Abstract
Melanoma is an exceptionally aggressive cancer with limited treatment options. As such, the idea that a minority of tumor cells, termed melanoma stem cells, are actually responsible for the progression of the disease offers up new possibilities for targeted therapies. However, reliable identification of these melanoma stem cells is complicated by the lack of clearly defined markers to distinguish them from the general tumor cell population. Additionally, there is evidence that under permissive conditions, a high proportion of melanoma cells are capable of forming tumors in mice. This review summarizes a number of the possible markers being considered for identifying melanoma stem cells, the potential role of transcription factors that regulate pluripotency and stem cell maintenance in melanoma, and evidence that may undermine the applicability of the cancer stem cell hypothesis to melanoma.
Keywords: Melanoma, Stem Cells, Melanoma Propagating Cells, Tumor-Initiating Cells, Review
2. CANCER STEM CELLS AND MELANOMA
The cancer stem cell hypothesis is built around the idea that only a subset of cancer cells is capable of maintaining and driving the progression of the disease. While the existence and role of cancer stem cells is well established in acute myeloid leukemia, cancer stem cell populations have also been identified in cancers of the colon, breast and brain (1-4). Thus, in many solid tumor cancers, cancer stem cells are now believed to be necessary for tumor formation, as well as resistance to chemotherapy and eventually escape from remission. The observation of putative cancer stem cells in other solid tumors has led researchers to investigate their existence in melanoma. While the qualifications for melanoma stem cells have generally been defined (tumorigenicity in xenograft, spheroid formation and self-renewal in non-adherent culture, some degree of plasticity/transdifferentiation potential), the markers used to identify these cells from the general tumor cell populations remain in debate. Reviewed here are a number of potential melanoma stem cell markers, the evidence supporting and disputing the cancer stem cell hypothesis in melanoma, and possible mechanisms of enhanced “stemness” in melanoma. Notably, the controversy regarding stem cells in melanoma has led many in the field to focus on the stem cell-like properties of the critical melanoma propagating cell/tumor-initiating cell population.
3. POTENTIAL MARKERS OF MELANOMA STEM CELLS
3.1. ABC transporters
ATP-binding cassette (ABC) transporters are a vast family of transmembrane proteins that exist in all organisms, from prokaryotes to humans. The transporters use ATP to actively transport toxic molecules out of cells (5). Interestingly, some of these transporters have been demonstrated to be expressed in highly tumorigenic subpopulations of melanoma, suggesting that they may be markers of melanoma stem cells.
Perhaps the most noteworthy ABC transporter identified as a potential marker is ABCB5. Expression of ABCB5 increases during melanoma progression in human tumor samples. Furthermore, ABCB5+ cells were able to resist treatment with doxorubicin (6) and were more tumorigenic than ABCB5− cells in xenograft assays. Suggestive that it may serve as a marker of a melanoma stem cell subpopulation, ABCB5+ melanoma cells expressed markers of undifferentiated cells, including CD133, CD166 and nestin, and had the ability to reestablish a heterogeneous tumor with both ABCB5+ and ABCB5− cells. Importantly, when animals were treated with antibodies against ABCB5, tumor growth in xenograft experiments was almost completely ablated due to antibody-dependent cell-mediated cytotoxicity in ABCB5+ cells (7). While a role for ABCB5+ melanoma cells in chemotherapy resistance and escape from remission would not be surprising, ABCB5 expression may also have traits beyond drug resistance that may help tumor formation in the first place. Recently, ABCB5+ cells were shown to be able to inhibit IL-2-dependent T-cell activation, thereby preventing efficient surveillance by the immune system (8). Also, ABCB5 allows melanoma cells to maintain hyperpolarized membranes and polyploid genomes, similar to that seen in skin progenitor cells, suggesting a direct role in the “stemness” of ABCB5+ cells (6, 9). It should be noted that not all xenografts using ABCB5+ cells produced tumors, suggesting other subpopulations may exist and that ABCB5 expression is not sufficient for tumor initiation (7). This is not surprising since ABCB5 is also expressed quite highly by normal melanocytes in addition to melanoma (10, 11).
While ABCB5+ cells were not analyzed for their ability to self-renew in culture, Keshet, et al demonstrated that a melanoma subpopulation expressing a different ABC transporter, MDR1, displayed enhanced self-renewal properties (12). MDR1+ cells exhibited less pigmentation than MDR1− cells, a sign of non-differentiation. Furthermore, MDR+ cells possessed the ability to continuously self-renew when grown in soft agar and expressed the pluripotency and self-renewal regulators, human telomerase reverse transcriptase (hTERT) and nanog. However, the tumorigenicity of MDR1+ cells in xenografts compared to MDR1− cells was not examined in this study. While MDR1 likely contributes to the resistance of melanoma to chemotherapy, its contribution to the “stemness” of melanoma cells remains unclear. It is important to note that while MDR1+ cells did exhibit cancer stem cell-like properties in vitro, the cells also co-expressed ABCB5 and ABCC2 mRNAs, suggesting that a number of ABC transporters may be expressed the same subpopulations (12).
