Abstract
Introduction
KCNJ11 gene activating mutations play a major role in the development of neonatal diabetes mellitus (NDM). KCNJ 11 gene encodes the Kir 6.2 subunit of ATP- sensitive potassium channel which is a critical regulator of pancreatic beta-cell insulin secretion.
Aim
To study KCNJ11 gene mutations in infants with NDM and the effect of sulfonylurea treatment on the glycemic control in patients with KCNJ11 gene mutation.
Subjects and methods
Thirty infants with NDM were screened for KCNJ11 gene mutations by DNA sequencing, insulin therapy was replaced by sulfonylurea treatment in patients with mutations.
Results
R201C heterozygous mutation was found in one patient who was successfully shifted from insulin therapy to sulfonylurea treatment, while E23k, I337V, and S385C polymorphisms were detected in 14 patients.
Conclusion
Screening for KCNJ 11 gene mutations could lead to identification of patients with mutations who can be successfully shifted from insulin therapy to sulfonylurea treatment improving their quality of life.
Keywords: KCNJ11 gene mutations, neonatal diabetes mellitus, sulfonylurea therapy versus insulin therapy
INTRODUCTION
Monogenic diabetes of infancy (MDI) or Neonatal diabetes mellitus (NDM) is a rare form of diabetes mellitus that is defined as persistent hyperglycemia, with onset within the first months of life requiring insulin management. The diagnosis of NDM is most often applied to diabetes with onset before 6 months of age; however, the age limit for NDM varies, and has been extended to 12 months of age (1). NDM may be transient diabetes in about 50% of patients that occurs within the first few weeks of life and resolves within a median of three months, or may be permanent in the other 50 % of patients (2).
Permanent neonatal diabetes is associated with defects in genes that play major roles in pancreatic beta cell development and function including mutations in KCNJ11 gene (encoding Kir 6.2 subunit), mutation in ABCC8 gene (encoding SUR1 subunit), both forming the ATP-sensitive potassium channel subunits and mutations in the insulin gene (INS) (3-9).
The determination of the underlying genetic cause has led to improved treatment, and it is necessary for genetic counselling in family planning. Although insulin is necessary for initial management of all patients with neonatal diabetes, there have been several reports of successful transition from insulin to sulfonylurea agents in patients with PNDM caused by mutation in the KCNJ11 gene (10,11).
We aimed to study KCNJ11 gene mutations in infants with NDM and study the effect of sulfonylurea treatment on glycemic control in relation to KCNJ11 gene mutations.
SUBJECTS AND METHODS
This is a cross sectional study that included thirty patients who were enrolled from Diabetic Endocrine and Metabolic Pediatric Unit (DEMPU) of Cairo University Mounira Children’s Hospital (Abu El Reesh). They were diagnosed to have NDM according to the ADA criteria for diagnosis of NDM (12). The study was approved by the Local Ethical Committee after informed consents were obtained from parents of all patients. Inclusion criteria were insulin-treated diabetic infants diagnosed within the first year of life. Exclusion criteria included patients who were diagnosed as being type 1 diabetes mellitus by auto-antibodies and HLA typing. Clinical data were recorded such as age at diagnosis, age at onset, family history, birth weight, gestational age. Physical examination and neurological finding were also recorded. The signs and symptoms at presentation, blood glucose level, and hemoglobin A1c% were obtained.
Genetic studies
DNA sequencing of KCNJ11 gene was done. All molecular analyses were carried out at the molecular unit of the Chemical pathology laboratories, Faculty of medicine, Cairo University.
DNA extraction
Genomic DNA was extracted from peripheral blood leukocytes using QiA amp DNA blood mini kit (Qiagen, Valencia, CA).
PCR amplification and sequencing of the KCNJ11 Gene: the small intronless KCNJ11 gene was amplified by PCR in three overlapping fragments of 564, 546 & 491 bp using three primers pairs as reported elsewhere (13). DNA amplification was done using Hybaid thermal cycler Promega Corporation, USA. The amplified products were detected on 1.5% agarose gel electrophoresis using electrophoresis apparatus (Pharmacia Eps500/400), and then visualized by ultraviolet trans-illumination (Promega, USA).
Sequence analysis of KCNJ 11 gene
Following amplification, the amplified products were then purified using QIA quick PCR purification kit (Qiagen), forward sequencing was performed using the Big Dye Terminator cycler sequencing Kit (Applied Biosystems, Warrington, USA), and reverse sequencing was done to confirm mutation. Reactions were analyzed on ABI 310 capillary DNA sequencer (Applied Biosystems).
DNA Sequences were compared to the human GenBank reference for KCNJ11 gene and using CLC sequence viewer 7.6 software.
