secretion215. Gastrin promotes histamine release from gastric body enterochromaffin-like (ECL) cells, which in turn enhances acid secretion from gastric parietal cells215. The CaSR is also expressed in gastric parietal cells and human studies have shown this GPCR to increase gastric acid secretion by influencing H+K+ATPase activity216,217.
Activation of the CaSR in the small intestine has also been found to improve post-prandial glucose tolerance in wild-type rats218. This effect of the GI-expressed CaSR on glucose tolerance may be mediated by a reduction in gastric emptying rate218, as well as through effects on GLP-1, which plays a key role in enhancing glucose-dependent insulin release219. Indeed, ex-vivo studies involving mouse intestine have shown that the CaSR is expressed in GLP-1-secreting L-cells and also that oligopeptides enhance GLP-1 secretion by activation of the CaSR219.
The GI-expressed CaSR also reduces food intake by modulating the secretion of entero-endocrine hormones210. Thus, the CaSR has been shown to inhibit the release of ghrelin and increase the secretion of PYY, which are neuropeptides involved in the hypothalamic regulation of appetite210. The CaSR may also exert these anorectic effects by increasing the secretion of GLP-1210, which in turn suppresses appetite following food ingestion, as well as by increasing CCK secretion from I-cells220, that leads to a delay in gastric emptying. CaSR activation may also cause emesis and studies involving mice have demonstrated that deoxynivalenol, which is an emesis-inducing toxin, acts by increasing CaSR-mediated secretion of CCK and PYY221. These findings may potentially explain the GI adverse effects such as nausea and vomiting, which are experienced by >30% of patients being treated with cinacalcet, which is used for pharmacological activation of the CaSR 146. Moreover, cinacalcet has been shown to impair gastric emptying in rats222, whereas, evocalcet, which is a recently reported orally active pyrrolidine-derived calcimimetic compound, does not alter gastric emptying in rats, and has also been shown to cause reduced emesis compared to cinacalcet in studies involving marmosets222.
Intestinal fluid secretion
Activation of the CaSR within colon epithelial cells has been shown in rodent studies to inhibit fluid secretion induced by secretagogues such as cholera toxin223. Cholera toxin causes secretory diarrhoea by increasing cyclic nucleotide generation within the colonic epithelium and also by stimulating the enteric nervous system (ENS) to release secretagogues such as vasoactive peptide224.
The colonic CaSR has been shown to counteract these effects by activating phosphodiesterases, which degrade the cyclic nucleotides generated by cholera toxin, and also by inhibiting the ENS223,224. These findings reveal the CaSR to be a potential therapeutic target for secretory diarrhoea caused by bacterial toxins223.
Intestinal barrier function and inflammation
Alterations in intestinal epithelial barrier function are implicated in the pathogenesis of inflammatory bowel disorders such as Crohn’s disease225. The CaSR is expressed in colonic epithelial cells as well as in colonic myofibroblasts, which are located at the basal surface of the epithelium and regulate epithelial barrier function226,227. Cellular studies involving colonic myofibroblasts have shown the CaSR to increase secretion of bone morphogenetic protein-2 (BMP-2), which promotes colonic epithelial barrier maturation227. The CaSR also inhibits tumour necrosis factor alpha (TNFα) secretion from colonic myofibroblasts228, and thus this GPCR may protect against intestinal inflammation. The epithelial CaSR also plays a key role in intestinal barrier function, and studies of intestinal epithelium-specific CaSR null mice have shown reduced transepithelial resistance in association with reduced colonic expression of tight junction proteins such as claudin-2229. This defect in epithelial barrier function was associated with an altered composition of the intestinal microbiome229 that comprised a decrease in the amount of beneficial lactobacilli, but an increase in Deferribacteraceae bacteria, which are linked to colitis229. This dysbiosis of the intestinal microbiota has been associated with pro-inflammatory responses in intestinal epithelium-specific CaSR null mice230. Indeed, in response to chemically induced colitis, these mice had more severe colitis with delayed recovery, when compared with the CaSR-expressing littermate controls229.
Pancreatic islets and glucose homeostasis
The CaSR is highly expressed in pancreatic islet α- and β-cells 2,231, and studies of isolated human islets and insulin-secreting cell lines have shown that CaSR activation leads to the upregulation of PLC and MAPK-mediated signalling responses in association with a transient rise in insulin and glucagon secretion231–233. Furthermore, studies involving an ADH1 mouse model, known as Nuclear flecks (Nuf), have shown that CaSR activation in heterozygous (Casr+/Nuf) and homozygous (CasrNuf/Nuf) mice is associated with impaired glucose tolerance, when compared to wild-type (Casr+/+) mice. This impaired glucose tolerance was ameliorated by calcilytic treatment7. Casr+/Nuf and CasrNuf/Nuf mice also had hypoinsulinaemia and reduced pancreatic islet mass, which was associated with reduced β-cell proliferation7. In addition, CasrNuf/Nuf mice had a lack of glucose-mediated suppression of glucagon secretion, which was associated with altered α-cell membrane depolarization7. These in vivo and ex vivo studies have highlighted roles for the CaSR in the regulation of pancreatic islet mass, and in α- and β-cell function.
Skin
Extracellular Ca2+ is required for the maintenance of an intact epidermal barrier, and in vitro studies have shown that Ca2+o plays a key role in keratinocyte differentiation234. Furthermore, Ca2+o mediates wound healing and skin re-epithelialisation following injury235. This effect is triggered by the epidermal CaSR, which upon activation promotes keratinocyte differentiation, survival, and adhesion236. In keeping with this, the barrier function of the skin in epidermis-specific CaSR null mice has been shown to be defective with impaired keratinocyte differentiation237. Moreover, mice with combined ablation of the CaSR and vitamin D receptor in keratinocytes were demonstrated to have delayed wound re-epithelialisation as a consequence of impaired keratinocyte adhesion and migration11. These findings reveal the importance of Ca2+ and vitamin D signalling for epidermal regeneration after injury11, analogous to the interactions between Ca2+ and vitamin D that have been reported for intestine function and colorectal tumourigenesis238.
Conclusions and future directions
The CaSR is a dimeric family C GPCR that signals via the G-proteins and beta-arrestin, and plays a pivotal role in bone and mineral metabolism by influencing parathyroid hormone secretion, urinary Ca2+ excretion, skeletal development, and lactation. Germline loss- and gain-of-function mutations of the CaSR or its intracellular partner proteins lead to inherited calcitropic disorders such as FHH types 1-3 and ADH types 1-2. Loss of parathyroid CaSR expression contributes to the development of primary and secondary hyperparathyroidism. The CaSR is also widely expressed in non-calcitropic tissues where it influence physiological processes such as nutrient sensing and the secretion of insulin and entero-endocrine hormones, neuronal and pulmonary development, vascular tone and wound healing. Moreover, pathophysiological alterations in CaSR expression or function are associated with cancers of the breast, prostate and colon, as well as with ischaemic brain injury, cardiovascular disease and asthma. The CaSR represents a pharmacological target for calcitropic disorders, and calcimimetic drugs are established as a medical therapy for hyperparathyroid disorders, and shown to be effective for symptomatic forms of FHH. Calcilytic drugs may represent a targeted therapy for ADH and have potential for other hypoparathyroid disorders. CaSR-targeted drugs are also being evaluated for non-calcitropic disorders, and novel strategies such as the use of inhaled calcilytics for asthma to minimize off-target effects, are being evaluated in pre-clinical models. However, a key challenge for the pharmaceutical industry is to develop compounds that can selectively influence biased signalling responses by the CaSR, thereby providing an approach for modulating CaSR function in a tissue- and disease-specific manner. The emergence of such therapies will aid the clinical management of non-calcitropic disorders without leading to off-target effects in calcitropic tissues.
Key points.
The CaSR is a family C G-protein coupled receptor that is expressed on the cell surface as a dimer, and signals via G-proteins and beta-arrestin
The CaSR regulates bone and mineral metabolism by influencing parathyroid hormone secretion, urinary Ca2+ excretion, skeletal development, and lactation
Germline CASR, GNA11 and AP2S1 mutations cause calcitropic disorders such as familial hypocalciuric hypercalcaemia (FHH) and/or autosomal dominant hypocalcaemia (ADH)
In non-calcitropic tissues, the CaSR influences biological processes that include: gastrointestinal nutrient sensing; secretion of insulin and entero-endocrine hormones; vascular tone; and wound healing
Abnormal expression or function of the CaSR is associated with: primary and secondary hyperparathyroidism; ischaemic brain injury; cardiovascular disease; asthma; and cancers of the breast, prostate and colon
CaSR-targeted calcimimetic and calcilytic drugs have therapeutic potential for calcitropic and non-calcitropic diseases
Acknowledgements
The authors are supported by: UK Medical Research Council programme grants (G9825289 and G1000467, to R.V.T.); Wellcome Trust Investigator Award (to R.V.T.); National Institute for Health Research Senior Investigator Award (to R.V.T.); European Commission Seventh Framework Programme (FP7-264663, to RV.T. and E.K.); Horizon 2020 Programme of the European Union (Project ID: 675228, to F.M.H., E.K., M-L. B., and R.V.T.); Austrian Science Fund (P29948 - B28, to E.K.), Vienna Science and Technology Fund (LS12-047, to E.K.); US National Institute of Health Research Award (R01AR067291, to W.C.); and Department of Veteran Affairs Merit Review Grant (I01 BX003453-01A2, to W.C.).