An additional ABC transporter that may mark melanoma stem cells is ABCG2. While not assessed independently for its ability to mark tumorigenic melanoma cells, ABCG2 was found to be co-expressed with CD133 in tumor-initiating melanoma cells (13).
3.2. CD133
CD133/prominin-1/AC133 is a transmembrane glycoprotein normally expressed on dermal-derived and hematopoietic stem cells, as well as endothelial progenitors, highlighting it as a marker of undifferentiated cells (14, 15). Since being identified as a marker of brain tumor-initiating cells (4), CD133 has been pursued as a marker of cancer stem cells in a variety of cancer types (16-18). As such, it is of little surprise that the melanoma field has examined CD133 as a marker of melanoma stem cells. Interestingly, while histological analysis found scattered expression of CD133 in patient samples, expression of CD133 did not correlate with prognosis. Additionally, nested CD133 staining could be seen in benign nevi, clusters of melanocytes that have undergone proliferation followed by subsequent senescent-like growth arrest (19, 20). This finding could be interpreted as the presence of stem cell-like populations in nevi, as well (21). Despite the lack of correlation with cancer grade, others have found CD133 to be expressed on tumorigenic subpopulations of melanoma (6, 7, 13). Of these studies, Monzani, et al found that CD133+ cells isolated from patients were able to efficiently form tumors in mice, whereas CD133− cells were not (13). Interestingly, cultured WM115 cells also expressed high levels of CD133. When WM115 cells were grown as non-adherent spheroids or xenografted into mice, CD133 expression became limited to a minority of tumor cells, similar to that seen in patient samples. When cells were isolated from the xenografts, CD133 expression was gradually restored in the cultured cells. The changes in CD133 expression observed in xenografts may reflect the high differentiation potential of CD133+ cells; however, the reestablishment of CD133+ cells in culture may indicate that expression of CD133 is readily switched on and off by melanoma cells depending on their environmental conditions.
An interesting complication to the use of CD133 as a marker for melanoma stem cells is its frequent co-expression with a number of the aforementioned ABC transporters. CD133 has been found to be frequently co-expressed with ABCB5 and ABCG2, with ABCG2 showing similar patterns of loss and re-expression under xenograft and cell culture conditions as seen with CD133 (6, 7, 13). In addition, CD133 also co-expresses with CD166/ALCAM, another potential cancer stem cell marker (22, 23). As of yet, efforts have not been made to resolve the tumorigenic capacity of cells with different co-expression patterns, i.e. CD133+/ABCB5+ versus CD133−/ABCB5+.
3.3. Nestin
Nestin is a type VI intermediate filament that is highly expressed in stem cells, especially those of the neural crest, as well as newly formed endothelial cells and myogenic precursors, which give rise to cardiac and smooth muscle (24-27). Interestingly, heterogeneous expression of nestin can be found in a variety of adult tissues raising the possibility that it corresponds with adult stem cells found in many tissues (26). Unlike possible melanoma stem cell markers CD133 and CD166, nestin expression has been shown to correlate with melanoma progression and prognosis, with increased expression seen in later stages of the disease and in patients with decreased 5-year survival rates (21, 28, 29). However, one possible complication with using nestin expression as a prognostic biomarker in tumor samples is its high expression in newly formed blood vessels, which are likely to be enriched in more aggressive tumors (24, 25, 29). Despite the likely contribution of nascent endothelial cells to nestin staining in tumors, melanoma cells in culture and those found in the peripheral blood of patients with melanoma have been shown to express high levels of nestin (30). As such, care must be taken when analyzing nestin expression in tumor samples as to which cells are contributing to the staining.
Similar to the ABC transporters found in potential melanoma stem cell populations, nestin is likely to contribute directly to aggressiveness of the disease. In agreement with the previous observations in melanoma, nestin expression was shown to increase in prostate cancer as the disease progressed to metastasis. Interestingly, knockdown of nestin in aggressive prostate cancer cell lines significantly impaired the ability of cells to migrate, invade and metastasize (31). Despite a clear contribution to prostate cancer cell mobility, the mechanism(s) by which nestin works is unclear. Nestin has been shown to heteropolymerize with vimentin, possibly influencing vimentin-bundle turnover, and may interact with microfilaments and microtubules through its long carboxyl-terminus (26, 27, 32). These interactions might make nestin a master orchestrator of cytoskeletal dynamics. While the contribution of nestin to melanoma aggression has not been explored, it is reasonable to assume that its role in melanoma will be similar to that in prostate cancer. This could easily expand the use of nestin from a biomarker to a target of therapeutics.
3.4. Other markers
Some additional markers have been explored in the hunt for the most reliable marker of tumorigenic melanoma cells. One early marker described is CD20/MS4A1, a membrane-spanning member of the 4A gene family that plays a role in the differentiation of B-cells into plasma cells (33). Fang, et al found that freshly isolated tumor cells, as well as some melanoma cell lines, gave rise to non-adherent spheroids when cultured in mouse embryo fibroblast (MEF)-conditioned embryonic stem cell media. These spheroid cells heterogeneously expressed CD20, while adherent cells were devoid of staining. As with many of the above markers, CD20+ cells alone appeared responsible for spheroid cell self-renewal and differentiation into CD20− cells. CD20+ cells also possessed a greater capacity for transdifferentiation into adipose, bone, and chondrocyte-like cells, hallmarking their pluripotency. Spheroid cells where found to be generally more tumorigenic than adherent cells (3-fold higher tumor formation); however, what is not clear from this study is whether CD20+ cells account for difference between the two groups of tumor cells (34). While other groups have looked at CD20 expression alongside other stem cell markers, correlation with tumor development has yet to be established (7, 35).
