Abstract
Objectives
To study the short and intermediate surgical, renal functional, and oncologic outcomes of multiplex partial nephrectomy (mPN) and standard partial nephrectomy (sPN) in the setting of a solitary kidney.
Patients and Methods
Review of a prospectively maintained database of patients undergoing solitary kidney partial nephrectomy at our institution was performed. Patients were stratified into two cohorts: mPN where 3 or more renal tumors were resected and sPN, where 1 or 2 tumors were resected. Perioperative, renal functional and oncological outcomes were compared.
Results
Ninety three patients with a solitary kidney underwent a total of 121 surgical procedures; 43 (35.5%) were sPN and 78 (64.4%) were mPN. The total and major (Clavien Grade III and IV) complication rates between sPN and mPN were similar (57.1% vs. 70.1%, p=0.2; 31.0% vs. 35.1%, p=0.3). At 12 months post-op, the percentage of patients with eGFR >45 was similar in each group (sPN 87.0%, mPN 73.7%; p=0.2), and long-term hemodialysis rates were 4.7% and 6.4%, respectively. Completion nephrectomy was performed in 2.3% of sPN and 2.6% of mPN. At a median follow up of 40.1 months, the metastasis rate was 8.6% in the sPN group and 4.1% in the mPN group (p=0.4).
Conclusions
Partial nephrectomy in the setting of a solitary kidney can effectively preserve renal function. The renal functional and oncologic outcomes were similar in sPN and mPN, with low HD rates and complication rates within the expected range of these operations. Three or more tumors in a solitary kidney should not be a contraindication for nephron sparing surgery.
Keywords: partial nephrectomy, renal cell carcinoma, multifocal kidney tumors, multiplex partial nephrectomy, nephron sparing surgery, solitary kidney
1. Introduction
Renal cell carcinoma (RCC) is a common malignancy with an estimated 64,000 new cases and 14,400 deaths in 2017.1 While most patients with RCC present with one tumor, a subset of patients presents with multifocal tumors, either due to known hereditary syndrome or for presumed sporadic RCC. Management of patients with multifocal tumors must weigh surgical complexity with nephron-preservation and cancer control. Multiplex partial nephrectomy (mPN)—the resection of 3 or more tumors from one kidney during a single operation is an alternative to radical nephrectomy in this patient population. This approach requires advanced surgical technique, and comes with the expectation of increased rates of intraoperative and postoperative complications.2–4 However, when feasible, mPN should be considered as an alternative to radical nephrectomy (RN), as patients with native renal function have higher quality of life (QOL) with equivalent oncologic outcomes.5,6
The challenge of weighing higher risks of complications to preserve renal function is heightened in patients with a solitary kidney. While partial nephrectomy may be optional in a patient with a contralateral kidney present, solitary kidney is still considered one of the strongest relative indications for partial nephrectomy. Patients with a genetic predisposition for bilateral, multifocal RCC—such as those with von Hippel-Lindau disease (VHL), Birt-Hogg-Dube syndrome (BHD), and hereditary papillary RCC (HPRCC)—often require multiple, bilateral kidney surgeries.7 After numerous surgeries, these patients are often left with a solitary kidney and renal function preservation becomes even more important.
Here we report the peri-operative and intermediate-term surgical, renal functional, and oncologic outcomes of partial nephrectomy (PN) in a solitary kidney. We compare these results between standard PN (sPN)—excision of 1 or 2 tumors from the same kidney—and mPN. To our knowledge, this is the largest cohort reported in the urologic literature to date.
2. Materials and Methods
2.1. Study Sample and Design
Retrospective review of a prospectively maintained institutional database of patients undergoing PN in a solitary kidney on an IRB-approved protocol between April 1992 and June 2017 was performed. Ninety-three patients undergoing 124 PNs were identified. Of these 121 surgeries had adequate clinical data and follow-up and were included in analysis. Surgeries were stratified into sPN, defined as excision of 1 or 2 tumors, and mPN, defined as removal of 3 or more tumors. Pathologically benign cysts and tumors were excluded from the tumor count at stratification. Additional analyses were performed comparing the initial mPN and repeat mPN.
