Abstract
Background:
Endoscopic and histologic healing are emerging as new therapeutic goals in ulcerative colitis (UC), as these endpoints are associated with less relapse, hospitalization and colectomy.
Aim:
We investigated the association of serum infliximab trough concentrations during maintenance therapy with endoscopic or histologic healing in UC.
Methods:
In this multi-center retrospective cohort study, we included consecutive patients with moderate-to-severe UC on infliximab maintenance therapy who had an endoscopic evaluation and underwent therapeutic drug monitoring within three months of the colonoscopy, between February 2008 and March 2016. Per event analysis was performed. Endoscopic healing was defined as Mayo endoscopic sub-score of ≤1. Histologic healing was defined as no or only focal mild active inflammation.
Results:
Seventy colonoscopies from 56 patients were evaluated. Infliximab trough concentrations (median [interquartile range]) were significantly higher in patients with endoscopic (11.3 [7.6–14.5] vs. 6.3 [0–9.8] μg/mL, p<0.001) or histologic (11.1 [6.7–14.5] vs. 6.7 [0–9.9] μg/mL, p=0.002) healing, respectively, compared to patients without healing. Receiver-operating characteristic analyses identified infliximab trough concentration thresholds of 7.5 [area under the curve (AUC): 0.758) and 10.5 (AUC: 0.721) μg/mL to be associated with endoscopic and histologic healing, respectively. Multiple logistic regression analysis identified infliximab trough concentration ≥7.5 [p=0.013; odds ratio (OR): 4.3; 95% confidence intervals (CI): 1.4–13.3] and ≥10.5 μg/mL [p=0.013; OR: 3.8; 95% CI: 1.3–11] as independent factors associated with endoscopic and histologic healing, respectively.
Conclusions:
This study demonstrated that infliximab trough concentrations during maintenance therapy are associated with endoscopic and histologic healing in patients with UC.
Keywords: Anti-TNF therapy, inflammatory bowel disease, antibodies to infliximab, therapeutic drug monitoring
INTRODUCTION
Anti-tumor necrosis factor (anti-TNF) therapies, particularly infliximab, have revolutionized the treatment of patients with moderate-to-severe ulcerative colitis (UC). Objective therapeutic outcomes, such as endoscopic and histologic healing, are currently considered as important target endpoints of anti-TNF therapy in UC, as these have been associated with lower rates of disease relapse, hospitalization and need for colectomy.1–6
Reactive therapeutic drug monitoring is emerging as the new standard-of-care for optimizing anti-TNF therapy in inflammatory bowel disease (IBD).7 Recent studies have demonstrated that proactive therapeutic drug monitoring of infliximab with drug titration to a therapeutic window is associated with favorable long-term therapeutic outcomes in IBD and may be superior to reactive therapeutic drug monitoring.8–10 Moreover, many exposure-response relationship studies have shown that higher serum anti-TNF drug concentrations are associated with better clinical outcomes in IBD, suggesting that it is maybe time to go beyond from a ‘treat-to-target’ to a ‘treat-to-trough’ therapeutic approch.11–22 Nevertheless, there are only limited data regarding therapeutic drug monitoring in UC and even less data regarding a therapeutic window to target for an important objective outcome, mucosal healing. To our knowledge, there are no data in UC with respect to anti-TNF therapeutic drug monitoring and histologic healing, which represents the ultimate form of mucosal healing.
The primary aim of the study was to investigate the association of serum infliximab trough concentrations during maintenance therapy with endoscopic or histologic healing in patients with moderate-to-severe UC. A secondary aim was to identify potential factors related to these therapeutic outcomes.