In contrast to the single marker/subpopulation studies mentioned before, Held, et al identified several subpopulations within several different murine melanoma models (36). Cells extracted from tumors fell into three subpopulations: CD34+/p75−, CD34−/p75−, and CD34−/p75+. Interestingly, each of these subpopulations had different tumor-forming properties and potential to reestablish tumor heterogeneity. The CD34+/p75− subpopulation exhibited the greatest ability to establish new tumors, but only gave rise to more CD34+/p75− cells. Interestingly, CD34−/p75− cells, which were less tumorigenic than CD34+/p75− cells, possessed the ability to reform a heterogeneous tumor. By contrast, CD34−/p75+ cells rarely formed tumors. These experiments utilized tumor cell transplantation between syngeneic mouse strains, which unlike the aforementioned xenograft experiments, maintain a normal immune system as well as the tissue microenvironment from the original tumor. This system may prevent unintentional selection against tumorigenic subpopulations that are poorly adapted to survival in the alien environments found in most xenografts.
In striking contrast to Held’s findings, Boiko, et al recently reported that in melanoma cells implanted in mice directly after isolation, cells expressing p75 (CD271) were considerably more tumorigenic than their CD271− counterparts. CD271+ cells frequently lacked markers of differentiated pigment cells, including MART1, TYR, and MAGE, further supporting the idea that melanoma stem cells are poorly differentiated. A major strength of this paper comes from the use of cells isolated from tumors of various stagings and from number of locations (37). This sample diversity lends strength to the usage of p75NGFR/CD271 as a broadly-applicable marker of tumorigenic cells. However, while CD271 expression was a reliable marker in tumors from a variety of origins, information regarding the mutations (i.e. B-RAF, NRAS, c-Kit) present in the tumor cells was lacking. It would be interesting to know whether CD271-expression correlates with certain genotypes. This might help rectify differences seen between human patients and mouse models, which are generally genetically uniform.
4. CANCER STEM CELLS, A TECHNICAL ARTIFACT?
Given all the efforts to identify markers that define a melanoma stem cell population, one of the most interesting and complicating observations in field was recently reported by Morrison and colleagues (35). They utilized selective conditions to enhance the efficiency of tumor take and formation, even to the point of single cell injections. In their experiments, they mixed melanoma cells with Matrigel prior to injection into highly immunocompromised NOD/SCID interleukin-2 receptor gamma chain null (Il2rg−/−) mice and extended the analysis time for tumor growth. Using these conditions, on average, 27% of melanoma cells injected directly from patients were able to form tumors. This is in sharp contrast to other groups that used no less than 100,000 cells to establish tumors (7, 34). Interestingly, when a number of potential melanoma stem markers (A2B5, CD44, CD49b, CD49d, CD49f, CD54, c-Kit, HNK1, L1CAM, CD133, CD166, MCAM, E-cadherin, N-cadherin, and p75 NGFR) were analyzed, no substantial difference in tumor formation could be seen between marker-positive and marker-negative cells. It would be interesting to know whether tumors from marker-positive or marker-negative cells formed equivalent tumors with regard to tumor invasion, angiogenesis, or resistance to chemotherapy between the resulting tumors. Differences in these properties may highlight a role for melanoma stem cells besides regulation of tumor growth. Nonetheless, these observations point away from the cancer stem cell hypothesis and towards the earlier, non-hierarchical hypothesis of tumor initiation.
So does this mean that the cancer stem cell hypothesis does not apply to melanoma after all? Not necessarily. While the above study shows convincingly that a high proportion of melanomas cells have the capability to form tumors given the right conditions, there are a number of possible explanations for the differences described by other groups. Firstly, the xenograft conditions utilized by the Morrison group may be so favorable that even non-aggressive cancer cells could proliferate to the point of forming tumors. While primary human melanocytes and mesenchymal stem cells were unable to form tumors in these conditions, it would be interesting to see whether weakly malignant cell types would display high tumorigenic properties. One can also imagine that under ideal conditions, tumor cell growth in a xenograft might parallel that of growth in a cell culture dish. Indeed, many tumor cells grow quite well in culture without the apparent need of cancer stem cells to keep the culture growing. Perhaps melanoma stem cells need the challenges of an inhospitable environment to demonstrate their importance. Cells may not need the drug efflux properties of ABC transporters or the pro-migratory properties of nestin for tumor initiation under these conditions. Another possible explanation is that as a tumor progresses to metastasis, the proportion of cancer stem cells is greatly enriched. As such, tumors taken from patients with advanced disease may simply consist of a very high proportion of tumorigenic cells. There is even evidence that primary tumors may be enriched for aggressive cells via self-seeding from distant metastases (38). Overall, the lack of association between tumor-formation and marker expression may simply reflect the need to identify more reliable markers for melanoma stem cells.
