Abstract
Introduction
Heart failure and dilated cardiomyopathy (DCM) in adults are rarely caused by hypoparathyroidism induced hypocalcemia.
Case report
Female patient, 40 years old, diabetic, with previous history of thyroidectomy for Graves’ disease, was hospitalized for syncope and symptoms of heart failure. ECG revealed sinus tachycardia, long QT, negative T from V1 up to V4. Chest X-ray, cardiac ultrasound and contrast cardiac MRI confirmed dilated left chambers, severe systolic dysfunction of the left ventricle (left ventricle ejection fraction=15%) due to diffuse hypokinesia and restrictive type of diastolic dysfunction. Patient status insignificantly improved with specific heart failure depletion treatment but important signs of hypocalcemia occurred. Low levels of total and ionic serum calcium were detected (total serum calcium 3.6 mg/dL, ionic calcium=2.2 mg/dL) along with low serum levels of parathormone (10 pg/mL) and high level of phosphatemia (6.4 mg/dL). After one month of parenteral treatment with calcium and oral vitamin D, hypocalcemic signs disappeared and heart failure significantly improved.
Conclusion
This rare adult condition is refractory to heart failure conventional therapy but promptly responds to restoration of normocalcemia. It is important to be aware of this pathophysiological setting, in order to treat it correctly.
Keywords: hypocalcemia, hypoparathyroidism, heart failure, dilated cardiomyopathy
INTRODUCTION
Calcium is an essential element for the normal ventricular systolic and diastolic function. Over the active process of myocardial membrane depolarization, there is a rapid inflow of calcium ions via active (voltage-gated) membrane calcium channels, and subsequent calcium ions’ release from sarcoplasmic reticulum (1). Then calcium binds with the troponin-tropomyosin complex, supporting the myocardial contraction: actin-myosin sliding and jointing. Relaxation occurs when calcium ions are actively pumped back into sarcoplasmic reticulum, tropomyosin resumes its shape and myosin slides back and uncouples actin (2).
Therefore, it seems obvious that hypocalcemia will affect cardiac contractility, and this was proved in current studies (3). Meanwhile, recent evidence suggests that vitamin D and parathormone (PTH) may have an independent role to play, also (4, 5).
Hypocalcemic heart failure is a rare finding in clinical practice. It was described especially in infants, due to severe vitamin D deficiency (6-8). Hypocalcemic ventricular dysfunction and dilatation in adulthood is substantially less found and described, being mainly related to hypoparathyroidism (9-11). This condition is refractory to heart failure conventional therapy, but if the etiology is recognized, it responds promptly to restoration of normocalcemia.
CASE REPORT
We report the case of a 40-year-old woman without cardiovascular history, who presents in Emergency Care Unit for syncope, progressive exertional dyspnea up to dyspnea at rest and paroxysmal nocturnal dyspnea. She is known with type 1 diabetes mellitus and with thyroidectomy for Graves’ disease for 6 years, with levothyroxin substitution treatment. Patient’s written approval was obtained in order to publish her case.
At admission the patient was conscious, in moderate distress, with normal body temperature, spontaneous peripheral oxygen saturation of 93%, blood pressure 100/60 mmHg equal in both arms, a regular ventricular rate of 100 beats per min, with a holosystolic IIIrd/IVth degree murmur in the axilla, protodiastolic left ventricle gallop, mild peripheral edema, bilateral lung stasis rales, moderate hepatomegaly (inferior liver limit at 3 cm below the costal edge).
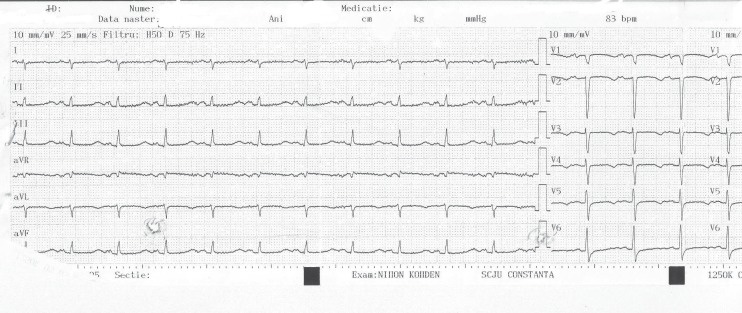
Her ECG revealed sinus tachycardia at 100/min, QRS axis at +90 degrees, long QT (QTc=640 ms), negative T from V1 up to V4 (Fig. 1).