MATERIALS AND METHODS
Study design, patient population and definitions
This was a multi-center retrospective cohort study. Consecutive patients with moderate-to-severe UC on infliximab maintenance therapy who had an endoscopic evaluation and underwent therapeutic drug monitoring within three months of the colonoscopy, between February 2008 and March 2016, were eligible for the study. Patients with an ostomy or ileal pouch-anal anastomosis were excluded. Patients underwent colonoscopy either for dysplasia surveillance or for assessment of disease activity in patients with IBD-related symptoms. Per event rather than per patient analysis was performed as follows. A patient could be analyzed more than once provided that at least 6 months elapsed between endoscopic evaluations and that each endoscopy correlated with an adjacent serum sample. Endoscopic healing was defined as a Mayo endoscopic sub-score of ≤1. Histologic healing was defined as no or only focal mild active inflammation. The colonoscopists were aware of the results at the time of the procedure in only 1/3 of the cases (25/70, 36%), as these endoscopies were done after the result of the therapeutic drug monitoring, while none of the pathologists were aware of any results of therapeutic drug monitoring. Although a standardized biopsy protocol could not be implemented due to the retrospective nature of the study, generally at our institutions, for dysplasia surveillance biopsies are typically done in a 4-quadrant fashion every 10 cm, and for disease activity biopsies are typically done by region of colon and per endoscopic activity. All clinical, endoscopic and histologic data were reviewed from the electronic medical records of the patients. The study was approved by the Institutional Review Boards of the Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, Massachusetts and the Department of Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia.
Infliximab concentration and antibodies to infliximab
Serum infliximab trough concentrations and antibodies to infliximab (ATI) during maintenance therapy were measured by Prometheus Laboratories (San Diego, CA). The study period overlapped with the use of two different infliximab assays, a drug-sensitive enzyme-linked immunosorbent assay (ELISA) until July 2012 followed by a drug-tolerant homogeneous mobility shift assay (HMSA).23 Infliximab concentrations of < 1 and 1.4 μg/mL and ATI < 3.1 U/mL and 1.7 μg/mL equivalents were considered as undetectable for the HMSA and ELISA, respectively.
Statistical analysis
Descriptive statistics were provided with medians and interquartile range (IQR) for continuous variables and frequencies and percentages for categorical variables. Receiver-operating characteristic (ROC) analyses were performed to identify infliximab trough concentration thresholds associated with endoscopic or histologic healing. Optimal thresholds were chosen using the Youden index, which maximizes the sum of the specificity and sensitivity of the ROC curve.16 Sensitivity (SN), specificity (SP), positive predictive value (PPV) and negative predictive value (NPV) were also calculated. Infliximab trough concentrations during maintenance therapy were compared between groups by a Mann-Whitney U-test. Serum infliximab trough concentrations were categorized also into quartiles. Rates of endoscopic or histologic healing were compared across infliximab trough concentration quartiles using a chi-square test (linear-by-linear association). The Kruskal-Wallis or chi-square test were used to compare continuous or discrete variables across quartile groups, respectively. The Mann-Whitney U-test and the chi-square or Fisher’s exact tests were used for univariable analysis to identify quantitative or categorical variables associated to endoscopic and histologic healing, respectively. These included age at diagnosis, age at infliximab initiation, sex, disease duration, pancolitis, smoking status, prior infliximab optimization, concomitant immunomodulators (thiopurines or methotrexate), body mass index (BMI), elevated C-reactive protein (CRP >5mg/L), albumin, type of assay, ATI and infliximab trough concentration as a categorical variable using as a cut-off the one derived from ROC analyses. To determine the independent effects of variables associated to endoscopic or histologic healing, a multivariable binary logistic regression was then performed including statistically significant variables from univariable analysis, based on the Backward Wald selection method. The results were expressed as odds ratio (OR) with 95% confidence intervals (CI) followed by the corresponding p-value. An incremental gain analysis was performed using 3 μg/mL increments of infliximab trough concentration vis-à-vis their respective rate of endoscopic or histologic healing. This analysis was used to define the range of maximal increase in the rate of endoscopic or histologic healing with any increase in drug concentration and a relevant therapeutic window, defined as the range of infliximab trough concentrations associated with an endoscopic or histologic healing rate of >70%. Results were considered statistically significant when p <0.05. All statistical analyses were performed using the SPSS 24.0 software (SPSS, Chicago, Illinois, USA) and GraphPad Prism version 5.03 for Windows (GraphPad Software, San Diego CA, USA).
RESULTS
Study population
Seventy colonoscopies [(surveillance colonoscopies, n=31 (44%)] from 56 patients [male, n=32 (57%)] with UC were evaluated. Forty-five patients had only one endoscopy, eight patients had two endoscopies and three patients had three endoscopies. Patients’ demographic and clinical characteristics are depicted in Table 1.