One further explanation may involve the possible reprogramming of “ordinary” melanoma cells into melanoma stem cells by altering the microenvironmental conditions. Melanoma cells are highly plastic cells, and as with all cancer cells, have a degree of genetic and epigenetic instability. As such, it is imaginable that melanoma cells might, perhaps in response to environmental cues, flip back and forth between differentiated and undifferentiated states. Notably, the use of co-injected Matrigel in the tumor formation assays is a potential influencing factor and the production of laminin (and/or other components enriched in Matrigel) in the cancer stem cell niche may be required for the propagation of the cancer stem cell population. While it has been established by other groups that tumors formed by marker-positive cells are heterogeneous for marker expression (7, 34), it would be interesting to know whether heterogeneous populations could be found in tumors spawned from marker-negative cells. As mentioned above, CD133 was found to be highly expressed in cultured WM115 cells, lost in all but a minority of cells in xenografts and spheroid culture conditions, and then re-expressed when cells were put back into normal culture (13). This suggests that melanoma cells may alter their stem cell-like properties depending on there culture conditions, and that differentiation is not strictly unidirectional. Additional support for the idea of a transient tumor-promoting subpopulation comes from Roesch and colleagues. Recently, they demonstrated that melanoma cells expressing high levels of the H3K4 demethylase JARID1B were essential for long-term tumor growth in xenografts. Interestingly, while JARID1B+ cells could give rise to both JARID1B+ and JARID1B− cells (in keeping with other cancer stem cell markers), JARID1B− cells could also give rise to JARID1B+ cells to a lesser extent, indicating a dynamic nature to melanoma stem cells (39). If, in fact, tumor cell reprogramming is occurring, what might be mediating it?
5. TRANSCRIPTION FACTORS: POSSIBLE ON-SWITCHES FOR MELANOMA STEM CELLS?
While the debate over which marker (or markers) best define the tumorigenic subpopulations of melanoma cells, one thing remains fairly clear in most cancers: the less differentiated the tumor, the worse the prognosis. Indeed, all the disputed markers of melanoma stem cells are typically found in primitive cell types, whether they are embryonic, neural crest, or melanocytic stem cells. So if the tumor-initiating cells in melanoma are largely undifferentiated cells, how do they get that way, and more importantly, how do we get them to stop being stem cell-like and promote terminal differentiation? Although some speculate that melanoma cells arise from transformed melanocytic stem cells, and therefore retain their pluripotent and self-renewing capabilities, an alternative hypothesis is that the oncogene-harboring cells of benign nevi are reprogrammed by the reacquisition of a handful of stem cell-related transcription factors.
Adult human cells, including fibroblasts and melanocytes, have been successfully transformed into induced pluripotent stem (iPS) cells, which possess most of the attributes of embryonic stem cells, by ectopically expressing the transcription factors Oct4, Sox2, plus either c-Myc and Klf4 or nanog and the RNA-binding protein, Lin28 (40-42). Interestingly, a number of these transcription factors have been found to be expressed in melanoma cells. Expression of Oct4 and nanog were both found to be enhanced in the spheroid forming fraction of C8161 cells, while nanog was found to co-expressed with MDR1, ABCB5 and ABCC2 in cells freshly derived from tumors (43, 44). C-Myc is frequently overexpressed in a variety of cancers, and in melanoma, has been shown to be required for evasion of oncogene-induced senescence (45). Sox2, like c-Myc, was recently shown to promote melanoma tumor growth in vivo (46). Depletion of Sox2 in A2058 cells significantly impaired tumor formation in xenografts. Additionally, high expression of Sox2 was shown to correlate with primary tumor thickness in patient samples (46). In another report, patients with Sox2-expressing tumors had a decreased 3-year median survival rate compared to those not expressing Sox2 (47).
How the expression patterns of the aforementioned pluripotency transcription factors relate to genotypes and the signaling pathways that regulate their expression requires further exploration. It is reasonable to assume that some of these transcription factors may be restricted to certain subsets of melanoma. Recently, we found that another pluripotency transcription factor, FOXD3, is lowly expressed in a number of melanoma cell lines. Typically, expression is detected in lines derived from metastases. FOXD3 is essential for the self-renewal and pluripotency of a number of primitive cell types, including embryonic and neural crest stem cells (48, 49). Disruption of MEK/ERK-signaling was shown to greatly enhance the expression of FOXD3, both at the mRNA and protein levels. Interestingly, although MEK-ERK1/2 signaling is elevated in most, if not all melanoma cell lines, only melanoma lines harboring oncogenic forms of B-RAF exhibited FOXD3 expression and regulation by MEK-ERK1/2 signaling (50). When overexpressed in melanoma cells, FOXD3 elicited a potent G0/G1 growth arrest (50). This reduction in growth is reminiscent of the dormancy phenotype observed in cancer stem cells. In a different study, ectopic expression of FOXD3 promoted the demethylation of silenced target promoters in MEFs (51). Since FOXD3 is an important part of a regulatory network with nanog and Oct4 in embryonic stem cells (52, 53), it is possible that endogenous FOXD3 promotes the un-silencing of these genes in melanoma. Whether FOXD3 in fact promotes the plasticity and self-renewal of mutant B-RAF-harboring melanoma remains to be seen, however, its enhanced expression in the presence of B-RAF/MEK inhibition may impact the response of patients to therapies that target this pathway.