Figure 1.
ECG at admission.
Laboratory analysis at admission revealed: elevated glycaemia (270 mg/dL), elevated liver enzymes, elevated N-terminal pro b-type natriuretic peptide (NT-proBNP), hypercholesterolemia, markers of myocardial necrosis within physiologic limits, with no rising pattern, elevated TSH (Table 1).
Table 1.
Biochemistry and hormonal values
| Day | Admission | Discharge |
| Glycemia (nv 60-110 mg/dL) | 270 | 109 |
| ALAT (nv<31 U/L) | 361 | 105 |
| ASAT (nv<32 U/L) | 463 | 83 |
| GGT (nv<40 U/L) | 909 | 503 |
| Uric acid (nv: 2-5.7 mg/dL) | 4.5 | |
| Serum cholesterol (nv: <200 mg/dL) | 322 | 213 |
| HDL-Cholesterol (nv>40 mg/dL) | 97 | 34 |
| LDL-Cholesterol (nv<100 mg/dL) | 223 | 163 |
| Triglycerides (nv:<150 mg/dL) | 187 | 116 |
| Creatinine (nv: <1 mg/dL | 0.91 | 1 |
| NT-proBNP (nv<86 pg/mL) | 900 | |
| CK-MB (nv<24) | 17 | 22 |
| Troponin T (nv<14 pg/mL) | 12.42 | 12.21 |
| Na (nv: 134-145 mEq/L) | 134 | 143 |
| K (nv: 3.3-5.1 mEq/L) | 5.3 | 4.5 |
| Anti dsDNA (nv: <100 UI/mL) | 54 | |
| Anti Ro antibodies: (nv: <7 U/mL) | 4.2 | |
| ANCA: (nv: <1/10) | 1/25 | |
| Total serum calcium (nv: 8.6-10 mg/dL) | 3.6 | 5.2 |
| Ionic serum calcium (nv: 3.82-4.82 mg/dL) | 2.2 | 2.9 |
| Serum magnesium (nv: 1.6-2.6 mg/dL) | 1.55 | 1.8 |
| Serum phosphorus (nv: 2.5-4.5 mg/dL) | 6.4 | 4.3 |
| Parathormone (nv: 15-65 pg/mL) | 10 | 6.7 |
| TSH (nv: 0.27-4.20 µUI/mL) | 15 | 5.6 |
| 25-OH-vitamin D (nv: 30-100 ng/mL) | 21.8 | 32 |
ALAT= Alanine aminotransferase; ASAT= Aspartate aminotransferase; CK-MB= Creatine kinase-MB; NT-proBNP = N-terminal pro b-type natriuretic peptide
dsDNA = anti-double stranded DNA antibodies; ANCA = anti-neutrophilic cytoplasmic antibodies. TSH= Thyroid stimulating hormone.
The chest X-ray exhibited signs of pulmonary stasis with hilum enlargement due to vascular elements involvement and an increased cardio-thoracic index.
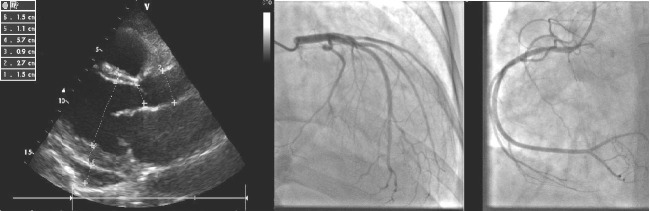
Cardiac ultrasound disclosed dilated left chambers (left ventricle end-diastolic diameter: 57 mm, left ventricle end-systolic diameter: 51 mm), left ventricle (LV) severe global systolic dysfunction (LVEF=15%) due to diffuse hypokinesia, restrictive type of diastolic dysfunction with elevated filling pressure indexes (E/E’=19), moderate functional mitral regurgitation, IIIrd degree tricuspid regurgitation, moderate pulmonary hypertension (peak systolic trans-tricuspid pressure gradient of 40 mmHg, systolic pulmonary artery pressure of 50mmHg), pericardial circumferential effusion of maximum 3 mm (Fig. 2). Angiocoronarography was performed, in order to exclude a plausible ischemic LV failure. This investigation revealed permeable epicardic coronary arteries, with no atherosclerotic lesions (Fig. 2).