Table 1.
Patients’ demographic and clinical characteristics.
| Patients’ demographic and clinical characteristics | N=56 |
|---|---|
| Male, (%) | 32 (57) |
| Age at diagnosis, median (IQR), y | 26 (22–41) |
| Age at infliximab initiation, median (IQR), y | 37 (28–50) |
| Disease duration: median (IQR), years | 4 (1–11) |
| Pancolitisa, (%) | 29 (52) |
| Smoking ever, (%) | 11 (20) |
| Prior infliximab optimization at first TDM, (%) | 35 (62) |
| Concomitant IMMb at first TDM, (%) | 18 (32) |
| BMI at first TDM, median (IQR), Kg/m2, (n=47) | 24.6 (23.3–28.1) |
| CRP >5 mg/L at first TDM, (%) | 3/10 (30) |
| Albumin at first TDM, median (IQR), g/dL, (n=17) | 3.9 (3.8–4.3) |
| Infliximab TC at first TDM, median (IQR), μg/mL | 6.9 (2.3–11.5) |
| ATI at first TDM, (%) | 8/55 (15) |
| Type of assay at first TDM: HMSA, (%) | 41 (73.2) |
according to Montreal classification for disease extension (E3);
thiopurines
BMI: body mass index; CRP: C-reactive protein; IMM: immunomodulators; IQR: interquartile range; TC: trough concentration; TDM: therapeutic drug monitoring; ATI: antibodies to infliximab; y: years; HMSA: homogeneous mobility shift assay.
Infliximab trough concentration and endoscopic healing
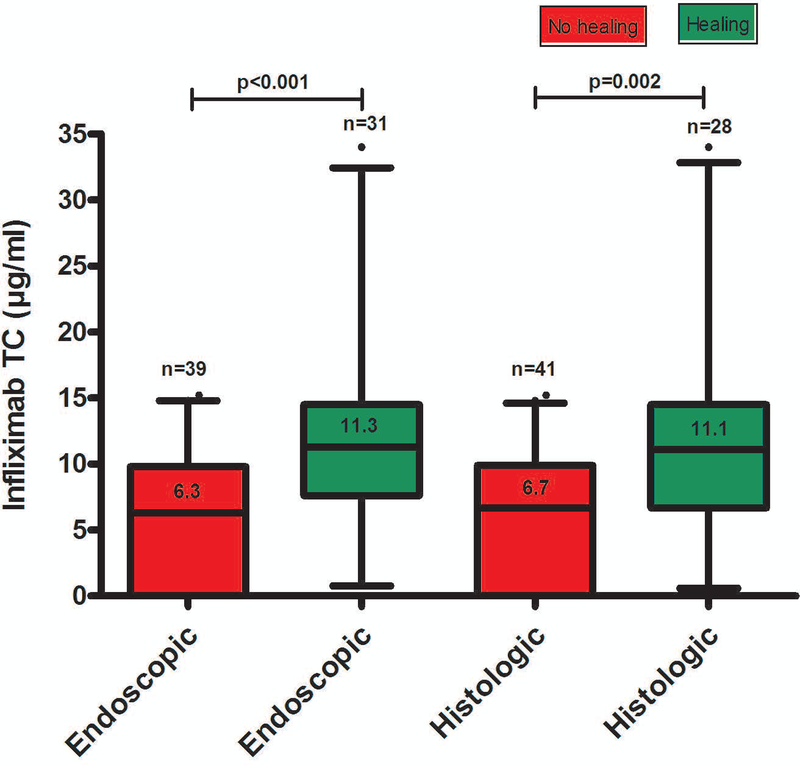
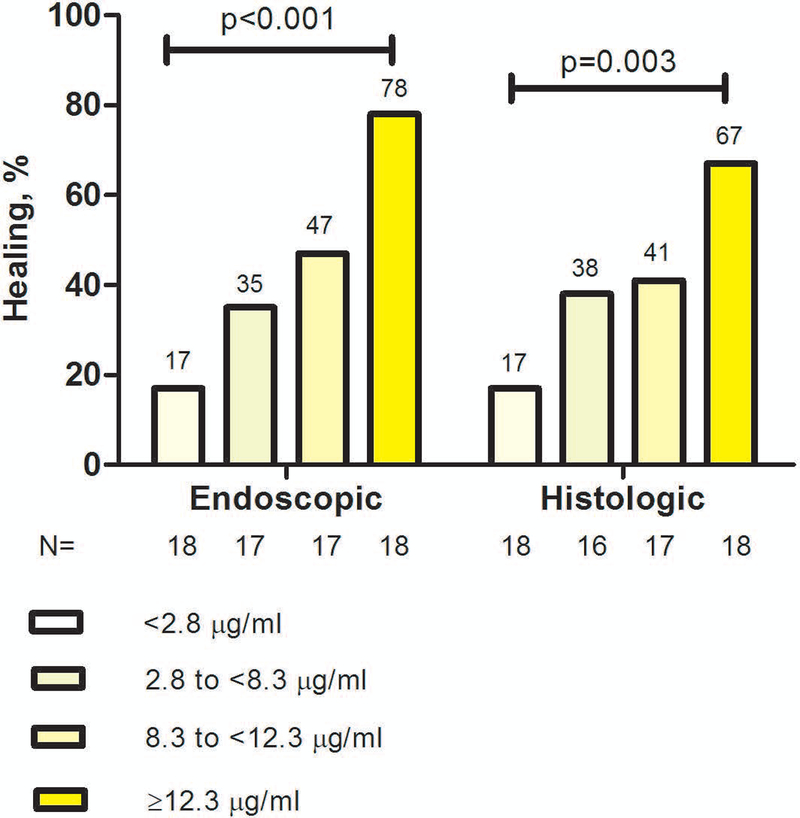
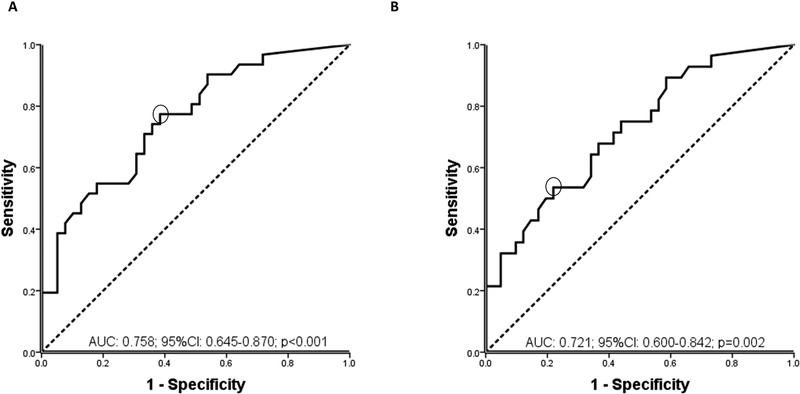
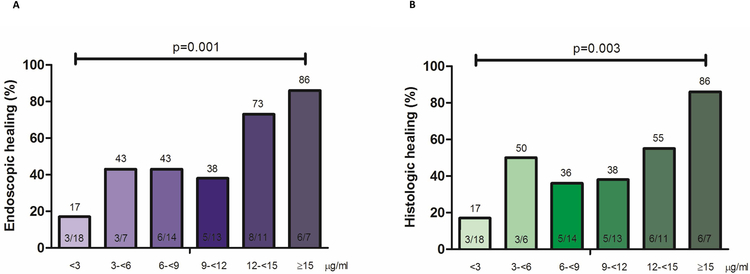
Endoscopic healing was found in 31/70 (44%) of colonoscopies. Serum infliximab trough concentration was statistically significantly higher in patients with endoscopic healing compared to those without (Figure 1). The relationship between infliximab trough concentration and endoscopic healing was further analyzed by dividing infliximab trough concentrations into quartiles. The higher infliximab trough concentration quartiles were associated with statistically significantly higher rates of endoscopic healing (Figure 2). Factors associated with the lowest quartile of infliximab trough concentration during maintenance therapy were male gender, low serum albumin levels, elevated serum CRP levels (>5 mg/L) and ATI positivity (supplementary Table 1). ROC analysis identified an infliximab trough concentration threshold of 7.5 μg/mL to be statistically significantly associated with endoscopic healing (SN 77%; SP 62%; PPV 62%; NPV 77%) (Figure 3A). In multivariable analysis, infliximab trough concentration ≥7.5 μg/mL was identified as the only variable independently associated with endoscopic healing [OR 4.3 (95% CI 1.4–13.3), p=0.013] (Table 2A). Based on incremental gain analysis, we identified an infliximab trough concentration therapeutic window of 12–15 μg/mL to be associated with an endoscopic healing rate over 70%, although the rate continued to increase beyond this threshold range (Figure 4A).