Statistical analysis
Chi-square test was used to compare allele and genotype frequency among different studied groups. Quantitative data were expressed as mean ± SD and compared using t-test when normally distributed, and as median and range using Mann Whitney U test when not normally distributed. P value <0.05 is considered significant. Statistics were done using SPSS V.18 (PSWAT).
RESULTS
Clinical presentation
Thirty infants who were diagnosed as NDM were included in the study; they were 18 (60%) males and 12 (40%) females. Twenty-six patients (86.7 %) presented at diagnosis with diabetic ketoacidosis (DKA). Six patients (20 %) had positive family history of type 2 diabetes. The median of gestational age was 36 months (range 28-39.5), the median of birth weight was 3000 gram (range 1000-4000). Twenty-eight cases were PNDM while only 2 cases were TNDM. The median of the dose of insulin therapy was 0.66 U/kg/day (range 0.35-1.1), while the median of HbA1c was 8.4 % (range 6.6-10.5). The results of KCNJ11 gene sequencing are listed in Table 1.
Table 1.
Results of KCNJ11 gene sequencing among the studied group
| R201C mutation n= 30 | E23K polymorphism n= 30 | I337V polymorphism n= 30 | S385C polymorphism n= 30 | |
| Wild type | 29/30 (96.7%) | 20/30 (66.7%) | 20/30 (66.7%) | 26/30 (86.7%) |
| Heterozygous | 1/30 (3.3%) | 8/30 (26.6%) | 8/30 (26.6 %) | 4/30 (13.3%) |
| Homozygous | 0/30 (0%) | 2/30 (6.7 %) | 2/30 (6.7%) | 0/30 (0%) |
E23K / I337V Combined heterozygous polymorphisms were detected in 10 patients (2 were homozygous and 8 were heterozygous), and 4 patients had S385C C>G (1154) heterozygous polymorphism. R201C heterozygous mutation was detected in one patient. Brief history of the case with KCNJ11 R201C mutation has been presented below:
A two months age full term male with a birth weight of 3500 gram was referred to the pediatric hospital with convulsions for which he received antiepileptic therapy. Ten days later, he developed diabetic ketoacidosis (DKA). Although the patient had normal birth weight, he was microcephalic. Follow- up of this patient revealed delay in both motor and mental development. This patient was diagnosed as developmental delay, epilepsy, and neonatal diabetes mellitus (DEND) syndrome.
Figure 1.
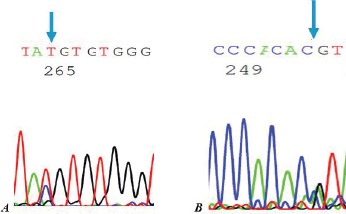
The sequence result of PCR products of KCNJ11. R201C [n: C>T (601)] heterozygous mutation, A: the forward strand & B: the reverse strand.
There was no family history of diabetes mellitus. HbA1c was 10%, he was treated with 0.87 U/kg/day of insulin. He was 12 months age at the time of the study. Mutational analysis for the KCNJ11 gene for this case revealed a C to T substitution at bp 601(c.601C>T) in heterozygote state, leading to the substitution of amino acid arginine by cysteine at codon 201, R201C.
After the results of KCNJ11 gene analysis, the patient was shifted from insulin therapy to sulfonylurea treatment over a period of 3-4 weeks until insulin was totally withdrawn and the patient was maintained on oral sulfonylurea (0.5-0.8 mg/kg/day) divided into 2-4 doses. Follow-up of this patient showed improved glycemic control (HbA1c decreased to 7.6%).
DISCUSSION
KCNJ11 mutations are typically dominant and fall into two categories; either direct reduction of the affinity of the ATP binding by mutations in the residues that form the ATP binding pocket or interfere with the access of the ATP to binding pocket (14). While in the second category the mutations can be located far from the ATP binding pocket itself (15,16). These mutations act by keeping the KATP channel open by reducing ATP sensitivity leading to hyperpolarization of the beta cells with reduced insulin secretion (17).
We studied KCNJ11 gene mutations in 30 infants with monogenic diabetes mellitus of infancy and the effect of sulfonylurea treatment on glycemic control in relation to KCNJ11 mutation.
R201 C mutation was detected in one male patient on insulin therapy, and insulin therapy was replaced by sulfonyl urea treatment, while the other patients showed KCNJ11 gene polymorphisms (E23K, I337V, and S385C).
Approximately 20% of individuals with KCNJ11 gene mutations and the majority of patients with R201C mutation have DEND syndrome (17, 18). At position 201 of KCNJ11 gene there is a CpG dinucleotide which represents a hotspot for mutations in eukaryotes gene (19). It is situated in the binding site for the ATP molecule which is known to decrease the sensitivity to ATP (4), and respond well to oral sulfonylurea treatment both in vivo and in vitro. Therefore, on switching the patient in our study from insulin therapy to sulfonylurea treatment, the glycemic control of the patient is improved.
