Abstract
Objective:
To evaluate the molecular subtypes of Mammary Paget’s disease (MPD) and the associated breast carcinomas.
Methods:
This retrospective study was carried out at King Khalid University Hospital and King Faisal Specialist Hospital, Riyadh, Saudi Arabia. Data from MPD patient cases from January 2010 to June 2016 were reviewed. The molecular subtypes were determined based on estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 (HER2) expression with immunohistochemical staining. The relative frequencies of the luminal A and B, HER2-enriched and basal-like molecular subtypes were calculated and compared for MPD and the associated breast carcinomas.
Results:
Among 22 patients with MPD, HER2-enriched was the most frequently occurring molecular subtype and was observed in 11 (50%) patients. Mammary Paget’s disease was classified as basal-like in 5 (22.7%) patients, and luminal A and B were each detected in 3 (13.6%) patients. The molecular subtype of MPD corresponded with the subtype of the associated breast carcinoma in 18 out of 20 patients (90%).
Conclusions:
The HER2-enriched subtype is the most frequently occurring molecular subtype in MPD. The molecular subtype of the associated breast carcinoma is usually similar to that of MPD. The molecular subtypes vary between MPD associated breast carcinoma and overall breast carcinoma. The HER2-enriched subtype is the most frequently occurring subtype of MPD associated breast carcinoma, while luminal subtypes are more common in overall breast carcinoma.
Breast carcinoma is well known for its heterogeneous molecular biology, clinical behavior and response to therapy.1,2 The increased molecular understanding of breast carcinoma has led to the identification of molecular subtypes and corresponding targeted therapies, which is a topic that is under intensive investigation.3,4 The histopathological classification of breast carcinoma is important for identifying its histologic variants but has limited clinical significance, as most breast carcinomas are categorized as ductal carcinoma not otherwise specified. A more significant classification is based on the DNA microarray signature of breast cancer cells.5 These intrinsic subtypes highlight the heterogeneity of breast cancer and more importantly influence the choice of therapy, the predicted outcomes of these therapies as well as the prognosis of the disease.6 Four molecular subtypes are referred to as luminal A and B, HER2-enriched and basal-like based on the expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2).7 Additionally, immunohistochemical (IHC) surrogate markers can reliably determine the molecular subtypes.8,9 Mammary Paget’s disease (MPD) is a rare manifestation that is seen in 1-4% cases of breast carcinoma.10,11 Although the hormone receptor status and frequency of the molecular subtypes of breast carcinoma have been extensively studied in different populations,12-14 their proportions in MPD and the associated breast carcinomas have not been widely studied.
Mammary Paget’s disease overexpresses markers associated with aggressive tumor behavior and is known to have higher expression of HER2.15 Furthermore, HER2 expression influences the motility-enhancing activity of tumor cells that are under the influence of the chemotactic factors secreted by epidermal keratinocytes.16 This may suggest that the HER2-positive molecular subtype of breast carcinoma may exhibit enhanced intraepidermal spread when presenting as MPD. In the present study, we determined and compared the relative frequency of the molecular subtypes in MPD and the associated breast carcinomas.
Methods
In this retrospective descriptive study, MPD cases were identified from surgical pathology records at King Khalid University Hospital and King Faisal Specialist Hospital & Research Center, Riyadh, Saudi Arabia. The clinical and demographic data were retrieved from patients’ electronic files. Two pathologists reviewed the retrieved slides to confirm the diagnoses and select appropriate sections from the nipple/skin and the underlying breast carcinoma for IHC staining. All the MPD cases retrieved from January 2010 to June 2016 were included in the study. Exclusion criteria included unavailability of the tissue blocks or inadequate number of Paget cells on re-sectioning for IHC. The study was approved by the Institutional Review Board.
