Abstract
Context
Schizophrenia is a chronic disease most frequently necessitating lifelong antipsychotic treatment. Selecting which antipsychotic is to be prescribed in an individual schizophrenia patient represents an important clinical decision that need to take into account efficacy and side effects.
Objective
Evaluating weight gain related with one year antipsychotic treatment in antipsychotic naive first-episode schizophrenia patients.
Design
This study is an analysis of weight gain associated with typical or atypical antipsychotics used in European First Episode Schizophrenia Trial (EUFEST) study.
Subjects and Methods
113 first episode naïve antipsychotic schizophrenia patients included in EUFEST - Romanian cohort, who were randomized to one of the 5 treatment arms. Weight was obtained at baseline, 3, 6, 9 and 12 months for the 5 antipsychotics (typical-Haloperidol; atypical-Olanzapine, Amisulpride, Ziprasidone, Quetiapine).
Results
There are no statistically significant differences between groups treated with typical or atypical antipsychotics or between any individual antipsychotics concerning weight gain during the study. Weight gain was the highest in the first 3 months (57.49%) for all the studied neuroleptics. At the end of the study, the less increase was observed with ziprasidone (3.87 kg) and the highest with olanzapine (9.83 kg).
Conclusion
Increase in weight has taken place for each individual neuroleptic, but also as a group (all neuroleptics) in the first three months (57.49%). Therefore, we should address the issue of weight gain with great care, especially in first period of antipsychotic administration, in order to fast deploy intervention tailored to maintain pre-treatment weight.
Keywords: antipsychotics, schizophrenia, weight gain
INTRODUCTION
Schizophrenia is a profoundly disabling illness. Among young adults in developed countries, it ranks near the top of causes of disability in both men and women. Because of the early onset, debilitating effects, and chronic evolution in most of the cases, schizophrenic disorders rank fifth among men and sixth among women as a leading cause of years lived with disability (1).
Patients suffering from schizophrenia live less to 15–25 years compared with the general population (2). Also people affected by schizophrenia have an important (2-3 times) increase in mortality compared to general population, all causes of death considered (3).
Both natural and unnatural (most unnatural causes are represented by suicide and various types of accidents, with an aggregated figure of unnatural death causes of schizophrenia being considered to be around 40% (4) sources of death are increased in schizophrenic patients compared with controls. Most likely causes of death in patients with schizophrenia are: heart diseases (3.4 times), diabetes (3.4 times), pneumonia, influenza and other respiratory ailments (11.6 times) (5). In this group of patients the major clinical risk factors for cardiac mortality are: hypertension, diabetes and cardiovascular disease.
Schizophrenia may trigger, in most cases, a cascade of socioeconomic and lifestyle factors that, in turn, can translate into adverse physical health outcomes.
The most important behavioral risk factors for cardiac and all-cause increased mortality in schizophrenia patients are: low physical activity, obesity, smoking, high rates of alcohol and drug abuse, difficulty in self-health evaluating, difficulty in accessing health care system, difficulty in therapeutical adherence. These factors play a role in explaining the excess deaths by natural causes in patients with schizophrenia (6).
Noteworthy, 3 out of 5 people affected by schizophrenia die from mostly preventable diseases. Improving life expectancy and nonetheless quality of life of patients with schizophrenia necessitates addressing all the above modifiable risk factors.
The discovery and introduction of antipsychotics represents a major breakthrough in treatment of schizophrenia. Recent meta-analyses (7) clearly indicated that long-term treatment with neuroleptics is associated with improved quality of life, decreased hospitalization, decreased aggressive behavior, and relapse prevention.
Most studies (8,9) indicate that patients not taking any antipsychotics have increased mortality too, and this fact may represent a very powerful argument for using long-term neuroleptic treatment in schizophrenia patients. Therefore using long term (mostly life-long) treatment with antipsychotics in schizophrenia represents a cornerstone in general management of patients with schizophrenia, although some authors consider important to identify putative subgroups of patients that would not need a long term treatment with antipsychotics (10).
First generation antipsychotics were discovered into the ‘60’s and represented for about 4 decades the standard of care for patients with schizophrenia, even though this class of drugs has a burden of a plethora of adverse effects, mainly bothersome extrapyramidal side effects (parkinsonism, dystonia, akatisia, tardive dyskinesia) but also sedation, lethargy, movement disorders, weight gain.