Our results are in agreement with Joshi and Phatarpekar, 2011, who described a case of neonatal diabetes mellitus who was proved to have KCNJ11 gene mutation and was successfully shifted from insulin therapy to oral sulfonylurea treatment on a dose of 0.2mg/kg/day (20).
Also, Dupont et al. 2012 described a 12 year-old Portuguese girl with PNDM due to R201C mutation in the KCNJ11 gene. Her medical history included prematurity and moderate developmental delay and showed a successful shift from insulin therapy to sulfonylurea treatment (21).
In conclusion, screening for KCNJ11 gene mutation could eventually lead to identification of those patients who will have the highest benefit from sulfonylurea treatment, where sulfonylurea treatment has become the treatment of choice for most cases with NDM due to KCNJ11 gene mutation. This has not only changed their life quality, but it has also marked clinical benefits; fluctuations of glucose level which is a common problem in neonatal patients are substantially reduced, and hypoglycemic episodes become less common. Also, plasma glucose levels are lower as indicated by a significant reduction in HbA1c, which decrease the risk of diabetic complications.
Conflict of interest
The authors declare that they have no conflict of interest concerning this article.
Acknowledgement
The study was supported by research grant from Cairo University for purchasing the kits. We wish to thank all patients and their families who participated in the study.
References
- 1.Iafusco D, Massa O, Pasquino B, Colombo C, Iughetti L, Bizzarri C, Mammı C, Lo Presti D, Suprani T, Schiaffini R, Colin G., Nichols L, Russo Grasso V, Meschi F, Bonfanti R, Brescianini S, Barbetti F. Minimal incidence of neonatal/infancy onset diabetes in Italy is 1:90,000 live births. Acta Diabetol. 2012;49(5):405–408. doi: 10.1007/s00592-011-0331-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polak M, Cave H. Neonatal diabetes mellitus: a disease linked to multiple mechanisms. Orphanet J Rare Dis. 2007;2:12. doi: 10.1186/1750-1172-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aguilar-Bryan L, Bryan J. Neonatal Diabetes Mellitus. Endocr Rev. 2008;29(3):265–291. doi: 10.1210/er.2007-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy R, Ellrad S, Hattersley AT. Clinical Implication of a molecular genetic classification of monogenic beta-cell diabetes. Nat Clin Pract Endocrinol Metab. 2008;4(4):200–213. doi: 10.1038/ncpendmet0778. [DOI] [PubMed] [Google Scholar]
- 5.Gloyn AL, Pearson ER, Antcliff JF, Gloyn AL, Pearson ER, Antcliff JF, Proks P, Bruining GJ, Slingerland AS, Howard N, Srinivasan S, Silva JM.C.L., Molnes J, Edghill EL, Frayling TM, Temple IK, Mackay D, Shield J. P.H, Sumnik Z, Van Rhijn A, Wales J. K.H, Clark P, Gorman S, Aisenberg J, Ellard S, Njølstad P. R., Ashcroft F. M., Hattersley A.T. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir 6.2 and permanent neonatal diabetes. N Engl J Med. 2004;350(18):1838–1849. doi: 10.1056/NEJMoa032922. [DOI] [PubMed] [Google Scholar]
- 6.Babenko A.P., Polak M, Cavé H, Pharm D, Busiah K, Czernichow P, Scharfmann R, Bryan J, Aguilar-Bryan L, Vaxillaire M, Froguel P. Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. N Engl J Med. 2006;355(5):456–466. doi: 10.1056/NEJMoa055068. [DOI] [PubMed] [Google Scholar]
- 7.Støy J, Edghill E, Flanagan S, Ye H, Paz V P, Pluzhnikov A, Below JE, Geoffrey Hayes M, Cox NJ, Lipkind G M, Lipton RB, Greeley S A W, Patch AM, Ellard S, Steiner D F, Hattersley AT, Philipson L H, Bell GI, Neonatal Diabetes International Collaborative Group Insulin gene mutations as a cause of permanent neonatal diabetes. Proc Natl Acad Sci. U S A. 2007;104(38):15040–15044. doi: 10.1073/pnas.0707291104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colombo C, Porzio O, Liu M, Massa O, Vasta M, Salardi S, Beccaria L, Monciotti C, Toni S, Pedersen O, Hansen T, Federici L, Pesavento R, Cadario F, Federici G, Ghirri P, Arvan P, Iafusco D, Barbetti F. Seven mutations in the human insulin gene linked to permanent neonatal/infancy-onset diabetes mellitus. J. Clin. Invest. 