Author Biographies
Dr Fadil Hannan is a Senior Clinical Lecturer in Musculoskeletal Biology at the University of Liverpool and Honorary Consultant Chemical Pathologist at the Royal Liverpool University Hospital, UK. His work focuses on elucidating the molecular, cellular and physiological role of the calcium-sensing receptor and partner proteins in mineral homeostasis.
Dr Enikö Kallay is Associate Professor at the Medical University of Vienna: She coordinates the EU-funded (Horizon 2020) Marie Sklodowska Curie European Training Network “CaSR Biomedicine. She studies the molecular and functional aspects of the CaSR in human colon cancer cells and its role in colorectal neoplastic transformation.
Dr Wenhan Chang, Ph.D., Professor of Medicine, investigates mechanisms controlling mineral and skeletal homeostasis with emphases on the actions of CaSR in physiological and pathological contexts. He and his colleague, Dr. Chia-Ling Tu, at UCSF generated floxed-CaSR mouse line that permitted the development of various tissue-specific CaSR knockout mouse models.
Dr Maria Luisa Brandi is Full Professor of Endocrinology at the University of Florence, and director of the Regional Program on Hereditary Endocrine Tumors and Bone Metabolic Unit. She is the President of the "Fondazione Italiana Ricerca sulle Malattie dell'Osso", a non-profit research organization devoted to bone metabolic disorders.
Rajesh Thakker is the May Professor of Medicine at the University of Oxford, and a Fellow of the Royal Society. He has pursued molecular, cellular, and physiological studies of mineral homeostasis disorders, and elucidated regulatory pathways downstream of the calcium-sensing receptor, as well evaluating therapies targeted to this receptor.
Footnotes
Competing interests:
F.M.H and R.V.T. have received grant funding from NPS/Shire Pharmaceuticals and GlaxoSmithKline for studies involving the use of calcium-sensing receptor allosteric inhibitors.
Contributor Information
Fadil M. Hannan, Department of Musculoskeletal Biology, Institute of Ageing and Chronic Disease, University of Liverpool, Liverpool, United Kingdom
Enikö Kallay, Department of Pathophysiology and Allergy Research, Medical University of Vienna, Vienna, Austria.
Wenhan Chang, Endocrine Research Unit, Veterans Affairs Medical Center, University of California, San Francisco, San Francisco, CA, USA.
Maria Luisa Brandi, Metabolic Bone Diseases Unit, Department of Surgery and Translational Medicine, University of Florence, Florence, Italy.
Rajesh V. Thakker, Academic Endocrine Unit, Radcliffe Department of Medicine, University of Oxford, Oxford, United Kingdom
References
- 1.Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001;81:239–297. doi: 10.1152/physrev.2001.81.1.239. [DOI] [PubMed] [Google Scholar]
- 2.Regard JB, Sato IT, Coughlin SR. Anatomical profiling of G protein-coupled receptor expression. Cell. 2008;135:561–571. doi: 10.1016/j.cell.2008.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hannan FM, Babinsky VN, Thakker RV. Disorders of the calcium-sensing receptor and partner proteins: insights into the molecular basis of calcium homeostasis. J Mol Endocrinol. 2016;57:R127–142. doi: 10.1530/JME-16-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hannan FM, Thakker RV. Calcium-sensing receptor (CaSR) mutations and disorders of calcium, electrolyte and water metabolism. Best Pract Res Clin Endocrinol Metab. 2013;27:359–371. doi: 10.1016/j.beem.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Ward DT, Brown EM, Harris HW. Disulfide bonds in the extracellular calcium-polyvalent cation-sensing receptor correlate with dimer formation and its response to divalent cations in vitro. J Biol Chem. 1998;273:14476–14483. doi: 10.1074/jbc.273.23.14476. [DOI] [PubMed] [Google Scholar]
- 6.Nemeth EF, Goodman WG. Calcimimetic and Calcilytic Drugs: Feats, Flops, and Futures. Calcif Tissue Int. 2016;98:341–358. doi: 10.1007/s00223-015-0052-z. [DOI] [PubMed] [Google Scholar]
- 7.Babinsky VN, et al. Mutant Mice With Calcium-Sensing Receptor Activation Have Hyperglycemia That Is Rectified by Calcilytic Therapy. Endocrinology. 2017;158:2486–2502. doi: 10.1210/en.2017-00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chattopadhyay N, et al. Calcium receptor expression and function in oligodendrocyte commitment and lineage progression: potential impact on reduced myelin basic protein in CaR-null mice. J Neurosci Res. 2008;86:2159–2167. doi: 10.1002/jnr.21662. [DOI] [PubMed] [Google Scholar]
- 9.Kim W, Wysolmerski JJ. Calcium-Sensing Receptor in Breast Physiology and Cancer. Front Physiol. 2016;7:440. doi: 10.3389/fphys.2016.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macleod RJ. CaSR function in the intestine: Hormone secretion, electrolyte absorption and secretion, paracrine non-canonical Wnt signaling and colonic crypt cell proliferation. Best Pract Res Clin Endocrinol Metab. 2013;27:385–402. doi: 10.1016/j.beem.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Oda Y, et al. Combined Deletion of the Vitamin D Receptor and Calcium-Sensing Receptor Delays Wound Re-epithelialization. Endocrinology. 2017;158:1929–1938. doi: 10.1210/en.2017-00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruat M, Traiffort E. Roles of the calcium sensing receptor in the central nervous system. Best Pract Res Clin Endocrinol Metab. 2013;27:429–442. doi: 10.1016/j.beem.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Schepelmann M, et al. The vascular Ca2+-sensing receptor regulates blood vessel tone and blood pressure. Am J Physiol Cell Physiol. 2016;310:C193–204. doi: 10.1152/ajpcell.00248.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yarova PL, et al. Calcium-sensing receptor antagonists abrogate airway hyperresponsiveness and inflammation in allergic asthma. Sci Transl Med. 2015;7 doi: 10.1126/scitranslmed.aaa0282. 284ra260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larsson SC, Burgess S, Michaelsson K. Association of Genetic Variants Related to Serum Calcium Levels With Coronary Artery Disease and Myocardial Infarction. JAMA. 2017;318:371–380. doi: 10.1001/jama.2017.8981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tennakoon S, Aggarwal A, Kallay E. The calcium-sensing receptor and the hallmarks of cancer. Biochim Biophys Acta. 2016;1863:1398–1407. doi: 10.1016/j.bbamcr.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 17.Kniazeff J, Prezeau L, Rondard P, Pin JP, Goudet C. Dimers and beyond: The functional puzzles of class C GPCRs. Pharmacol Ther. 2011;130:9–25. doi: 10.1016/j.pharmthera.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Chang W, et al. Complex formation with the Type B gamma-aminobutyric acid receptor affects the expression and signal transduction of the extracellular calcium-sensing receptor. Studies with HEK-293 cells and neurons. J Biol Chem. 2007;282:25030–25040. doi: 10.1074/jbc.M700924200. [DOI] [PubMed] [Google Scholar]
- 19.Cheng Z, et al. Type B gamma-aminobutyric acid receptors modulate the function of the extracellular Ca2+-sensing receptor and cell differentiation in murine growth plate chondrocytes. Endocrinology. 2007;148:4984–4992. doi: 10.1210/en.2007-0653. [DOI] [PubMed] [Google Scholar]
- 20.Gama L, Wilt SG, Breitwieser GE. Heterodimerization of calcium sensing receptors with metabotropic glutamate receptors in neurons. J Biol Chem. 2001;276:39053–39059. doi: 10.1074/jbc.M105662200. [DOI] [PubMed] [Google Scholar]
- 21.Felder CB, Graul RC, Lee AY, Merkle HP, Sadee W. The Venus flytrap of periplasmic binding proteins: an ancient protein module present in multiple drug receptors. AAPS PharmSci. 1999;1:E2. doi: 10.1208/ps010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herberger AL, Loretz CA. Vertebrate extracellular calcium-sensing receptor evolution: selection in relation to life history and habitat. Comp Biochem Physiol Part D Genomics Proteomics. 2013;8:86–94. doi: 10.1016/j.cbd.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Geng Y, et al. Structural mechanism of ligand activation in human calcium-sensing receptor. Elife. 2016;5 doi: 10.7554/eLife.13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang C, et al. Structural basis for regulation of human calcium-sensing receptor by magnesium ions and an unexpected tryptophan derivative co-agonist. Science Advances. 2016;2:e1600241. doi: 10.1126/sciadv.1600241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.On JS, Chow BK, Lee LT. Evolution of parathyroid hormone receptor family and their ligands in vertebrate. Front Endocrinol (Lausanne) 2015;6:28. doi: 10.3389/fendo.2015.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goolam MA, et al. Roles of intraloops-2 and -3 and the proximal C-terminus in signalling pathway selection from the human calcium-sensing receptor. FEBS Lett. 2014;588:3340–3346. doi: 10.1016/j.febslet.