Abstract
Objectives:
To evaluate p63 expression pattern in Saudi colorectal cancer (CRC) patients and correlate that with clinicopathological parameters and its role in carcinogenesis and prognosis.
Methods:
Archival tumor samples were analyzed by immunohistochemistry for p63 expression in 324 consecutive Saudi patients diagnosed with CRC between January 2006 and December 2017 at the Pathology Department of a tertiary care Hospital, Madinah, Saudi Arabia.
Results:
P63 over-expression was absent in normal mucosa, while 12.5% cases of adenoma showed its over-expression. In CRC, p63 expression was high in 24.1% of cases. There were no significant correlations between p63 expression and gender, tumor location, tumor size, and tumor histologic differentiation. However, high p63 expression revealed a significant correlation with age (p=0.035), tumor type (p=0.004), American Joint Committee on Cancer stage (p=0.046), lymph node metastasis (p=0.006), lymphovascular invasion (p=0.006), distant metastasis (p=0.049) high Ki67 expression (p=0.000) and K-ras expression (p=0.002). The Kaplan-Meier analysis revealed a shorter period of survival with p63 over-expression (p<0.001). The Cox-regression model analysis showed that p63 over-expression was an independent prognostic marker in CRC (p=0.000).
Conclusion:
P63 expression increased from normal to adenoma to carcinoma sequence. Moreover, p63 cytoplasmic expression seems to be related to high Ki67 indexing, K-ras expression, advanced tumor stage and poor clinical outcome of CRC. These findings suggest a significant role of cytoplasmic p63 expression in tumor progression and prognosis.
Colorectal cancer (CRC) is the third leading cancer in men and the second leading cancer in women throughout the world. There is a wide geographical variation in the incidence of CRC across the world; approximately 55% of CRC cases occur in developed countries, while the lowest incidence has been noted in Africa and Asia.1,2 Previous data on CRC within Saudi Arabia predict a potentially alarming increase in CRC in the forthcoming decades.3 Colorectal cancer is the second most common cancer after breast cancer in the general Saudi population. In the Madinah region, CRC is the number one cancer in men and the third most common cancer in women after breast and thyroid cancer.4 Moreover, in a recent study from the Madinah region, CRC is reported to present in advanced stages with aggressive behavior.5 The p63 belongs to the p53 gene family and is a transcription factor that maps to the long arm of chromosome 3. It transactivates p53 target genes and induces apoptosis after expression. The p63 encodes 2 main groups of splicing variants. The splice variant that has an NH2-terminal is known as TAp63 and has properties similar to p53. The group without the NH2-terminal is called ΔNp63. Mutations of ΔNp63 are ‘dominant-negative’ meaning that they promote growth and survival by competing with binding sites of p53.6 ΔNp63a was found to cause accumulation and signaling of b-catenin, supporting the oncogenic function of p63.7 The medical literature; however, suggests that the function of p63, although tissue-specific, is quite controversial. The ΔN isoform of p63 is upregulated in many cancers such as head and neck, esophagus, lung, gastric, pancreatic, extrahepatic bile duct, and Merkel cell carcinomas and is reported to act as an oncogene in these cancers.7 Previous study8 reported that the ΔN isoform is usually downregulated in prostate, breast, bladder, and ovarian cancers. Furthermore, a combined analysis of real time polymerase chain reactions and immunohistochemistry (IHC) have revealed that ΔNp63 isoform expression is progressively reduced in the advanced stages of certain cancers, namely, breast, prostate, urothelial and bladder cancers, and has disappeared in the majority of invasive cancers and nodal metastases.8-10 An extensive literature search has revealed only a few studies available on the expression of p63 in CRC,11-13 and there is not a single research article on this topic from Saudi Arabia. The objective of our research was to estimate the frequency of p63 expression in CRC specimens from the Madinah region of Saudi Arabia and its clinicopathological correlation.
Methods
The present study was a retrospective study involving archival tumor blocks and clinicopathological data that did not involve any patient’s personal information or have any implication on the management protocol. Hence, according to the principles of the Helsinki Declaration, no ethical approval was required for our study. The study included 324 consecutive cases of CRC diagnosed at the Pathology Department of a tertiary care hospital in the Madinah region of Saudi Arabia over a period of 12 years (January 2006 to December 2017). The clinicopathological data, including gender, age, tumor type, size, site, grade, lymphovascular invasion, lymph node status, and the American Joint Committee on Cancer (AJCC) stage were retrieved from the patients’ records. Forty cases of colorectal adenomas and 20 cases of normal colonic mucosa were also included in the present study. Normal colonic mucosa were taken from the normal appearing mucosa away from the tumor and the adenoma samples were taken from different patients. Only histopathologically confirmed invasive CRC cases who underwent total colectomies, hemicolectomies, or wide local excision were included. In situ lesions, recurrences, biopsies, benign lesions and metastases were excluded.