6. FINAL THOUGHTS
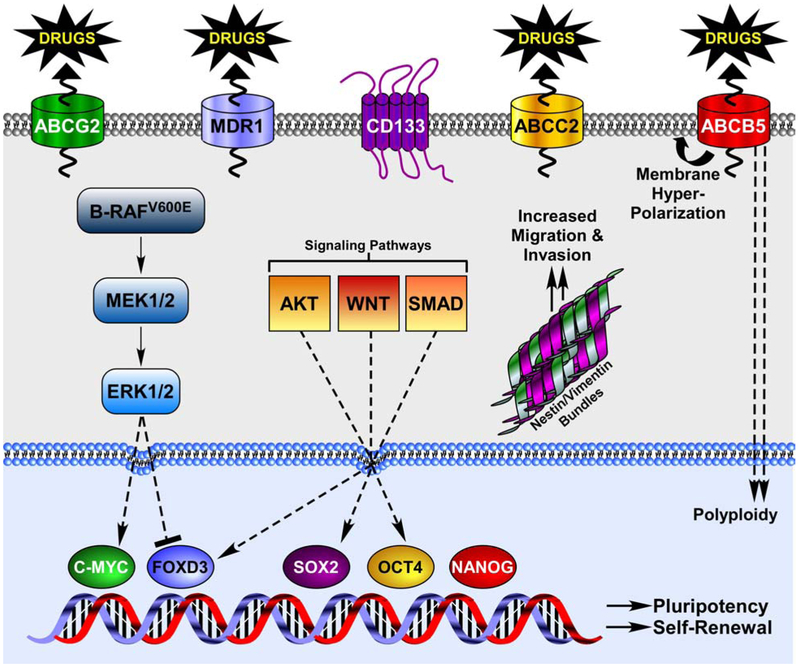
The cancer stem cell hypothesis is an inviting concept for the melanoma field. The ability to halt cancer progression by targeting a small subset of the tumor cell population would be extremely advantageous, especially for a disease with a high propensity to spread and resist conventional therapies, as is the case with malignant melanoma. While the potential payoff for targeting putative melanoma stem cells is clear, several obstacles remain. First, bona fide markers must be established for melanoma stem cell populations. As it stands, many potential markers have been observed in highly tumorigenic subpopulations, including ABCB5, ABCG2, MDR1, CD133, CD20, CD40, and nestin (Figure 1). However, even when marker-positive populations are shown to be significantly more aggressive than their marker-negative brethren, reasonably large numbers (100,000 cells) of marker-positive cells are often used in xenograft assays. Such large inoculations could easily accommodate novel tumorigenic subpopulations within the marker-positive groups. Additionally, many of these markers have been found to be co-expressed on the same cell. It would be interesting to see if the tumorigenic potential of marker-positive subpopulations can be further graded by their co-expression of other known markers. This certainly seems to be the case with CD34 and p75 in melanoma mouse models (36).
Figure 1.
Stem cell markers and regulation of pluripotency transcription factors in melanoma.
Secondly, while validation of the true melanoma stem cell subpopulation(s) will sharpen the focus of investigators as to which cells to work with in vivo and in vitro, novel targeted therapies will require an understanding of signaling pathways that distinguish melanoma stem cells from the general population. It is likely that transcription factors like c-Myc, Oct4, nanog, Sox2, and FOXD3 will play some part in the plasticity and self-renewal of melanoma cells, as they do in normal stem cells. What is less clear is which cellular pathways regulate the expression and activity of these transcription factors in melanoma. Expression of FOXD3, Sox2, and Oct4 are regulated by the Smad, Wnt, and PI3-kinase pathways in stem cells, making these pathways potential candidates in melanoma (54) (Figure 1). Targeting pathways that drive stem cell transcription factors has the potential to rob melanoma cells of their self-renewal capabilities. A factor that may be overlooked in the melanoma stem cell studies is the genotypes of the melanomas being analyzed. Some transcription factors, like FOXD3 in mutant B-RAF-harboring melanoma, may be restricted to cells with specific mutations. Similarly, differences in marker expression observed by different groups may reflect different oncogenic insults (B-RAF, N-Ras, c-Kit) in the cell cultures and patient tumors used. This highlights the need for careful genetic profile in the future of cancer treatment.
Finally, the possibility that most, if not all, melanoma cells can form tumors under the right conditions remains a concern (35). If this is in fact the case in patients, then all tumor cells must be targeted by therapy. However, there is still the possibility that all tumorigenic cells require stem cell-like properties to promote disease. By understanding how these stem cell-like properties are developed and maintained in melanoma, it might be possible to prevent bulk tumor cells from reverting into tumorigenic melanoma stem cells, while simultaneously nullifying the existing melanoma stem cell population. In this way, advanced disease may be effectively treated without the need for complete elimination of tumor cells.