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 27.Leach K, et al. Towards a structural understanding of allosteric drugs at the human calcium-sensing receptor. Cell Res. 2016;26:574–592. doi: 10.1038/cr.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Breitwieser GE. The calcium sensing receptor life cycle: trafficking, cell surface expression, and degradation. Best Pract Res Clin Endocrinol Metab. 2013;27:303–313. doi: 10.1016/j.beem.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Zhuang X, Northup JK, Ray K. Large putative PEST-like sequence motif at the carboxyl tail of human calcium receptor directs lysosomal degradation and regulates cell surface receptor level. J Biol Chem. 2012;287:4165–4176. doi: 10.1074/jbc.M111.271528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nesbit MA, et al. Mutations in AP2S1 cause familial hypocalciuric hypercalcemia type 3. Nat Genet. 2013;45:93–97. doi: 10.1038/ng.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conigrave AD, Ward DT. Calcium-sensing receptor (CaSR): pharmacological properties and signaling pathways. Best Pract Res Clin Endocrinol Metab. 2013;27:315–331. doi: 10.1016/j.beem.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Thomsen AR, Smajilovic S, Brauner-Osborne H. Novel strategies in drug discovery of the calcium-sensing receptor based on biased signaling. Curr Drug Targets. 2012;13:1324–1335. doi: 10.2174/138945012802429642. [DOI] [PubMed] [Google Scholar]
- 33.Nesbit MA, et al. Mutations affecting G-protein subunit alpha11 in hypercalcemia and hypocalcemia. N Engl J Med. 2013;368:2476–2486. doi: 10.1056/NEJMoa1300253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hofer AM, Brown EM. Extracellular calcium sensing and signalling. Nat Rev Mol Cell Biol. 2003;4:530–538. doi: 10.1038/nrm1154. [DOI] [PubMed] [Google Scholar]
- 35.Kifor O, et al. Regulation of MAP kinase by calcium-sensing receptor in bovine parathyroid and CaR-transfected HEK293 cells. Am J Physiol Renal Physiol. 2001;280:F291–302. doi: 10.1152/ajprenal.2001.280.2.F291. [DOI] [PubMed] [Google Scholar]
- 36.Tfelt-Hansen J, et al. Calcium-sensing receptor stimulates PTHrP release by pathways dependent on PKC, p38 MAPK, JNK, and ERK1/2 in H-500 cells. Am J Physiol Endocrinol Metab. 2003;285:E329–337. doi: 10.1152/ajpendo.00489.2002. [DOI] [PubMed] [Google Scholar]
- 37.Thomsen AR, Hvidtfeldt M, Brauner-Osborne H. Biased agonism of the calcium-sensing receptor. Cell Calcium. 2012;51:107–116. doi: 10.1016/j.ceca.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 38.Pi M, Spurney RF, Tu Q, Hinson T, Quarles LD. Calcium-sensing receptor activation of rho involves filamin and rho-guanine nucleotide exchange factor. Endocrinology. 2002;143:3830–3838. doi: 10.1210/en.2002-220240. [DOI] [PubMed] [Google Scholar]
- 39.Grant MP, Stepanchick A, Cavanaugh A, Breitwieser GE. Agonist-driven maturation and plasma membrane insertion of calcium-sensing receptors dynamically control signal amplitude. Sci Signal. 2011;4:ra78. doi: 10.1126/scisignal.2002208. [DOI] [PubMed] [Google Scholar]
- 40.Breitwieser GE. Pharmacoperones and the calcium sensing receptor: exogenous and endogenous regulators. Pharmacol Res. 2014;83:30–37. doi: 10.1016/j.phrs.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 41.Hannan FM, et al. Adaptor protein-2 sigma subunit mutations causing familial hypocalciuric hypercalcaemia type 3 (FHH3) demonstrate genotype-phenotype correlations, codon bias and dominant-negative effects. Hum Mol Genet. 2015;24:5079–5092. doi: 10.1093/hmg/ddv226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Howles SA, et al. Cinacalcet for Symptomatic Hypercalcemia Caused by AP2S1 Mutations. N Engl J Med. 2016;374:1396–1398. doi: 10.1056/NEJMc1511646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gorvin CM, et al. AP2? Mutations Impair Calcium-Sensing Receptor Trafficking and Signaling, and Show an Endosomal Pathway to Spatially Direct G-Protein Selectivity. Cell Rep. 2018;22:1054–1066. doi: 10.1016/j.celrep.2017.12.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alam MU, et al. Calcification is associated with loss of functional calcium-sensing receptor in vascular smooth muscle cells. Cardiovasc Res. 2009;81:260–268. doi: 10.1093/cvr/cvn279. [DOI] [PubMed] [Google Scholar]
- 45.Kim JY, et al. Calcium-sensing receptor (CaSR) as a novel target for ischemic neuroprotection. Ann Clin Transl Neurol. 2014;1:851–866. doi: 10.1002/acn3.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Babinsky VN, et al. Allosteric Modulation of the Calcium-Sensing Receptor Rectifies Signaling Abnormalities Associated with G-protein alpha-11 Mutations causing Hypercalcemic and Hypocalcemic Disorders. J Biol Chem. 2016;291:10876–10885. doi: 10.1074/jbc.M115.696401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gorvin CM, et al. Cinacalcet Rectifies Hypercalcemia in a Patient With Familial Hypocalciuric Hypercalcemia Type 2 (FHH2) Caused by a Germline Loss-of-Function Galpha11 Mutation. J Bone Miner Res. 2017 doi: 10.1002/jbmr.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gorvin CM, et al. Gα11 mutation in mice causes hypocalcemia rectifiable by calcilytic therapy. JCI Insight. 2017;2:e91103. doi: 10.1172/jci.insight.91103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davey AE, et al. Positive and negative allosteric modulators promote biased signaling at the calcium-sensing receptor. Endocrinology. 2012;153:1232–1241. doi: 10.1210/en.2011-1426. [DOI] [PubMed] [Google Scholar]
- 50.Fox J, Lowe SH, Conklin RL, Petty BA, Nemeth EF. Calcimimetic compound NPS R-568 stimulates calcitonin secretion but selectively targets parathyroid gland Ca(2+) receptor in rats. J Pharmacol Exp Ther. 1999;290:480–486. [PubMed] [Google Scholar]
- 51.Gorvin CM, et al. A calcium-sensing receptor mutation causing hypocalcemia disrupts a transmembrane salt bridge to activate β-arrestin biased signaling. Science Signaling. 2018 doi: 10.1126/scisignal.aan3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hannan FM, Olesen MK, Thakker RV. Calcimimetic and calcilytic therapies for inherited disorders of the calcium-sensing receptor signalling pathway. Br J Pharmacol. 2017 doi: 10.1111/bph.14086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brown EM. Extracellular Ca2+ sensing, regulation of parathyroid cell function, and role of Ca2+ and other ions as extracellular (first) messengers. Physiol Rev. 1991;71:371–411. doi: 10.1152/physrev.1991.71.2.371. [DOI] [PubMed] [Google Scholar]
- 54.Conigrave AD. The Calcium-Sensing Receptor and the Parathyroid: Past, Present, Future. Front Physiol. 2016;7:563. doi: 10.3389/fphys.2016.00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang W, Tu C, Chen TH, Bikle D, Shoback D. The extracellular calcium-sensing receptor (CaSR) is a critical modulator of skeletal development. Sci Signal. 2008;1:ra1. doi: 10.1126/scisignal.1159945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fan Y, et al. Interrelated role of Klotho and calcium-sensing receptor in parathyroid hormone synthesis and parathyroid hyperplasia. Proc Natl Acad Sci U S A. 2018;115:E3749–E3758. doi: 10.1073/pnas.1717754115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hendy GN, Canaff L. Calcium-Sensing Receptor Gene: Regulation of Expression. Front Physiol. 2016;7:394. doi: 10.3389/fphys.2016.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Riccardi D, Brown EM. Physiology and pathophysiology of the calcium-sensing receptor in the kidney. Am J Physiol Renal Physiol. 2010;298:F485–499. doi: 10.1152/ajprenal.00608.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sato T, et al. Parathyroid hormone controls paracellular Ca(2+) transport in the thick ascending limb by regulating the tight-junction protein Claudin14. Proc Natl Acad Sci U S A. 2017;114:E3344–E3353. doi: 10.1073/pnas.1616733114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Loupy A, et al. PTH-independent regulation of blood calcium concentration by the calcium-sensing receptor. J Clin Invest. 2012;122:3355–3367. doi: 10.1172/JCI57407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Riccardi D, Valenti G. Localization and function of the renal calcium-sensing receptor. Nat Rev Nephrol. 2016;12:414–425. doi: 10.1038/nrneph.2016.59. [DOI] [PubMed] [Google Scholar]
- 62.Toka HR, et al. Deficiency of the calcium-sensing receptor in the kidney causes parathyroid hormone-independent hypocalciuria. J Am Soc Nephrol. 2012;23:1879–1890. doi: 10.1681/ASN.2012030323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Toka HR. New functional aspects of the extracellular calcium-sensing receptor. Curr Opin Nephrol Hypertens. 2014;23:352–360. doi: 10.1097/01.mnh.0000447016.21228.e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Graca JA, et al. Comparative expression of the extracellular calcium-sensing receptor in the mouse, rat, and human kidney. Am J Physiol Renal Physiol. 2016;310:F518–533. doi: 10.1152/ajprenal.00208.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ba J, Brown D, Friedman PA. Calcium-sensing receptor regulation of PTH-inhibitable proximal tubule phosphate transport. Am J Physiol Renal Physiol. 2003;285:F1233–1243. doi: 10.1152/ajprenal.00249.2003. [DOI] [PubMed] [Google Scholar]