Immunohistochemical staining was performed on formalin-fixed, paraffin-embedded tissue sections using a Ventana automated immunohistochemistry system (BenchMark ULTRA Roche Diagnostics, USA). Sections were stained for ER, PR, and HER2. All reagents were procured from Roche Diagnostics. All primary antibodies were of rabbit origin. The primary monoclonal antibodies were anti-ER (clone SP1), anti-PR (clone 1E2), and anti-HER2 (clone 4B5). A DAB detection system (ultraView Universal kit) was used for detecting the primary antibodies. Appropriate positive and negative controls were included. For ER and PR, nuclear staining in more than 1% of the cells was considered positive. The percentage of membranous positive tumor cells was recorded for HER2. The staining was further graded as mild (1+), moderate (2+) and strong (3+) based on the intensity of the staining according to the guidelines of the College of American Pathologists.17 Only cases with 3+ scores were considered positive, representing intense circumferential membranous staining in more than 10% of the malignant cells. Fluorescent in situ hybridization (FISH) was not performed. All HER2 cases scored ≤2+ were assumed to be negative.13 The molecular subtypes were defined based on the IHC markers as follows: luminal A (ER positive, PR positive or negative, and HER2 negative), luminal B (ER positive, PR positive or negative, and HER2 positive), HER2-enriched (ER and PR negative, and HER2 positive), and basal-like (ER, PR and HER2 negative).7
Statistical analysis was performed using the IBM SPSS Statistics for Windows, Version 22.0 (Armonk, NY: IBM Corp.). Frequencies and percentages were calculated for all nominal variables. The Chi-square test was used to compare categorical variables for the molecular subtypes. A p-value of <0.05 was considered statistically significant.
Results
Initially, 27 cases of MPD were identified. Five cases were excluded because of technical problems (namely, unavailable blocks or an absence or inadequate number of Paget cells on the IHC sections). In addition, in 2 cases of underlying in situ carcinoma, immunohistochemistry was not possible due to the unavailability of the tissue blocks. Thus, 22 cases of MPD and 20 cases of the associated breast carcinomas were included in the final analysis.
All patients were female. The age of the patients ranged from 33 to 82 years, with an average of 51.7 years. All cases of MPD were associated with some form of breast carcinoma. Out of the 22 cases, 6 (27%) were associated with ductal carcinoma in situ (DCIS), and 16 (73%) were associated with invasive ductal carcinoma (IDC). Six of the IDC cases also had an in situ component. The histological grade was available in 18 cases. Fifteen (94%) out of the 16 IDC cases were high grade, while one of the 2 available DCIS cases was graded as high and the other was graded as intermediate.
Upon immunostaining, positive staining for ER was observed in 70-90% of the cells with moderate to strong intensity in 6/22 (27%) samples of MPD and in 6/20 (30%) samples of an associated breast carcinoma. The Paget cells did not show any positivity for PR in any of the samples. Progesterone receptor was positive in 5 (25%) breast carcinoma samples with an intensity of staining ranging from weak to moderate in 10-60% of the cells. In 22 of the MPD samples, HER2 was classified as positive (3+) in 14 (64%) and negative (≤2+) in 8 (36%) samples. In 20 of the samples of associated breast carcinomas, HER2 was scored as positive in 13 (65%) and negative in 7 (35%) samples. The HER2 scores were different in 3 patients between the MPD and associated breast carcinoma samples. The difference was limited to one grade (3+ and 2+) in 2 patients: the grade was higher in the MPD sample in one patient and was higher in the associated breast carcinoma sample in one patient. In the third case, the HER2 grade was 0 in the MPD sample and 2+ in the associated breast carcinoma sample.
