Abstract
Introduction
The presence of Hürthle cells (HC) in fine needle thyroid biopsy (FNAB) is a real concern for a cytologist and also for an endocrinologist. We aimed to demonstrate if the presence of HC is associated with specific cytological features in FNAB results.
Material and Methods
This retrospective study analyzed 89 patients diagnosed with thyroid nodules, with FNAB; were two groups of patients: the study group A (HC+) (22 patients) with HC and control group B (HC-) (67 patients) with no HC; for both groups we analyzed the presence of 9 cytomorphologic features: overall cellularity, background colloid, lymphocyte infiltration, chronic inflammation, large nucleoli, small nucleoli, syncytial infiltration, nuclear pleomorphism/atypia, cellular pleomorphism.
Results
We found no statistical differences between age and gender. Nodules with diameter greater than 2 cm were present, more frequently in the group without HC, 43 (64.18%). The presence of HC is correlated with cellular pleomorphism (p=0.042) and nuclear pleomorphism (p < 0.0001) with no correlation between the other investigated parameters. The presence of colloid was correlated with the absence of HC (p= 0.014). In group with HC was a positive correlation with cellular pleomorphism and fibrosis. In the presence of fibrosis, HC was correlated with nuclear pleomorphism (p=0.03). In the group with HC without fibrosis there are more characteristic the sets with positive nuclear pleomorphism, positive large nucleoli and negative small nucleoli (p= 0.002).
Conclusions
The presence of HC in FNAB results is associated with colloid in small amounts, associated with nodules smaller than 2 cm, correlated with cellular pleomorphism and nuclear pleomorphism. Fibrosis can be a protective feature against malignancy because cellular parameters were not significantly associated with HC except the cellular pleomorphism.
Keywords: Hurthle cells, cytomorphologic features, thyroid, FNAB
INTRODUCTION
In the thyroid, by altered mitochondria result Hürthle cells (HC) which are enlarged epithelial cells with abundant eosinophilic granular cytoplasm. In general these cells can be found in histological sections of thyroid glands affected with Hashimoto’s thyroiditis (HT), but also in toxic and nontoxic nodular goiter (1). The terms oncocyte, HC, and oxyphilic cell are now used to indicate similar cells, specific features, in different anatomic locations. HC is properly used only to describe cells of thyroid follicular origin (2). HC are also named oncocytic or oxyphilic cells for describing follicular-derived epithelial cells with oncocytic cytology. The large amount of mitochondria is considered a result of mitochondrial DNA encoding for mitochondrial enzymes, leading to proliferation through stimulation of transcription factors encoded by the nucleus (3).
These cells can be found throughout the body, including the kidney, salivary glands, parathyroids and thyroid gland. Classical description of these cells contains: a large (10–15μ), polygonal cell with clear cell borders, abundant eosinophilic finely granular cytoplasm, a large hyperchromatic round to oval nucleus, and a prominent nucleolus and a lot of mitochondria in the cytoplasm (3, 4). The World Health Organization (WHO) accepts the term oncocytic or oncocyte (5). The WHO classifies HC adenoma (HCA) and HC carcinoma (HCC) as oncocytic variants of follicular adenoma and follicular cell carcinoma. HCC is by distinct genetic profile and clinical evolution more aggressive compared with PTC and FTC (3, 6, 7).
Fine-needle aspiration biopsy (FNAB) is an important procedure for differentiating benign from malignant thyroid nodules, recommended in the diagnosis of thyroid nodules. The accuracy of FNAB by Mayo Clinic methodology is about 95%. It is safe, inexpensive and reliable. It has no serious complications and can be easily performed. We used the Mayo Clinic technique for ultrasound guided fine needle aspiration biopsy (FNAB) because it used 6 punctions with 27-gauge needle. Another technique, fine needle non-aspiration (FNNA) biopsy, avoids aspiration but still permits cytological review of thyroid masses. FNAB decreased surgery by 50% in patients with thyroid nodules (8-12).