Figure legend 1.
Distribution of serum infliximab trough concentrations during maintenance therapy based on endoscopic or histologic healing in patients with moderate-to-severe ulcerative colitis. Box plots (5–95%) show the median (solid line within box), interquartile range (upper and lower box boundaries), standard deviation (whiskers) and outliers (black dot).
TC: trough concentration
Figure legend 2.
Rates of endoscopic and histologic healing by infliximab serum trough concentration quartiles during maintenance therapy in patients with moderate-to-severe ulcerative colitis
p-value (chi-square test, linear-by-linear association).
Figure legend 3.
Receiver operator curve (ROC) analysis for infliximab serum trough concentrations during maintenance therapy stratifying patients with moderate-to-severe ulcerative colitis with and without endoscopic (A) or histologic (B) healing.
AUC: area under the curve, CI: confidence interval.
Table 2.
Variables associated with endoscopic (A) and histologic (B) healing in patients with moderate-to-severe ulcerative colitis treated with infliximab.
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| Variables | p | OR | 95%CI | p | OR | 95%CI |
| A. Endoscopic healing | ||||||
| Infliximab TC ≥7.5 μg/mL | 0.002 | 5.5 | 1.9–15.8 | 0.013 | 4.3 | 1.4–13.3 |
| ATI | 0.008 | 0.8 | 0.7–0.9 | |||
| B. Histologic healing | ||||||
| Infliximab TC ≥ 10.5 μg/mL | 0.010 | 4.1 | 1.4–11.7 | 0.013 | 3.8 | 1.3–11 |
| ATI | 0.018 | 0.8 | 0.7–0.9 | |||
TC: trough concentration; ATI: antibodies to infliximab.
Figure legend 4.
Incremental gain in endoscopic (A) and histologic (B) healing rates in relation to serum infliximab trough concentrations during maintenance therapy in patients with moderate-to-severe ulcerative colitis. Increments of 3 μg/mL infliximab trough concentration were used to define the range of maximal increase in the rate of endoscopic or histologic healing with any increase in drug concentration during maintenance therapy.
p-value (chi-square test, linear-by-linear association).
Infliximab trough concentration and histologic healing
Histologic healing was found in 28/69 (41%) of colonoscopies. Serum infliximab trough concentration was statistically significantly higher in patients with histologic healing compared to those without (Figure 1). Higher infliximab trough concentration quartiles were associated with statistically significantly higher rates of histologic healing (Figure 2). ROC analysis identified an infliximab trough concentration threshold of 10.5 μg/mL to be statistically significantly associated with histologic healing (SN 54%; SP 78%; PPV 63%; NPV 71%) (Figure 3B). In multivariable analysis, infliximab trough concentration ≥10.5 μg/mL was identified as the only variable independently associated with histologic healing [OR 3.8 (95% CI 1.3–11), p=0.013] (Table 2A). Based on incremental gain analysis, no plateau for histologic healing by infliximab trough concentration was reached, and only at infliximab trough concentration above 15 μg/mL did histologic healing rates exceed 70% (Figure 4B).
DISCUSSION
This multi-center retrospective cohort study demonstated that higher infliximab trough concentrations during maintenance therapy were associated with endoscopic and histologic healing in patients with moderate-to-severe UC. To date, this is the largest study evaluating the association between anti-TNF concentrations and mucosal healing in UC. More importantly, to our knowledge this study is the first to investigate anti-TNF drug concentrations for the endpoint of histologic healing. Specifically, infliximab trough concentration thresholds of 7.5 and 10.5 μg/ml were found to be independently associated with endoscopic and histologic healing, respectively, with even higher concentrations associated with more desirable rates of both endoscopic and histologic healing.