- 66.Kantham L, et al. The calcium-sensing receptor (CaSR) defends against hypercalcemia independently of its regulation of parathyroid hormone secretion. Am J Physiol Endocrinol Metab. 2009;297:E915–923. doi: 10.1152/ajpendo.00315.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bland R, Walker EA, Hughes SV, Stewart PM, Hewison M. Constitutive expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in a transformed human proximal tubule cell line: evidence for direct regulation of vitamin D metabolism by calcium. Endocrinology. 1999;140:2027–2034. doi: 10.1210/endo.140.5.6683. [DOI] [PubMed] [Google Scholar]
- 68.Topala CN, et al. Activation of the Ca2+-sensing receptor stimulates the activity of the epithelial Ca2+ channel TRPV5. Cell Calcium. 2009;45:331–339. doi: 10.1016/j.ceca.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 69.Renkema KY, et al. The calcium-sensing receptor promotes urinary acidification to prevent nephrolithiasis. J Am Soc Nephrol. 2009;20:1705–1713. doi: 10.1681/ASN.2008111195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Atchison DK, Ortiz-Capisano MC, Beierwaltes WH. Acute activation of the calcium-sensing receptor inhibits plasma renin activity in vivo. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1020–1026. doi: 10.1152/ajpregu.00238.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dvorak-Ewell MM, et al. Osteoblast extracellular Ca2+ -sensing receptor regulates bone development, mineralization, and turnover. J Bone Miner Res. 2011;26:2935–2947. doi: 10.1002/jbmr.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goltzman D, Hendy GN. The calcium-sensing receptor in bone--mechanistic and therapeutic insights. Nat Rev Endocrinol. 2015;11:298–307. doi: 10.1038/nrendo.2015.30. [DOI] [PubMed] [Google Scholar]
- 73.Rodriguez L, Cheng Z, Chen TH, Tu C, Chang W. Extracellular calcium and parathyroid hormone-related peptide signaling modulate the pace of growth plate chondrocyte differentiation. Endocrinology. 2005;146:4597–4608. doi: 10.1210/en.2005-0437. [DOI] [PubMed] [Google Scholar]
- 74.Cheng I, et al. Identification and localization of the extracellular calcium-sensing receptor in human breast. J Clin Endocrinol Metab. 1998;83:703–707. doi: 10.1210/jcem.83.2.4558. [DOI] [PubMed] [Google Scholar]
- 75.VanHouten J, et al. The calcium-sensing receptor regulates mammary gland parathyroid hormone-related protein production and calcium transport. J Clin Invest. 2004;113:598–608. doi: 10.1172/JCI18776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.VanHouten JN, Neville MC, Wysolmerski JJ. The calcium-sensing receptor regulates plasma membrane calcium adenosine triphosphatase isoform 2 activity in mammary epithelial cells: a mechanism for calcium-regulated calcium transport into milk. Endocrinology. 2007;148:5943–5954. doi: 10.1210/en.2007-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hinnie J, Bell E, McKillop E, Gallacher S. The prevalence of familial hypocalciuric hypercalcemia. Calcif Tissue Int. 2001;68:216–218. doi: 10.1007/s002230001201. [DOI] [PubMed] [Google Scholar]
- 78.Christensen SE, et al. Discriminative power of three indices of renal calcium excretion for the distinction between familial hypocalciuric hypercalcaemia and primary hyperparathyroidism: a follow-up study on methods. Clinical endocrinology. 2008;69:713–720. doi: 10.1111/j.1365-2265.2008.03259.x. [DOI] [PubMed] [Google Scholar]
- 79.Eastell R, et al. Diagnosis of asymptomatic primary hyperparathyroidism: proceedings of the Fourth International Workshop. J Clin Endocrinol Metab. 2014;99:3570–3579. doi: 10.1210/jc.2014-1414. [DOI] [PubMed] [Google Scholar]
- 80.Marx SJ. Letter to the editor: Distinguishing typical primary hyperparathyroidism from familial hypocalciuric hypercalcemia by using an index of urinary calcium. J Clin Endocrinol Metab. 2015;100:L29–30. doi: 10.1210/jc.2014-4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Volpe A, Guerriero A, Marchetta A, Caramaschi P, Furlani L. Familial hypocalciuric hypercalcemia revealed by chondrocalcinosis. Joint, bone, spine : revue du rhumatisme. 2009;76:708–710. doi: 10.1016/j.jbspin.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 82.Pearce SH, et al. Calcium-sensing receptor mutations in familial hypocalciuric hypercalcaemia with recurrent pancreatitis. Clin Endocrinol (Oxf) 1996;45:675–680. doi: 10.1046/j.1365-2265.1996.750891.x. [DOI] [PubMed] [Google Scholar]
- 83.Hannan FM, et al. Identification of 70 calcium-sensing receptor mutations in hyper- and hypo-calcaemic patients: evidence for clustering of extracellular domain mutations at calcium-binding sites. Hum Mol Genet. 2012;21:2768–2778. doi: 10.1093/hmg/dds105. [DOI] [PubMed] [Google Scholar]
- 84.Pollak MR, et al. Mutations in the human Ca(2+)-sensing receptor gene cause familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Cell. 1993;75:1297–1303. doi: 10.1016/0092-8674(93)90617-y. [DOI] [PubMed] [Google Scholar]
- 85.Pidasheva S, D'Souza-Li L, Canaff L, Cole DE, Hendy GN. CASRdb: calcium-sensing receptor locus-specific database for mutations causing familial (benign) hypocalciuric hypercalcemia, neonatal severe hyperparathyroidism, and autosomal dominant hypocalcemia. Hum Mutat. 2004;24:107–111. doi: 10.1002/humu.20067. [DOI] [PubMed] [Google Scholar]
- 86.Savas-Erdeve S, et al. Treatment experience and long-term follow-up data in two severe neonatal hyperparathyroidism cases. J Pediatr Endocrinol Metab. 2016;29:1103–1110. doi: 10.1515/jpem-2015-0261. [DOI] [PubMed] [Google Scholar]
- 87.Pallais JC, Kifor O, Chen YB, Slovik D, Brown EM. Acquired hypocalciuric hypercalcemia due to autoantibodies against the calcium-sensing receptor. N Engl J Med. 2004;351:362–369. doi: 10.1056/NEJMoa040008. [DOI] [PubMed] [Google Scholar]
- 88.Song L, et al. Glucocorticoid-responsive lymphocytic parathyroiditis and hypocalciuric hypercalcemia due to autoantibodies against the calcium-sensing receptor: a case report and literature review. Eur J Endocrinol. 2017;177:K1–K6. doi: 10.1530/EJE-17-0172. [DOI] [PubMed] [Google Scholar]
- 89.Huang Y, Breitwieser GE. Rescue of calcium-sensing receptor mutants by allosteric modulators reveals a conformational checkpoint in receptor biogenesis. J Biol Chem. 2007;282:9517–9525. doi: 10.1074/jbc.M609045200. [DOI] [PubMed] [Google Scholar]
- 90.White E, McKenna J, Cavanaugh A, Breitwieser GE. Pharmacochaperone-mediated rescue of calcium-sensing receptor loss-of-function mutants. Mol Endocrinol. 2009;23:1115–1123. doi: 10.1210/me.2009-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leach K, Gregory KJ. Molecular insights into allosteric modulation of Class C G protein-coupled receptors. Pharmacol Res. 2017;116:105–118. doi: 10.1016/j.phrs.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 92.Leach K, et al. Impact of clinically relevant mutations on the pharmacoregulation and signaling bias of the calcium-sensing receptor by positive and negative allosteric modulators. Endocrinology. 2013;154:1105–1116. doi: 10.1210/en.2012-1887. [DOI] [PubMed] [Google Scholar]
- 93.Makita N, et al. An acquired hypocalciuric hypercalcemia autoantibody induces allosteric transition among active human Ca-sensing receptor conformations. Proc Natl Acad Sci U S A. 2007;104:5443–5448. doi: 10.1073/pnas.0701290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gorvin CM, et al. G-Protein Subunit-alpha11 Loss-of-Function Mutation, Thr54Met, Causing Familial Hypocalciuric Hypercalcemia Type 2 (FHH2) J Bone Miner Res. 2016;31:1200–1206. doi: 10.1002/jbmr.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vargas-Poussou R, et al. Familial hypocalciuric hypercalcemia types 1 and 3 and primary hyperparathyroidism: similarities and differences. J Clin Endocrinol Metab. 2016 doi: 10.1210/jc.2015-3442. jc20153442. [DOI] [PubMed] [Google Scholar]
- 96.McMurtry CT, et al. Significant developmental elevation in serum parathyroid hormone levels in a large kindred with familial benign (hypocalciuric) hypercalcemia. Am J Med. 1992;93:247–258. doi: 10.1016/0002-9343(92)90229-5. [DOI] [PubMed] [Google Scholar]
- 97.Fujisawa Y, et al. Identification of AP2S1 mutation and effects of low calcium formula in an infant with hypercalcemia and hypercalciuria. J Clin Endocrinol Metab. 2013;98:E2022–2027. doi: 10.1210/jc.2013-2571. [DOI] [PubMed] [Google Scholar]
- 98.Hendy GN, et al. Codon Arg15 Mutations of the AP2S1 Gene: Common Occurrence in Familial Hypocalciuric Hypercalcemia Cases Negative for Calcium-Sensing Receptor (CASR) Mutations. J Clin Endocrinol Metab. 2014;99:E1311–1315. doi: 10.1210/jc.2014-1120. [DOI] [PubMed] [Google Scholar]