Surgical technique has been previously described. In brief, the kidney was completely mobilized within Gerota’s fascia. Intraoperative ultrasound was used to define tumors. The renal hilum was clamped only for hilar and/or endophytic tumors. Tumor enucleation was performed by circumferentially incising the renal capsule. A plane was defined between the tumor pseudocapsule and renal parenchyma. Renorraphies were performed in the standard fashion.
2.2. Study Variables
Demographic and diagnostic data were collected on all patients. These included age, sex, race, height, weight, and past medical and surgical history. Operative and post-operative data collected include approach, operative time, estimated blood loss (EBL), urine output (UOP), blood transfusions, complications, and discharge day. Follow-up time was calculated from date of PN to day of last follow-up. In patients with multiple surgeries, follow-up time was calculated from date of first PN to subsequent PN. Long term follow-up data included complications, creatinine, use of hemodialysis (HD), and metastases. The estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI 2009 formula.8 HD included temporary “bridge” HD prior to resumption of adequate renal function and long-term HD. For statistical purposes, patients who initiated HD after completion RN or subsequent RN were not included as those requiring HD. Pathologic confirmation was required for diagnosis of metastatic disease. Cytoreductive surgery was defined as metastatic disease diagnosed pre-operatively or during the procedure. Patients undergoing cytoreductive surgery were not included in the post-op metastasis rate.
2.3. Statistical Methods
Statistical analyses were performed using SPSS software. Categorical variables were compared using Chi-square test, and continuous variables were compared using the Mann-Whitney U test. Kaplan-Meier was used to estimate post-operative metastatis-free survival, and Log Rank test was used to compare groups. Statistical significance was defined as two-sided p value being less than 0.05.
3. Results
3.1. Patient demographics and operative characteristics
A cohort of 93 patients (median [IQR] age 53.8 [17.1]) underwent a total of 121 operations; 35.5% of the procedures were sPN (n=43) and 64.%% were mPN (n=78). Patient ethnicity, sex, and diagnoses are shown in Table 1. Table 2 depicts operative characteristics and follow-up time. Patients undergoing mPN were more likely to have had a prior ipsilateral PN (50.0% vs. 27.0%, p=0.02) and have larger tumors (median [IQR] 3.6 cm [1.5] vs. 2.6 cm [2.1], p=0.001). More sPNs were performed robotically compared to mPN (14 [32.6%] vs. 7 [9.1], p<0.01). mPN resulted in higher estimated blood loss (EBL) (median 2.5 [3.3] L vs. 1.4 [1.7] L, p=0.001) and more transfusions (median 5.0 [7.0] vs. 1.0 [4.0[ units, p<0.001). The vast majority of all cases were done without renal hilar occlusion accounting for the high EBL values in both groups.
Table 1.
Patient Characteristics
| No. Patients/Operations | 93/124 |
| Median Age (IQR) | 53.8 (17.1) |
| Sex (%) | |
| Male | 54 (58.1) |
| Female | 39 (41.9) |
| Race (%) | |
| Caucasian | 75 (80.6) |
| African-American | 11 (11.8) |
| Other | 7(7.5) |
| Median BMI (IQR), kg/m2 | 29.2 (8.9) |
| Diagnosis (%) | |
| VHL | 44 (50.0) |
| Sporadic RCC | 27 (30.7) |
| HLRCC | 5 (5.7) |
| HPRC | 5 (5.7) |
| BHD | 2(2.3) |
| Other | 5 (5.7) |
VHL=von Hippel-Lindau; RCC- renal cell carcinoma; HLRCC= hereditary leiomyomatosis and renal cell carcinoma; HPRC=hereditary papillary RCC; BHD= Birt-Hogg-Dube
Table 2.