OBJECTIVE
The aim of our study was to evaluate de incidence of HC in the result of FNAB performed by Mayo Clinic method, using a performed ultrasound guide for thyroid biopsy in Sibiu county. We also want to identify a set of criteria for the cytological diagnosis of HC nodules by FNAB.
MATERIALS AND METHODS
This retrospective cross-sectional clinical study was conducted between 2013-2015 and enrolled 89 patients who were diagnosed with uni-nodular or multinodular goiter, with a recent FNAB. All subjects were evaluated after having given their informed consent for FNAB and for study.
The main criteria to include patients in the study was the result of FNAB performed for uni-nodular or multinodular goiter with suspicious ultrasound description. None had a personal history of external beam radiation treatment.
FNAB was performed using Mayo Clinic technique (12) with aspiration technique by 27G needle with 10ml syringe, by ultrasound guide. After fixing with Gimafix spray, smears were stained with Giemsa stain and wet ethanol stained with hematoxylin and eosin. FNAB results were classified as benign, suspicious, malignant and non-diagnostic cytology. Separately we evaluated the presence of HC.
Depending on the presence or absence of HC we analyzed two groups of patients: the study group A (HC+) included 22 patients with Hürthle cells in FNAB and the control group B (HC-) 67 patients with no Hürthle cells.
For both groups we analyzed the presence of 9 cytomorphologic features frequently described by the cytologist: overall cellularity, background colloid, lymphocyte infiltration, chronic inflammation, large nucleoli, small nucleoli, syncytial infiltration, nuclear pleomorphism/atypia, cellular pleomorphism.
Even with ultrasonographic guidance, the minimal tumour size detectable by FNAB was around 5 mm (13).
Statistical analysis
Statistical analysis was performed using a commercially available statistical software package (SPSS 10.0) for Windows (Microsoft). Comparison of categorical variables was performed using student t test and Likelihood ratio. A p value <0.05 was considered significant.
RESULTS
The study included 89 patients (77 female, 12 male), all with thyroid nodules. Mean age of patients was 49.5 years (range: 15-82 years). The thyroid nodules ranged in size from 1-4.8 cm (maximum diameter). All patients had a clear fine-needle aspiration biopsy results.
Female gender predominates in study group and this fact revealed the high prevalence of this thyroid pathology at women (86.52%).
The cytological examinations of FNA materials found 22 (24.72%) cases with HC and 67 (75.28%) cases without HC. Only 6 patients present specific features of HCC in cytological samples associated with HC.
The demographic features of the patients were similar between the two FNA groups (group A versus group B) and are presented in Table 1. Analysis of the two groups demonstrated no statistical differences between the age of the groups. Also, we do not find any correlation between gender distribution in study groups (p=0.47), but it can be observed that in male are more frequently present (83.3%) in non-Hürthle cell group than in women (74%) (Table 1). All the other parameters, including age, size of nodules, did not reach statistically significant results.
Table 1.
Distribution of clinical parameters in 2 groups
| Variable | Group A (HC +) n= 22 | Group B (HC -) n= 67 | p value |
| Gender | |||
| Male (n / %) | 2/ 16.7 | 10/ 83.3 | 0.471 |
| Women (n / %) | 20/ 26.0 | 57/74 | |
| Mean age (years) | 52.68±13.453 | 48.49±15.145 | 0.227 |
| Nodule size (cm) | 1.932±0.679 | 2.258±0.870 | 0.076 |
| ≤ 2 cm (n/%) | 13/ 59.09 | 24/ 35.82 | 0.056 |
| > 2 cm (n/%) | 9/ 40.91 | 43/ 64.18 | |
| ≤ 2 cm (mean±std) | 1.93±0.68 | 2.22±0.82 | 0.110 |
| > 2 cm (mean±std) | 2.62±0.48 | 2.93±0.72 | 0.146 |
*p value <0.05 was considered significant
The nodular diameter of 2 cm was chosen as the cutoff point; nodules smaller than 2 cm in the Hürthle group (A) were reported in 13 cases (59.09%) while 24 (35.82%) cases in the group B. Nodules with diameter greater than 2 cm were present, statistically significant more frequently in the group without HC, 43 (64.18%). In group without HC the nodules greater than 2 cm were more frequent than nodules smaller than 2 cm.