Schizophrenia represents a very heterogeneous condition (genetic, clinical presentation, severity, treatment response and so on) most probably representing several different diseases (11). The main clinical manifestations of this syndrome are: positive symptoms (delusions, hallucinations), negative symptoms (avolition, anhedonia, social withdrawal) and cognitive impairment (diminished attention, memory, cognition etc). First generation antipsychotics (such as haloperidol and chlorpromazine) are mainly efficacious on positive symptoms, which is a major drawback because both negative symptoms and cognitive impairment represent in fact the major source of disability in schizophrenia (12). Atypical antipsychotics were initially viewed as a major improvement in the treatment of schizophrenic patients because this class of medication was seen as having a better side effect profile (less/absent extrapyramidal symptoms) and improved efficacy in treatment of negative symptoms and cognitive impairment. Unfortunately recent studies challenged (13) the initial optimism that atypical antipsychotics are indeed associated with an improvement in negative or cognitive symptoms. Moreover, soon after introduction of second generation antipsychotics it became clear that this class also has its own significant side effects. A very important side effect associated with second generation antipsychotics is weight gain (14).
This side-effect represents a major concern in the treatment of schizophrenia because weight gain is a major contributor to increased morbidity (15), including, but probably not limited to: glucose intolerance, and diabetes mellitus (16), metabolic syndrome (17), sleep apnea (18) and cardiovascular diseases (19). All of these conditions can lead to increased mortality (20). Furthermore, fear of weight gain is one of the main factors contributing to poor compliance found in antipsychotic treatment (21), so it may adversely affect the clinical outcome (22).
For overweight and obese people it is very difficult to lose weight and afterward to maintain a lower weight (23). It is definitively far more difficult for a person with schizophrenia to lose weight when he/she is struggling also with cognitive impairment, negative symptoms, frequently with a poor support of social network, scant social conditions and with a rather meager access to the health system. It is therefore very important to try to use (if possible) medication with the lowest risk for weight gain. This may raise the question about how much weight gain each individual neuroleptic is associated with in order to make the best clinical decision for people with schizophrenia.
According to several meta-analyses (24) most neuroleptics contribute to weight gain. Particularly clozapine and olanzapine were associated with severe weight gain, whereas aripiprazole, amisulpride and ziprasidone appeared almost weight neutral (25).
Studies in drug-naïve schizophrenia patients are the more informative studies of this kind as weight outcomes are not influenced by the level of overweight due to a previous neuroleptic use or deleterious lifestyle changes connected with cognitive impairments or other harmful behaviors coupled with a longer evolution of illness (overeating, sedentary lifestyle, etc.).
Our study looks into weight changes in first episode psychosis neuroleptic native patients diagnosed with schizophrenia and treated with antipsychotics for a duration of 1 year. The neuroleptics studied in our paper are both first generation antipsychotics (haloperidol) and second-generation antipsychotics (olanzapine, quetiapine, amisulpride and ziprasidone).
We know so far that the general efficacy of antipsychotics is equal both individually and between classes of antipsychotics (13), that is why in clinical activity, therapeutic decision is based frequently just on side effects, of which weight gain plays an important part (26). Moreover, an increasing number of patients outside schizophrenia are treated with antipsychotics, recent studies showing that over 50% of patients having prescribed an antipsychotic being treated so off-label (27).
METHODS
Data analysed in our paper are drawn from European First Episode Schizophrenia Trial study and represent a secondary analysis of data about Romanian patients (N=113).
The main purpose of EUFEST study (28) was to compare atypical antipsychotics (amisulpride 200–800 mg/day, olanzapine 5–20 mg/day, quetiapine 200–750 mg/day, ziprasidone 40–160 mg/day) with low doses of typical neuroleptics (haloperidol 1–4 mg/day) in terms of effectiveness. The measure for effectiveness in EUFEST is all-cause treatment discontinuation, defined as the time to discontinuation of the study drug to which patients were originally randomized.
Investigators from 50 centres in Europe and Israel participated into this trial. Eligible patients were 18– 40 years of age and met DSM- IV criteria for schizophrenia, schizophreniform, or schizoaffective disorder confirmed by the Mini International Neuropsychiatric Interview Plus.
Inclusion criteria were: 18– 40 years of age and DSM- IV criteria for schizophrenia, schizophreniform, or schizoaffective disorder confirmed by the Mini International Neuropsychiatric Interview Plus, first episode of disease with no more than 2 years elapsed between the onset of positive symptoms and recruitment into the trial and previous use of antipsychotic drugs of less than 2 weeks during the preceding year and less than 6 weeks lifetime. The investigators invited eligible patients to participate after providing information orally and in writing about the trial. After complete description of the study to the subjects, written informed consent was obtained. The trial complied with the Declaration of Helsinki and was approved by the Ethics Committees of the participating centres.
Patients did not meet exclusion criteria in this study if: no more than 2 years elapsed since the onset of positive symptoms; any antipsychotic have not been used exceeding 2 weeks in the previous year or 6 weeks lifetime; patients had not a known intolerance to one of the study drugs; and patients did not meet any of the contraindications for any of the study drugs as mentioned in the (local) package insert texts.