Operation Characteristics
| Operation | sPN | mPN | Total | p value |
|---|---|---|---|---|
| n (%) | 43 (35.5) | 78 (64.5) | 121 | |
| Median Tumor Number (IQR) | 1.0 (1.0) | 9.0 (10.5) | 4.0 (9.0) | p<0.001 |
| Median Largest Tumor (IQR), cm | 2.6(2.1) | 3.6(1.5) | 3.5 (2.0) | p=0.001 |
| Prior Ipsilateral PN (%) | 12 (27.9) | 39 (50.0) | 51 (42.1) | p=0.02 |
| Median Time from PN (IQR), mo | 71.0 (67.8) | 80.6 (86.5) | 76.3 (75.6) | p=0.5 |
| Cytoreductive Procedures (%) | 8 (18.6) | 4(5.1) | 12 (9.9) | p=0.03 |
| Right Sided PN (%) | 21 (50.0) | 37 (48.1) | 58 (48.7) | p=0.9 |
| Robotic Approach (%) | 14 (32.6) | 7(9.1) | 21 (17.5) | p<0.01 |
| Median EBL (IQR), L | 1.4 (1.7) | 2.5 (3.3) | 2.0(3.2) | p=0.001 |
| Median UOP (IQR), mL | 450.0 (385.0) | 500.0 (439.0) | 470.0 (413.8) | p=0.4 |
| Clamp Used (%) | 17 (44.7) | 10 (26.3) | 27 (35.5) | p=0.2 |
| Median Clamp Time (IQR), mins | 24.0 (30.0) | 42.0 (29.0) | 35.0 (32.0) | p=0.3 |
| Median Op Time (IQR), mins | 315.0 (163.8) | 420.5 (169.3) | 410.0 (164.3) | p<0.01 |
| Median No. Units Transfused (IQR) | 1.0 (4.0) | 5.0 (7.0) | 4.0(7.0) | p<0.001 |
| Median POD Discharge (IQR) | 7.0 (3.0) | 8.0(5.5) | 7.5 (5.0) | p<0.001 |
| Median Follow Up Time (IQR), mo | 35.9 (71.1) | 42.5 (76.2) | 40.1 (74.5) | p=0.5 |
Tumor Number- Number of tumors with a cancer diagnosis on pathology resected, cysts and benign masses removed were excluded; Largest Tumor- greatest diameter of resected tumors as measured by pathology; Time from PN- months from date of prior PN on ipsilateral kidney; Cytoreductive procedures- PN performed on patients with metastatic disease; EBL=estimated blood loss; UOP=urine output; Op Time=operation time; No. Units Transfused- packed red blood cell units given during operation; Follow Up Time- months from date of surgery to date of urology or nephrology clinic visit or date of next PN
3.2. Post-operative complications
Table 3 describes post-operative complications of 119 procedures with available data. The overall complication rate was 65.5%, and the major complication (Clavien Grade III and IV) rate was 33.6%. There were no Class V complications. The total and major complication rates between sPN and mPN were similar (57.1% vs. 70.1%, p=0.2; 31.0% vs. 35.1%, p=0.3). Additionally, there was no significant difference in complication rate between initial mPN and repeat mPN (Total: 71.8% vs. 68.4%, p=0.8; Major: 30.8% vs. 39.5%, p=0.5), nor between initial sPN and repeat sPN (Total: 51.6% vs. 72.8%, p=0.3; Major: 25.8% vs. 45.5%, p=0.4. Within the sPN cohort, 20 (46.7%) patients had urologic complcations, and 45 (58.4%) mPN patients had urologic complications (p=0.3). Each urologic complication is listed in Table 3.
Table 3.