In Table 2, we analyzed the distribution of several cytological parameters in the HC and non-HC nodules category as previously proposed in other papers. All the cases were reviewed based on the cytological parameters proposed.
Table 2.
The distribution of histopathologic features of the thyroid nodules in 2 groups
| Variable (n/%) | Group A (HC +) n= 22 | Group B (HC -) n= 67 | p value | |
| Colloid | + | 14/ 63.6 | 59/ 88.1 | 0.014* |
| - | 8/ 36.4 | 8/ 11.9 | ||
| Fibrosis | + | 8/ 36.4 | 25/ 37.3 | 0.936 |
| - | 14/ 63.6 | 42/ 62.7 | ||
| Hypercellularity | + | 15/ 68.2 | 47/ 70.1 | 0.862 |
| - | 7/ 31.8 | 20/ 29.9 | ||
| Cellular pleomorphism | + | 14/ 63.6 | 26/ 38.8 | 0.042* |
| - | 8/ 36.4 | 41/ 61.2 | ||
| Syncytial infiltrate | + | 4/ 18.2 | 11/ 16.4 | 0.849 |
| - | 18/ 81.8 | 56/ 83.6 | ||
| Lymphocytic infiltrate | + | 12/ 54.5 | 23/ 34.3 | 0.095 |
| - | 10/ 45.5 | 44/ 65.7 | ||
| Nuclear pleomorphism | + | 8/ 36.4 | 2/ 3.0 | < 0.0001* |
| - | 14/ 63.6 | 65/ 97.0 | ||
| Large nucleoli | + | 4/ 18.2 | 9/ 13.4 | 0.592 |
| - | 18/ 81.8 | 58/ 86.6 | ||
| Small nucleoli | + | 2/ 9.1 | 2/ 3.0 | 0.264 |
| - | 20/ 90.9 | 65/ 97.0 | ||
Legend: + present, - absent ; * p value <0.05 was considered significant
We supported the evidence that the presence of HC is correlated with cellular pleomorphism and nuclear pleomorphism, but we did not find any correlation between the other investigated parameters (Table 2). In group with HC the presence of cellular pleomorphism was more frequent than the absence of this cytological parameter (63.6 % compared with 36.4%).
Also the presence of colloid was significant statistically correlated with the absence of HC (p=0.014). The study of cytologic features examined showed a statistical difference between the group with HC and the group without HC: background colloid was present more frequently (88.1%) in the group without CH (p=0.014) compared with the group with HC in samples (Fig. 1).
Figure 1.
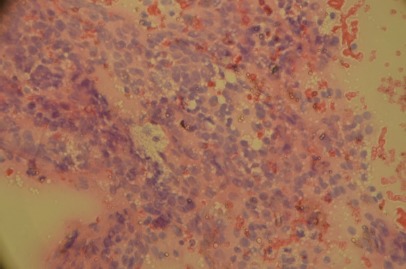
Hürthle cell cluster size increased slightly, with the most voluminous cytoplasm looking fine granular, eosinophilic eccentric and slightly enlarged nuclei. May-Grunwald-Giemsa (MGG) staining. Original magnification 400x.
We correlated the presence/absence of fibrosis in groups with some specific cytological parameters.
The data demonstrated that in the group with HC exists a positive correlation between the presence of cellular pleomorphism and fibrosis. In the presence of fibrosis HC was correlated with nuclear pleomorphism (p=0.03) (Table 3). In absence of fibrosis we demonstrated no correlation between cellular pleomorphism, hypercellularity, syncytial infiltrate and the presence of HC (Table 3).
Table 3.