Immunohistochemical procedure
Tissue cores were extracted from archival blocks of the primary CRC and used in the construction of tissue mini arrays (TmAs) using Elkablawy and Albasri’s TmA technique14 as follows: a semiautomatic metal puncher with a 5 mm punch tip was used as a TmA manual kit to punch and extract the tissue cores from pre-warmed paraffin blocks from the main donor tissue blocks. The cores were manually transferred, organized, and attached to a standard block mold with glue and filled with liquid paraffin to build TmA blocks. We then cut 4 micrometer-thick sections of tissue from the TmA blocks, mounted these on poly-l-lysine-coated slides and subjected them to IHC using the Avidin Biotin detection system, as directed by manufacturer guidelines. We used a mouse anti-p63 monoclonal antibody (clone 4A4 diluted 1:200 in blocking solution, Ventana Inc., Tucson, AZ, United States). Sections were double stained with other primary antibodies (anti-Ki67 and K-ras antibodies diluted 1:100 and K-rasantibodies diluted 1:200 in blocking solutions; Ventana Inc., Tucson, AZ, United States). An automatic immunostainer (Ventana Bench Mark XT; Ventana Inc., Tucson, AZ, United States) was used to perform the IHC.The sections were deparaffinized which is followed by cell conditioning. Antigen retrieval was carried out on the machine which utilized Tris based reaction buffer concentrate (pH 7.6 ± 0.2) which is followed by primary antibody application for 32 minutes at 37°C, then the visualization was performed by Avidin Biotin detection system. A case of normal prostatic tissue was used as a positive control for p63. Negative controls were achieved by replacing the primary antibody with serum.
Interpretation of immunohistochemical staining
We observed a uniform cytoplasmic expression of p63 in CRC and adenoma cases, but neither nuclear nor stromal p63 expression was noted in any of the examined cases. Interestingly, contrary to the cytoplasmic staining pattern in the adenoma and CRC cases, the control sample of normal prostatic tissue showed the usual strong nuclear expression in the basal layer; thus, excluding the possibility of misinterpretation. Hence, for the purposes of interpretation of this unusual cytoplasmic expression, we considered tumor cells to be positive for p63 when a distinct cytoplasmic yellow to brown staining was identified. This distinct immunostaining pattern was independently reviewed by 2 pathologists and an average score was taken according to the method described by Albasri et al.15 The p63 expression level was calculated by combining an estimate of the percentage of immunoreactive cells (a quantitative score) with an estimate of the staining intensity (a staining intensity score). The quantitative percentage score was assigned as follows: score 0 was assigned for negative or no staining, score 1 was given when 1% - 10% of cells showed positive staining, score 2 was assigned when 11% - 50% of cells showed positive staining, score 3 was given when 51% - 70% cells were positively stained, and score 4 was assigned when 71% - 100% of positively stained cells were observed. Staining intensity was rated on a scale of 0 to 3 as follows: 0 = negative (no staining), 1 = weak, 2 = moderate, and 3 = strong. The final expression score was stated as follows: ‘-’ for a score of 0, ‘+’ for scores 1–3, ‘++’ for scores 4 to 6 and ‘+++’ for scores >6. For statistical analysis, we combined the cases that scored ‘-’ and ‘+’ as low scores and compared them to the cases that scored ‘++’ and ‘+++’ as high scores.12,15
Statistical analysis
Data was statistically analyzed by using the Statistical Package for Social Sciences version 22.0 (BM Corp, Armonk, NY, United States). The reproducibility of the inter-observer variations was evaluated by correlation analysis between the 2 data sets. The association of the p63 expression with the clinicopathological parameters of the patient was determined by using the Chi-squared test and Fisher’s exact test. Cumulative patient survival was assessed using the Kaplan-Meier method. Finally, the logrank test was used to compare survival curves. The COX-2 proportional hazard linear regression model was performed using a forward stepwise procedure, to look for independent factors independently associated with survival. A p-value less than 0.05 was considered significant for all statistical analyses.