These findings are in line with previous studies demonstrating a positive association between higher serum anti-TNF trough concentrations and improved therapeutic outcomes in IBD patients.11–22 A key concept reinforced by these data is that drug concentration thresholds may differ depending on the outcome of interest. For instance, an analysis of the large randomized controlled trials, ACT-1 and ACT-2, in UC identified a target infliximab trough concentration of 3.7 μg/ml during maintenance therapy (week 30) for the endpoint of clinical response (week 30),13 whereas Ungar et al recently demonstrated that higher infliximab concentrations (6–10 μg/mL) were needed to achieve a mucosal healing rate of 80–90% in patients with IBD.14 We observed that higher infliximab trough concentrations of at least 12–15 μg/mL were associated with endoscopic healing in over 70% of UC patients. These values are somewhat higher than those reported by Ungar et al.14 However, our population was restricted to patients with UC only, in contrast to the study by Ungar et al, in which most patients had Crohn’s disease (CD). It is quite possible that a higher infliximab concentration may be required in UC due to high inflammatory burden in the colon with the potential for fecal loss of infliximab.24–27 Similarly, higher infliximab concentrations were associated with healing in perianal fistulizing CD, as Yarur et al. identified an infliximab trough concentration cut-off of ≥10.1 μg/mL associated with fistula healing and proposed that even higher concentrations (>20.2 μg/mL) must be targeted before changing to other therapeutic options.19 Also of importance in our study is that the rates of endoscopic healing did not reach a plateau with respect to increasing infliximab trough concentrations and instead continued to increase with higher drug concentrations. One explanation is that higher concerntrations may be required for some patients to attain this objective endpoint. It is also possible, however, that a more healed mucosa allows for the attainment of higher infliximab concentrations.
A novel aspect of our study was establishing an association between infliximab concentrations and histologic healing. In fact, we identified that infliximab trough concentrations associated with histologic healing were numerically higher than those associated with endoscopic healing and also that fairly high infliximab concentrations (>15 μg/mL) were associated with more satisfactory rates of histologic healing above 70%. As with mucosal healing, the rates of histologic healing did not reach a plateau with respect to increasing infliximab trough concentrations and continued to increase with higher drug concentrations, which again implyies either that higher concentrations may be required for certain patients to attain this most rigorous endpoint or that attainment of this more rigorous endpoint allows for higher infliximab concnetrations to be achieved. The totality of the evidence to date thus seems to indicate that while higher infliximab trough concentrations are associated with clinical response/remission in IBD, even higher concentrations are associated with more objective endpoints of biochemical remission or mucosal healing, with the highest concentrations associated with the most difficult objective endpoints of fistula healing or histologic healing.13, 14, 17, 19
In this study, several factors were identified to be associated with low infliximab trough concentration quartiles during maintenance therapy: male gender, a higher disease activity (lower serum albumin, elevated serum CRP) and ATI. These variables have been previously shown to associate with a rapid clearance of anti-TNF drugs.27–29
Limitations of this study include its retrospective nature and the lack of central reading of endoscopies. However, the overwhelming majority of endoscopies included in this study were performed by experts in IBD. The definition of histologic healing used in this study was selected based on simplicity and clinical relevance, as no histologic index available for UC has been developed via a formal validation process and it is not even clear which histologic features are most predictive of healing or response to therapy.30 Another possible limitation is that due to the evolution of laboratory technology over time, two different therapeutic drug monitoring assays were used, although infliximab concentrations were comparable when measured by ELISA or HMSA.31 Finally, due to the absence of longitudinal data, it remains unclear if high serum concentrations are necessary to induce deep remission or if deep remission is associated with high drug concentrations because of reduced drug clearance and fecal loss. Consequently, only association and not causality can be established.
In conclusion, this study indicates that relatively high infliximab trough concentrations during maintenance therapy are associated with endoscopic and histologic healing in patients with moderate-to-severe UC, and importantly that infliximab trough concentrations may vary depending on the therapeutic outcome of interest. Nevertheless, before incorporating these results into therapeutic algorithms, large prospective longitudinal studies are warranted to establish causality between higher anti-TNF drug concentrations and objective therapeutic outcomes in IBD.