Correlation between cellular component and fibrosis in 2 groups
| Variable (n/%) | Group A (HC +) n= 22 | Group B (HC -) n= 67 | p value | |
| With fibrosis | ||||
| n=8 | n=25 | |||
| Cellular Pleomorphism | + | 6/75.0 | 8/32.0 | 0.031* |
| - | 2/25.0 | 17/68.0 | ||
| Hypercellularity | + | 6/75.0 | 17/68.0 | 0.704 |
| - | 2/25.0 | 8/32.0 | ||
| Syncytial infiltrate | + | 0/0.0 | 5/20.0 | 0.081 |
| - | 8/100.0 | 20/80.0 | ||
| Absence of fibrosis | ||||
| n=14 | n=42 | |||
| Cellular Pleomorphism | + | 8/57.1 | 18/42.9 | 0.354 |
| - | 6/42.9 | 24/57.1 | ||
| Hypercellularity | + | 9/64.3 | 30/71.4 | 0.618 |
| - | 5/35.7 | 12/28.6 | ||
| Scyncytial infiltrate | + | 6/42.9 | 12/28.6 | 0.329 |
| - | 8/57.1 | 30/71.4 | ||
Legend: + present, - absent ; * p value <0.05 was considered significant
Stepwise multivariate analysis was used to identify the set of parameters that provided the best separation between the 2 groups. The first group of sets include nuclear characteristics (nuclear polymorphism, large and small nucleoli) (Fig. 2). The analysis revealed that in the group with HC without fibrosis there are more characteristic the sets with positive nuclear pleomorphism, positive large nucleoli and negative small nucleoli (p= 0.002, test Likelihood ratio) (Table 4).
Figure 2.
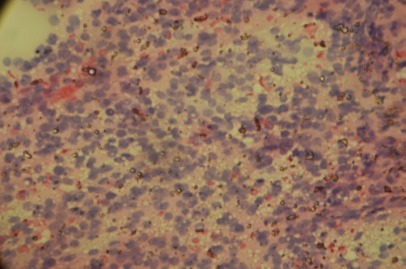
Hurthle cells with larger nuclei slightly enlarged nucleoli, with finely granular cytoplasm. May-Grunwald-Giemsa (MGG) staining. Original magnification 400x.
Table 4.
Correlation between nuclear component and fibrosis
| Variable (n/%) | Group A (HC +) n= 22 | Group B (HC -) n= 67 | p value | |
| With fibrosis | ||||
| n=8 | n=25 | |||
| Large nucleoli | + | 0/0 | 3/12.0 | 0.185 |
| - | 8/100.0 | 22/88.0 | ||
| Small nucleoli | + | 0/0 | 0/0.0 | - |
| - | 8/100.0 | 25/100.0 | ||
| Nuclear polymorphism | + | 1/12.5 | 0/0.0 | 0.087 |
| - | 7/87.5 | 25/100.0 | ||
| Absence of fibrosis | ||||
| n=14 | n=42 | |||
| Large nucleoli | + | 4/28.6 | 6/14.3 | 0.245 |
| - | 10/71.4 | 36/85.7 | ||
| Small nucleoli | + | 2/14.3 | 2/4.8 | 0.263 |
| - | 12/85.7 | 40/95.2 | ||
| Nuclear polymorphism | + | 7/50.0 | 2/4.8 | <0.0001* |
| - | 7/50.0 | 40/95.2 | ||
Legend: + present, - absent ; * p value <0.05 was considered significant
The same analysis was made for cellular component (hypercellularity, cellular pleomorphism, syncytial infiltrate, lymphocytic infiltrate). The data did not act out of any correlation for any possible combinations.
DISCUSSION
The presence of HC lesions is still a concern for a cytologist. A lot of studies published till now try to identify some correlation between cytological features and the presence of malignancy associated with HC.
The aim of our study was to demonstrate if the presence of HC is associated with specific cytological features in FNAB results.
Mean age of patients was 49.5 years (range: 15-82 years), with no statistical difference between the age in the groups; the presence of HC is not influenced by age or gender but we observed the predominance of women in this group (women 26%, men 16.7%). Similar data was reported by Elliott DD et al. in 2006, with a significant frequency of benign HC neoplasia in women (72 patients) compared with 11 males (14).