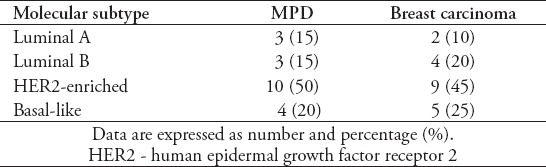
Overall, out of the 22 MPD samples, 3 were classified as luminal A and 3 were classified as luminal B, 11 were classified as HER2-enriched (Figure 1) and 5 were classified as basal-like (Figure 2). The molecular subtype was determined in 20 samples of the associated breast carcinoma. The frequency of the molecular subtypes in the 20 MPD and associated breast carcinomas is shown in Table 1. Molecular subtypes differed in 2 (10%) instances between the MPD and associated breast carcinoma samples. In both cases, the difference was limited to one grade (3+ and 2+) for HER2 staining, with one patient having a higher grade in the MPD sample and one patient having a higher grade in the associated breast carcinoma sample.
Figure 1.

Human epidermal growth factor receptor-2 (HER2)-enriched molecular subtype of MPD. A) Estrogen and B) progesterone receptors were negative and C) HER2 was overexpressed in 100% of the Paget cells (immunostaining, original magnification 20x objective).
Figure 2.
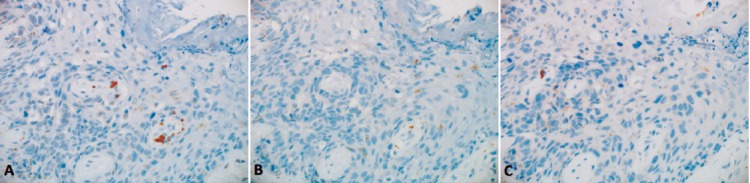
Basal-like subtype of MPD. A) Estrogen and B) progesterone receptors and C) HER2 were negative in the Paget cells. (immunostaining, original magnification 20X objective).
Table 1.
Molecular subtypes in Mammary Paget’s disease (MPD) and the associated breast carcinomas (N=20).
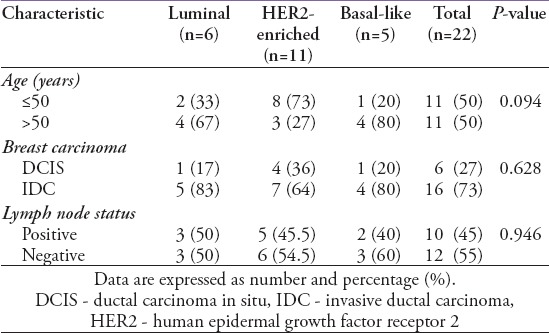
Some clinicopathological characteristics are compared among the different molecular subtypes of MPD in Table 2. In 11 (50%) patients, the age of the patient was ≤50 years; 8 of these samples were HER2-enriched. In the >50 years age group, 3 out of the 11 samples were HER2-enriched. This difference was not statistically significant. Furthermore, there was no statistically significant difference among the frequency of the molecular subtypes in DCIS and IDC or the positive or negative lymph node status.
Table 2.
Clinicopathological characteristics among the Mammary Paget’s disease molecular subtypes.
Discussion
We used a limited panel of IHC stains to determine the molecular subtype of 22 MPD samples and 20 samples of an associated breast carcinoma. Mammary Paget’s disease was associated with breast carcinoma in all of the cases. This is consistent with earlier reports.18,19 Mammary Paget’s disease was thought to arise from multipotent cells in the epidermis from the terminal portion of the lactiferous ducts in studies in which MPD was reported without an underlying breast carcinoma.20 Alternatively, these cases of MPD may represent extensions from breast carcinomas that were too small to be detected by the available technologies.21 In fact, despite an increase in the incidence of breast carcinoma, the incidence of MPD is decreasing due to the earlier detection of the former, prior to its spread to the epidermis, emphasizing the association of MPD and breast carcinoma.22
In this study, 6 (27%) MPD patients had associated DCIS and 16 (73%) had associated IDC. In a cohort of 55 patients with MPD, Onoe et al found DCIS in 23 (42%) patients and IDC in 32 (58%) patients.23 Song et al found an associated breast carcinoma in 57 out of 66 patients with MPD. Twelve (21%) out of 57 associated breast carcinomas were DCIS, while 45 (79%) were IDC.24 Ninety-four percent of our IDC samples were high grade. Lester et al reported high nuclear grade in 93% of MPD-associated carcinomas, while Kothari et al reported high grade in all evaluated cases of IDC.25,26 In concordance with these reports, we found that MPD was associated with high-grade IDC, which was congruent with its aggressive clinical behavior.