Supplementary Material
ACKNOWLEDGEMENTS
Declaration of personal interests: A.S.C: received consultancy fees from AbbVie, Janssen, Takeda, Ferring, Miraca, AMAG, and Pfizer; M.T.O: received consultancy fees from Janssen, AbbVie, UCB, Takeda, Pfizer, Merck, and Lycera, and received research grant support from UCB; The remaining authors disclose no conflicts of interest.
Declaration of funding interests: K.P. is supported by Ruth L. Kirschstein NRSA Institutional Research Training Grant 5T32DK007760-18.
REFERENCES
- 1.Levesque BG, Sandborn WJ, Ruel J, et al. Converging goals of treatment of inflammatory bowel disease from clinical trials and practice. Gastroenterology 2015; 148: 37–51. [DOI] [PubMed] [Google Scholar]
- 2.Arias MT, Vande Casteele N, Vermeire S, et al. A panel to predict long-term outcome of infliximab therapy for patients with ulcerative colitis. Clin Gastroenterol Hepatol 2015; 13: 531–8. [DOI] [PubMed] [Google Scholar]
- 3.Laharie D, Filippi J, Roblin X, et al. Impact of mucosal healing on long-term outcomes in ulcerative colitis treated with infliximab: a multicentre experience. Aliment Pharmacol Ther 2013; 37: 998–1004. [DOI] [PubMed] [Google Scholar]
- 4.Colombel JF, Rutgeerts P, Reinisch W, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology 2011; 141: 1194–201 [DOI] [PubMed] [Google Scholar]
- 5.Tursi A, Elisei W, Picchio M, et al. Histological inflammation in ulcerative colitis in deep remission under treatment with infliximab. Clin Res Hepatol Gastroenterol 2015; 39: 107–113. [DOI] [PubMed] [Google Scholar]
- 6.Patil DT, Moss AC, Odze RD. Role of histologic inflammation in the natural history of ulcerative colitis. Gastrointest Endosc Clin N Am 2016; 26: 629–40. [DOI] [PubMed] [Google Scholar]
- 7.Papamichael K, Cheifetz AS. Therapeutic drug monitoring in IBD: the new standard-of-care for anti-TNF therapy. Am J Gastroenterol 2017; 112: 673–6. [DOI] [PubMed] [Google Scholar]
- 8.Papamichael K, Chachu KA, Vajravelu RK, et al. Improved long-term outcomes of patients with inflammatory bowel disease receiving proactive compared with reactive monitoring of serum concentrations of infliximab. Clin Gastroenterol Hepatol 2017;15:1580–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology 2015; 148: 1320–9. [DOI] [PubMed] [Google Scholar]
- 10.Vaughn BP, Martinez-Vazquez M, Patwardhan VR, Moss AC, Sandborn WJ, Cheifetz AS. Proactive therapeutic concentration monitoring of infliximab may improve outcomes for patients with inflammatory bowel disease: results from a pilot observational study. Inflamm Bowel Dis 2014; 20: 1996–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seow CH, Newman A, Irwin SP, et al. Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut 2010; 59: 49–54. [DOI] [PubMed] [Google Scholar]
- 12.Cornillie F, Hanauer SB, Diamond RH, et al. Postinduction serum infliximab trough level and decrease of C-reactive protein level are associated with durable sustained response to infliximab: a retrospective analysis of the ACCENT I trial. Gut 2014; 63: 1721–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adedokun OJ, Sandborn WJ, Feagan BG, et al. Association between serum concentration of infliximab and efficacy in adult patients with ulcerative colitis. Gastroenterology 2014; 147: 1296–307. [DOI] [PubMed] [Google Scholar]
- 14.Ungar B, Levy I, Yavne Y, et al. Optimizing anti-TNF-α therapy: serum levels of infliximab and adalimumab are associated with mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2016; 14: 550–7.e2. [DOI] [PubMed] [Google Scholar]
- 15.Yarur AJ, Jain A, Hauenstein SI, et al. Higher adalimumab levels are associated with histologic and endoscopic remission in patients with Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis 2016; 22: 409–15. [DOI] [PubMed] [Google Scholar]