We analyzed the presence of 9 cytomorphologic features frequently described by a cytologist: overall cellularity, background colloid, lymphocyte infiltration, chronic inflammation, large nucleoli, small nucleoli, syncytial infiltration, nuclear pleomorphism / atypia, cellular pleomorphism. Elliott DD presented a study performed to distinguish neoplastic HC lesions by cytomorphologic analysis based on 14 cytologic features with highly predictive of HC neoplasm: non-macrofollicular architecture, absence of colloid, absence of inflammation and presence of transgressing blood vessels (14).
The polymorphism, presence of inflammatory cells, in particular lymphocytes, a honeycomb or macrofollicular arrangement of the HC, absent or inconspicuous nucleolus in the HC and abundant colloid are usual lesions in nonneoplastic HC nodules by FNAB (15). Our data demonstrated that the presence of HC in FNAB is not associated with nodules with diameter greater than 2 cm, because with statistical significant HC were more frequent in the group without HC, 43 (64.18%). Similar data with association of HC in nodules smaller than 2 cm was published by Rossi et al. in 2013 (16).
Some data revealed that a nodule with HC in a Hashimoto thyroiditis or in multinodular goiter has similar cytological changes like in HC neoplasm (HCN). In Hashimoto disease and in multinodular colloid goiter there were identified numerous lymphocytes or a large amount of colloid in the needle aspirate (17-19).
Our results demonstrated that the presence of oncocytic cells is correlated with cellular pleomorphism and nuclear pleomorphism, but no correlation between the other investigated parameters like fibrosis, hypercellularity, syncytial infiltrate, lymphocytic infiltrate, large nucleoli or small nucleoli.
Some papers demonstrated that HC adenoma (HCA) and carcinoma (HCC) present in FNAB similar cytological findings including sheets and clusters of polygonal epithelial cells with abundant, granular, eosinophilic or basophilic cytoplasm, oval nuclei with regular nuclear contours and conspicuous or inconspicuous nucleoli. HCC is characterized by the presence of syncytial clusters of HC with or without prominent nuclei and abundant naked tumor cell nuclei (20).
Studies that described in HCC overall cellularity, absent colloid, and extensive HC cellularity do not identify clear cytologic elements to exclude neoplasia. In contrast with this in benign forms of HC are rare described this cytomorphologic features (3).
In our study the presence of colloid was significant statistically correlated with the absence of HC (p= 0.014), and can sustain that data published by Cannon et al. in 2011, but opposite to Rossi et al. who demonstrated a little amount of colloid in benign cases and absent in malignant forms (3, 16).
In a large study published in 2011, Cannon demonstrated that the absence of background colloid was the fourth most statistically significant cytomorphologic feature, directly correlated with histopathologic trabecular, solid, or microfollicular morphology (3). In our study we demonstrated a statistical difference between the group with HC and the group without HC: background colloid was present more frequently (88.1%) in group without CH (p=0.014) compared with the group with HC.
Gonzalez et al. analyzed 38 Hürthle cell nodules on FNAB demonstrating that the architectural arrangement and colloid were not useful diagnostic features (21). Nuclear pleomorphism (enlargement, hyperchromasia, and prominent nucleoli), mitoses, solid and trabecular architecture and scant colloid are elements that can be different in benign and malignant oncocytic lesions (22).
Cannon et al. demonstrated in a study that nuclear atypia such as nucleolar prominence, N/C ratio, and nuclear pleomorphism were not statistically significant in the cells of nonneoplastic compared with neoplastic nodules of the thyroid gland. For this reason, nuclear atypia is not a useful histologic or cytologic criterion for assessing nodules of endocrine organs (3). Our data demonstrated that nuclear pleomorphism is significant statistically correlated with the presence of HC (36.4%, p<0.0001). Renshaw A.A. et al. showed among 64 benign cytological samples the limited nuclear irregularities and nuclear pleomorphisms as a consequence of inflammatory changes in HT (22).