Results
Clinicopathological characteristics of the cases
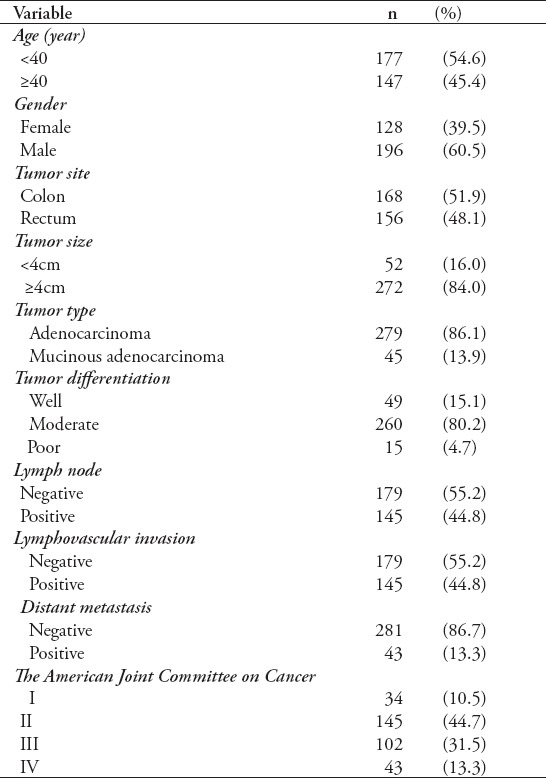
A cohort of 324 patients was included in the study. There were 196 male cases (60.5%) and 128 female cases (39.5%) giving a male to female ratio of 1.5:1. The age of the studied cases ranged from 22 to 96 years with a mean age of 56.9 years. Left-sided tumors were more common than right-sided tumors and were seen in 63.5% of cases. Tumor size was ≥4 cm in 84% of the studied cases. The most common histologic type was adenocarcinoma, seen in 279 cases (86.1%). Most tumors were moderately differentiated (80.2%). Most patients were diagnosed with stage II (44.7%) and stage III (31.5%) cancers according to the AJCC. Approximately 44.8% of cases had positive lymph node metastasis and lymphovascular invasion, and distant metastasis were seen in 30.3% of cases. Table 1 summarizes the clinicopathological characteristics of the 324 CRC cases.
Table 1.
The clinicopathological features of 324 CRC cases.
Expression profiles of p63
Evaluation of p63 protein expression on TmA sections containing normal colonic mucosa revealed a complete lack of p63 expression. In contrast, p63 expression was observed in the cytoplasm of 12.5% (5/40) of cases of colorectal adenoma. In CRC, high cytoplasmic p63 expression was noted in 24.1% (78/324) of cases and low cytoplasmic p63 expression was noted in 75.9% (246/324) of cases. The immunohistochemical profile of our study is shown in Figure 1.
Figure 1.
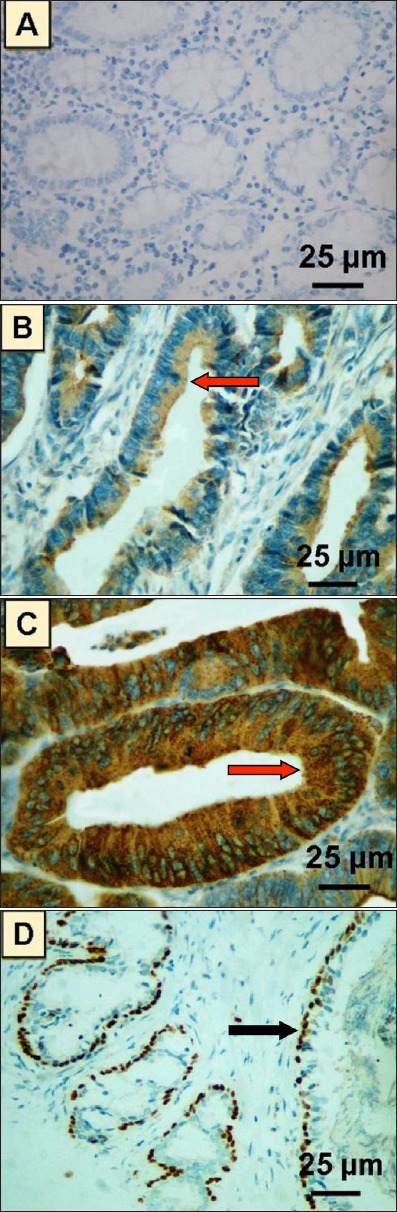
Photomicrograph of different patterns of p63 expression by immunohistochemistry in A) normal colonic mucosa showing a negative expression of p63, B) colorectal adenoma showing cytoplasmic expression of p63 in the tumor cells, C) colorectal carcinoma showing intense cytoplasmic expression of p63 in tumor cells. Red arrows indicate cytoplasmic expression of p63, and D) a control sample from normal prostate gland showing intense selective nuclear expression of p63 in basal cell layer. Black arrow indicates nuclear expression of p63.