2008;118(6):2148–2156. doi: 10.1172/JCI33777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellard S, Flanagan SE, Girard CA, Patch AM, Harries LW, Parrish A, Edghill EL, Mackay DJ, Proks P, Shimomura K, Haberland H, Carson DJ, Shield JP, Hattersley AT, Ashcroft FM. Permanent neonatal diabetes caused by dominant, recessive, or compound heterozygous SUR1 mutations with opposite functional effects. Am J Hum Genet. 2007;81(2):375–382. doi: 10.1086/519174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan Y-M, Laffel LMB. Transition from insulin to glyburide in a 4-month-old girl with neonatal diabetes caused by a mutation in KCNJ11. Pediaric Diabetes. 2007;8(4):235–238. doi: 10.1111/j.1399-5448.2007.00231.x. [DOI] [PubMed] [Google Scholar]
- 11.Vakili R, Ghahraman M, Ghaemi N, Faraji B, Hashemi poor M, Ahmadi E, Abbaszadegan MR, Saeidi M, Naghibzadeh B. Clinical and Molecular Genetic Analysis of Iranian Neonatal Diabetes Cases Demonstrating Mutations in KCNJ11 gene. Iranian Journal of Neonatology. 2012;3(2):85–90. [Google Scholar]
- 12.American Diabetes Association Classification and diagnosis of diabetes. Diabetes Care. 2015;38(Suppl. 1):S8–S16. doi: 10.2337/dc15-S005. [DOI] [PubMed] [Google Scholar]
- 13.Klupa T, Edghill EL, Nazim J, Sieradzki J, Ellard S, Hattersley AT, Malecki MT. The identification of a R201H mutation in KCNJ11 which encodesKir 6.2, and successful transfer to sustained release sulfonylurea therapy in a subject with neonatal diabetes: evidence for heterogeneity of beta cell function among carriers of the R201H mutation. Diabetologia. 2005;48(5):1029–1031. doi: 10.1007/s00125-005-1731-5. [DOI] [PubMed] [Google Scholar]
- 14.Koster JC, Koster JC, Cadario F, Peruzzi C, Colombo C, Nichols CG, Barbetti F. The G53D mutation in Kir 6.2 (KCNJ11) is associated with neonatal diabetes and motor dysfunction in adulthood that is improved with sulfonylurea therapy. 2008;93(3):1054–1061. doi: 10.1210/jc.2007-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Proks P, Antcliff JF, Lippiat J, Gloyn AL, Hattersley AT, Ashcroft FM. Molecular basis of Kir 6.2 mutations associated with neonatal diabetes or neonatal diabetes plus neurological features. Proc Nat Acad Sci. 2004;101(50):17539–17544. doi: 10.1073/pnas.0404756101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flanagan SE, Edghill EL, Gloyn AL, Ellard S, Hattersley AT. Mutations in KCNJ11, which encodes Kir 6.2, are a common cause of diabetes diagnosed in the first 6 months of life, with the phenotype determined by genotype. Diabetologia. 2006;49(6):1190–1197. doi: 10.1007/s00125-006-0246-z. [DOI] [PubMed] [Google Scholar]
- 17.Gloyn AL, Siddiqui J, Ellard S. Mutations in the genes encoding the pancreatic ß-cell KATP channel subunits Kir 6.2 (KCNJ11) and SUR1 (ABCC8) in diabetes mellitus and hyperinsulinism. Hum Mutat. 2006;27(3):220–231. doi: 10.1002/humu.20292. [DOI] [PubMed] [Google Scholar]
- 18.De León D, Stanley C. Permanent Neonatal Diabetes Mellitus. In: Pagon RA, Adam MP, Bird TD, Dolan CR, Fong CT, Smith RJH, Stephens K, editors. Gene-review. Seattle: Copyright, University of Washington; 1993-2014. Updated 2014. [PubMed] [Google Scholar]
- 19.Hattersley AT, Ashcroft FM. Activating mutations in Kir 6.2 and neonatal diabetes: new clinical syndromes, new scientific insights, and new therapy. Diabetes. 2005;54(9):2503–2513. doi: 10.2337/diabetes.54.9.2503. [DOI] [PubMed] [Google Scholar]
- 20.Joshi R, Phatarpekar A. Neonatal diabetes mellitus due to L233F mutation in the KCNJ11 gene. World J Pediatr. 2011;7(4):371–372. doi: 10.1007/s12519-011-0254-z. [DOI] [PubMed] [Google Scholar]
- 21.Dupont J, Pereira C, Medeira A, Duarte R, Ellard S, Sampaio Permanent neonatal diabetes mellitus due to KCNJ11 mutation in a Portuguese family: transition from insulin to oral sulfonylureas. J Ped Endocrinol Metab. 2012;25(3-4):367–370. doi: 10.1515/jpem-2011-0191. [DOI] [PubMed] [Google Scholar]