In our study the correlation of the fibrosis in groups with some specific cytological parameters demonstrated in group with HC a positive correlation between presence of cellular pleomorphism and fibrosis. We showed a positive correlation between fibrosis and nuclear polymorphism (p=0.03) (Table 3). In absence of fibrosis we demonstrated no correlation between cellular pleomorphism, hypercellularity, syncytial infiltrate and the presence of HC.
In the group with HC without fibrosis are more characteristic the sets with positive nuclear polymorphism, positive large nucleoli and negative small nucleoli. We found in cases without fibrosis a significant statistical association between HC and nuclear pleomorphism (p<0.0001). Evaluation of cellular component (hypercellularity, cellular pleomorphism, syncytial infiltrate, lymphocytic infiltrate) in the presence of fibrosis demonstrated a significant association of the cellular pleomorphism and HC (p=0.03). In the absence of fibrosis we cannot identify a correlation between cellular component and the presence of HC. In cases with fibrosis the incidence of nuclear changes is not important, demonstrating that fibrosis can be a protective feature against malignancy. In the presence of fibrosis all cellular parameters were not significantly associated with HC except for the cellular pleomorphism (p=0.031).
In conclusion, we can observe that the presence of HC in FNAB results is relatively frequent and the presence of this type of cell is associated with colloid in small amounts, but not associated with nodules with diameter greater than 2 cm, and is correlated with cellular pleomorphism and nuclear pleomorphism. Also we demonstrated that fibrosis can be a protective feature against malignancy because cellular parameters were not significantly associated with HC except for the cellular pleomorphism.
Conflict of interest
The authors declare that they have no conflict of interest concerning this article.
References
- 1.Sood N, Nigam JS. Correlation of Fine Needle Aspiration Cytology Findings with Thyroid Function Test in Cases of Lymphocytic Thyroiditis. J Thyroid Res. 2014;2014:1–5. doi: 10.1155/2014/430510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wakely PE. Oncocytic and oncocyte-like lesions of the head and neck. Ann Diagn Pathol. 2008;12:222–230. doi: 10.1016/j.anndiagpath.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Cannon J. The significance of Hurthle cells in Thyroid Disease. The Oncologist. 2011;16:1380–1387. doi: 10.1634/theoncologist.2010-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montone KT, Baloch ZW, LiVolsi VA. The thyroid Hürthle (oncocytic) cell and its associated pathologic conditions: A surgical pathology and cytopathology review. Arch Pathol Lab Med. 2008;132:1241–1250. doi: 10.5858/2008-132-1241-TTHOCA. [DOI] [PubMed] [Google Scholar]
- 5.Baloch ZW, LiVolsi VA, Asa SL. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: A synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008;36:425–437. doi: 10.1002/dc.20830. [DOI] [PubMed] [Google Scholar]
- 6.Giorgadze T, Rossi ED, Fadda G. Does fine-needle aspiration diagnosis of “Hürthle-cell neoplasm/follicular neoplasm with oncocytic features” denote increased risk of malignancy. Diagn Cytopathol. 2004;31:307–312. doi: 10.1002/dc.20132. [DOI] [PubMed] [Google Scholar]
- 7.Cibas ES, Ali SZ. The Bethesda System for Reporting Thyroid Cytopathology. Thyroid. 2009;19:1159–1165. doi: 10.1089/thy.2009.0274. [DOI] [PubMed] [Google Scholar]
- 8.Gurkan DE, Kiyak G, Bozkurt B. Correlation of thyroid fine-needle aspiration with final histopathology: a case series. Minerva Chirurgica. 2013;68:191–197. [PubMed] [Google Scholar]