Correlation of p63 expression with clinicopathological parameters
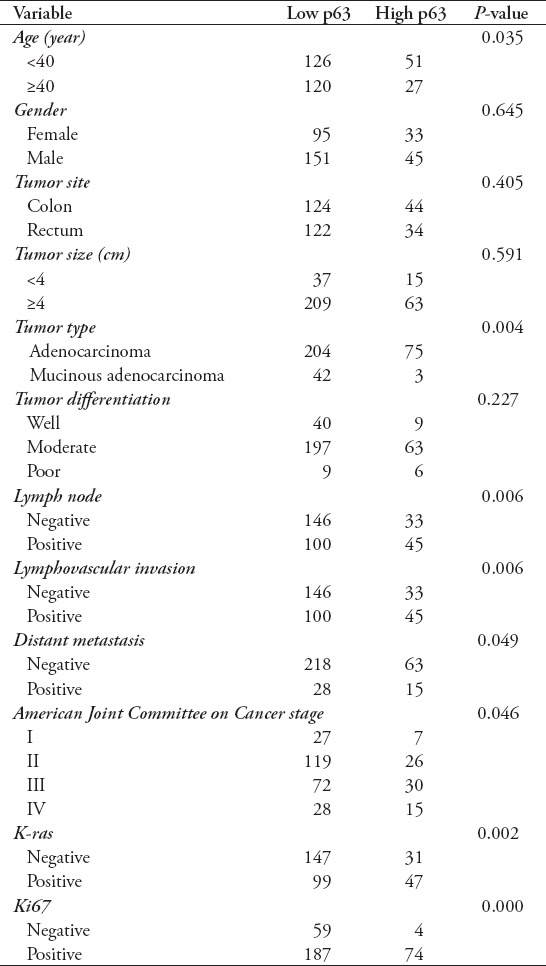
Expression of p63 did not show any significant correlation with gender, tumor site, tumor size, or tumor differentiation. However, p63 expression revealed a significant correlation with age (p=0.035), tumor type (p=0.004), AJCC stage (p=0.046), lymph node metastasis (p=0.006), lymphovascular invasion (p=0.006), distant metastasis (p=0.049), high Ki67 expression (p=0.000), and K-ras expression (p=0.002). The summary of the correlation of p63 over-expression with clinicopathological variables is depicted in Table 2.
Table 2.
Correlation of p63 expression with the clinicopathological variables in colorectal cancer (CRC) cases.
Univariate and multivariate long-term survival analysis
Significantly shorter survival was observed in patients with high p63 expression than inpatients with low p63 expression (p<0.001). Among the independent prognostic indicators, high p63 expression (p=0.000), AJCC (p<0.001), lymph node metastasis (p<0.001), lymphovascular invasion (p<0.001), distant metastasis (p<0.001), histological tumor grade (p<0.001), Ki67 (p=0.000), and K-ras expression (p=0.000) were found to be significant independent prognostic indicators, as calculated by multivariate analysis of the COX regression model. All the significant features associated with survival (p<0.05) including hazard ratio (HR) and confidence interval (CI) for the 324 CRC patients in the study are depicted in Table 3, while Figure 2 shows the overall survival curves for p63 immunoprofiles.
Table 3.
Test statistics for equality of survival distribution for prognostic factors examined in 324 colorectal carcinomas. A Univariate Kaplan-Meier and multivariate- Cox proportional hazards regressionapproach to cancer-specific mortality.
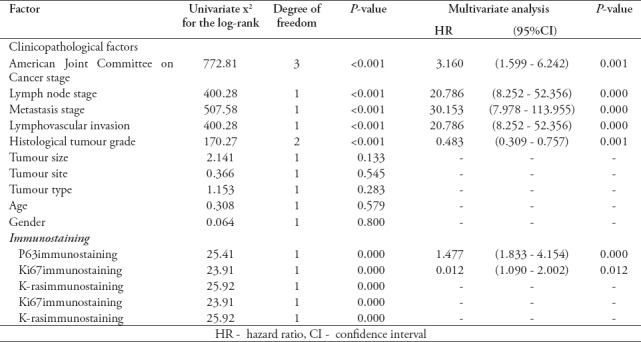
Figure 2.
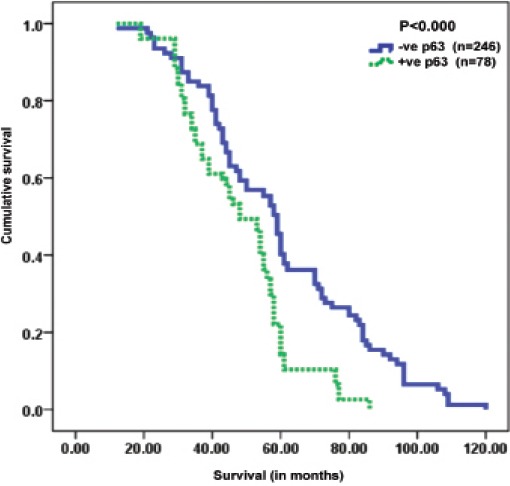
Kaplan-meier survival curves showing significant overall lower survival functions for high p63 expression in colorectal cancer cases.
Discussion
Tumor protein p63, a member of the p53 gene family, has a well-established role in diagnostic pathology as a myoepithelial marker; it is routinely used in histopathology laboratories for the diagnosis of prostatic carcinoma and invasive breast carcinoma. Despite its established role in these tumors, the widespread role of p63 in carcinogenesis is somewhat controversial and is mainly attributed to the presence of various isoforms and posttranslational modifications of p63. The spectrum of p63 expression has been widely studied in several cancers, especially squamous cell carcinomas.8,9 However, the role of p63 in the CRC is not well documented and few studies on p63 expression in CRC are available, particularly from our country and region. Our study is the first of its kind in Saudi Arabia, where we observed a cytoplasmic expression of p63 instead of the usual nuclear expression. An extensive literature search revealed that there are few studies on p63 expression in CRC. Although p63 was discovered in 1997, it was not until late 2006 that Carneiro et al11 from Brazil analyzed the expression of p63 in the CRC. In 2012, Guo et al12 from China reported the role of p63 expression in the prognosis of CRC. More recently, in 2014, Bahnassy et al13 from Egypt analyzed the prognostic and predictive value of p53 family proteins (including p63) in CRC carcinogenesis. Although, the pattern of staining was not clearly mentioned in the study carried ou by Guo et al12 from China, the rest of the other studies mentioned above revealed a nuclear pattern of p63 expression, contrary to our findings of intense cytoplasmic staining of p63 in the CRC and adenoma cases.