Figure 2.
Cardiac ultrasound and angiocoronarography (left and right coronary arteries without atherosclerosis).
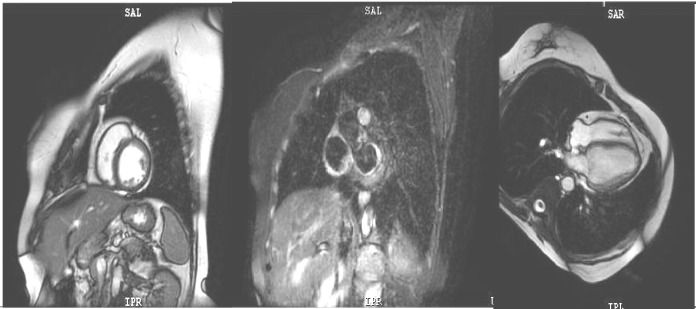
Cardiac MRI was also performed, proving homogeneous myocardial perfusion and a late contrast enhanced aspect within normal limits (Fig. 3).
Figure 3.
Cardiac MRI: homogeneous myocardial perfusion within normal limits.
Treatment was initiated with loop diuretic (furosemide 120 mg/24 h i.v., decreasing progressively to a daily dose of 40 mg p.o.), ACE inhibitor (ramipril 2.5 mg/ day), beta-blocker (carvedilol up to 6.25 mg/day), anti-aldosterone diuretic (spironolactone 25 mg/day), atorvastatin 20 mg/day, insulin and levothyroxine (titered up to 150 microg/day).
Giving the circumstances of LV dilatation with severe systolic dysfunction and the presence of long QT – we presumed an episode of tachyarrhythmia as a cause for syncope. Repeated 24 hours ECG monitoring showed no significant arrhythmias.
Patient’s cardiac status was very slowly improving under treatment, with partial remission of the congestive signs, 6 kg loss in weight, residual dyspnea at moderate effort, but she started to complain of important nocturnal muscular cramps and parestesia. Along hospitalization, she started to exhibit typical hypocalcemic signs of neuromuscular hyperexcitability: Chvostek, Weiss, Trousseau positive signs, psychomotor agitation and convulsions.
Laboratory blood tests disclosed severe hypocalcemia: total serum calcium of 3.6 mg/dL, ionic calcium=2.2 mg/dL. So, we considered hypocalcaemia pre-existent to the actual cardiac distress, based not only on the anamnesis but also on the long QT segment at admission, that it was curiously symptomless before, and that it was exacerbated by the diuretic treatment.
Parathormone testing was assessed, along with phosphoremia, and they revealed severe primary hypoparathyroidism (Table 1).
Further investigation was performed: cerebral CT scan that revealed calcifications of the basal ganglia. (Fig. 4).
Figure 4.
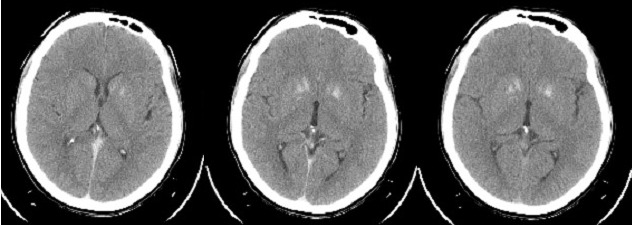
Cerebral CT scan revealing calcification of the basal ganglia.
Parenteral treatment with gluconic calcium was started: 950 mg of calcium (1 ampoule) at 6 hours, followed by oral treatment – lactic calcium 6000 mg/day and calcitriol 0.5 µg per day was associated. The following days patient’s clinical and serological evolution improved, hypocalcemic signs have disappeared in several days.
