Abstract
Context
Cardiomyopathy is the most frequent cardiovascular complication in acromegaly.
Objective
We aimed to compare some echocardiographic markers in acromegaly patients with controls and find a correlation with disease duration, disease activity, levels of growth hormone (GH) and insulin-like growth factor 1 (IGF-1).
Design
We conducted a cross-sectional case-control study for the period of 2008-2012.
Subjects and methods
Acromegaly patients altogether 146 (56 men and 90 women), were divided into four groups according to disease activity and the presence of arterial hypertension (AH). The control group included 83 subjects, matching the patient groups by age, gender and presence of AH. GH was measured by an immunofluorometric method, while IGF-1 by IRMA method. All patients and controls were subjected to one- and two-dimensional transthoracic echocardiography, color and pulse Doppler.
Results
We found a thickening of the left ventricular walls and an increase in the left ventricular mass. However, these changes were not statistically significant in all groups and no correlation with disease duration could be demonstrated. As markers of diastolic dysfunction, increased deceleration time and isovolumetric relaxation were registered, which were dependent mainly on age in a binary logistic regression analysis, but not GH or IGF-1. Using absolute values, ejection and shortening fractions were increased in some groups. Using cut-off values, a higher percentage of systolic dysfunction was demonstrated in patients compared to their corresponding controls. Engagement of the right heart ventricle was also found – increased deceleration time and decreased e/a tric ratio.
Conclusions
In conclusion, functional impairments of both ventricles were present, with a predominance of left ventricular diastolic dysfunction.
Keywords: acromegaly, acromegaly cardiomyopathy, echocardiography, GH, IGF-1
INTRODUCTION
Acromegaly is characterized by a high frequency of cardiovascular complications, playing a significant role in increased mortality in these patients (1-4). Approximately 50% of patients with active acromegaly die from cardiovascular disease (5, 6), and their mortality is 1.79-1.9 times higher compared to biochemically controlled patients (7). In contrast to the past decades, currently, neoplasms stand out as another major cause of death (8, 9). The main cardiovascular complications in acromegaly are acromegalic cardiomyopathy, particularly concentric biventricular hypertrophy, and arterial hypertension (AH) (3-5, 10-13). Left ventricular hypertrophy is found in up to 90% of autoptic cases (14). Arterial hypertension, found in more than one-third of acromegaly patients, is an additional factor predisposing to left ventricular hypertrophy and could further aggravate it (4, 5, 10, 15, 16). In rare cases, ventricular dilation could be observed (5). Amongst functional impairments, the most frequent one is diastolic dysfunction, while systolic dysfunction is a rare event (5, 11, 12, 17, 18). Right ventricular functional impairments are present as well. Apart from disease activity, disease duration is another factor related to the higher frequency of complications (19-21).
The aim of the present study was to compare some echocardiographic indicators between patients with acromegaly and control groups and find a correlation between these indicators and disease activity, disease duration and levels of GH and IGF-1.
MATERIALS AND METHODS
We conducted a cross-sectional case-control study for the period of 2008-2012 year.
The study included 229 subjects, 83 controls and 146 (56 men and 90 women) acromegaly patients with a mean age of 50.59 ± 12.48 years. Patients were divided into groups, according to gender, disease activity and presence of arterial hypertension, presented in Table 1. The controls matched the patient groups by age, height, weight, gender and the presence of hypertension. We have included only patients without concomitant pathology and if it was present (hypopituitarism, primary hypothyroidism or hyperthyroidism), it was properly medically compensated. We have excluded patients with decompensated concomitant pathology. The study protocol was approved by the local ethics committee. Each subject (patient or control) signed a written informed consent. The study was done in agreement with Helsinki declaration for studies in human subjects and was approved by the institutional ethics committee.
Table 1.