Baseline data were obtained between 4 weeks before and 1 week after randomization on demographics, diagnoses, current medication, psychopathology (Positive and Negative Syndrome Scale - PANSS), severity of illness (clinical global impression - CGI), overall psychosocial functioning (global assessment of functioning scale - GAF), extrapyramidal symptoms (St Hans rating scale - SHRS), depression (Calgary Depression Schizophrenia Scale - CDSS), neurocognitive performance (Trail Making A [time], Flexibility Index [Trail Making B–A time], Wechsler Adult Intelligence Scale Digit-symbol Coding, Purdue pegboard, Rey Auditory Verbal Learning Test), and quality of life (Manchester Short Assessment of Quality of Life - MANSA).All scales have been given to the included patients at Baseline, 1, 2, 3, 6, 9, and 12 months. Weight was obtained at baseline, 3, 6, 9 and 12 months.
In this article we used the term ‘schizophrenia’ for diagnostics of schizophrenia, schizophreniform and schizoaffective disorder.
Statistical analysis
We used descriptive analysis to characterize demographics and repartition into treatment arms. Mean, standard deviation (SD), and sample size are provided for continuous variables. Discrete variables are described using frequencies and percentages. All statistical tests were 2 tailed; α (level of significance) was 5%. Discrete variables were analysed with a χ2 test. The differences between different treatment arms (Haloperidol, Olanzapine, Amisulpride, Quetiapine, Ziprasidone) were assessed using ANOVA function corrected with Bonferonni t-tests for normal distributed values and with Kruskal-Wallis for non-normal distributed values. Comparison between categorical independent data and categorical dependent data has been done using multinomial regression. All data were analysed using Statistical Package for Social Sciences (SPSS) version 21.
RESULTS
The EUFEST study in Romania included 113 patients, which were randomized to one of the treatment arms. From the 113 initial randomized, 61 (54%) are women and 52 (46%) men.
We analyzed differences between treatment arms regarding socio-economic status, demographics or clinical data. As shown in Table 1 there are no statistically significant (p>0.05) differences at baseline between patients with first episode schizophrenia treated with first generation antipsychotics (haloperidol) or atypical antipsychotics (olanzapine, quetiapine, amisulpride, ziprasidone).
Table 1.
Demographic and clinical characteristics of EUFEST patients according to treatment arms. N= number of patients
| Antipsychotic | Haloperidol | Olanzapine | Quetiapine | Amisulpride | Ziprasidone | p |
| N | 18 | 27 | 18 | 26 | 24 | N/A |
| Marital status (%) | 88.9 | 81.5 | 77.8 | 84.6 | 66.7 | .377 |
| Gender (% male) | 61.1 | 44.4 | 55.6 | 50 | 25 | .126 |
| Current occupation (%,yes) | 44.4 | 55.6 | 44.4 | 65.4 | 50 | .785 |
| Patient education (years) | 11.94 (1.73) |
12.52 (3.60) | 12.61 (2.03) |
12.42 (2.85) |
12.63 (2.76) |
.829 |
| Patient age at randomization –years M(SD) | 25.66 (5.98) |
26.87 (6.20) |
27.04 (6.13) |
26.91 (5.43) |
27.81 (6.86) |
.906 |
| Height (cm) -baseline | 166.39 (9.85) | 170.07 (9.55) | 170.56 (9.51) | 169.04 (9.60) | 165.96 (5.99) | .228 |
| Weight (kg) -baseline | 59.83 (12.83) | 64 (11.85) | 63.72 (14.04) | 60.26 (9.62) | 60.96 (11.39) | .737 |
| PANSS total scale | 91.61 (20.09) | 88.48 (15.44) | 98.82 (26.33) | 86.85 (14.49) | 87.50 (16.13) | .589 |
| Visit 1 CDSS total sum score | 4.61 (6.64) | 5.30 (5.74) | 5.29 (6.60) | 4.88 (5.21) | 3.54 (4.45) | .751 |
| Visit 1 MANSA total mean score | 3.68 (.88) | 3.92 (1.03) | 3.92 (1.23) | 4.16 (.93) | 4.34 (.89) | .232 |
| Visit 1 GAF score (Mean+/-SD) | 39.06 (13.56) | 40.67 (12.71) | 37.65 (17.05) | 38.24 (12.52) | 38.33 (10.26) | .926 |
Regarding weight at baseline the data were non-normal distributed (Shapiro-Wilk p=.001), therefore we eliminated the outliers (94, 98, 100, 125 kg) from the analysis. After eliminating the outliers the data about weight gain at baseline were normally distributed (Shapiro-Wilk p=.181). The data at 3, 6, 9 and 12 months were not normal distributed, but we did not eliminate the outliers because the figures obtained represent the weight gain after treatment therefore in these cases it would be important to keep the outliers for further analysis.