- 99.Hovden S, Rejnmark L, Ladefoged SA, Nissen PH. AP2S1 and GNA11 mutations - not a common cause of familial hypocalciuric hypercalcemia. Eur J Endocrinol. 2017;176:177–185. doi: 10.1530/EJE-16-0842. [DOI] [PubMed] [Google Scholar]
- 100.Gorvin CM, et al. Large-scale exome datasets reveal a new class of adaptor-related protein complex 2 sigma subunit (AP2sigma) mutations, located at the interface with the AP2 alpha subunit, that impair calcium-sensing receptor signalling. Hum Mol Genet. 2018;27:901–911. doi: 10.1093/hmg/ddy010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kelly BT, et al. A structural explanation for the binding of endocytic dileucine motifs by the AP2 complex. Nature. 2008;456:976–979. doi: 10.1038/nature07422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pearce SH, et al. A familial syndrome of hypocalcemia with hypercalciuria due to mutations in the calcium-sensing receptor. N Engl J Med. 1996;335:1115–1122. doi: 10.1056/NEJM199610103351505. [DOI] [PubMed] [Google Scholar]
- 103.Vargas-Poussou R, et al. Functional characterization of a calcium-sensing receptor mutation in severe autosomal dominant hypocalcemia with a Bartter-like syndrome. J Am Soc Nephrol. 2002;13:2259–2266. doi: 10.1097/01.asn.0000025781.16723.68. [DOI] [PubMed] [Google Scholar]
- 104.Watanabe S, et al. Association between activating mutations of calcium-sensing receptor and Bartter's syndrome. Lancet. 2002;360:692–694. doi: 10.1016/S0140-6736(02)09842-2. [DOI] [PubMed] [Google Scholar]
- 105.Yamamoto M, Akatsu T, Nagase T, Ogata E. Comparison of hypocalcemic hypercalciuria between patients with idiopathic hypoparathyroidism and those with gain-of-function mutations in the calcium-sensing receptor: is it possible to differentiate the two disorders? J Clin Endocrinol Metab. 2000;85:4583–4591. doi: 10.1210/jcem.85.12.7035. [DOI] [PubMed] [Google Scholar]
- 106.Li D, et al. Autosomal dominant hypoparathyroidism caused by germline mutation in GNA11: phenotypic and molecular characterization. J Clin Endocrinol Metab. 2014;99:E1774–1783. doi: 10.1210/jc.2014-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mannstadt M, et al. Germline mutations affecting Galpha11 in hypoparathyroidism. N Engl J Med. 2013;368:2532–2534. doi: 10.1056/NEJMc1300278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Piret SE, et al. Identification of a G-Protein Subunit-alpha11 Gain-of-Function Mutation, Val340Met, in a Family with Autosomal Dominant Hypocalcemia Type 2 (ADH2) J Bone Miner Res. 2016;31:1207–1214. doi: 10.1002/jbmr.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jensen AA, et al. Functional importance of the Ala(116)-Pro(136) region in the calcium-sensing receptor. Constitutive activity and inverse agonism in a family C G-protein-coupled receptor. J Biol Chem. 2000;275:29547–29555. doi: 10.1074/jbc.M910023199. [DOI] [PubMed] [Google Scholar]
- 110.Hu J, Spiegel AM. Structure and function of the human calcium-sensing receptor: insights from natural and engineered mutations and allosteric modulators. J Cell Mol Med. 2007;11:908–922. doi: 10.1111/j.1582-4934.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hu J, et al. A region in the seven-transmembrane domain of the human Ca2+ receptor critical for response to Ca2+ J Biol Chem. 2005;280:5113–5120. doi: 10.1074/jbc.M413403200. [DOI] [PubMed] [Google Scholar]
- 112.Dore AS, et al. Structure of class C GPCR metabotropic glutamate receptor 5 transmembrane domain. Nature. 2014;511:557–562. doi: 10.1038/nature13396. [DOI] [PubMed] [Google Scholar]
- 113.Zhang C, et al. Role of Ca2+ and L-Phe in regulating functional cooperativity of disease-associated "toggle" calcium-sensing receptor mutations. PLoS One. 2014;9:e113622. doi: 10.1371/journal.pone.0113622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kemp EH, et al. Activating autoantibodies against the calcium-sensing receptor detected in two patients with autoimmune polyendocrine syndrome type 1. Journal of Clinical Endocrinology and Metabolism. 2009;94:4749–4756. doi: 10.1210/jc.2009-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kifor O, et al. Activating antibodies to the calcium-sensing receptor in two patients with autoimmune hypoparathyroidism. Journal of Clinical Endocrinology and Metabolism. 2004;89:548–556. doi: 10.1210/jc.2003-031054. [DOI] [PubMed] [Google Scholar]
- 116.Li Y, et al. Autoantibodies to the extracellular domain of the calcium sensing receptor in patients with acquired hypoparathyroidism. Journal of Clinical Investigation. 1996;97:910–914. doi: 10.1172/JCI118513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tenhola S, et al. Impaired growth and intracranial calcifications in autosomal dominant hypocalcemia caused by a GNA11 mutation. Eur J Endocrinol. 2016;175:211–218. doi: 10.1530/EJE-16-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hannan FM, et al. A homozygous inactivating calcium-sensing receptor mutation, Pro339Thr, is associated with isolated primary hyperparathyroidism: correlation between location of mutations and severity of hypercalcaemia. Clin Endocrinol (Oxf) 2010;73:715–722. doi: 10.1111/j.1365-2265.2010.03870.x. [DOI] [PubMed] [Google Scholar]
- 119.Cetani F, et al. No evidence for mutations in the calcium-sensing receptor gene in sporadic parathyroid adenomas. J Bone Miner Res. 1999;14:878–882. doi: 10.1359/jbmr.1999.14.6.878. [DOI] [PubMed] [Google Scholar]
- 120.Hosokawa Y, Pollak MR, Brown EM, Arnold A. Mutational analysis of the extracellular Ca(2+)-sensing receptor gene in human parathyroid tumors. J Clin Endocrinol Metab. 1995;80:3107–3110. doi: 10.1210/jcem.80.11.7593409. [DOI] [PubMed] [Google Scholar]
- 121.Newey PJ, et al. Whole-exome sequencing studies of nonhereditary (sporadic) parathyroid adenomas. J Clin Endocrinol Metab. 2012;97:E1995–2005. doi: 10.1210/jc.2012-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Corbetta S, et al. Calcium-sensing receptor expression and signalling in human parathyroid adenomas and primary hyperplasia. Clin Endocrinol (Oxf) 2000;52:339–348. doi: 10.1046/j.1365-2265.2000.00933.x. [DOI] [PubMed] [Google Scholar]
- 123.Kifor O, et al. Reduced immunostaining for the extracellular Ca2+-sensing receptor in primary and uremic secondary hyperparathyroidism. J Clin Endocrinol Metab. 1996;81:1598–1606. doi: 10.1210/jcem.81.4.8636374. [DOI] [PubMed] [Google Scholar]
- 124.Cetani F, et al. Parathyroid expression of calcium-sensing receptor protein and in vivo parathyroid hormone-Ca(2+) set-point in patients with primary hyperparathyroidism. J Clin Endocrinol Metab. 2000;85:4789–4794. doi: 10.1210/jcem.85.12.7028. [DOI] [PubMed] [Google Scholar]
- 125.Mingione A, et al. Filamin A is reduced and contributes to the CASR sensitivity in human parathyroid tumors. J Mol Endocrinol. 2017;58:91–103. doi: 10.1530/JME-16-0184. [DOI] [PubMed] [Google Scholar]
- 126.Hjalm G, MacLeod RJ, Kifor O, Chattopadhyay N, Brown EM. Filamin-A binds to the carboxyl-terminal tail of the calcium-sensing receptor, an interaction that participates in CaR-mediated activation of mitogen-activated protein kinase. J Biol Chem. 2001;276:34880–34887. doi: 10.1074/jbc.M100784200. [DOI] [PubMed] [Google Scholar]
- 127.Varshney S, et al. Methylation status of the CpG islands in vitamin D and calcium-sensing receptor gene promoters does not explain the reduced gene expressions in parathyroid adenomas. J Clin Endocrinol Metab. 2013;98:E1631–1635. doi: 10.1210/jc.2013-1699. [DOI] [PubMed] [Google Scholar]
- 128.Corbetta S, et al. R990G polymorphism of the calcium-sensing receptor and renal calcium excretion in patients with primary hyperparathyroidism. Eur J Endocrinol. 2006;155:687–692. doi: 10.1530/eje.1.02286. [DOI] [PubMed] [Google Scholar]
- 129.Yamauchi M, et al. Association of polymorphic alleles of the calcium-sensing receptor gene with the clinical severity of primary hyperparathyroidism. Clin Endocrinol (Oxf) 2001;55:373–379. doi: 10.1046/j.1365-2265.2001.01318.x. [DOI] [PubMed] [Google Scholar]
- 130.Rodriguez M, Nemeth E, Martin D. The calcium-sensing receptor: a key factor in the pathogenesis of secondary hyperparathyroidism. Am J Physiol Renal Physiol. 2005;288:F253–264. doi: 10.1152/ajprenal.00302.2004. [DOI] [PubMed] [Google Scholar]
- 131.Cunningham J, Locatelli F, Rodriguez M. Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol. 2011;6:913–921. doi: 10.2215/CJN.06040710. [DOI] [PubMed] [Google Scholar]
- 132.Brown AJ, Ritter CS, Finch JL, Slatopolsky EA. Decreased calcium-sensing receptor expression in hyperplastic parathyroid glands of uremic rats: role of dietary phosphate. Kidney Int. 1999;55:1284–1292. doi: 10.1046/j.1523-1755.1999.00386.x. [DOI] [PubMed] [Google Scholar]
- 133.Mizobuchi M, et al. Calcimimetic compound upregulates decreased calcium-sensing receptor expression level in parathyroid glands of rats with chronic renal insufficiency. J Am Soc Nephrol. 2004;15:2579–2587. doi: 10.1097/01.ASN.0000141016.20133.33. [DOI] [PubMed] [Google Scholar]