Post-Operative Complications
| Operation | sPN n=42 | mPN n=77 | Total n=119 | p value |
|---|---|---|---|---|
| n=42 | n=77 | n=119 | ||
| Total (%) | 24 (57.1) | 54 (70.1) | 78 (65.5) | p=0.2 |
| Major Complications (%) | 13 (31.0) | 27 (35.1) | 40 (33.6) | p=0.3 |
| Urologic Complications (%) | 20 (47.6) | 45 (58.4) | 65 (54.6) | p=0.3 |
| Median Number of Urologic Complications (IQR) | 0 (1) | 1 (2) | 1 (1) | p=0.07 |
| Breakdown of Urologic Complications Adrenal insufficiency | 0 | 1 | 1 | |
| AKI | 1 | 5 | 6 | |
| Bleeding | 5 | 16 | 21 | |
| Delayed bowel injury | 0 | 2 | 2 | |
| Fluid overload | 4 | 11 | 15 | |
| Pneumothorax | 1 | 1 | 2 | |
| Renal artery thrombosis | 0 | 1 | 1 | |
| Rhabdomyolysis | 0 | 1 | 1 | |
| Ureteral obstruction | 1 | 2 | 3 | |
| Urinary leak | 10 | 25 | 35 | |
| Urinary retention | 2 | 4 | 6 | |
| UTI/Pyelonephritis | 0 | 7 | 7 | |
| Would dehiscence | 0 | 1 | 1 | |
| Wound infection | 0 | 1 | 1 | |
Major Complications- Clavien Grade III and IV; There were no Class V complications. In patients with multiple complications, only the highest Clavien Grade complication was tallied for total and major complication rates. Urologic complications were tallied on a per procedure basis for complication rate and individually for median number of urologic complications.
3.3. Renal functional outcomes
Renal functional outcomes are shown in Table 4. While patients undergoing mPN have a higher creatinine at 12 months post-operation (1.5 [0.6] vs. 1.3 [0.6], p=0.03), both operations resulted in similar eGFR by 3 months post-operation (52.0 [18.5] vs. 47.0 [25.0], p=0.2). The percentage of patients with eGFR > 45 was not statisitically different between groups at 3 months (sPN 87.5% vs. mPN 68.2%, p=0.07) and 12 months post operation (sPN 87.0% vs. mPN 73.7%, p=0.2). The rate of long-term HD was 5.8% (4.7% in the sPN stratification and 6.4% in the mPN stratification [p=1.0]). In the initial mPN group, the long-term HD rate was 5.1%, whereas in the repeat mPN group the rate was 7.7% (p=1.0). The completion nephrectomy rate—defined as a radical nephrectomy that was performed during an attempted PN—was 2.5% overall, 2.3% of sPN, and 2.6% of mPN. All of these cases were in patients who had prior ipsilateral partial nephrectomies. Completion nephrectomies were performed as a result of either a complication (i.e. renal vascular injury) or because of oncologic necessity (unrecognized segmental vein tumor thrombus).