Because an important number (N=20) of patients did not finish the study (non-completers), we compare if they differ from the group who complete (completers) the study regarding initial weight because if there were significant differences between the 2 groups about initial weight this might be an important bias. There are no differences between the 2 groups (completers mean initial weight=62.58kg +/-12.73kg, non-completers 65.20kg +/-18.81kg, p=. 461) therefore the results can be generalized to our cohort of patients. Results for completers are presented separately for each antipsychotic in Table 2 and Graphic 1.
Graphic 1.
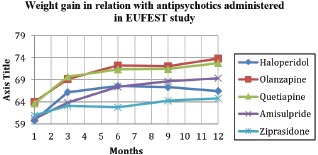
Weight gain in relation with antipsychotics administered in EUFEST study - completers.
Table 2.
Weight in relation with antipsychotics administered in EUFEST (completers)
| Antipsychotic | Month kg. (SD) | Total increase | ||||
| 0 | 3 | 6 | 9 | 12 | Kg (%) | |
| Haloperidol | 59.83 (12.83) | 66.27 (11.65) | 67.60 (10.49) | 67.40 (10.76) | 66.53 (11.57) | 6.7 (11.20) |
| Olanzapine | 64 (11.84) | 69.21 (12.06) | 72.26 (11.38) | 72.14 (10.52) | 73.83 (11.04) | 9.83 (15.36) |
| Quetiapine | 63.72 (14.03) | 69.75 (16.79) | 71.38 (18.33) | 71.54 (18.14) | 72.79 (17.94) | 9.07 (14.23) |
| Amisulpride | 60.26 (9.62) | 63.87 (9.95) | 67.5 (10.72) | 68.7 (11.54) | 69.37 (11.12) | 9.11 (15.11) |
| Ziprasidone | 60.96 (11.38) | 63.14 (10.52) | 62.79 (10.03) | 64.33 (10.45) | 64.83 (10.14) | 3.87 (6.35) |
There are no statistically significant differences between groups concerning weight gain during the study, i.e. at 3 (p=. 382), 6 (p=.123), 9 (p=.282) and 12 months (p=.113).
The bulk of weight increase appeared during the first 3 months for all the studied neuroleptics (Haloperidol: 6.44 kg -96% from the total weight gain, Olanzapine 5.21 kg -53% from the total weight gain, Quetiapine 6.03 kg -66.48% from the total weight gain, Amisulpride 3.61 kg -39.62% from the total weight gain and Ziprasidone 2.18 kg -56,33% from the total weight gain). Regarding the entire group of patients treated with antipsychotics as a group, the mean weight at baseline was 61.83kg (11.79) and at 3 months the mean increased to 66.32kg (12.21). There was an increase in weight of 4.49 kg, representing an overall increase in weight during the first 3 months of 7.26%.
Thereafter the increase continues but at a slower pace (Graphic 2).
Graphic 2.
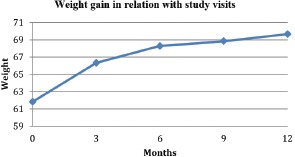
Weight gain in relation with study visits in EUFEST – Romanian cohort.
For the entire study weight increase during the 12 months was highest for olanzapine (9.83 kg) and lowest for ziprasidone (3.87 kg).
For the entire group of patients (completers and non-completers) treated with neuroleptics the total augment in weight took place during the first 3 months (7.81 kg weight gain at 12 months with 4.49 kg increase at 3 months representing 57.49% from the total weight gain). The weight gain for entire population treated with neuroleptics (both typicals and atypicals) is 12.63% (7.81kg). Results for the whole cohort of treated patients are presented in Graph 2.
DISCUSSION
Our analysis of Romanian first episode schizophrenia patients included in EUFEST study followed up weight changes in relation with neuroleptics assigned in naive neuroleptic patients for a total duration of 1 year. First episode patients with schizophrenia do not come with the risk of associating lifestyle changes, or unhealthy behaviors that occur later in the course of schizophrenia, which makes the analysis of weight gain in relation with antipsychotic administration in this specific population of patients more valuable and informative. Moreover, the patients were not exposed to previous antipsychotics or other psychotropic drugs associated with weight gain, therefore the results are not biased by this factor. By and large the results from our study are in concordance with literature data. Overwhelmingly literature data suggest that basically all antipsychotics are associated with prominent weight gain in antipsychotic-naive and first-episode patients (29). Again in accordance to data from literature (30) our results indicate that most of weight gains appeared early (months) during the course of treatment and continued for the rest of the study period without a plateau but at a slower pace.
According to an important meta-analysis (25) most antipsychoties are associated with a statistically significant increase in weight post- baseline, with the exception of amisulpride, aripiprazole, asenapine, sertindole, ziprasidone, which showed no statistically significant, weight change.