General, hormonal and treatment characteristics in patients with acromegaly
| Males | Females | |||||||
| G1 n=6 |
G2 n=16 |
G3 n=18 |
G4 n=16 |
G1 n=15 |
G2 n=26 |
G3 n=18 |
G4 n=31 |
|
| GH (mean±SD) | 1.92±1.57 | 2.61±1.26 | 28.46±25.49 | 31.58±31.26 | 3.39±2.3 | 2.59±1.66 | 27.38±32.3 | 18.24±16.14 |
| IGF1 (mean±SD) | 24.85±14.5 | 24.21±10.8 | 79.02±33.3 | 86.46±46.4 | 26.6±14.09 | 22.64±11 | 75.5±31.8 | 64.5±36.6 |
| Age yrs (mean±SD) | 48.17±14.8 | 53.2±14 | 46.6±13 | 49.3±14.4 | 42.3±13.5 | 57.5±6.3 | 37.4±12 | 55.4±7 |
| Weight kgs (mean±SD) | 87.8±12.1 | 93.1±18.8 | 97.6±24.4 | 96.1±17.8 | 79.1±17.7 | 80.1±13.8 | 71.9±10.4 | 82.2±16.7 |
| Height cm (mean±SD) | 175.2±5.4 | 175.6±8.7 | 180.3±12.3 | 179.1±10.6 | 168.0±6.8 | 161.7±6.7 | 166.8±5.8 | 163.4±6 |
| Treatment | ||||||||
| TSA (n) | 4 | 11 | 2 | 4 | 6 | 12 | 3 | 6 |
| TSA+Ro (n) | 2 | 2 | 0 | 0 | 3 | 6 | 1 | 3 |
| Primary DA (n) | 0 | 1 | 1 | 2 | 0 | 0 | 1 | 1 |
| TSA+DA (n) | 0 | 2 | 2 | 3 | 3 | 2 | 4 | 10 |
| TSA+SSA (n) | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| TSA+DA+SSA (n) | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| TSA+Ro+ DA (n) | 0 | 0 | 0 | 1 | 2 | 6 | 2 | 2 |
| TSA+Ro+SSA (n) | 0 | 0 | 2 | 1 | 1 | 0 | 1 | 0 |
| TSA+Ro+DA+SSA (n) | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| AH controlled under therapy (n) | 11 | 11 | 20 | 24 | ||||
G1 - normotensive patients with controlled disease; G2 - hypertensive patients with controlled disease; G3 - normotensive patients with active disease; G4 - hypertensive patients with active disease; n – number of patients; GH – growth hormone, measured in mIU/L, conversion factor to ng/ml is 3; IGF-1 – insulin-like growth factor 1, measured in nmol/L; yrs- years; kgs – kilograms; cm – centimeters; TSA – transsphenoidal adenomectomy; Ro – radiotherapy; DA – Dopamine agonist; SSA – somatostatin analog; AH – arterial hypertension.
We used the patients’ history, as well as old photos to determine disease onset. The mean duration from the start of the disease to diagnosis was approximately 8 years.
Disease control and activity were determined according to the criteria from the 2000-year guideline (22). GH and IGF-1 values, as well as the treatment approach (Table 1).
Disease duration was determined by the time between acromegaly onset and the last follow-up for active patients, and the time of achievement of remission in biochemically controlled patients.
The arterial pressure was measured by a mercury manometer in a sitting position, three times over 5-minute intervals (mean value), and it was followed-up at each medical visit. Hypertensive patients were defined by the presence of arterial pressure above 140/90 mmHg or use of antihypertensive drugs. All patients with arterial hypertension were under antihypertensive treatment by the last follow-up and the control rate is presented in Table 1. The following classes of drugs were used: calcium channel blockers, angiotensin convertase enzyme inhibitors, angiotensin-II receptor antagonists, diuretics, beta-blockers and combinations between them.
All hypertensive control patients were well controlled under medical treatment, the same classes of drugs being used as in the study group patients.
Laboratory analysis
Hormonal analysis
The hormonal analysis was performed in a certified centralized laboratory (clinical, steroid and radioimmunological laboratory of the Clinical Center of Endocrinology).
Serum GH concentration was determined by a solid-phase two-site immunofluorometric assay based on a direct sandwich technique with two monoclonal antibodies directed against two different epitopes of the human GH molecule (DELFIA; Perkin Elmer Life and Analytical Sciences, Wallac Oy, Finland). The sensitivity of this assay was < 0.03 mIU/L. The intra- and inter-assay coefficients of variation were 3.9% and 5.0%, respectively.
Serum IGF-I was measured by highly sensitive and specific immunoradiometric assay after acid–alcohol extraction (Immunotech; Beckman Coulter Co., France). Analytical sensitivity was < 0.26 nmol/L. The intra- and inter-assay coefficients of variation were 6.3% and 6.8%, respectively.