Table 4.
Peri-Operative Renal Functional Outcomes
| Operation | sPN | mPN | Total | p value |
|---|---|---|---|---|
| n=43 | n=78 | n=121 | ||
| Median Creatinine (IQR), mg/dL | ||||
| Pre-Op | 1.3 (0.6) | 1.3 (0.5) | 1.3 (0.5) | p=0.4 |
| Discharge | 1.3 (0.7) | 1.9 (1.0) | 1.7 (1.0) | p<0.001 |
| 3 months Post-Op | 1.4(0.6) | 1.5 (0.7) | 1.6 (0.6) | p=0.04 |
| 12 months Post-Op | 1.3 (0.6) | 1.5 (0.6) | 1.4 (0.6) | p=0.03 |
| Median eGFR (IQR), mL/min/1.73 m2 | ||||
| Pre-Op | 59.0 (30.0) | 57.0 (29.5) | 57.0 (28.0) | p=0.5 |
| Discharge | 53.5 (19.0) | 36.0 (19.0) | 40.0 (27.5) | p<0.001 |
| 3 months Post-Op | 52.0 (18.5) | 47.0 (25.0) | 48.0 (23.0) | p=0.2 |
| 12 months Post-Op | 54.5 (25.5) | 47.5 (21.3) | 50.5 (21.8) | p=0.07 |
| eGFR> 45 | ||||
| 3 mo | n=24 | n=44 | n=68 | |
| 21 (87.5) | 30 (68.2) | 51 (75.0) | p=0.07 | |
| 12 mo | n=23 | n=38 | n=61 | |
| 20 (87.0) | 28 (73.7) | 48 (78.7) | p=0.2 | |
| HD (%) | ||||
| Total | 3 (7.0) | 8 (10.3) | 11 (9.1) | p=0.8 |
| Temporary/Bridge | 1 (2.3) | 4(5.1) | 5 (4.1) | p=0.7 |
| Long Term | 2 (4.7) | 5 (6.4) | 7 (5.8) | p=1.0 |
| Subsequent RN (%) | ||||
| Total | 4(9.3) | 7 (9.0) | 11 (9.1) | |
| Completion RN | 1 (2.3) | 2(2.6) | 3 (2.5) | |
| Salvage RN | 3 (7.0) | 5 (6.4) | 8(6.6) | |
Creatinine- measured in mg/dL; eGFR=estimated glomerular filtration rate calculated using the CKD- EPI equation, measured in mL/min per 1.73 m2. HD=hemodialysis; Subsequent RN=radical nephrectomy of solitary kidney; Completion RN- RN resulted from intra- or post-op complication of PN; Salvage RN- RN was planned for oncologic or transplant purposes
3.4. Oncologic outcomes
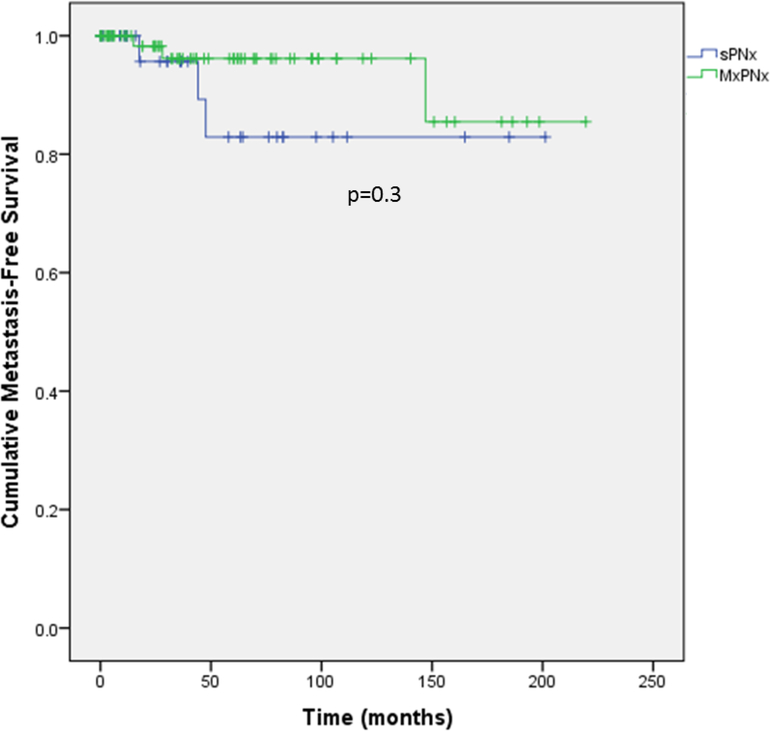
Table 5 and Supplemental Figure 1 depict oncologic outcomes. The overall post-PN metastasis rate was 5.5%. There were 3 (8.6%) cases of metastases following sPN and 3 (4.1%) after mPN (median follow-up 40.1 months). The mean (95% confidence interval) times to metastasis were 173.6 (144.5, 202.4) months in sPN patients and 204.3 (186.8, 221.7) months in the mPN group (p=0.3).
Table 5.