However, these findings are not supported by a recent 3-year study of treatment with quetiapine, ziprasidone, and aripiprazole in patients with a first psychotic episode which showed important weight gain associated with these antipsychotics, the proportion of patients gaining ≥7% baseline weight was 23% for ziprasidone, 32% for quetiapine, and 45% for aripiprazole (31).
The results of our study are also in agreement with the results from the original EUFEST study which showed that antipsychotics considered body weight neutral (amisulpride, ziprasidone, and low-dose haloperidol), were associated with relevant weight gain (9.7, 4.8 and 6.3 kg respectively) (13).
It is considered that antipsychotics induce changes in appetite and food intake, mostly because of interaction with serotoninergic (32), histaminergic (33) and dopaminergic (34) neurotransmitter systems inducing alteration in appetite and food intake.
It is to note that the methodological differences between studies and the pharmacogenomics differences between different antipsychotics (including sex and races differences) may be the cause of the heterogeneity of data related to antipsychotic weight gain increase (AIWG).
The mechanism of drug-induced weight gain is mostly unclear; receptor-binding profiles of these drugs have been implicated but this may be only a partial and incomplete biological explanation.
Genetic factors are among the strongest risk factors influencing antipsychotic weight gain (35).
Similar to other complex phenotypes, AIWG is likely to be polygenic with each polymorphism making a minor contribution to the overall phenotype. Furthermore, risk factors may vary between antipsychotic medications.
The extent to which pharmacokinetic genes may predict AIWG is still rather unclear. Recent data (36) suggests that genetic determinates of variable drug metabolism (CYP450, MDR1, flavin-containing mono-oxygenase 3) have little impact on the clinical use of antipsychotic medication.
From a pharmacodynamics approach the serotonin receptor genes-HTR2C has been the most extensively studied gene in AIWG with conflicting findings (37).
All antipsychotics target to a certain degree D2 dopamine receptors. Disruption in the dopamine D2 receptor gene (DRD2) was reported to be associated with obesity (35). Traditionally histamine may have been considered a compelling candidate for treatment-emergent weight gain; however, only one study has examined histamine receptors 1, 2 and 3 obtaining rather unexpectedly negative findings (38). However, Jassim (39) examined the five-receptor subunits of hypothalamic AMP-activated protein kinase (AMPK1) using tag-SNPs. Of the markers examined, only the rs10074991 polymorphism of the PRKAA1 subunit (AMPK) was associated with weight gain.
Other systems under genetic regulation, which arguably participate in AIWG, are: the G-protein system, proteins implicated in synaptic signaling, Leptin (one of the most important appetite regulators), the melancortin-4 receptor, Brain-derived neurotrophic factor, Neuropeptide Y, Pro-melanin-concentrating hormone, Ghrelin, Endocannabinoids, Cellular lipogenesis pathways, High-density and low-density lipoprotein pathways, Tumor necrosis factor alpha and so on (for a more comprehensive review see (40)).
Another advantage of our study was that we were able to comparatively analyze a first generation antipsychotic (haloperidol) with second generation antipsychotics (olanzapine, quetiapine, amisulpride and ziprasidone), second generation antipsychotics between them and all individual neuroleptics between all neuroleptics making the full results more complex.
Furthermore, our analysis also looked into which period of time weight changes occur. There are 5 measurement points in the present study, allowing us to see which is the period most likely associated with the risk for weight gain.
Our study has shown that all antipsychotics are associated with an important increase in weight in first episode schizophrenia patients, the bulk of this increase appearing during the first 3 months. We did not find any statistically significant differences between antipsychotics regarding this side-effect during the entire study or at any time point, although ziprasidone has been associated with the lowest weight gain.
Antipsychotic treatment in schizophrenia is essential. The results of our analysis in EUFEST study show that we can expect increase in weight in administration of any antipsychotic. Thus, we suggest that: a) it is mandatory to monitor increase in weight in all schizophrenia patients treated with any antipsychotics; b) clinicians must provide education regarding prevention of weight increase to all their patients treated with any antipsychotic in long term starting from the very beginning of treatment; c) there should be a constant care regarding identification and implementation of pharmacological measures designed for prevention of weight gain in the case of this group of patients.
Increase in weight has taken place for each neuroleptic, regardless of class (typical versus atypical) and as a group (all neuroleptics) in the first three months. The most important consequence is that the fast increase in weight in such a short period of time makes the prevention method to be of highest importance, because afterwards, the loss of weight will be difficult to achieve, if not impossible. Moreover, it is of outmost importance to identify and target predictors (clinical, paraclinical) associated with weight gain with the existing pharmacological prevention methods.