Patient was discharged after one month with important improvement of cardiac symptoms (heart failure regression from IVth NYHA class to IInd NYHA class), improvement of LVEF (30%) with no hypocalcemic manifestations and with the recommendations to continue cardiologic treatment and lactic calcium supplementation up to 6 g per day along with 2 µg of alpha-calcidolum. She was advised to monitor her serum calcium levels every two weeks in the first two months, along with regular kidney ultrasound, and every three months afterwards.
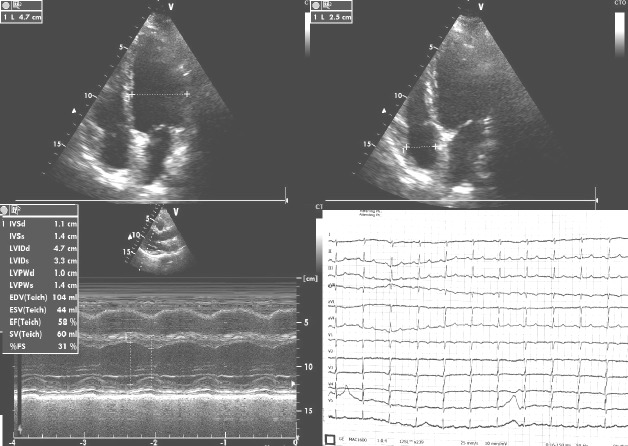
One year after the above-mentioned discharge, the patient was under daily medication with 3 g lactic calcium, 2 µg of alpha-calcidolum, 12.5 mg carvedilol and 150 µg levo-thyroxine, and complained only of dyspnea at intense efforts with no hypocalcemic symptoms. She had normal values of calcemia and 25-OH-vitamin D. Control cardiac ultrasound and ECG were performed and they both were within normal limits (Fig. 5).
Figure 5.
Normal cardiac ultrasound and ECG one year after discharge.
DISCUSSION
Identifying the etiology of DCM in order to establish the optimal therapy is of great importance, due to the fact that certain types are reversible (ethanolic DCM is reversible when alcohol consumption stops, tachyarrhythmia induced DCM is reversible when tachycardia disappears). In the meantime, genetic primary dilative cardiomyopathy has no etiological treatment, only some promising new therapies: stem cells transplant, genic therapy and micro-RNA (12).
Even if epicardial coronaries are permeable we still could not exclude the coronary heart disease: hyperglycemia may induce ischemic disorder due to lesions of the microvascularisation. These lesions cannot be revealed either by coronarography or by angio CT scan (13).
Dysglycemia and insulin resistance may directly damage the atrial and ventricular myocardium by inflammatory mechanisms (14). Medical literature sustain that it is reasonable for the cardiovascular phenotype to get worse as glucose impairment is more severe, hypothesizing that geometry and function of the heart are gradually affected by progressive deterioration in glucose metabolism (15).
Our patient has no familial history of DCM and no recent occurrence of fever. Nevertheless, clinical manifestations as well as the young age of our patient can induce the suspicion of a previous myocarditis evolving towards DCM. Anyway, cardiac MRI proved homogeneous myocardial perfusion and a late contrast enhanced aspect within normal limits.
Another hypothesis in our case was a form of cardiac damage due to an autoimmune disease (16). Arguments were: the feminine gender, the young age and the presence of already two autoimmune disorders: type 1 diabetes mellitus and Graves’ disease. To rule out this possibility we performed dosage of anti-double stranded DNA antibodies (anti dsDNA antibodies), anti Ro antibodies and ANCA (anti-neutrophilic cytoplasmic antibodies) – all of them negative (see Table 1). More than this, there was no clinical evidence for a systemic disorder.
Tachyarrhythmic cardiopathy was not taken into account – because the hyperthyroidism of Graves’disease, that usually induces sustained tachycardia, was solved 6 years ago and the patient’s thyroid status at admission was hypothyroidism. The tachyarrhythmic dilation of LV usually improves several months after controlling the ventricular rate (17).
Neuromuscular symptoms completely remitted after calcemia normalized. Investigation of hypocalcemia in our patient revealed the diagnosis of primary hypoparathyroidism, confirmed both by serum values of total and ionic calcium, serum parathormone and by basal ganglia calcification in CT.