Ultimately, if the cancer stem cell hypothesis holds up to the scrutiny and rigors of scientific investigation in melanoma, the consequences for effective therapies could be monumental. In the time being, however, it remains crucial to develop therapies that will target all cancer cells in the event that the quest to unmask the melanoma stem cell never reaches fruition.
7. ACKNOWLEDGEMENTS
The Aplin lab is supported by grants from the American Cancer Society (RSG-08-03-01-CSM), the National Institutes of Health (R01-GM067893 and R01-CA125103), and the Pennsylvania Department of Health (AF0301). Additional funding was provided by the Joanna M. Nicolay Melanoma Foundation. We are very grateful to Dr. Michele Weiss for providing the illustration.
Abbreviations:
- ABC transporter
ATP-binding cassette transporter
- hTERT
human telomerase reverse transcriptase
- MEF
mouse embryo fibroblast
- Il2rg−/−
interleukin-2 receptor gamma chain null Il2rg−/−
- iPS cells
induced pluripotent stem cells
8. REFERENCES
- 1.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ and Clarke MF: Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA, 100, 3983–3988 (2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonnet D and Dick JE: Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 3, 730–737 (1997) [DOI] [PubMed] [Google Scholar]
- 3.O'Brien CA, Pollett A, Gallinger S and Dick JE: A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature, 445, 106–110 (2007) [DOI] [PubMed] [Google Scholar]
- 4.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB: Identification of human brain tumour initiating cells. Nature, 432, 396–401 (2004) [DOI] [PubMed] [Google Scholar]
- 5.Jones PM and George AM: The ABC transporter structure and mechanism: perspectives on recent research. Cell Mol Life Sci, 61, 682–699 (2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frank NY, Margaryan A, Huang Y, Schatton T, Waaga-Gasser AM, Gasser M, Sayegh MH, Sadee W and Frank MH: ABCB5-mediated doxorubicin transport and chemoresistance in human malignant melanoma. Cancer Res, 65, 4320–4333 (2005) [DOI] [PubMed] [Google Scholar]
- 7.Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, Zhan Q, Jordan S, Duncan LM, Weishaupt C, Fuhlbrigge RC, Kupper TS, Sayegh MH and Frank MH: Identification of cells initiating human melanomas. Nature, 451, 345–349 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schatton T, Schutte U, Frank NY, Zhan Q, Hoerning A, Robles SC, Zhou J, Hodi FS, Spagnoli GC, Murphy GF and Frank MH: Modulation of T-cell activation by malignant melanoma initiating cells. Cancer Res, 70, 697–708 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frank NY, Pendse SS, Lapchak PH, Margaryan A, Shlain D, Doeing C, Sayegh MH and Frank MH: Regulation of progenitor cell fusion by ABCB5 P-glycoprotein, a novel human ATP-binding cassette transporter. J Biol Chem, 278, 47156–47165 (2003) [DOI] [PubMed] [Google Scholar]
- 10.Chen KG, Szakács G, Annereau J-P, Rouzaud F, Liang X-J, Valencia JC, Nagineni CN, Hooks JJ, Hearing VJ and Gottesman MM: Principal expression of two mRNA iso forms (ABCB 5α and ABCB 5β ) of the ATP-binding cassette transporter gene ABCB 5 in melanoma cells and melanocytes. Pigment Cell Res, 18, 102–112 (2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heimerl S, Bosserhoff A, Langmann T, Ecker J and Schmitz G: Mapping ATP-binding cassette transporter gene expression profiles in melanocytes and melanoma cells. Melanoma Res, 17, 265–273 (2007) [DOI] [PubMed] [Google Scholar]
- 12.Keshet GI, Goldstein I, Itzhaki O, Cesarkas K, Shenhav L, Yakirevitch A, Treves AJ, Schachter J, Amariglio N and Rechavi G: MDR1 expression identifies human melanoma stem cells. Biochem Biophys Res Comm, 368, 930–936 (2008) [DOI] [PubMed] [Google Scholar]
- 13.Monzani E, Facchetti F, Galmozzi E, Corsini E, Benetti A, Cavazzin C, Gritti A, Piccinini A, Porro D, Santinami M, Invernici G, Parati E, Alessandri G and La Porta CAM: Melanoma contains CD133 and ABCG2 positive cells with enhanced tumourigenic potential. Eur J Cancer, 43, 935–946 (2007) [DOI] [PubMed] [Google Scholar]
- 14.Belicchi M, Pisati F, Lopa R, Porretti L, Fortunato F, Sironi M, Scalamogna M, Parati EA, Bresolin N and Torrente Y: Human skin-derived stem cells migrate throughout forebrain and differentiate into astrocytes after injection into adult mouse brain. J Neurosci Res, 77, 475–486 (2004) [DOI] [PubMed] [Google Scholar]
- 15.Shmelkov SV, St.Clair R, Lyden D and Rafii S: AC133/CD133/Prominin-1. Int J Biochem Cell Biol, 37, 715–719 (2005) [DOI] [PubMed] [Google Scholar]