Limitations
An important limitation of our study consists in the relatively small number of patients in the analyzed cohort, however this is the largest cohort of patients from one country in EUFEST study and the biggest study of this kind in Romania. Another important fact, which constitutes both a limitation and an essential advantage in our study, is the fact that patients are neuroleptic-naive, which could not enable us to generalize data to patients with chronic disease.
Conclusion
All neuroleptics (haloperidol, olanzapine, quetiapine, amisulpride, ziprasidone) studied in EUFEST produced a weight increase in Romanian cohort of first episode schizophrenia patients (7.81 kg representing 12.63% from the initial weight) and the differences between them were not statistically significant.
At the end of the study, the less increase was observed with ziprasidone (3.87 kg) and the highest with olanzapine (9.83 kg).
This analysis of the complex relations over time between neuroleptics and weight gain in people with first episode schizophrenia in EUFEST study shows that the highest weight gain is in the first 3 months for all neuroleptics studied in EUFEST – Romanian cohort. The weight gain in the first 3 months was highest for haloperidol and lowest for ziprasidone, but no statistical differences were found between neuroleptics. The total weight gain in the Romanian cohort of first episode patients was 7.81 kg at the end of the study, from which 57.49 % (4.49kg) occurred in the first three months. There is a stringent need of other studies that should identify risk factors associated with weight gain, together with potential subgroups of patients in which weight gain is most frequently observed. Moreover, there should be a constant interest to identify the best prevention methods for weight increase in subgroups of patients at greater risk for weight gain due to antipsychotic treatment, either psycho-educational, pharmacological, or a combination of those.
Conflict of interest
The authors declare that they have no conflict of interest concerning this article.
Acknowledgements
All the authors had an equal contribution and have similar rights. All the authors approved the final version of this article.
References
- 1.Lora A, Kohn R, Levav I, McBain R, Morrise J, Saxenae S. Service availability and utilization and treatment gap for schizophrenic disorders: a survey in 50 low- and middle-income countries. Bulletin of the World Health Organization. 2012;90(1):47–54B. doi: 10.2471/BLT.11.089284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang CK, Hayes RD, Perera G, Broadbent MT, Fernandes AC, Lee WE, Hotopf M, Stewart R. Life expectancy at birth for people with serious mental illness and other major disorders from a secondary mental health care case register in London. PLoS One. 2011;6(5) doi: 10.1371/journal.pone.0019590. e19590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tiihonen J, Lonnqvist J, Wahlbeck K, Klaukka T, Niskanen L, Tanskanen A, Haukka J. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study) Lancet. 2009;374(9690):620–627. doi: 10.1016/S0140-6736(09)60742-X. [DOI] [PubMed] [Google Scholar]
- 4.Bushe CJ, Taylor M, Haukka J. Mortality in schizophrenia: a measurable clinical endpoint. J Psychopharmacol. 2010;24(4 Suppl):17–25. doi: 10.1177/1359786810382468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parks J., Svendsen D, Singer P, Foti ME. Morbidity and Mortality in People with Serious Mental Illness. NASMHPD Medical Directors Council Publications and Reports 2006 (Accessed October 2006, at site http://www.nasmhpd.org/content/morbidity-and-mortality-people-serious-mental-illness).
- 6.Brown S, Kim M, Mitchell C, Inskip H. Twenty-five year mortality of a community cohort with schizophrenia. The British Journal of Psychiatry. 2010;196(2):116–121. doi: 10.1192/bjp.bp.109.067512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leucht S, Tardy M, Komossa K, Heres S, Kissling W, Salanti G, Davis JM. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet. 2012;379(9831):2063–2071. doi: 10.1016/S0140-6736(12)60239-6. [DOI] [PubMed] [Google Scholar]