Despite ethnic, geographical and age-dependent variations, the majority of breast carcinomas express ER, with proportions ranging from 51% to 77%.12,27 Estrogen receptor positivity is less frequent in MPD- and MPD-associated breast carcinoma. In our cohort, ER was positive in 27% of samples of MPD and in 30% of samples of an associated breast carcinoma. These findings agree with several earlier reports.15,28,29 However, other studies reported a lower frequency of ER-positive MPD. In a cohort of 28 patients, Lester et al25 found that 18 patients had MPD associated with DCIS and 10 with IDC. All of the cases of MPD that were associated with DCIS were negative for ER, while 30% of the MPD cases that were associated with IDC were ER-positive. In our study, one out of 6 patients with MPD and underlying DCIS was positive for ER. On the other hand, five out of 16 patients (31%) with MPD and underlying IDC were positive for ER. Sek et al19 also reported a lower rate of ER-positive MPD. Sek et al19 used a tissue microarray (TMA) instead of standard IHC stains. The use of a TMA may have yielded a lower positive rate due to tumor heterogeneity, as smaller tissue sections are examined in TMA techniques. Furthermore, the authors used a different primary antibody and detection system than was used in our study. As importantly, Sek et al19 used a higher cutoff limit of 10% for ER positivity than was used in our study. We considered samples to be ER positive if 1% or more tumor cells stained positively.30
Overall, breast carcinoma overexpresses HER2 in 13-30% of cases.31 Overexpression of HER2 is higher in MPD and the associated breast carcinomas and ranges from 60 to 80%.28,29,32,33 Likewise, in our study, HER2 was overexpressed (score 3+) in 14 (64%) of the samples of MPD. Some studies reported a higher proportion of HER2-positive patients, in excess of 90%.15,19,32 Leigl et al32 reported overexpression of HER2 in 56 out of 58 (97%) patients with MPD. Similarly, Sek et al19 reported 96% and Fu et al15 reported 93% HER2-positivity in the MPD patients. This higher proportion of positive patients may be attributed to differences in the staining techniques and subjective scoring. Furthermore, we considered the 2+ (equivocal) score to be negative. It is probable that our 2+ HER2 cases would have been reclassified if we did molecular analysis, increasing the proportion of the HER2-enriched subtype.
The molecular subtypes of MPD generally correspond to their associated breast carcinoma.19,25,29 In this study, the molecular subtypes were congruent in 18 out of 20 patients. In 2 patients, the difference was due to HER2 staining and was limited to one grade (3+ and 2+), with one patient having a higher score in the MPD sample and one patient having a higher score in the associated breast carcinoma sample. This result could be explained by tumor heterogeneity, or alternatively it could represent technical difficulties associated with HER2 determination.34
The molecular subtypes of breast carcinoma that were observed in our population corresponded to the other parts of Asia. In India, luminal A has been reported as the most common molecular subtype of breast carcinoma followed by the basal-like subtype with no significant difference in the younger versus older women.12,35 Studies by Kurebayashi et al36 from Japan (63%) and Rahmawati et al37 from Indonesia also revealed a high prevalence of luminal A subtype (41.3%).
The distribution of the molecular subtypes in MPD from this study and the molecular subtypes in MPD and breast carcinoma as a whole from the literature are depicted in Figure 3.
Figure 3.