In our present study, we observed a predominantly cytoplasmic staining for p63 in tumor cells, which is a rare expression pattern for a protein that normally exhibits strong nuclear staining. It has previously been reported that the isoforms TAp63 (alpha, beta, and gamma subisoforms) are nuclear in location and considered to be tumor suppressor genes. In contrast, ΔNp63 (delta) is the cytoplasmic isoform and is considered an oncogene.6 Ye et al16 have recently confirmed these findings in their research on p63 expression in esophageal squamous cell carcinoma. They found that although both isoforms were expressed, ΔNp63 was dominant in their study. They observed that the silencing of p63 induced more severe knockdown of ΔNp63 protein levels than that of TAp63, thus strongly implicating a role for ΔNp63 in the progression of cancers. This mislocalization and imbalance in the isoforms of p63 may amend the stability and functions of p63 and thus disrupt cell cycle arrest and apoptosis. The cytoplasmic shift of p63 suggests an alteration in the cell cycle pathway, converting a tumor suppressor gene into an oncogene and leading to cancer progression. This may have prognostic significance in the carcinogenesis.16 Like p53, the nuclear localization of the p63 protein is crucial for its role as a transcription factor. An alteration in its localization from nucleus to cytoplasm may lead to disruption of cell cycle checkpoint regulations and mechanisms of apoptosis, thus contributing to the initiation and progression of carcinogenesis.17,18 The cytoplasmic shuffling of p53 is reported to be associated with advanced cancer presentation and poor survival in patients with CRC, inflammatory breast carcinoma, lung carcinoma, and prostate carcinoma.19-22 The exact mechanism of p63 cytoplasmic shuttling remains unclear, but several mechanisms have been proposed. One such mechanism explains that this shift could arise from disruptions in the nuclear transport pathway, like those mediated by the murine double minute2 (Mdm2) gene, where p63-induced apoptosis is reported to be reduced thus leading to oncogenic expression.23 In our study, we evaluated the expression of p63 proteins, using IHC, in a large, well-characterized cohort of CRC cases. This is the largest study to date pertaining to p63 expression in CRC, and we found that 24.1% of CRC cases expressed high levels of p63 in the cytoplasm of tumor cells. Our study is the first of its kind to consider the cytoplasmic expression of p63 in the CRC. This cytoplasmic expression of p63 was distinctly upregulated from normal to adenoma to carcinoma sequence, suggesting that p63 plays a role in CRC carcinogenesis and tumor progression. The study carried out by Carneiro et al11 also showed the upregulation of p63 expression in CRC but they reported a nuclear staining pattern. Recent reports of p63 expression in colorectal tissues are controversial, even though all of them used the same 4A4 monoclonal antibody to p63.24-26 According to manufacturer guidelines, this particular 4A4 clone of p63 antibody can detect all 6 known isoforms of p63, including the ΔN isoform.27 However, a few studies who used the same 4A4 monoclonal antibody reported that p63 was undetectable in CRC.9,28 Carneiro et al11 observed p63 expression in 20% of adenoma and 26% of CRC in their series. Bahnassy et al13 reported a downregulation of p63 expression, with a higher 73.3% frequency of p63 expression in adenoma compared to 38.8% in adenocarcinoma. In our present study, we observed p63 expression in 12.5% of adenoma cases and 24.1% of CRC cases, clearly indicating an upregulation of p63 in our cohort.
In this study, we did not find any statistically significant correlation of p63 expression with gender, tumor size, or tumor location. Similar observations were made by a group of scientists from China.12 In contrast, we found a statistically significant correlation of p63 expression with variables of poor prognosis such as age, advanced AJCC stages, tumor type, lymph node metastasis, lymphovascular invasion, and distant metastasis. There is a lack of data on the comparison of our CRC findings. Moreover, none of the previous studies showed a distinct cytoplasmic expression of p63 in CRC cases. However, few studies are available in the English literature that have analyzed the clinicopathological correlation of p63 expression in CRC. A research study by Guo et al12 on p63 expression in CRC did not find any significant correlation between pathological tumor stages and vascular invasion. In contrast, Bahnassy et al13 have reported a significant correlation between tumor stage, lymphovascular invasion, and tumor recurrence. However, due to the distinct cytoplasmic expression of p63 in our cases, we could not compare our findings against this type of clinicopathological correlation in CRC cases in other recent and past literature. Nevertheless, a similar kind of observation between clinicopathological findings and cytoplasmic p63 expression has been observed by several scientists in the adenocarcinoma of the lung and prostate gland.21,22,29 Several reports have also mentioned a positive correlation between p63 expression and the grading of other neoplasms such as head and neck squamous cell carcinoma, lung, and breast carcinomas.24,30-32 Our findings of significant correlation between cytoplasmic p63 expression and clinicopathological parameters in CRC cases are in accordance with these studies and prove that cytoplasmic p63 expression can be considered a marker of tumor proliferation and differentiation in the CRC.