The acquired hypoparathyroidism was due to a lesion of the parathyroid glands’ vasculature during thyroidectomy and not to accidental parathyroidectomy, according to the histopathological examination.
Hypoparathyroidism is an uncommon condition due to a large spectrum of etiologies: congenital or acquired. Congenital causes include Di George syndrome (parathyroid hypoplasia, cardiac, renal and skeletal disorders associated with neurocognitive impairment and thymus hypoplasia), autoimmune polyendocrine syndrome type 1 (adrenal insufficiency, candidiasis and hypoparathyroidism), inherited genetic mutations of the pre-proPTH gene. Acquired causes include surgery (during thyroidectomy, neck dissection, parathyroidectomy), autoimmune hypoparathyroidism alone or part of an autoimmune polyglandular syndrome or isolated non-genetic hypoparathyroidism (very rare) (18).
PTH plays an important role in cardiac physiology not only due to calcium homeostasis but also directly, as recent molecular studies revealed (19). In adult cardiomyocytes, PTH directly induces a calcium influx, by activating G-protein signaling. Calcium influx will activate protein kinase C which, interfering with adrenoreceptor B, will attenuate contractility, and not induce a direct contractile effect, as presumed. PTH exerts changes in cardiac myocyte proliferation and hypertrophy too (5). In the meantime, PTH decreases the calcium influx in vascular smooth muscle cells, with subsequent vasodilation (20).
Although the exact mechanisms are not known, hypocalcemia seems to reduce cardiac contractility, while PTH itself seems to have a positive chronotropic effect on cardiomyocytes (21).
A peculiarity of our case was the possible long evolution of subclinical hypoparathyroidism as the patient suffered thyroidectomy 6 years ago and she was never suspected with hypoparathyroidism. We found only one similar case, with such a late revealed hypoparathyroidism after thyroid surgery, in medical literature (10).
In conclusion, especially because of the rarity of hypocalcemia induced dilated cardiomyopathy in adults, this etiology must be suspected in a cardiac patient with neuro-muscular signs as parestesia, neuromuscular hyperexcitability or convulsions.
Early calcium supplementation, usually along with vitamin D, reduces and even reverses ventricular hypertrophy and dilatation as well as conduction abnormalities (5), as it was our case.
Conflict of interest
All authors equally contributed to this paper and they declare no conflict of interest.
References
- 1.Luo M, Anderson ME. Ca2+ Cycling in Heart Failure. Circ. Res. 2013;113(6):690–708. doi: 10.1161/CIRCRESAHA.113.301651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262(5134):740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- 3.Sauter TC, Lindner G, Ahmad SS, Leichtle AB, Fiedler GM, Exadaktylos AK, Haider DG. Calcium Disorders in the Emergency Department: Independent Risk Factors for Mortality. PLoS ONE. 2015;10(7) doi: 10.1371/journal.pone.0132788. e0132788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Priya S, Siddiqi Z, Karoli R, Fatima J, Gupta S, Mishra R. Study of Vitamin D Status in Patients with Dilated Cardiomyopathy at a Teaching Hospital in North India. JCEcho. 2016;26(3):89–93. doi: 10.4103/2211-4122.187959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown SJ, Ruppe MD, Tabatabai LS. The Parathyroid Gland and Heart Disease. Methodist Debakey Cardiovasc J. 2017;13(2):49–54. doi: 10.14797/mdcj-13-2-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moges T, Shiferaw Y, Heye T. Maternal vitamin D deficiency: A Culprit for Hypocalcaemia Induced Myocardial Failure in a Four-Month Old Infant: A Case Report From Tikur Anbessa Specialized Hospital, Ethiopia. Ethiop J Health Sci. 2017;27(3):299–304. doi: 10.4314/ejhs.v27i3.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar M, Saikia D, Kumar V, Tomar R. Vitamin D deficiency presenting with cardiogenic shock in an infant. Ann Pediatr Cardiol. 2011;4:207–209. doi: 10.4103/0974-2069.84668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yilmaz O, Olgun H, Ciftel M, Kilic O, Kartal I, Iskenderoglu NY, Laloglu F, Ceviz N. Dilated cardiomyopathy secondary to rickets-related hypocalcaemia: eight case reports and a review of the literature. Cardiol Young. 2015;25(2):261–266. doi: 10.1017/S1047951113002023. [DOI] [PubMed] [Google Scholar]