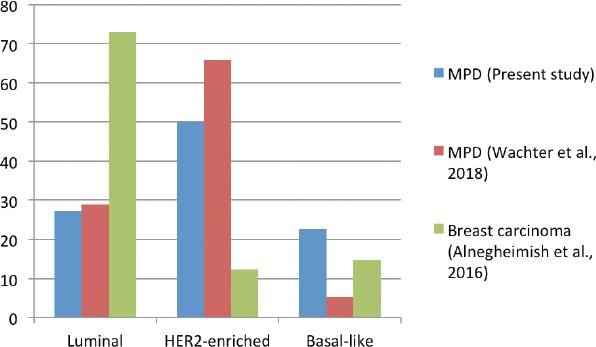
Distribution of the molecular subtypes in Mammary Paget’s disease (MPD) in the present study and in MPD29 and breast carcinoma12 from the literature. HER2 - human epidermal growth factor receptor 2
A comparison of the studies revealed significant differences in the distribution of the molecular subtypes between MPD and breast carcinoma as a whole. The luminal subtype was the most prevalent molecular subtype of breast carcinoma. On the other hand, the HER2-enriched subtype was dominant in both studies among the MPD patients. The higher proportion of the HER2-enriched molecular subtype is related to poor prognosis in MPD.26,28 In MPD studies, the distribution of the molecular subtypes was generally comparable. Taken together, HER2-enriched subtypes (HER2-enriched and luminal B) represented 64% of our MPD sample cohort, as compared to a study by Wachter et al that observed that 86% of MPD samples were HER2-enriched. On the other hand, 5 (23%) of our MPD samples were classified as basal-like, in contrast a study by Wachter et al29 that found only 2 (10%) basal-like MPD samples. Both of these differences underpin the limitation of our HER2 scoring in the 5 cases classified as 2+ by IHC staining.
Study limitations
A limitation of our study was the small number of cases due to the low incidence of MPD. However, this number allowed for the evaluation of statistical significance, and our results clearly demonstrated an increased frequency of HER2-positive molecular subtypes. We did not use Ki67 as a marker of proliferative index. Furthermore, we could not perform molecular analysis for the HER2 equivocal cases based on IHC and considered them to be negative. This was due to the limited availability of retrieved tissue samples of MPD. From a technical standpoint, unless tissue is selected by laser microdissection, immunohistochemical staining is considered preferable over molecular analysis due to the scattered nature of the Paget cells in the epidermis.29 For this reason, the proportion of HER2-enriched molecular subtypes in MPD could be even higher than the figure reported in this study.
In conclusion, this study shows that the HER2-enriched subtype is the most frequent molecular subtype in MPD. Mammary Paget’s disease was associated with breast carcinoma in all of the cases and was more likely to be associated with high grade IDC. The molecular subtypes of the underlying breast carcinomas are usually similar to that of MPD. Molecular subtypes vary between MPD and associated breast carcinomas and breast carcinoma as a whole. The HER2-enriched subtype is the most frequent subtype in MPD and associated breast carcinomas as opposed to the luminal subtype in breast carcinoma as a whole.
Footnotes
References
- 1.Arpino G, Milano M, De Placido S. Features of aggressive breast cancer. Breast. 2015;24:594–600. doi: 10.1016/j.breast.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Alabdulkarim B, Hassanain M, Bokhari A, AlSaif A, Alkarji H. Age distribution and outcomes in patients undergoing breast cancer resection in Saudi Arabia. A single-institute study. Saudi Med J. 2018;39:464–469. doi: 10.15537/smj.2018.5.21993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prat A, Pineda E, Adamo B, Galván P, Fernández A, Gaba L, et al. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast. 2015;24(Suppl 2):S26–S35. doi: 10.1016/j.breast.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 6.Viale G. The current state of breast cancer classification. Ann Oncol. 2012;23(Suppl 10):x207–x210. doi: 10.1093/annonc/mds326. [DOI] [PubMed] [Google Scholar]