- 16.Miki J, Furusato B, Li H, Gu Y, Takahashi H, Egawa S, Sesterhenn IA, McLeod DG, Srivastava S and Rhim JS: Identification of putative stem cell markers, CD133 and CXCR4, in hTERT-immortalized primary nonmalignant and malignant tumor-derived human prostate epithelial cell lines and in prostate cancer specimens. Cancer Res, 67, 3153–3161 (2007) [DOI] [PubMed] [Google Scholar]
- 17.Ferrandina G, Bonanno G, Pierelli L, Perillo A, Procoli A, Mariotti A, Corallo M, Martinelli E, Rutella S, Paglia A, Zannoni G, Mancuso S and Scambia G: Expression of CD133–1 and CD133–2 in ovarian cancer. Int J Gynecol Cancer, 18, 506–514 (2008) [DOI] [PubMed] [Google Scholar]
- 18.Wright MH, Calcagno AM, Salcido CD, Carlson MD, Ambudkar SV and Varticovski L: Brca1 breast tumors contain distinct CD44+/CD24− and CD133+ cells with cancer stem cell characteristics. Breast Cancer Res, 10, R10 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, Majoor DM, Shay JW, Mooi WJ and Peeper DS: BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature, 436, 720–724 (2005) [DOI] [PubMed] [Google Scholar]
- 20.Gray-Schopfer VC, Cheong SC, Chong H, Chow J, Moss T, Abdel-Malek ZA, Marais R, Wynford-Thomas D and Bennett DC: Cellular senescence in naevi and immortalisation in melanoma: a role for p16? Br J Cancer, 95, 496–505 (2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein WM, Wu BP, Zhao S, Wu H, Klein-Szanto AJP and Tahan SR: Increased expression of stem cell markers in malignant melanoma. Mod Pathol, 20, 102–107 (2006) [DOI] [PubMed] [Google Scholar]
- 22.Dalerba P, Dylla SJ, Park I-K, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, Shelton AA, Parmiani G, Castelli C and Clarke MF: Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci, 104, 10158–10163 (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horst D, Kriegl L, Engel J, Kirchner T and Jung A: Prognostic Significance of the cancer stem cell markers CD133, CD44, and CD166 in colorectal cancer. Cancer Invest, 27, 844–850 (2009) [DOI] [PubMed] [Google Scholar]
- 24.Amoh Y, Bouvet M, Li L, Tsuji K, Moossa A, Katsuoka K and Hoffman R: Visualization of nascent tumor angiogenesis in lung and liver metastasis by differential dual-color fluorescence imaging in nestin-linked-GFP mice. Clin Exp Metastasis, 23, 315–322 (2006) [DOI] [PubMed] [Google Scholar]
- 25.Amoh Y, Yang M, Li L, Reynoso J, Bouvet M, Moossa AR, Katsuoka K and Hoffman RM: Nestin-linked green fluorescent protein transgenic nude mouse for imaging human tumor angiogenesis. Cancer Res, 65, 5352–5357 (2005) [DOI] [PubMed] [Google Scholar]
- 26.Gilyarov A: Nestin in central nervous system cells. Neurosci Behav Physiol, 38, 165–169 (2008) [DOI] [PubMed] [Google Scholar]
- 27.Michalczyk K and Ziman M: Nestin structure and predicted function in cellular cytoskeletal organisation. Histol Histopathol. , 20, 665–671 (2005) [DOI] [PubMed] [Google Scholar]
- 28.Tanabe K, Amoh Y, Kanoh M, Takasu H, Sakai N, Sato Y and Katsuoka K: Prognostic significance of the hair follicle stem cell marker nestin in patients with malignant melanoma. Eur J Dermatol, 20, 283–288 (2010) [DOI] [PubMed] [Google Scholar]
- 29.Brychtova S, Fiuraskova M, Hlobilkova A, Brychta T and Hirnak J: Nestin expression in cutaneous melanomas and melanocytic nevi. J Cutan Pathol, 34, 370–375 (2007) [DOI] [PubMed] [Google Scholar]
- 30.Fusi A, Ochsenreither S, Busse A, Rietz A and Keilholz U: Stem cell marker nestin expression in peripheral blood of patients with melanoma. Br J Dermatol., [Epub ahead of print] (2010) [DOI] [PubMed] [Google Scholar]
- 31.Kleeberger W, Bova GS, Nielsen ME, Herawi M, Chuang A-Y, Epstein JI and Berman DM: Roles for the stem cell associated intermediate filament nestin in prostate cancer migration and metastasis. Cancer Res, 67, 9199–9206 (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steinert PM, Chou Y-H, Prahlad V, Parry DAD, Marekov LN, Wu KC, Jang S-I and Goldman RD: A high molecular weight intermediate filament-associated protein in BHK-21 cells is nestin, a type VI intermediate filament protein. limited co-assembly in vitro to form heteropolymers with type III vimentin and type IV alpha - internexin. J Biol Chem, 274, 9881–9890 (1999) [DOI] [PubMed] [Google Scholar]
- 33.Perosa F, Favoino E, Caragnano MA, Prete M and Dammacco F: CD20: A target antigen for immunotherapy of autoimmune diseases. Autoimmun Rev, 4, 526–531 (2005) [DOI] [PubMed] [Google Scholar]
- 34.Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE and Herlyn M: A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res, 65, 9328–9337 (2005) [DOI] [PubMed] [Google Scholar]