A statistically significant observation was noted among the cytoplasmic p63 expression, high Ki67 indexing, and K-ras expression. These observations provide major evidence supporting the role of cytoplasmic p63 expression with that of tumor proliferation and poor patient outcomes. This emphasizes the shift from tumor suppressor gene proteins, leading to various cellular events and alterations in function, to oncogenes.17,18 Similar findings were observed in previously published reports showing the prognostic value of Ki67 in laryngeal squamous cell carcinoma.33 Ki67 immunostaining and K-ras mutations in CRC have been reported to play an important role in detecting the biological aggressiveness of CRC.34 Moreover, a large study of Kirsten Ras mutations from 13 different countries by the Colorectal Cancer Collaborative Group Study (RASCAL Study) indicated that the presence of K-ras mutation is associated with poor patient outcomes.35 Lan et al36 reported the role of PI3K/PTEN/AKT and RAS/RAF/MAPK pathways in the metastasis and recurrence of CRC. Similarly, Shen et al37 observed the role of RAS/RAF/MAPK signaling pathway in the progression of gastric cancer and it is activated by an oncogene cyclin D1 and they concluded that it is associated with poor patient outcome. Although, none of them found any role of p63 in the activation of RAS/RAF/MAPK pathway. However, our findings in univariate and multivariate analysis support the idea of cytoplasmic shuffling of p63 is associated with worse patient survival and high cytoplasmic p63 expression acts as an independent risk factor in the progression of CRC. Similar findings of cytoplasmic p63 expression being an independent risk factor have been reported in lung and prostate cancers in previous studies.21,22
Study limitations
This study has a few limitations, First, the sample was restricted to a single tertiary care government institution, which might limit the extension of results to the general population in the Madinah region. Second, include relatively low number of cases. Third, lack of a standardised scoring system or uniformly accepted threshold for cytoplasmic positivity due to high variability for P63 protein expression reported by different authors. Lastly, the heterogeneous staining patterns of P63 due to using TMA technique.
In conclusion, we studied the expression of p63 in a large cohort of CRC cases and found its expression to correlate with poor prognostic parameters. Only a few studies have been published in the recent medical literature and our study is the first to report cytoplasmic localization of p63 in CRC cases, in contrast to previously reported nuclear localization. This observation suggests that the localization shift from nucleus to cytoplasm may be an important mechanism for the oncogenic effect of p63 in the CRC. Further studies to investigate the mechanism and significance of this localization shift of p63 are strongly recommended.
Acknowledgment
We extend our sincere thanks to The Deanship of Scientific Research, Taibah University, Madinah, Saudi Arabia, for their constant encouragement and support. We would also like to thank Scribendi, the Editing and Proofreading Services for English Documents (https://www.scribendi.com/) for the English Language editing.
Footnotes
References
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN. Cancer incidence and mortality worldwide. Lyon (FR): International Agency for Research on Cancer; 2012. [Google Scholar]
- 2.Bray F, Ren JS, Masuyer E, Ferlay J. Estimates of global cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2013;132:1133–1145. doi: 10.1002/ijc.27711. [DOI] [PubMed] [Google Scholar]
- 3.Ibrahim EM, Zeeneldin AA, El-Khodary TR, Al-Gahmi AM, Bin Sadiq BM. Past, present and future of colorectal cancer in the Kingdom of Saudi Arabia. Saudi J Gastroenterol. 2008;14:178–182. doi: 10.4103/1319-3767.43275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saudi Health Council. Annual Cancer Incidence Report for Saudi Cancer Registry (SCR) [(Updated 2014; Accessed 2018 November 11)]. Available from https://nhic.gov.sa/eServices/Documents/2014.pdf .