- 134.Gogusev J, et al. Depressed expression of calcium receptor in parathyroid gland tissue of patients with hyperparathyroidism. Kidney Int. 1997;51:328–336. doi: 10.1038/ki.1997.41. [DOI] [PubMed] [Google Scholar]
- 135.Canadillas S, et al. Calcium-sensing receptor expression and parathyroid hormone secretion in hyperplastic parathyroid glands from humans. J Am Soc Nephrol. 2005;16:2190–2197. doi: 10.1681/ASN.2004080657. [DOI] [PubMed] [Google Scholar]
- 136.Mathias RS, Nguyen HT, Zhang MY, Portale AA. Reduced expression of the renal calcium-sensing receptor in rats with experimental chronic renal insufficiency. J Am Soc Nephrol. 1998;9:2067–2074. doi: 10.1681/ASN.V9112067. [DOI] [PubMed] [Google Scholar]
- 137.Coe FL, Parks JH, Moore ES. Familial idiopathic hypercalciuria. N Engl J Med. 1979;300:337–340. doi: 10.1056/NEJM197902153000703. [DOI] [PubMed] [Google Scholar]
- 138.Lerolle N, et al. No evidence for point mutations of the calcium-sensing receptor in familial idiopathic hypercalciuria. Nephrol Dial Transplant. 2001;16:2317–2322. doi: 10.1093/ndt/16.12.2317. [DOI] [PubMed] [Google Scholar]
- 139.Vezzoli G, et al. Influence of calcium-sensing receptor gene on urinary calcium excretion in stone-forming patients. J Am Soc Nephrol. 2002;13:2517–2523. doi: 10.1097/01.asn.0000030077.72157.d2. [DOI] [PubMed] [Google Scholar]
- 140.Vezzoli G, et al. Decreased transcriptional activity of calcium-sensing receptor gene promoter 1 is associated with calcium nephrolithiasis. J Clin Endocrinol Metab. 2013;98:3839–3847. doi: 10.1210/jc.2013-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mayr B, Schnabel D, Dorr HG, Schofl C. GENETICS IN ENDOCRINOLOGY: Gain and loss of function mutations of the calcium-sensing receptor and associated proteins: current treatment concepts. Eur J Endocrinol. 2016;174:R189–208. doi: 10.1530/EJE-15-1028. [DOI] [PubMed] [Google Scholar]
- 142.Nemeth EF. Calcimimetic and calcilytic drugs: just for parathyroid cells? Cell Calcium. 2004;35:283–289. doi: 10.1016/j.ceca.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 143.Nemeth EF, Shoback D. Calcimimetic and calcilytic drugs for treating bone and mineral-related disorders. Best Pract Res Clin Endocrinol Metab. 2013;27:373–384. doi: 10.1016/j.beem.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 144.Wada M, et al. The calcimimetic compound NPS R-568 suppresses parathyroid cell proliferation in rats with renal insufficiency. Control of parathyroid cell growth via a calcium receptor. J Clin Invest. 1997;100:2977–2983. doi: 10.1172/JCI119851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Walter S, et al. Pharmacology of AMG 416 (Velcalcetide), a novel peptide agonist of the calcium-sensing receptor, for the treatment of secondary hyperparathyroidism in hemodialysis patients. J Pharmacol Exp Ther. 2013;346:229–240. doi: 10.1124/jpet.113.204834. [DOI] [PubMed] [Google Scholar]
- 146.Block GA, et al. Effect of Etelcalcetide vs Cinacalcet on Serum Parathyroid Hormone in Patients Receiving Hemodialysis With Secondary Hyperparathyroidism: A Randomized Clinical Trial. JAMA. 2017;317:156–164. doi: 10.1001/jama.2016.19468. [DOI] [PubMed] [Google Scholar]
- 147.Alexander ST, et al. Critical Cysteine Residues in Both the Calcium-Sensing Receptor and the Allosteric Activator AMG 416 Underlie the Mechanism of Action. Mol Pharmacol. 2015;88:853–865. doi: 10.1124/mol.115.098392. [DOI] [PubMed] [Google Scholar]
- 148.Rus R, et al. Novel inactivating mutations of the calcium-sensing receptor: the calcimimetic NPS R-568 improves signal transduction of mutant receptors. J Clin Endocrinol Metab. 2008;93:4797–4803. doi: 10.1210/jc.2008-1076. [DOI] [PubMed] [Google Scholar]
- 149.Festen-Spanjer B, Haring CM, Koster JB, Mudde AH. Correction of hypercalcaemia by cinacalcet in familial hypocalciuric hypercalcaemia. Clin Endocrinol (Oxf) 2008;68:324–325. doi: 10.1111/j.1365-2265.2007.03027.x. [DOI] [PubMed] [Google Scholar]
- 150.Timmers HJ, Karperien M, Hamdy NA, de Boer H, Hermus AR. Normalization of serum calcium by cinacalcet in a patient with hypercalcaemia due to a de novo inactivating mutation of the calcium-sensing receptor. J Intern Med. 2006;260:177–182. doi: 10.1111/j.1365-2796.2006.01684.x. [DOI] [PubMed] [Google Scholar]
- 151.Gannon AW, Monk HM, Levine MA. Cinacalcet monotherapy in neonatal severe hyperparathyroidism: a case study and review. J Clin Endocrinol Metab. 2014;99:7–11. doi: 10.1210/jc.2013-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Atay Z, et al. Novel homozygous inactivating mutation of the calcium-sensing receptor gene (CASR) in neonatal severe hyperparathyroidism-lack of effect of cinacalcet. Bone. 2014;64:102–107. doi: 10.1016/j.bone.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 153.Nemeth EF, et al. Calcilytic compounds: potent and selective Ca2+ receptor antagonists that stimulate secretion of parathyroid hormone. J Pharmacol Exp Ther. 2001;299:323–331. [PubMed] [Google Scholar]
- 154.Fitzpatrick LA, et al. The effects of ronacaleret, a calcium-sensing receptor antagonist, on bone mineral density and biochemical markers of bone turnover in postmenopausal women with low bone mineral density. J Clin Endocrinol Metab. 2011;96:2441–2449. doi: 10.1210/jc.2010-2855. [DOI] [PubMed] [Google Scholar]
- 155.Halse J, et al. A phase 2, randomized, placebo-controlled, dose-ranging study of the calcium-sensing receptor antagonist MK-5442 in the treatment of postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2014;99:E2207–2215. doi: 10.1210/jc.2013-4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hannan FM, et al. The Calcilytic Agent NPS 2143 Rectifies Hypocalcemia in a Mouse Model With an Activating Calcium-Sensing Receptor (CaSR) Mutation: Relevance to Autosomal Dominant Hypocalcemia Type 1 (ADH1) Endocrinology. 2015;156:3114–3121. doi: 10.1210/en.2015-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Letz S, et al. Novel activating mutations of the calcium-sensing receptor: the calcilytic NPS-2143 mitigates excessive signal transduction of mutant receptors. J Clin Endocrinol Metab. 2010;95:E229–233. doi: 10.1210/jc.2010-0651. [DOI] [PubMed] [Google Scholar]
- 158.Letz S, et al. Amino alcohol- (NPS-2143) and quinazolinone-derived calcilytics (ATF936 and AXT914) differentially mitigate excessive signalling of calcium-sensing receptor mutants causing Bartter syndrome Type 5 and autosomal dominant hypocalcemia. PLoS One. 2014;9:e115178. doi: 10.1371/journal.pone.0115178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Dong B, et al. Calcilytic Ameliorates Abnormalities of Mutant Calcium-Sensing Receptor (CaSR) Knock-in Mice Mimicking Autosomal Dominant Hypocalcemia (ADH) J Bone Miner Res. 2015;30:1980–1993. doi: 10.1002/jbmr.2551. [DOI] [PubMed] [Google Scholar]
- 160.Ramnitz M, et al. Treatment of Autosomal Dominant Hypocalcemia with the Calcilytic NPSP795. J Bone Miner Res. 2015;30(Suppl 1) doi: 10.1002/jbmr.3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bandyopadhyay S, Tfelt-Hansen J, Chattopadhyay N. Diverse roles of extracellular calcium-sensing receptor in the central nervous system. J Neurosci Res. 2010;88:2073–2082. doi: 10.1002/jnr.22391. [DOI] [PubMed] [Google Scholar]
- 162.Bravo-Sagua R, Mattar P, Diaz X, Lavandero S, Cifuentes M. Calcium Sensing Receptor as a Novel Mediator of Adipose Tissue Dysfunction: Mechanisms and Potential Clinical Implications. Front Physiol. 2016;7:395. doi: 10.3389/fphys.2016.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Brennan SC, et al. Calcium sensing receptor signalling in physiology and cancer. Biochim Biophys Acta. 2013;1833:1732–1744. doi: 10.1016/j.bbamcr.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 164.Riccardi D, Kemp PJ. The calcium-sensing receptor beyond extracellular calcium homeostasis: conception, development, adult physiology, and disease. Annu Rev Physiol. 2012;74:271–297. doi: 10.1146/annurev-physiol-020911-153318. [DOI] [PubMed] [Google Scholar]
- 165.Ahearn TU, et al. Calcium-Sensing Receptor Tumor Expression and Lethal Prostate Cancer Progression. J Clin Endocrinol Metab. 2016;101:2520–2527. doi: 10.1210/jc.2016-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mihai R, Stevens J, McKinney C, Ibrahim NB. Expression of the calcium receptor in human breast cancer--a potential new marker predicting the risk of bone metastases. Eur J Surg Oncol. 2006;32:511–515. doi: 10.1016/j.ejso.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 167.Casala C, et al. The calcium-sensing receptor is silenced by genetic and epigenetic mechanisms in unfavorable neuroblastomas and its reactivation induces ERK1/2-dependent apoptosis. Carcinogenesis. 2013;34:268–276. doi: 10.1093/carcin/bgs338. [DOI] [PubMed] [Google Scholar]