Echocardiography
All participants in the study were subjected to one- (1P-TTE) and two-dimensional (2P-TTE) transthoracic echocardiography, color and pulse Doppler, performed by two highly-qualified cardiologists-echocardiographists that were “blind” to the protocol of the study and subject’s data.
The echocardiographic examination was performed according to the requirements of the American Society of Echocardiography (ASE), in left-sided, parasternal and apical position with 2.5 MHz Phased Array transducer, pulse Doppler frequency of 2.5 MHz, device Aloka SSD-4000.
The following parameters were measured on M-mode tracing: left ventricular internal end-systolic and end-diastolic diameters (LVIDS and LVIDD), end-systolic and end-diastolic interventricular septum thickness (IVSTS and IVSTD) and posterior wall thickness in systole and diastole (PWTS and PWTD). Left atrium (LA) diameter, maximum aortic diameter (AO), telediastolic volume (TDV), telesystolic volume (TSV), and stroke volume (SV) were presented as indices (Xi=X/BSA).
Left ventricular mass (LVM), presented as index (LVMi) was calculated by Penn cube method according to Devereux’s formula (1986) (23):
LVMi (g/m2)=(1.04x(IVST+LVID+PWT)3 - (LVID)3 - 13.6)/BSA.
LVMi>110 g/m2 in women and >134 g/m2 in men were considered as cut-off points for left ventricular hypertrophy (24).
Assessment of the left ventricular systolic function was mainly based on the ejection fraction (EF) and shortening fraction (EFS) with normal values respectively: EF>50% and EFS from 30 to 45%.
Diastolic function of the left ventricle was assessed by the isovolumetric relaxation time (IVRT) and deceleration time (DTE). E-wave deceleration time reflects how rapidly the flow velocity declines in early diastole and normal values were defined by 199±32 msec. IVRT reflects the time between the left ventricular inflow and the left ventricular outflow profiles and normal values were defined by 69±12 msec. Impaired left ventricular relaxation was defined by DTE>240 msec and IVRT >100 msec.
Diastolic dysfunction of the right ventricle was assessed pulsed Doppler tricuspid inflow as early (e) and atrial (a) peak velocity ratio (e/a) (tric e/a) and deceleration time of the right ventricle (tricDT) (25).
Statistical analysis
We used descriptive methods and methods of statistical inference. A P value <0.05 was considered statistically significant. Normality of data distribution was tested with the Kolmogorov-Smirnov test and Shapiro-Wilk test. In order to compare the means of two independent groups, we used parametric t-test in normally distributed data and non-parametric Mann-Whitney test in non-normally distributed data. Correlation between variables was evaluated by Spearman’s correlation coefficient. We used multivariate binary logistic regression analysis in order to find variables predicting systolic and diastolic dysfunction, and left ventricular hypertrophy.
RESULTS