- 5.Albasri A, Yosef H, Hussainy AS, Sultan SA, Alhujaily A. Histopathological features of colorectal cancer in Al-Madinah region of Saudi Arabia:8 years experience. Asian Pac J Cancer Prev. 2014;15:3133–3137. doi: 10.7314/apjcp.2014.15.7.3133. [DOI] [PubMed] [Google Scholar]
- 6.Soussi T, Wiman KG. TP53:an oncogene in disguise. Cell Death Differ. 2015;22:1239. doi: 10.1038/cdd.2015.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yougong C, Shijie P, Yimin F, Zhi-Xiong L, Chenghua X. double dealing tale of p63:an oncogene or a tumor suppressor. Cell Mol Life Sci. 2018;75:965–973. doi: 10.1007/s00018-017-2666-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Massion PP, Taflan PM, Jamshedur Rahman SM, Yildiz P, Shyr Y, Edgerton ME, et al. Significance of p63 amplification and overexpression in lung cancer development and prognosis. Cancer Res. 2003;63:7113–7121. [PubMed] [Google Scholar]
- 9.Reis-Filho JS, Simpson PT, Martins A, Preto A, Gärtner F, Schmitt FC. Distribution of p63, cytokeratins 5/6 and cytokeratin 14 in 51 normal and 400 neoplastic human tissue samples using TARP-4 multi-tumor tissue microarray. Virchows Arch. 2003;443:122–132. doi: 10.1007/s00428-003-0859-2. [DOI] [PubMed] [Google Scholar]
- 10.Bergholz J, Xiao ZX. Role of p63 in development, tumorigenesis and cancer progression. Cancer Microenviron. 2012;5:311–322. doi: 10.1007/s12307-012-0116-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carneiro FP, Ramalho LN, Britto-Garcia S, Ribeiro-Silva A, Zucoloto S. Immunohistochemical expression of p16, p53, and p63 in colorectal adenomas and adenocarcinomas. Dis Colon Rectum. 2006;49:588–594. doi: 10.1007/s10350-006-0515-4. [DOI] [PubMed] [Google Scholar]
- 12.Guo HQ, Huang GL, Liu OF, Liu YY, Yao ZH, Yao SN, et al. p63 Expression is a prognostic factor in colorectal cancer. Int J Biol Markers. 2012;27:212–218. doi: 10.5301/JBM.2012.9581. [DOI] [PubMed] [Google Scholar]
- 13.Bahnassy AA, Zekri AR, Salem SE, Abou-Bakr AA, Sakr MA, Abdel-Samiaa AG, et al. Differential expression of p53 family proteins in colorectal adenomas and carcinomas:Prognostic and predictive values. Histol Histopathol. 2014;29:207–216. doi: 10.14670/HH-29.207. [DOI] [PubMed] [Google Scholar]
- 14.Elkablawy MA, Albasri AM. High quality tissue miniarray technique using a conventional TV/radio telescopic antenna. Asian Pac J Cancer Prev. 2015;16:1129–1133. doi: 10.7314/apjcp.2015.16.3.1129. [DOI] [PubMed] [Google Scholar]
- 15.Albasri AM, Elkablawy MA, Hussainy AS, Yousif HM, Alhujaily AS. Impact of cyclooxygenase-2 over-expression on the prognosis of colorectal cancer patients:An experience from Western Saudi Arabia. SaudiMed J. 2018;39:773–780. doi: 10.15537/smj.2018.8.22837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye S, Lee KB, Park MH, Lee JS, Kim SM. p63 regulates growth of esophageal squamous carcinoma cells via the Akt signaling pathway. Int J Oncol. 2014;44:2153–2159. doi: 10.3892/ijo.2014.2374. [DOI] [PubMed] [Google Scholar]
- 17.Fabbro M, Henderson BR. Regulation of tumor suppressors by nuclear-cytoplasmic shuttling. Exp Cell Res. 2003;282:59–69. doi: 10.1016/s0014-4827(02)00019-8. [DOI] [PubMed] [Google Scholar]
- 18.Hood JK, Silver PA. Diverse nuclear transport pathways regulate cell proliferation and oncogenesis. Biochim Biophys Acta. 2000;1471:M31–M41. doi: 10.1016/s0304-419x(00)00018-4. [DOI] [PubMed] [Google Scholar]
- 19.Prall F, Hühns M. Quantitative evaluation of TP53 immunohistochemistry to predict gene mutations:lessons learnt from a series of colorectal carcinomas. Hum Pathol. 2019;84:246–253. doi: 10.1016/j.humpath.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Inoue T, Stuart J, Leno R, Maki CG. Nuclear import and export signals in control of the p53-related protein p73. J Biol Chem. 2002;277:15053–15060. doi: 10.1074/jbc.M200248200. [DOI] [PubMed] [Google Scholar]
- 21.Narahashi T, Niki T, Wang T, Goto A, Matsubara D, Funata N, et al. Cytoplasmic localization of p63 is associated with poor patient survival in lung adenocarcinoma. Histopathology. 2006;49:349–57. doi: 10.1111/j.1365-2559.2006.02507.x. [DOI] [PubMed] [Google Scholar]
- 22.Dhillon PK, Barry M, Stampfer MJ, Perner S, Fiorentino M, Fornari A, et al. Aberrant cytoplasmic expression of p63 and prostate cancer mortality. Cancer Epidemiol Biomarkers Prev. 2009;18:595–600. doi: 10.1158/1055-9965.EPI-08-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stindt MH, Muller PA, Ludwig RL, Kehrloesser S, Dötsch V, Vousden KH. Functional interplay between MDM2, p63/p73 and mutant p53. Oncogene. 2015;34:4300–4310. doi: 10.1038/onc.2014.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lo Muzio L, Santarelli A, Caltabiano R, Rubini C, Pieramici T, Trevisiol L, et al. p63 overexpression associates with poor prognosis in head and neck squamous cell carcinoma. Hum Pathol. 2005;36:187–194. doi: 10.1016/j.humpath.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Koga F, Kawakami S, Kumagai J, Takizawa T, Ando N, Arai G, et al. Impaired Delta Np63 expression associates with reduced beta-catenin and aggressive phenotypes of urothelial neoplasms. Br J Cancer. 2003;88:740–747. doi: 10.1038/sj.bjc.6600764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sethi I, Romano RA, Gluck C, Smalley K, Vojtesek B, Buck MJ, Sinha S. A global analysis of the complex landscape of isoforms and regulatory networks of p63 in human cells and tissues. BMC Genomics. 2015;16:584. doi: 10.1186/s12864-015-1793-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agilent Dako. Monoclonal Mouse Anti-Human p63 Protein. Clone 4A4 (product information) [[Accessed 2018 November 13]]. Available from http://www.dako.com/download.pdf?objectid=112041003 .