Oncologic Outcomes
| Operation | sPN | mPN | Total | p value |
|---|---|---|---|---|
| n=35 | n=74 | n=109 | ||
| Post-Op Metastases (%) | 3 (8.6) | 3 (4.1) | 6(5.0) | p=0.4 |
| Mean* Time to Metastasis (95% CI), mo | 173.6 (144.5, 202.4) | 204.3 (186.8, 221.7) | 199.4 (183.5, 215.4) | p=0.3 |
| Median Follow Up Time (IQR), mo | 35.9 (71.1) | 42.5 (76.2) | 40.1 (74.5) | p=0.5 |
Metastases were pathologic diagnoses. Cytoreductive procedures were excluded.
Median time to metastasis not reached
Figure 1.
Kaplan-Meier curve of metastasis-free survival. Events were defined as pathological diagnosis of metastatic disease.
4. Discussion
The standard treatment of solitary RCC tumors 4 centimeters or less is PN.9 In patients with multiple tumors, mPN and/or bilateral PN are also preferred to RN as results have shown higher QOL and overall survival in those with native renal function and no difference in oncologic outcomes. 5,6 For these reasons PN is a crucial treatment option in patients with a solitary kidney. However, these studies were of patients with both renal units functioning. In solitary kidneys, sPN has become common practice, though mPN in solitary kidneys is less common and the outcomes of this procedure are not as well known as a result.10–12 In this retrospective cohort study evaluating surgical and renal functional outcomes of sPN and mPN in a solitary kidney, we demonstrate that mPN is as effective as sPN in preserving native renal function without affecting oncologic outcomes.
The long-term HD rate for all procedures was 5.8%. Understandably, this is higher than the 2.1% long-term HD rate in patients with both kidneys undergoing PN for T1a tumors.13 However, our result is similar to prior studies on sPN in solitary kidneys, which report a long-term HD rates ranging from 2.7%−6.1%.14,15 Though mPN patients have a lower eGFR at discharge compared to patients undergoing sPN, by 12 months post-op, there is no significant difference in eGFR. This suggests that in a solitary kidney, mPN is equally effective in preserving native renal function as sPN. In addition, there were equivalent proportions of patients in both cohorts who remained off long-term HD and patients with eGFR >45 at 12 months post-op. Given these data, the presence of multiple tumors should not deter one from PN in order to avoid HD, even in a solitary kidney
While preventing long-term HD is the ultimate reason for performing nephron sparing surgery, complications are another important concern. Our overall complication rate was 65.5%, and the major complication rate was 33.6%. Prior studies of sPN in a solitary kidney report a 42.6–62.5% complication rate.10,12,16 Notably, 42.1% of PNs in our cohort were repeat ipsilateral PNs. In repeat PN patients, Johnson et al. reported an overall complication rate of 78.4% and a major complication rate of 19.6%, 17 and Bratslavsky et al. reported a major complication rate of 46% in patients undergoing a third ipsilateral PN patients.18 Our rates of major and minor complications fall within these levels which reinforces the previously published data on the complexity of these cases. In a subgroup analysis, Abdelhafefz et al. reported complications in 30.7% of patients with multifocal tumors or imperative indication for nephron sparing surgery—defined as a single kidney or pre-op creatinine >1.2 mg/dL—.19 While this is lower than our complication rate, it is important to consider that the median number of tumors excised in our entire cohort was 4 with a mean of 9 tumors per kidney in the mPN group, whereas the cases reported by Abdelhafefz et al were predominantly solitary tumors and included in the subgroup due to solitary kidney or high creatinine. By contrast, Liu et all demonstrated that when PN was performed on kidneys with greater than 20 tumors, both the intra-op and post-op complication rate was greater than 50%.2 Our data in context with these previously published series emphasize the importance of counseling not only the patient and the patient families about the complexity of these operations, but also preparing surgical colleagues and hospital support staff so that these complications are perceived as expected parts of the normal recovery from these challenging operations.