- 168.Kim W, et al. Calcium-Sensing Receptor Promotes Breast Cancer by Stimulating Intracrine Actions of Parathyroid Hormone-Related Protein. Cancer Res. 2016;76:5348–5360. doi: 10.1158/0008-5472.CAN-15-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mamillapalli R, VanHouten J, Zawalich W, Wysolmerski J. Switching of G-protein usage by the calcium-sensing receptor reverses its effect on parathyroid hormone-related protein secretion in normal versus malignant breast cells. J Biol Chem. 2008;283:24435–24447. doi: 10.1074/jbc.M801738200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hernandez-Bedolla MA, Carretero-Ortega J, Valadez-Sanchez M, Vazquez-Prado J, Reyes-Cruz G. Chemotactic and proangiogenic role of calcium sensing receptor is linked to secretion of multiple cytokines and growth factors in breast cancer MDA-MB-231 cells. Biochim Biophys Acta. 2015;1853:166–182. doi: 10.1016/j.bbamcr.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 171.Saidak Z, et al. Extracellular calcium promotes the migration of breast cancer cells through the activation of the calcium sensing receptor. Exp Cell Res. 2009;315:2072–2080. doi: 10.1016/j.yexcr.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 172.Silver IA, Murrills RJ, Etherington DJ. Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp Cell Res. 1988;175:266–276. doi: 10.1016/0014-4827(88)90191-7. [DOI] [PubMed] [Google Scholar]
- 173.Sanders JL, et al. Extracellular calcium-sensing receptor expression and its potential role in regulating parathyroid hormone-related peptide secretion in human breast cancer cell lines. Endocrinology. 2000;141:4357–4364. doi: 10.1210/endo.141.12.7849. [DOI] [PubMed] [Google Scholar]
- 174.Boudot C, et al. Overexpression of a functional calcium-sensing receptor dramatically increases osteolytic potential of MDA-MB-231 cells in a mouse model of bone metastasis through epiregulin-mediated osteoprotegerin downregulation. Oncotarget. 2017 doi: 10.18632/oncotarget.16999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.de Torres C, et al. The calcium-sensing receptor and parathyroid hormone-related protein are expressed in differentiated, favorable neuroblastic tumors. Cancer. 2009;115:2792–2803. doi: 10.1002/cncr.24304. [DOI] [PubMed] [Google Scholar]
- 176.Rodriguez-Hernandez CJ, et al. Cinacalcet inhibits neuroblastoma tumor growth and upregulates cancer-testis antigens. Oncotarget. 2016;7:16112–16129. doi: 10.18632/oncotarget.7448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bernichtein S, et al. Vitamin D3 Prevents Calcium-Induced Progression of Early-Stage Prostate Tumors by Counteracting TRPC6 and Calcium Sensing Receptor Upregulation. Cancer Res. 2017;77:355–365. doi: 10.1158/0008-5472.CAN-16-0687. [DOI] [PubMed] [Google Scholar]
- 178.Yang W, et al. Calcium intake and risk of colorectal cancer according to expression status of calcium-sensing receptor (CASR) Gut. 2017 doi: 10.1136/gutjnl-2017-314163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chakrabarty S, Radjendirane V, Appelman H, Varani J. Extracellular calcium and calcium sensing receptor function in human colon carcinomas: promotion of E-cadherin expression and suppression of beta-catenin/TCF activation. Cancer Res. 2003;63:67–71. [PubMed] [Google Scholar]
- 180.Aggarwal A, et al. The calcium-sensing receptor: A promising target for prevention of colorectal cancer. Biochimica et biophysica acta. 2015 doi: 10.1016/j.bbamcr.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Aggarwal A, et al. The calcium-sensing receptor suppresses epithelial-to-mesenchymal transition and stem cell-like phenotype in the colon. Mol Cancer. 2015;14:61. doi: 10.1186/s12943-015-0330-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rey O, et al. Negative cross-talk between calcium-sensing receptor and beta-catenin signaling systems in colonic epithelium. The Journal of biological chemistry. 2012;287:1158–1167. doi: 10.1074/jbc.M111.274589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.MacLeod RJ. Extracellular calcium-sensing receptor/PTH knockout mice colons have increased Wnt/beta-catenin signaling, reduced non-canonical Wnt signaling, and increased susceptibility to azoxymethane-induced aberrant crypt foci. Lab Invest. 2013;93:520–527. doi: 10.1038/labinvest.2013.51. [DOI] [PubMed] [Google Scholar]
- 184.Fetahu IS, et al. Calcium-sensing receptor silencing in colorectal cancer is associated with promoter hypermethylation and loss of acetylation on histone 3. International journal of cancer. 2014;135:2014–2023. doi: 10.1002/ijc.28856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hizaki K, et al. Epigenetic inactivation of calcium-sensing receptor in colorectal carcinogenesis. Mod Pathol. 2011;24:876–884. doi: 10.1038/modpathol.2011.10. [DOI] [PubMed] [Google Scholar]
- 186.Fetahu IS, et al. miR-135b- and miR-146b-dependent silencing of calcium-sensing receptor expression in colorectal tumors. International journal of cancer. 2016;138:137–145. doi: 10.1002/ijc.29681. [DOI] [PubMed] [Google Scholar]
- 187.Ruat M, Molliver ME, Snowman AM, Snyder SH. Calcium sensing receptor: molecular cloning in rat and localization to nerve terminals. Proc Natl Acad Sci U S A. 1995;92:3161–3165. doi: 10.1073/pnas.92.8.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yano S, Brown EM, Chattopadhyay N. Calcium-sensing receptor in the brain. Cell Calcium. 2004;35:257–264. doi: 10.1016/j.ceca.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 189.Chattopadhyay N, et al. Calcium receptor stimulates chemotaxis and secretion of MCP-1 in GnRH neurons in vitro: potential impact on reduced GnRH neuron population in CaR-null mice. Am J Physiol Endocrinol Metab. 2007;292:E523–532. doi: 10.1152/ajpendo.00372.2005. [DOI] [PubMed] [Google Scholar]
- 190.Tharmalingam S, Wu C, Hampson DR. The calcium-sensing receptor and integrins modulate cerebellar granule cell precursor differentiation and migration. Dev Neurobiol. 2016;76:375–389. doi: 10.1002/dneu.22321. [DOI] [PubMed] [Google Scholar]
- 191.Vizard TN, et al. Regulation of axonal and dendritic growth by the extracellular calcium-sensing receptor. Nat Neurosci. 2008;11:285–291. doi: 10.1038/nn2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hiyama TY, Noda M. Sodium sensing in the subfornical organ and body-fluid homeostasis. Neurosci Res. 2016;113:1–11. doi: 10.1016/j.neures.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 193.Conley YP, et al. Evidence supporting a role for the calcium-sensing receptor in Alzheimer disease. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:703–709. doi: 10.1002/ajmg.b.30896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Armato U, et al. Calcium-sensing receptor antagonist (calcilytic) NPS 2143 specifically blocks the increased secretion of endogenous Abeta42 prompted by exogenous fibrillary or soluble Abeta25-35 in human cortical astrocytes and neurons-therapeutic relevance to Alzheimer's disease. Biochim Biophys Acta. 2013;1832:1634–1652. doi: 10.1016/j.bbadis.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 195.Kim JY, Kim N, Yenari MA, Chang W. Mild Hypothermia Suppresses Calcium-Sensing Receptor (CaSR) Induction Following Forebrain Ischemia While Increasing GABA-B Receptor 1 (GABA-B-R1) Expression. Transl Stroke Res. 2011;2:195–201. doi: 10.1007/s12975-011-0082-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kapoor A, et al. An idiopathic epilepsy syndrome linked to 3q13.3-q21 and missense mutations in the extracellular calcium sensing receptor gene. Ann Neurol. 2008;64:158–167. doi: 10.1002/ana.21428. [DOI] [PubMed] [Google Scholar]
- 197.Stepanchick A, McKenna J, McGovern O, Huang Y, Breitwieser GE. Calcium sensing receptor mutations implicated in pancreatitis and idiopathic epilepsy syndrome disrupt an arginine-rich retention motif. Cell Physiol Biochem. 2010;26:363–374. doi: 10.1159/000320560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Molostvov G, et al. Extracellular calcium-sensing receptor is functionally expressed in human artery. Am J Physiol Renal Physiol. 2007;293:F946–955. doi: 10.1152/ajprenal.00474.2006. [DOI] [PubMed] [Google Scholar]
- 199.Schreckenberg R, Schluter KD. Calcium sensing receptor expression and signalling in cardiovascular physiology and disease. Vascul Pharmacol. 2018 doi: 10.1016/j.vph.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 200.Molostvov G, Fletcher S, Bland R, Zehnder D. Extracellular calcium-sensing receptor mediated signalling is involved in human vascular smooth muscle cell proliferation and apoptosis. Cell Physiol Biochem. 2008;22:413–422. doi: 10.1159/000185484. [DOI] [PubMed] [Google Scholar]
- 201.Greenberg HZ, et al. Stimulation of calcium-sensing receptors induces endothelium-dependent vasorelaxations via nitric oxide production and activation of IKCa channels. Vascul Pharmacol. 2016;80:75–84. doi: 10.1016/j.vph.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Raggi P, et al. The ADVANCE study: a randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol Dial Transplant. 2011;26:1327–1339. doi: 10.1093/ndt/gfq725. [DOI] [PubMed] [Google Scholar]