- 8.Weinmann S, Read J, Aderhold V. Influence of antipsychotics on mortality in schizophrenia: systematic review. Schizophr Res. 2009;113(1):1–11. doi: 10.1016/j.schres.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 9.Suvisaari J, Partti K, Peralä J, Viertiö S, Saarni SE, Lönnqvist J, Saarni SI, Härkänen T. Mortality and its determinants in people with psychotic disorder. Psychosom Med. 2013;75(1):60–67. doi: 10.1097/PSY.0b013e31827ad512. [DOI] [PubMed] [Google Scholar]
- 10.Matei VP, Mihailescu AI, Davidson M. Is non-pharmacological treatment an option for certain schizophrenia patients? Psychiatr Danub. 2014;26(4):308–313. [PubMed] [Google Scholar]
- 11.Arnedo J, Svrakic DM, Del Val C, Romero-Zaliz R, Hernandez-Cuervo H, Molecular Genetics of Schizophrenia Consortium. Fanous AH, Pato MT, Pato CN, de Erausquin GA, Cloninger CR, Zwir I. Uncovering the hidden risk architecture of the schizophrenias: Confirmation in three independent genome-wide association studies. The American Journal of Psychiatry. 2015;172(2):139–153. doi: 10.1176/appi.ajp.2014.14040435. [DOI] [PubMed] [Google Scholar]
- 12.Velligan DI, Mahurin RK, Diamond PL, Hazleton BC, Eckert SL, Miller AL. The functional significance of symptomatology and cognitive function in schizophrenia. Schizophr Res. 1997;25(1):21–31. doi: 10.1016/S0920-9964(97)00010-8. [DOI] [PubMed] [Google Scholar]
- 13.Kahn RS, Fleischhacker WW, Boter H, Davidson M, Vergouwe Y, Keet IP, Gheorghe MD, Rybakowski JK, Galderisi S, Libiger J, Hummer M, Dollfus S, López-Ibor JJ, Hranov LG, Gaebel W, Peuskens J, Lindefors N, Riecher-Rössler A, Grobbee DE, EUFEST study group Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomized clinical trial. Lancet. 2008;371(9618):1085–1097. doi: 10.1016/S0140-6736(08)60486-9. [DOI] [PubMed] [Google Scholar]
- 14.McEvoy JP, Meyer JM, Goff DC, Nasrallah HA, Davis SM, Sullivan L, Meltzer HY, Hsiao J, Scott Stroup T, Lieberman JA. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophrenia Research. 2005;80(1):19–32. doi: 10.1016/j.schres.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 15.Wirshing DA, Wirshing WC, Kysar L, Berisford MA, Goldstein D, Pashdag J, Mintz J, Marder SR. Novel antipsychotics: comparison of weight gain liabilities. Journal of Clinical Psychiatry. 1999;60(6):358–363. [PubMed] [Google Scholar]
- 16.Hedenmalm K, Hagg S, Stahl M, Mortimer O, Spigset O. Glucose intolerance with atypical antipsychotics. Drug Saf. 2002;25(15):1107–1116. doi: 10.2165/00002018-200225150-00005. [DOI] [PubMed] [Google Scholar]
- 17.Scheen AJ, De Hert MA. Abnormal glucose metabolism in patients treated with antipsychotics. Diabetes and Metabolism. 2007;33(3):169–175. doi: 10.1016/j.diabet.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Wirshing DA, Pierre JM, Wirshing WC. Sleep apnea associated with antipsychotic-induced obesity. Journal of Clinical Psychiatry. 2002;63(4):369–370. doi: 10.4088/jcp.v63n0415f. [DOI] [PubMed] [Google Scholar]
- 19.Robinson DG, Woerner MG, Delman HM, Kane JM. Pharmacological treatments for first-episode schizophrenia. Schizophrenia Bulletin. 2005;31(3):705–722. doi: 10.1093/schbul/sbi032. [DOI] [PubMed] [Google Scholar]
- 20.Kurzthaler I, Fleischhacker WW. The clinical implications of weight gain in schizophrenia. Journal of Clinical Psychiatry. 2001;62(Suppl 7):32–37. [PubMed] [Google Scholar]
- 21.Perkins DO. Predictors of noncompliance in patients with schizophrenia. Journal of Clinical Psychiatry. 2002;63(12):1121–1128. doi: 10.4088/jcp.v63n1206. [DOI] [PubMed] [Google Scholar]
- 22.Allison DB, Casey DE. Antipsychotic-induced weight gain: a review of the literature. Journal of Clinical Psychiatry. 2001;62(Suppl 7):22–31. [PubMed] [Google Scholar]
- 23.Dobbs R, Sawers C, Thompson F, Manyika J, Woetzel J, Child P, McKenna S, Spatharou A. How the world could better fight obesity. McKinsey & Company. 2014. (Accessed November 2014, at site http://www.mckinsey.com/insights/economic_studies/how_the_world_could_better_fight_obesity).