- 16.Papamichael K, Van Stappen T, Vande Casteele N, et al. Infliximab concentration thresholds during induction therapy are associated with short-term mucosal healing in patients with ulcerative colitis. Clin Gastroenterol Hepatol 2016; 14: 543–9. [DOI] [PubMed] [Google Scholar]
- 17.Ward MG, Warner B, Unsworth N, et al. Infliximab and adalimumab drug levels in Crohn’s disease: contrasting associations with disease activity and influencing factors. Aliment Pharmacol Ther 2017; 46: 150–61. [DOI] [PubMed] [Google Scholar]
- 18.Yarur AJ, Kubiliun MJ, Czul F, et al. Concentrations of 6-thioguanine nucleotide correlate with trough levels of infliximab in patients with inflammatory bowel disease on combination therapy. Clin Gastroenterol Hepatol 2015; 13: 1118–24. [DOI] [PubMed] [Google Scholar]
- 19.Yarur AJ, Kanagala V, Stein DJ, et al. Higher infliximab trough levels are associated with perianal fistula healing in patients with Crohn’s disease. Aliment Pharmacol Ther 2017; 45: 933–40. [DOI] [PubMed] [Google Scholar]
- 20.Papamichael K, Baert F, Tops S, et al. Post-induction adalimumab concentration is associated with short-term mucosal healing in patients with ulcerative colitis. J Crohns Colitis 2017; 11: 53–9. [DOI] [PubMed] [Google Scholar]
- 21.Papamichael K, Rivals-Lerebours O, Billiet T, et al. Long-term outcome of patients with ulcerative colitis and primary non-response to infliximab. J Crohns Colitis 2016; 10: 1015–23. [DOI] [PubMed] [Google Scholar]
- 22.Peyrin-Biroulet L, Reinisch W, Colombel JF, et al. Clinical disease activity, Creactive protein normalisation and mucosal healing in Crohn’s disease in the SONIC trial. Gut 2014; 63: 88–95 [DOI] [PubMed] [Google Scholar]
- 23.Wang SL, Ohrmund L, Hauenstein S, et al. Development and validation of a homogeneous mobility shift assay for the measurement of infliximab and antibodies-to-infliximab concentrations in patient serum. J Immunol Methods 2012; 382: 177–88. [DOI] [PubMed] [Google Scholar]
- 24.Brandse JF, van den Brink GR, Wildenberg ME, et al. Loss of infliximab into feces is associated with lack of response to therapy in patients with severe ulcerative colitis. Gastroenterology 2015; 149: 350–5. [DOI] [PubMed] [Google Scholar]
- 25.Dotan I, Ron Y, Yanai H, et al. Patient factors that increase infliximab clearance and shorten half-life in inflammatory bowel disease: a population pharmacokinetic study. Inflamm Bowel Dis 2014; 20: 2247–59. [DOI] [PubMed] [Google Scholar]
- 26.Seah D, De Cruz P. Review article: the practical management of acute severe ulcerative colitis. Aliment Pharmacol Ther 2016; 43: 482–513. [DOI] [PubMed] [Google Scholar]
- 27.Ungar B, Mazor Y, Weisshof R, et al. Induction infliximab levels among patients with acute severe ulcerative colitis compared with patients with moderately severe ulcerative colitis. Aliment Pharmacol Ther 2016; 43: 1293–9. [DOI] [PubMed] [Google Scholar]
- 28.Fasanmade AA, Adedokun OJ, Ford J, et al. Population pharmacokinetic analysis of infliximab in patients with ulcerative colitis. Eur J Clin Pharmacol 2009; 65: 1211–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ordas I, Feagan BG, Sandborn WJ. Therapeutic drug monitoring of tumor necrosis factor antagonists in inflammatory bowel disease. Clin Gastroenterol Hepatol 2012; 10: 1079–87. [DOI] [PubMed] [Google Scholar]
- 30.Bryant RV, Winer S, Travis SP, et al. Systematic review: histological remission in inflammatory bowel disease. Is ‘complete’ remission the new treatment paradigm? An IOIBD initiative. J Crohns Colitis 2014; 8: 1582–97. [DOI] [PubMed] [Google Scholar]
- 31.Steenholdt C, Bendtzen K, Brynskov J, et al. Clinical implications of measuring drug and anti-drug antibodies by different assays when optimizing infliximab treatment failure in Crohn’s disease: post hoc analysis of a randomized controlled trial. Am J Gastroenterol 2014; 109: 1055–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.