- 35.Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM and Morrison SJ: Efficient tumour formation by single human melanoma cells. Nature, 456, 593–598 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Held MA, Curley DP, Dankort D, McMahon M, Muthusamy V and Bosenberg MW: Characterization of melanoma cells capable of propagating tumors from a singlecCell. Cancer Res, 70, 388–397 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boiko AD, Razorenova OV, van de Rijn M, Swetter SM, Johnson DL, Ly DP, Butler PD, Yang GP, Joshua B, Kaplan MJ, Longaker MT and Weissman IL: Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature, 466, 133–137 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim M-Y, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH-F, Norton L and Massagué J: Tumor self-seeding by circulating cancer cells. Cell, 139, 1315–1326 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roesch A, Fukunaga-Kalabis M, Schmidt EC, Zabierowski SE, Brafford PA, Vultur A, Basu D, Gimotty P, Vogt T and Herlyn M: A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell, 141, 583–594 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II and Thomson JA: Induced pluripotent stem cell lines derived from human somatic cells. Science, 318, 1917–1920 (2007) [DOI] [PubMed] [Google Scholar]
- 41.Takahashi K and Yamanaka S: Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126, 663–676 (2006) [DOI] [PubMed] [Google Scholar]
- 42.Utikal J, Maherali N, Kulalert W and Hochedlinger K: Sox2 is dispensable for the reprogramming of melanocytes and melanoma cells into induced pluripotent stem cells. J Cell Sci, 122, 3502–3510 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keshet GI, Goldstein I, Itzhaki O, Cesarkas K, Shenhav L, Yakirevitch A, Treves AJ, Schachter J, Amariglio N and Rechavi G: MDR1 expression identifies human melanoma stem cells. Biochem Biophys Res Commun, 368, 930–936 (2008) [DOI] [PubMed] [Google Scholar]
- 44.Strizzi L, Abbott DE, Salomon DS and Hendrix MJC: Potential for Cripto-1 in defining stem cell-like characteristics in human malignant melanoma. Cell Cycle, 7, 1931–1935 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhuang D, Mannava S, Grachtchouk V, Tang WH, Patil S, Wawrzyniak JA, Berman AE, Giordano TJ, Prochownik EV, Soengas MS and Nikiforov MA: C-MYC overexpression is required for continuous suppression of oncogene-induced senescence in melanoma cells. Oncogene, 27, 6623–6634 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laga AC, Lai C-Y, Zhan Q, Huang SJ, Velazquez EF, Yang Q, Hsu M-Y and Murphy GF: Expression of the embryonic stem cell transcription factor SOX2 in human skin: Relevance to melanocyte and merkel cell biology. Am J Pathol., 176, 903–913 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schoenhals M, Kassambara A, Vos JD, Hose D, Moreaux J and Klein B: Embryonic stem cell markers expression in cancers. Biochem Biophys Res Comm, 383, 157–162 (2009) [DOI] [PubMed] [Google Scholar]
- 48.Hanna LA, Foreman RK, Tarasenko IA, Kessler DS and Labosky PA: Requirement for Foxd3 in maintaining pluripotent cells of the early mouse embryo. Genes Dev, 16, 2650–2661 (2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kos R, Reedy MV, Johnson RL and Erickson CA: The winged-helix transcription factor FoxD3 is important for establishing the neural crest lineage and repressing melanogenesis in avian embryos. Development, 128, 1467–1479 (2001) [DOI] [PubMed] [Google Scholar]
- 50.Abel EV and Aplin AE: FOXD3 is a mutant B-RAF-regulated inhibitor of G1/S progression in melanoma cells. Cancer Res, 70, 2891–2900 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu J, Watts JA, Pope SD, Gadue P, Kamps M, Plath K, Zaret KS and Smale ST: Transcriptional competence and the active marking of tissue-specific enhancers by defined transcription factors in embryonic and induced pluripotent stem cells. Genes Dev, 23, 2824–2838 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pan G, Li J, Zhou Y, Zheng H and Pei D: A negative feedback loop of transcription factors that controls stem cell pluripotency and self-renewal. FASEB J, 20, 1730–1732 (2006) [DOI] [PubMed] [Google Scholar]
- 53.Pan G and Thomson JA: Nanog and transcriptional networks in embryonic stem cell pluripotency. Cell Res, 17, 42–49 (2007) [DOI] [PubMed] [Google Scholar]
- 54.Lee MY, Lim HW, Lee SH and Han HJ: Smad, PI3K/Akt, and Wnt-dependent signaling pathways are involved in BMP-4-induced ESC self-renewal. Stem Cells, 27, 1858–1868 (2009) [DOI] [PubMed] [Google Scholar]