Our data indicate that the complication rates of sPN and mPN were similar. The overall complication rate for sPN and mPN was 57.1% and 70.1%, respectively. While mPN seems to have a higher rate, this failed to achieve statistical significance. Although this is the largest series reported thusfar, the numbers are still relatively small and the lack of significance is likely due to insufficient power to detect this difference which is very likely real. More surprisingly, no difference in the major complication rates was noted with 31.0% in sPN and 35.1% in mPN. Additionally, there was higher EBL during mPN compared to sPN (2.5 L vs. 1.4 L). However, we report low rates of clamp usage, particularly in mPNs (44.7% of sPNs and 26.3% of mPNs). These results demonstrate that the presence of multiple tumors in a solitary kidney is not a contraindication to performing nephron sparing surgery.
We assessed oncologic outcomes of nephron sparing surgery in solitary kidneys. At a median follow-up of 40.1 months, the overall post-op metastasis rate was 5.5%. This rate falls well within the range of previously reported rates of 1.9%−8.1%. 11,20,21 Notably, 70% of patients in our cohort have a hereditary cancer syndrome. Various studies of renal tumors in VHL and BHD report metastasis rates of 6.7%−14.3%.22–24 The metastasis rate in our mPN group of 4.5% is similar to those reported in these studies. These suggest that in patients with solitary kidney, regardless of number of tumors, performing nephron sparing surgery to avoid renal replacement therapy does not compromise oncologic success.
This study is not without limitations. This is a single-institution, retrospective study with all the potential inherent biases of that study design. Given that a much higher proportion of our patient population has hereditary RCC compared to most practices these findings may not be as generalizable to standard practices. Additionally, as noted previously, despite being one of the largest cohorts of mPN in a solitary kidney, the small number of procedures decreased the power of the study to identify differences in outcomes between sPN and mPN. Finally, our cohort includes a small number of procedures performed 25 years ago or more. We cannot control for improvements surgical technique and other treatment options which could affect patients selected for PN.
5. Conclusions
PN in a solitary kidney effectively preserves native renal function, often obviating the need for long-term HD associated with radical nephrectomy in this population.. Renal functional and oncologic outcomes were similar for both sPN and mPN. The overall need for HD is low and the complication rates, though high, are within expected ranges for these operations. Thus, three or more tumors in a solitary kidney should not be a contraindication for nephron sparing surgery.
Highlights.
Patients with hereditary kidney cancer may present with multiple tumors in a solitary kidney.
Multiplex partial nephrectomy in solitary kidneys can offer renal preservation without sacrificing oncologic efficacy.
Multiplex partial nephrectomy in solitary kidneys can associated with high complications rate, including urine leak and bleeding, but renal loss is rare.
Acknowledgments
Financial Funding:
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
This research was also made possible through the National Institutes of Health Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from Pfizer Inc., The Doris Duke Charitable Foundation, The Alexandria Real Estate Equities, Inc. and Mr. and Mrs. Joel S. Marcus, and the Howard Hughes Medical Institute, as well as other private donors. For a complete list, please visit the Foundation website at: http://fnih.org/work/education-training-0/medical-researchscholars-program
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A: Cancer Statistics, 2017. CA Cancer J Clin 67:7–30, 2017 [DOI] [PubMed] [Google Scholar]