- 203.Sun YH, et al. Calcium-sensing receptor induces rat neonatal ventricular cardiomyocyte apoptosis. Biochem Biophys Res Commun. 2006;350:942–948. doi: 10.1016/j.bbrc.2006.09.142. [DOI] [PubMed] [Google Scholar]
- 204.Sun J, Murphy E. Calcium-sensing receptor: a sensor and mediator of ischemic preconditioning in the heart. Am J Physiol Heart Circ Physiol. 2010;299:H1309–1317. doi: 10.1152/ajpheart.00373.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Marz W, et al. Alanine to serine polymorphism at position 986 of the calcium-sensing receptor associated with coronary heart disease, myocardial infarction, all-cause, and cardiovascular mortality. J Clin Endocrinol Metab. 2007;92:2363–2369. doi: 10.1210/jc.2006-0071. [DOI] [PubMed] [Google Scholar]
- 206.Brennan SC, et al. The extracellular calcium-sensing receptor regulates human fetal lung development via CFTR. Sci Rep. 2016;6 doi: 10.1038/srep21975. 21975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Finney BA, et al. Regulation of mouse lung development by the extracellular calcium-sensing receptor, CaR. J Physiol. 2008;586:6007–6019. doi: 10.1113/jphysiol.2008.161687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yamamura A, et al. Enhanced Ca(2+)-sensing receptor function in idiopathic pulmonary arterial hypertension. Circ Res. 2012;111:469–481. doi: 10.1161/CIRCRESAHA.112.266361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Geibel JP, Hebert SC. The functions and roles of the extracellular Ca2+-sensing receptor along the gastrointestinal tract. Annu Rev Physiol. 2009;71:205–217. doi: 10.1146/annurev.physiol.010908.163128. [DOI] [PubMed] [Google Scholar]
- 210.Alamshah A, et al. l-phenylalanine modulates gut hormone release and glucose tolerance, and suppresses food intake through the calcium-sensing receptor in rodents. Int J Obes (Lond) 2017;41:1693–1701. doi: 10.1038/ijo.2017.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Tang L, et al. The Extracellular Calcium-Sensing Receptor in the Intestine: Evidence for Regulation of Colonic Absorption, Secretion, Motility, and Immunity. Front Physiol. 2016;7:245. doi: 10.3389/fphys.2016.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Engelstoft MS, et al. Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Mol Metab. 2013;2:376–392. doi: 10.1016/j.molmet.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Reimann F, Tolhurst G, Gribble FM. G-protein-coupled receptors in intestinal chemosensation. Cell Metab. 2012;15:421–431. doi: 10.1016/j.cmet.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 214.Spreckley E, Murphy KG. The L-Cell in Nutritional Sensing and the Regulation of Appetite. Front Nutr. 2015;2:23. doi: 10.3389/fnut.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Feng J, et al. Calcium-sensing receptor is a physiologic multimodal chemosensor regulating gastric G-cell growth and gastrin secretion. Proc Natl Acad Sci U S A. 2010;107:17791–17796. doi: 10.1073/pnas.1009078107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ceglia L, Harris SS, Rasmussen HM, Dawson-Hughes B. Activation of the calcium sensing receptor stimulates gastrin and gastric acid secretion in healthy participants. Osteoporos Int. 2009;20:71–78. doi: 10.1007/s00198-008-0637-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Dufner MM, et al. The calcium-sensing receptor acts as a modulator of gastric acid secretion in freshly isolated human gastric glands. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1084–1090. doi: 10.1152/ajpgi.00571.2004. [DOI] [PubMed] [Google Scholar]
- 218.Muramatsu M, et al. Activation of the gut calcium-sensing receptor by peptide agonists reduces rapid elevation of plasma glucose in response to oral glucose load in rats. Am J Physiol Gastrointest Liver Physiol. 2014;306:G1099–1107. doi: 10.1152/ajpgi.00155.2014. [DOI] [PubMed] [Google Scholar]
- 219.Diakogiannaki E, et al. Oligopeptides stimulate glucagon-like peptide-1 secretion in mice through proton-coupled uptake and the calcium-sensing receptor. Diabetologia. 2013;56:2688–2696. doi: 10.1007/s00125-013-3037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Liou AP, et al. The extracellular calcium-sensing receptor is required for cholecystokinin secretion in response to L-phenylalanine in acutely isolated intestinal I cells. Am J Physiol Gastrointest Liver Physiol. 2011;300:G538–546. doi: 10.1152/ajpgi.00342.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wu W, Zhou HR, Pestka JJ. Potential roles for calcium-sensing receptor (CaSR) and transient receptor potential ankyrin-1 (TRPA1) in murine anorectic response to deoxynivalenol (vomitoxin) Arch Toxicol. 2017;91:495–507. doi: 10.1007/s00204-016-1687-x. [DOI] [PubMed] [Google Scholar]
- 222.Kawata T, et al. A novel calcimimetic agent, evocalcet (MT-4580/KHK7580), suppresses the parathyroid cell function with little effect on the gastrointestinal tract or CYP isozymes in vivo and in vitro. PLoS One. 2018;13:e0195316. doi: 10.1371/journal.pone.0195316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Geibel J, et al. Calcium-sensing receptor abrogates secretagogue-induced increases in intestinal net fluid secretion by enhancing cyclic nucleotide destruction. Proc Natl Acad Sci U S A. 2006;103:9390–9397. doi: 10.1073/pnas.0602996103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Cheng SX. Calcium-sensing receptor inhibits secretagogue-induced electrolyte secretion by intestine via the enteric nervous system. Am J Physiol Gastrointest Liver Physiol. 2012;303:G60–70. doi: 10.1152/ajpgi.00425.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Konig J, et al. Human Intestinal Barrier Function in Health and Disease. Clin Transl Gastroenterol. 2016;7:e196. doi: 10.1038/ctg.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Beltinger J, et al. Human colonic subepithelial myofibroblasts modulate transepithelial resistance and secretory response. Am J Physiol. 1999;277:C271–279. doi: 10.1152/ajpcell.1999.277.2.C271. [DOI] [PubMed] [Google Scholar]
- 227.Peiris D, Pacheco I, Spencer C, MacLeod RJ. The extracellular calcium-sensing receptor reciprocally regulates the secretion of BMP-2 and the BMP antagonist Noggin in colonic myofibroblasts. Am J Physiol Gastrointest Liver Physiol. 2007;292:G753–766. doi: 10.1152/ajpgi.00225.2006. [DOI] [PubMed] [Google Scholar]
- 228.Kelly JC, Lungchukiet P, Macleod RJ. Extracellular Calcium-Sensing Receptor Inhibition of Intestinal EpithelialTNF Signaling Requires CaSR-Mediated Wnt5a/Ror2 Interaction. Front Physiol. 2011;2:17. doi: 10.3389/fphys.2011.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Cheng SX, et al. Epithelial CaSR deficiency alters intestinal integrity and promotes proinflammatory immune responses. FEBS Lett. 2014;588:4158–4166. doi: 10.1016/j.febslet.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Owen JL, Cheng SX, Ge Y, Sahay B, Mohamadzadeh M. The role of the calcium-sensing receptor in gastrointestinal inflammation. Semin Cell Dev Biol. 2016;49:44–51. doi: 10.1016/j.semcdb.2015.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Squires PE, et al. The extracellular calcium-sensing receptor on human beta-cells negatively modulates insulin secretion. Diabetes. 2000;49:409–417. doi: 10.2337/diabetes.49.3.409. [DOI] [PubMed] [Google Scholar]
- 232.Gray E, et al. Activation of the extracellular calcium-sensing receptor initiates insulin secretion from human islets of Langerhans: involvement of protein kinases. J Endocrinol. 2006;190:703–710. doi: 10.1677/joe.1.06891. [DOI] [PubMed] [Google Scholar]
- 233.Straub SG, Kornreich B, Oswald RE, Nemeth EF, Sharp GW. The calcimimetic R-467 potentiates insulin secretion in pancreatic beta cells by activation of a nonspecific cation channel. J Biol Chem. 2000;275:18777–18784. doi: 10.1074/jbc.M000090200. [DOI] [PubMed] [Google Scholar]
- 234.Elsholz F, Harteneck C, Muller W, Friedland K. Calcium--a central regulator of keratinocyte differentiation in health and disease. Eur J Dermatol. 2014;24:650–661. doi: 10.1684/ejd.2014.2452. [DOI] [PubMed] [Google Scholar]
- 235.Lansdown AB. Calcium: a potential central regulator in wound healing in the skin. Wound Repair Regen. 2002;10:271–285. doi: 10.1046/j.1524-475x.2002.10502.x. [DOI] [PubMed] [Google Scholar]
- 236.Tu CL, Bikle DD. Role of the calcium-sensing receptor in calcium regulation of epidermal differentiation and function. Best Pract Res Clin Endocrinol Metab. 2013;27:415–427. doi: 10.1016/j.beem.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Bikle DD, Jiang Y, Nguyen T, Oda Y, Tu CL. Disruption of Vitamin D and Calcium Signaling in Keratinocytes Predisposes to Skin Cancer. Front Physiol. 2016;7:296. doi: 10.3389/fphys.2016.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Aggarwal A, Kallay E. Cross Talk between the Calcium-Sensing Receptor and the Vitamin D System in Prevention of Cancer. Front Physiol. 2016;7:451. doi: 10.3389/fphys.2016.00451. [DOI] [PMC free article] [PubMed] [Google Scholar]