- 28.Glickman JN, Yang A, Shahsafaei A, McKeon F, Odze RD. Expression of p53-related protein p63 in the gastrointestinal tract and in esophageal metaplastic and neoplastic disorders. Hum Pathol. 2001;11:1157–1165. doi: 10.1053/hupa.2001.28951. [DOI] [PubMed] [Google Scholar]
- 29.Ferronika P, Triningsih FX, Ghozali A, Moeljono A, Rahmayanti S, Shadrina AN, et al. p63 cytoplasmic aberrance is associated with high prostate cancer stem cell expression. Asian Pac J Cancer Prev. 2012;13:1943–1948. doi: 10.7314/apjcp.2012.13.5.1943. [DOI] [PubMed] [Google Scholar]
- 30.Ribeiro-Silva A, ZambelliRamalho LN, Britto Garcia S, Zucoloto S. The relationship between p63 and p53 expression in normal and neoplastic breast tissue. Arch Pathol Lab Med. 2003;3:336–340. doi: 10.5858/2003-127-0336-TRBPAP. [DOI] [PubMed] [Google Scholar]
- 31.Wang BY, Gil J, Kaufman D, Gan L, Kohtz DS, Burstein DE. P63 in pulmonary epithelium, pulmonary squamous neoplasms, and other pulmonary tumors. Hum Pathol. 2002;33:921–926. doi: 10.1053/hupa.2002.126878. [DOI] [PubMed] [Google Scholar]
- 32.Xia W, Chen JS, Zhou X, Sun PR, Lee DF, Liao Y, Zhou BP, Hung MC. Phosphorylation/cytoplasmic localization of p21Cip1/WAF1 is associated with HER2/neu overexpression and provides a novel combination predictor for poor prognosis in breast cancer patients. Clin Cancer Res. 2004;10:3815–3824. doi: 10.1158/1078-0432.CCR-03-0527. [DOI] [PubMed] [Google Scholar]
- 33.Re M, Zizzi A, Ferrante L, Stramazzotti D, Goteri G, Gioacchini FM, Olivieri F, Magliulo G, Rubini C. p63 and Ki-67 immunostainings in laryngeal squamous cell carcinoma are related to survival. European Archives of Oto-Rhino-Laryngology. 2014;271:1641–1651. doi: 10.1007/s00405-013-2833-1. [DOI] [PubMed] [Google Scholar]
- 34.Melling N, Kowitz CM, Simon R, Bokemeyer C, Terracciano L, Sauter G, Izbicki JR, Marx AH. High Ki67 expression is an independent good prognostic marker in colorectal cancer. Journal of Clinical Pathology. 2016;69:209–214. doi: 10.1136/jclinpath-2015-202985. [DOI] [PubMed] [Google Scholar]
- 35.Andreyev HJ, Norman AR, Cunningham D, Oates J, Dix BR, Iacopetta BJ, Young J, Walsh T, Ward R, Hawkins N, et al. Kirsten ras mutations in patients with colorectal cancer:the 'RASCAL II'study. Br J Cancer. 2001;85:692–696. doi: 10.1054/bjoc.2001.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen B, Li M, Wang H, Xin L, Xie J. Expression and clinical significance of the RAS/RAF/MAPK cell signaling pathway in gastric cancer. Int J Clin Exp Med. 2018;11:11682–11689. [Google Scholar]
- 37.Lan YT, Jen Kou L, Lin CH, Yang SH, Lin CC, Wang HS, Chen WS, Lin TC, Jiang JK, Chang SC. Mutations in the RAS and PI3K pathways are associated with metastatic location in colorectal cancers. J Surg Oncol. 2015;111:905–910. doi: 10.1002/jso.23895. [DOI] [PubMed] [Google Scholar]