- 9.Darrat YH, Gress TW, Thompson EA, Wehner PS, Charles M. Cardiomyopathy Secondary to Thyroid and Parathyroid Dysfunction: Case Report and Literature Review. MJM. 2016;2(1):3. [Google Scholar]
- 10.Elikowski W, Małek-Elikowska M, Lachowska-Kotowska P. Severe reversible hypocalcemic cardiomyopathy diagnosed 36 years after subtotal thyroidectomy - a case report. Pol Merkur Lekarski. 2017;43(253):26–31. [PubMed] [Google Scholar]
- 11.Azzoug S, Diab N, Chentli F. Reversible Cardiomyopathy Related to Hypoparathyroidism in a Subject with Fahr’s Syndrome. Acta Endo. 2011;7(1):101–110. [Google Scholar]
- 12.Batra CM, Agarwal R. Hypocalcemic Cardiomyopathy and Pseudohypoparathyroidism Due to Severe Vitamin D Deficiency. J Assoc Physicians India. 2016;64:74–76. [PubMed] [Google Scholar]
- 13.Kibel A, Selthofer-Relatic K, Drenjancevic I, Bacun T, Bosnjak I, Kibel D, Gros M. Coronary microvascular dysfunction in diabetes mellitus. J Int Med Res. 2017;45(6):1901–1929. doi: 10.1177/0300060516675504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jia G, Hill MA, Sowers JR. Diabetic Cardiomyopathy. An Update of Mechanisms Contributing to This Clinical Entity. Circ. Res. 2018;122:624–638. doi: 10.1161/CIRCRESAHA.117.311586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Capaldo B, Di Bonito P, Iaccarino M, Roman MJ, Lee ET, Devereux RB, de Simone G. Cardiovascular Characteristics in Subjects With Increasing Levels of Abnormal Glucose Regulation. The Strong Heart Study. Diabetes Care. 2013;36(4):992–997. doi: 10.2337/dc12-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fairweather DL, Petri MA, Coronado MJ, Cooper LT., Jr Autoimmune heart disease: role of sex hormones and autoantibodies in disease pathogenesis. Expert Rev Clin Immunol. 2012;8(3):269–284. doi: 10.1586/eci.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gopinathannair R, Etheridge SP, Marchlinski FE, Spinale FG, Lakkireddy D, Olshansky B. Arrhythmia-Induced Cardiomyopathies: Mechanisms, Recognition, and Management. J. Am. Coll. Cardiol. 2015;66(15):1714–1728. doi: 10.1016/j.jacc.2015.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bilezikian JP, Khan A, Potts JT, Jr, Brandi ML, Clarke BL, Shoback D, Jüppner H, D’Amour P, Fox J, Rejnmark L, Mosekilde L, Rubin MR, Dempster D, Gafni R, Collins MT, Sliney J, Sanders J. Hypoparathyroidism in the adult: epidemiology, diagnosis, pathophysiology, target-organ involvement, treatment, and challenges for future research. J Bone Miner Res. 2011;26(10):2317–2337. doi: 10.1002/jbmr.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalinowski L, Dobrucki LW, Malinsk T. Nitric oxide as a second messenger in parathyroid hormone-related protein signaling. J. Endocrinol. 2001;170:433–440. doi: 10.1677/joe.0.1700433. [DOI] [PubMed] [Google Scholar]
- 20.Greenberg HZ, Shi J, Jahan KS, Martinucci MC, Gilbert SJ, Vanessa Ho WS, Albert AP. Stimulation of calcium-sensing receptors induces endothelium-dependent vasorelaxations via nitric oxide production and activation of IKCa channels. Vasc. Pharmacol. 2016;80:75–84. doi: 10.1016/j.vph.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bansal B, Bansal M, Bajpai P, Garewal HK. Hypocalcemic cardiomyopathy-different mechanisms in adult and pediatric cases. J Clin Endocrinol Metab. 2014;99(8):2627–2632. doi: 10.1210/jc.2013-3352. [DOI] [PubMed] [Google Scholar]