- 2.Fadahunsi AT, Sanford T, Linehan WM, et al. : Feasibility and outcomes of partial nephrectomy for resection of at least 20 tumors in a single renal unit. J Urol 185:49–53, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hankins RA, Walton-Diaz A, Truong H, et al. : Renal functional outcomes after robotic multiplex partial nephrectomy: the National Cancer Institute experience with robotic partial nephrectomy for 3 or more tumors in a single kidney. Int Urol Nephrol 48:1817–1821, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maurice MJ, Ramirez D, Nelson RJ, et al. : Multiple Tumor Excisions in Ipsilateral Kidney Increase Complications After Partial Nephrectomy. J Endourol 30:1200–1206, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Arnold ML, Thiel DD, Diehl N, et al. : Comparison of baseline quality of life measures between renal cell carcinoma patients undergoing partial versus radical nephrectomy. BMC Urol 13:52, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parker PA, Swartz R, Fellman B, et al. : Comprehensive assessment of quality of life and psychosocial adjustment in patients with renal tumors undergoing open, laparoscopic and nephron sparing surgery. J Urol 187:822–6, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bratslavsky G, Linehan WM: Long-term management of bilateral, multifocal, recurrent renal carcinoma. Nat Rev Urol 7:267–75, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levey AS, Stevens LA, Schmid CH, et al. : A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–12, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell S, Uzzo RG, Allaf ME, et al. : Renal Mass and Localized Renal Cancer: AUA Guideline. J Urol, 2017 [DOI] [PubMed] [Google Scholar]
- 10.Bhindi B, Mason RJ, Haddad MM, et al. : Outcomes After Cryoablation Versus Partial Nephrectomy for Sporadic Renal Tumors in a Solitary Kidney: A Propensity Score Analysis. Eur Urol 73:254–259, 2018 [DOI] [PubMed] [Google Scholar]
- 11.Haber GP, Lee MC, Crouzet S, et al. : Tumour in solitary kidney: laparoscopic partial nephrectomy vs laparoscopic cryoablation. BJU Int 109:118–24, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Zargar H, Bhayani S, Allaf ME, et al. : Comparison of perioperative outcomes of robot-assisted partial nephrectomy and open partial nephrectomy in patients with a solitary kidney. J Endourol 28:1224–30, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Sun M, Bianchi M, Hansen J, et al. : Chronic kidney disease after nephrectomy in patients with small renal masses: a retrospective observational analysis. Eur Urol 62:696–703, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Thompson RH, Frank I, Lohse CM, et al. : The impact of ischemia time during open nephron sparing surgery on solitary kidneys: a multi-institutional study. J Urol 177:471–6, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Verhoest G, Patard JJ, Oger E, et al. : Predictive factors of chronic kidney disease stage V after partial nephrectomy in a solitary kidney: a multi-institutional study. Urol Oncol 32:28.e21–6, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Xiaobing W, Wentao G, Guangxiang L, et al. : Comparison of radiofrequency ablation and partial nephrectomy for tumor in a solitary kidney. BMC Urol 17:79, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson A, Sudarshan S, Liu J, et al. : Feasibility and outcomes of repeat partial nephrectomy. J Urol 180:89–93; discussion 93, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bratslavsky G, Liu JJ, Johnson AD, et al. : Salvage partial nephrectomy for hereditary renal cancer: feasibility and outcomes. J Urol 179:67–70, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Abdelhafez M, Bastian A, Rausch S, et al. : Laparoscopic versus Open Partial Nephrectomy: Comparison of Overall and Subgroup Outcomes. Anticancer Res 37:261–265, 2017 [DOI] [PubMed] [Google Scholar]
- 20.Olweny EO, Park SK, Tan YK, et al. : Radiofrequency ablation versus partial nephrectomy in patients with solitary clinical T1a renal cell carcinoma: comparable oncologic outcomes at a minimum of 5 years of follow-up. Eur Urol 61:1156–61, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Thompson RH, Atwell T, Schmit G, et al. : Comparison of partial nephrectomy and percutaneous ablation for cT1 renal masses. Eur Urol 67:252–9, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Duffey BG, Choyke PL, Glenn G, et al. : The relationship between renal tumor size and metastases in patients with von Hippel-Lindau disease. J Urol 172:63–5, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Matin SF, Ahrar K, Wood CG, et al. : Patterns of intervention for renal lesions in von Hippel-Lindau disease. BJU Int 102:940–5, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Pavlovich CP, Grubb RL 3rd, Hurley K, et al. : Evaluation and management of renal tumors in the Birt-Hogg-Dube syndrome. J Urol 173:1482–6, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Walther MM, Choyke PL, Glenn G, et al. : Renal cancer in families with hereditary renal cancer: prospective analysis of a tumor size threshold for renal parenchymal sparing surgery. J Urol 161:1475–9, 1999 [DOI] [PubMed] [Google Scholar]