- 7.Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, et al. Personalizing the treatment of women with early breast cancer:highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Research. 2005;11:678–685. doi: 10.1158/1078-0432.CCR-04-2421. [DOI] [PubMed] [Google Scholar]
- 9.Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736–750. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaudary MA, Millis RR, Lane EB, Miller NA. Paget's disease of the nipple:a ten-year review including clinical, pathological, and immunohistochemical findings. Breast Cancer Res Treat. 1986;8:139–46. doi: 10.1007/BF01807702. [DOI] [PubMed] [Google Scholar]
- 11.Paget J. On disease of the mammary areola preceding cancer of the mammary gland. Cancer J Clin. 1971;21:303–304. [Google Scholar]
- 12.Alnegheimish NA, Alshatwi RA, Alhefdhi RM, Arafah MM, AlRikabi AC, Husain S. Molecular subtypes of breast carcinoma in Saudi Arabia. A retrospective study. Saudi Med J. 2016;37:506–512. doi: 10.15537/smj.2016.5.15000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sengal AT, Haj-Mukhtar NS, Elhaj AM, Bedri S, Kantelhardt EJ, Mohamedani AA. Immunohistochemistry defined subtypes of breast cancer in 678 Sudanese and Eritrean women;hospitals based case series. BMC Cancer. 2017;17:804. doi: 10.1186/s12885-017-3805-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.AlZaman AS, Mughal SA, AlZaman YS, AlZaman ES. Correlation between hormone receptor status and age, and its prognostic implications in breast cancer patients in Bahrain. Saudi Med J. 2016;37:37–42. doi: 10.15537/smj.2016.1.13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu W, Lobocki CA, Silberberg BK, Chelladurai M, Young SC. Molecular Markers in Paget Disease of the Breast. J Surg Oncol. 2001;77:171–178. doi: 10.1002/jso.1090. [DOI] [PubMed] [Google Scholar]
- 16.Dittmar T, Husemann A, Schewe Y, Nofer JR, Niggemann B, Zänker KS, et al. Induction of cancer cell migration by epidermal growth factor is initiated by specific phosphorylation of tyrosine 1248 of c-erbB-2 receptor via EGFR. FASEB J. 2002;16:1823–1825. doi: 10.1096/fj.02-0096fje. [DOI] [PubMed] [Google Scholar]
- 17.Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, et al. Human epidermal growth factor receptor 2 testing in breast cancer:American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J Clin Oncol. 2018;36:2105–2122. doi: 10.1200/JCO.2018.77.8738. [DOI] [PubMed] [Google Scholar]
- 18.Merrill AY, White A, Howard-McNatt M. Paget's Disease of the Breast:An Institutional Review and Surgical Management. Am Surg. 2017;83:e96–98. [PubMed] [Google Scholar]
- 19.Sek P, Zawrocki A, Biernat W, Piekarski JH. HER2 molecular subtype is a dominant subtype of mammary Paget's cells. An immunohistochemical study. Histopathology. 2010;57:564–571. doi: 10.1111/j.1365-2559.2010.03665.x. [DOI] [PubMed] [Google Scholar]
- 20.Mai KT, Yazdi HM, Perkins DG. Mammary Paget's disease:evidence of diverse origin of the disease with a subgroup of Paget's disease developing from the superficial portion of lactiferous duct and a discontinuous pattern of tumor spread. Pathol Int. 1999;49:956–961. doi: 10.1046/j.1440-1827.1999.00976.x. [DOI] [PubMed] [Google Scholar]
- 21.Sandoval-Leon AC, Drews-Elger K, Gomez-Fernandez CR, Yepes MM, Lippman ME. Paget's disease of the nipple. Breast Cancer Res Treat. 2013;141:1–12. doi: 10.1007/s10549-013-2661-4. [DOI] [PubMed] [Google Scholar]
- 22.Chen CY, Sun LM, Anderson BO. Paget disease of the breast:changing patterns of incidence, clinical presentation, and treatment in the U.S. Cancer. 2006;107:1448–1458. doi: 10.1002/cncr.22137. [DOI] [PubMed] [Google Scholar]