- 24.Klemp M, Tvete IF, Skomedal T, Gaasemyr J, Natvig B, Aursnes I. A review and Bayesian meta-analysis of clinical efficacy and adverse effects of 4 atypical neuroleptic drugs compared with haloperidol and placebo. J Clin Psychopharmacol. 2011;31(6):698–704. doi: 10.1097/JCP.0b013e31823657d9. [DOI] [PubMed] [Google Scholar]
- 25.Bak M, Fransen A, Janssen J, van Os J, Drukker M. Almost all antipsychotics result in weight gain: a meta-analysis. PLoS One. 2014;9(4) doi: 10.1371/journal.pone.0094112. e94112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Citrome L. A review of the pharmacology, efficacy and tolerability of recently approved and upcoming oral antipsychotics: an evidence-based medicine approach. CNS Drugs. 2013;27(11):879–911. doi: 10.1007/s40263-013-0105-7. [DOI] [PubMed] [Google Scholar]
- 27.Marston L, Nazareth I, Petersen I, Walters K, Osborn DP. Prescribing of antipsychotics in UK primary care: a cohort study. BMJ Open. 2014;4(12) doi: 10.1136/bmjopen-2014-006135. e006135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fleischhacker WW, Keet IP, Kahn RS, EUFEST Steering Committee The European First Episode Schizophrenia Trial (EUFEST): rationale and design of the trial. Schizophr Res. 2005;78(2-3):147–156. doi: 10.1016/j.schres.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 29.De Hert M, Detraux J, van Winkel R, Yu W, Correll CU. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2011;8(2):114–126. doi: 10.1038/nrendo.2011.156. [DOI] [PubMed] [Google Scholar]
- 30.Bushe CJ, Slooff CJ, Haddad PM, Karagianis JL. Weight change from 3-year observational data: findings from the worldwide schizophrenia outpatient health outcomes database. J Clin Psychiatry. 2012;73(6):749–755. doi: 10.4088/JCP.11m07246. [DOI] [PubMed] [Google Scholar]
- 31.Perez-Iglesias R, Ortiz-Garcia De La Foz V, Martinez Garcia O, Amado JA, Garcia-Unzueta MT, Ayesa-Arriola R, Suarez-Pinilla P, Tabares-Seisdedos R, Crespo-Facorro B. Comparison of metabolic effects of aripiprazole, quetiapine and ziprasidone after 12 weeks of treatment in first treated episode of psychosis. Schizophr Res. 2014;159(1):90–94. doi: 10.1016/j.schres.2014.07.045. [DOI] [PubMed] [Google Scholar]
- 32.Starrenburg FC, Bogers JP. How can antipsychotics cause Diabetes Mellitus? Insights based on receptor-binding profiles, humoral factors and transporter proteins. Eur Psychiatry. 2009;24(3):164–170. doi: 10.1016/j.eurpsy.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Kim DH, Maneen MJ, Stahl SM. Building a better antipsychotic: receptor targets for the treatment of multiple symptom dimensions of schizophrenia. Neurotherapeutics. 2009;6(1):78–85. doi: 10.1016/j.nurt.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panariello F, De Luca V, de Bartolomeis A. Weight gain, schizophrenia and antispychotics: new findings from animal model and pharmacogenomic studies. Schizophr Res Treatment. 2011 doi: 10.1155/2011/459284. vol. 2011, Article ID 459284, 16 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muller DJ, Kennedy JL. Genetics of antipsychotic treatment emergent weight gain in schizophrenia. Pharmacogenomics. 2006;7(6):863–887. doi: 10.2217/14622416.7.6.863. [DOI] [PubMed] [Google Scholar]
- 36.Grossman I, Sullivan PF, Walley N, Liu Y, Dawson JR, Gumbs C, Gaedigk A, Leeder JS, McEvoy JP, Weale ME, Goldstein DB. Genetic determinants of variable metabolism have little impact on the clinical use of leading antipsychotics in the CATIE study. Genet Med. 2008;10(10):720–729. doi: 10.1097/GIM.0b013e3181863239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCarthy S, Mottagui-Tabar S, Mizuno Y, Sennblad B, Hoffstedt J, Arner P, Wahlestedt C, Andersson B. Complex HTR2C linkage disequilibrium and promoter associations with body mass index and serum leptin. Hum Genet. 2005;117(6):545–557. doi: 10.1007/s00439-005-1328-6. [DOI] [PubMed] [Google Scholar]
- 38.Ujike H, Nomura A, Morita Y, Morio A, Okahisa Y, Kotaka T, Kodama M, Ishihara T, Kuroda S. Multiple genetic factors in olanzapine-induced weight gain in schizophrenia patients: a cohort study. J Clin Psychiatry. 2008;69(9):1416–1422. doi: 10.4088/jcp.v69n0909. [DOI] [PubMed] [Google Scholar]
- 39.Jassim G, Ferno J, Theisen FM, Haberhausen M, Christoforou A, Havik B, Gebhardt S, Remschmidt H, Mehler-Wex C, Hebebrand J, Lehellard S, Steen VM. Association study of energy homeostasis genes and antipsychotic-induced weight gain in patients with schizophrenia. Pharmacopsychiatry. 2011;44(1):15–20. doi: 10.1055/s-0030-1263174. [DOI] [PubMed] [Google Scholar]
- 40.Lett TA, Wallace TJ, Chowdhury NI, Tiwari AK, Kennedy JL, Muller DJ. Pharmacogenetics of antipsychotic-induced weight gain: review and clinical implications. Mol Psychiatry. 2012;17(3):242–266. doi: 10.1038/mp.2011.109. [DOI] [PubMed] [Google Scholar]