- 9.Rathi M, Ahmad F, Budania SK, Awasthi S, Kumar A, Dutta S. Cytomorphological aspects of Hashimoto’s thyroiditis: our experience at a Tertiary center. Clinical Medicine Insights. Pathology. 2014;7:1–5. doi: 10.4137/CPath.S13580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar SS, Negi VS, Siddaraju N. Cytomorphologic study of Hashimoto’s thyroiditis and its serologic correlation: a study of 150 cases. Acta Cytologica. 2009;53(5):507–516. doi: 10.1159/000325377. [DOI] [PubMed] [Google Scholar]
- 11.Gayathri B, Kalyani R, Harendra KM, Krishna PK. Fine needle aspiration cytology of Hashimoto’s thyroiditis – A diagnostic pitfall with review of literature. J Cytol. 2011;28:210–213. doi: 10.4103/0970-9371.86353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gharib H. Fine-needle aspiration biopsy of thyroid nodules: advantages, limitation, and effect. Mayo Clin Proc. 1994;69:44–49. doi: 10.1016/s0025-6196(12)61611-5. [DOI] [PubMed] [Google Scholar]
- 13.Mikosch P, Gallowitsch HJ, Kresnik E, Jester J, Wurtz FG, Kerschbaumer K, Unterweger O, Dinges HP, Lind P. Value of ultrasound-guided fine-needle aspiration biopsy of thyroid nodules in an endemic goitre area. Eur J Nucl Med. 2000;27:62–69. doi: 10.1007/pl00006664. [DOI] [PubMed] [Google Scholar]
- 14.Elliott DD, Pitman MB, Bloom L, Faquin WC. Fine-needle aspiration biopsy of Hürthle cell lesions of the thyroid gland: A cytomorphologic study of 139 cases with statistical analysis. Cancer. 2006;108:102–109. doi: 10.1002/cncr.21716. [DOI] [PubMed] [Google Scholar]
- 15.Auger M. Hürthle cells in fine-needle aspirates of the thyroid: A review of their diagnostic criteria and significance. Cancer Cytopathol. 2014;122:241–249. doi: 10.1002/cncy.21391. [DOI] [PubMed] [Google Scholar]
- 16.Rossi ED, Martini M, Straccia P, Raffaelli M, Pennacchia I, Marrucci E, Lombardi CP, Pontecorvi A, Fadda G. The cytologic category of oncocytic (Hurthle) cell neoplasm mostly includes low-risk lesions at histology: an institutional experience. Eur J Endocrinol. 2013;169:649–655. doi: 10.1530/EJE-13-0431. [DOI] [PubMed] [Google Scholar]
- 17.Pu RT, Yang J, Wasserman PG, Bhuiya T, Griffith KA, Michael CW. Does Hurthle cell lesion/neoplasm predict malignancy more than follicular lesion/neoplasm on thyroid fine-needle aspiration? Diagn Cytopathol. 2006;34:330–334. doi: 10.1002/dc.20440. [DOI] [PubMed] [Google Scholar]
- 18.Kini SR. 2nd ed. Philadelphia: Wolters Kluwer/Lippincott Williams and Wilkins; 2011. Color Atlas of Differential Diagnosis in Exfoliative and Aspiration Cytopathology; pp. 123–124. [Google Scholar]
- 19.Canberk S, Griffin AC, Goyal A. Oncocytic follicular nodules of the thyroid with or without chronic lymphocytic thyroiditis: An institutional experience. CytoJournal. 2013;10:2–3. doi: 10.4103/1742-6413.106686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen GK, Lee MW, Ginsberg J, Wragg T, Bilodeau D. Fine-needle aspiration of the thyroid: an overview. CytoJournal. 2005;2:12–13. doi: 10.1186/1742-6413-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez JL, Wang HH, Ducatman BS. Fine-needle aspiration of Hurthle cell lesions. A cytomorphologic approach to diagnosis. Am J Clin Pathol. 1993;100:231–235. doi: 10.1093/ajcp/100.3.231. [DOI] [PubMed] [Google Scholar]
- 22.Renshaw AA. Hürthle cell carcinoma is a better gold standard than Hürthle cell neoplasm for fine-needle aspiration of the thyroid: Defining more consistent and specific cytologic criteria. Cancer. 2002;96:261–266. doi: 10.1002/cncr.10797. [DOI] [PubMed] [Google Scholar]