- 23.Onoe S, Kinoshita T, Tamura N, Nagao T, Kuno H, Hojo T, et al. Feasibility of breast conserving surgery for Paget's disease. Breast. 2011;20:515–518. doi: 10.1016/j.breast.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Song Q, Jin Y, Huang T, Zhang JH. Diagnosis and treatment of Paget's disease of the breast:an analysis of 72 cases. Int J Clin Exp Med. 2015;8:19616–19620. [PMC free article] [PubMed] [Google Scholar]
- 25.Lester T, Wang J, Bourne P, Yang Q, Fu L, Tang P. Different panels of markers should be used to predict mammary Paget's disease associated with in situ or invasive ductal carcinoma of the breast. Ann Clin Lab Sci. 2009;39:17–24. [PubMed] [Google Scholar]
- 26.Kothari AS, Beechey-Newman N, Hamed H, Fentiman IS, D'Arrigo C, Hanby AM, et al. Paget disease of the nipple:a multifocal manifestation of higher-risk disease. Cancer. 2002;95:1–7. doi: 10.1002/cncr.10638. [DOI] [PubMed] [Google Scholar]
- 27.Yip CH, Rhodes A. Estrogen and progesterone receptors in breast cancer. Future Oncol. 2014;10:2293–2301. doi: 10.2217/fon.14.110. [DOI] [PubMed] [Google Scholar]
- 28.Ling H, Hu X, Xu XL, Liu ZB, Shao ZM. Patients with nipple-areola Paget's disease and underlying invasive breast carcinoma have very poor survival:a matched cohort study. PLoS One. 2013;8:e61455. doi: 10.1371/journal.pone.0061455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wachter DL, Wachter PW, Fasching PA, Beckmann MW, Hack CC, Riener MO, et al. Characterization of molecular subtypes of paget disease of the breast using immunohistochemistry and in situ hybridization. Arch Pathol Lab Med. 2019;143:206–211. doi: 10.5858/arpa.2017-0578-OA. [DOI] [PubMed] [Google Scholar]
- 30.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version) Arch Pathol Lab Med. 2010;134:e48–72. doi: 10.5858/134.7.e48. [DOI] [PubMed] [Google Scholar]
- 31.Dai X, Xiang L, Li T, Bai Z. Cancer hallmarks, biomarkers and breast cancer molecular subtypes. J Cancer. 2016;7:1281–1294. doi: 10.7150/jca.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liegl B, Horn LC, Moinfar F. Androgen receptors are frequently expressed in mammary and extramammary Paget's disease. Mod Pathol. 2005;18:1283–1288. doi: 10.1038/modpathol.3800437. [DOI] [PubMed] [Google Scholar]
- 33.Bianco MK, Vasef MA. HER-2 gene amplification in Paget disease of the nipple and extramammary site:a chromogenic in situ hybridization study. Diagn Mol Pathol. 2006;15:131–135. doi: 10.1097/01.pdm.0000213456.30151.5b. [DOI] [PubMed] [Google Scholar]
- 34.Chen C, Peng J, Xia HS, Yang GF, Wu QS, Chen LD, et al. Quantum dots-based immunofluorescence technology for the quantitative determination of HER2 expression in breast cancer. Biomaterials. 2009;30:2912–2918. doi: 10.1016/j.biomaterials.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 35.Gupta P, Rai NN, Agarwal L, Namdev S. Comparison of molecular subtypes of carcinoma of the breast in two different age groups:a single institution experience. Cureus. 2018;10:e2834. doi: 10.7759/cureus.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurebayashi J, Moriya T, Ishida T, Hirakawa H, Kurosumi M, Akiyama F, et al. The prevalence of intrinsic subtypes and prognosis in breast cancer patients of different races. Breast. 2007;16(Suppl 2):S72–7. doi: 10.1016/j.breast.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 37.Rahmawati Y, Setyawati Y, Widodo I, Ghozali A, Purnomosari D. Molecular subtypes of indonesian breast carcinomas - lack of association with patient age and tumor size. Asian Pac J Cancer Prev. 2018;19:161–166. doi: 10.22034/APJCP.2018.19.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]