The mean values of the echocardiographic variables and comparison with the corresponding control groups are presented at Tables 2 and 3 for the males and females respectively.
Table 2.
Comparison of some echocardiographic markers between the male patient groups and their corresponding control groups
| Males |
Group1 (N=6) |
Control1 (N=6) |
p |
Group2 (N=16) |
Control2 (N=16) |
p |
Group3 (N=18) |
Control3 (N=18) |
p |
Group4 (N=16) |
Control4 (N=16) |
p |
| ivstd | 13±2.5 | 12±0.9 | 0.105 | 13.7±1.8 | 13.6±0.8 | 0.439 | 13±3.4 | 12±1.3 | 0.251 | 13.7±2.5 | 13.9±0.9 | 0.543 |
| lvidd | 46.7±10.5 | 50.2±3.9 | 0.777 | 45.8±8.7 | 48.8±3.7 | 0.218 | 51.5±5.6 | 49.6±2.7 | 0.524 | 49.7±5 | 51±3.7 | 0.284 |
| pwtd | 12.2±2.3 | 11.3±1.4 | 0.425 | 12.8±1.2 | 13.1±0.7 | 0.359 | 12.7±2.5 | 11.8±1.3 | 0.292 | 13.2±1.5 | 12.9±0.9 | 0.435 |
| ivsts | 17.4±3.3 | 17.3±1.2 | 0.810 | 18.9±2.4 | 17.6±0.9 | 0.005 | 17.1±4.2 | 16.4±3.4 | 0.774 | 18.5±2.1 | 16.9±1.1 | 0.026 |
| lvids | 32±7.6 | 33.7±3.4 | 0.608 | 29.2±7 | 30.1±3.9 | 0.513 | 33.7±6.4 | 31.7±3.6 | 0.276 | 31.4±4.8 | 31.8±4.1 | 0.619 |
| pwts | 19.6±3.6 | 18.5±1 | 0.003 | 20.3±3 | 17.8±1.2 | 0.006 | 18.6±5 | 18.6±5 | 1.000 | 20.7±3.3 | 18.8±2.6 | 0.079 |
| la | 38.6±4.3 | 37.2±1.5 | 0.351 | 40.3±5.9 | 38.3±2.3 | 0.300 | 42.8±6.5 | 36±2.5 | 0.000 | 40.7±5.7 | 37.8±3.7 | 0.062 |
| ao | 37.5±4.5 | 37.2±1.7 | 0.016 | 39.1±3.7 | 37.9±1.1 | 0.337 | 39.4±4.9 | 36.4±2.5 | 0.082 | 40.4±5.9 | 36.9±2.9 | 0.048 |
| ef | 66.1±5 | 62.5±5.7 | 0.759 | 68.8±9.6 | 72.7±7.3 | 0.117 | 63.4±9.2 | 69.2±7.4 | 0.069 | 64.4±10.2 | 66.1±4.3 | 0.843 |
| efs | 36.4±3.3 | 38.8±3.4 | 0.150 | 42.2±7.7 | 39.8±3.2 | 0.473 | 35.9±6.5 | 36.3±3.4 | 0.972 | 38.5±3.6 | 37.9±2.1 | 0.553 |
| lvm | 272.1±94.8 | 224±32 | 0.083 | 301.7±81.9 | 264.9±50.2 | 0.170 | 310.2±143.7 | 230.8±43.1 | 0.060 | 296.4±120.2 | 283.8±39.4 | 0.664 |
| lvm index | 150.8±32.7 | 110±17 | 0.04 | 142.2±38.9 | 123.8±19.5 | 0.135 | 138.9±54.4 | 105±18 | 0.037 | 132.4±42.5 | 127.6±23.1 | 0.477 |
| dte | 251.5±48 | 188.7±12.9 | 0.006 | 275±48.5 | 223.8±34.6 | 0.003 | 226.1±80.9 | 193.2±15.4 | 0.023 | 234.4±61.5 | 201±19 | 0.002 |
| ivrt | 126.9±27.1 | 105.2±9.3 | 0.136 | 118.1±24.5 | 116±18.1 | 0.719 | 102.5±24.6 | 118.7±22.9 | 0.056 | 118.2±29.4 | 120.8±20.8 | 0.921 |
| tric e | 0.4±0.1 | 0.6±0.1 | 0.004 | 0.5±0.1 | 0.6±0.1 | 0.041 | 0.5±0.1 | 0.5±0.1 | 0.201 | 0.5±0.1 | 0.6±0.1 | 0.109 |
| tric a | 0.3±0.1 | 0.6±0.1 | 0.001 | 0.3±0.1 | 0.6±0.1 | 0.000 | 0.4±0.2 | 0.5±0.1 | 0.106 | 0.4±0.1 | 0.5±0.1 | 0.005 |
| tric dt | 227.2±36.6 | 190.3±10.8 | <0.001 | 269.1±39.9 | 253.8±42.4 | 0.336 | 210.7±69.7 | 223.7±47.4 | 0.826 | 221.4±54 | 192.9±52.6 | 0.062 |
| TDV | 108.4±41.3 | 127.8±29.3 | 0.824 | 106±37.4 | 116.8±26 | 0.152 | 124.7±36.2 | 128.7±26 | 0.303 | 112.1±26.9 | 128.1±25.9 | 0.043 |
| TSV | 37.9±20.1 | 54.2±12.6 | 0.481 | 31.9±12.8 | 28±7.2 | 0.428 | 45.9±17.6 | 34±11.4 | 0.032 | 38.5±13.1 | 34.4±13.8 | 0.196 |
| SV | 70.6±22.1 | 75.2±29.3 | 0.887 | 74.1±25.2 | 88.8±23.7 | 0.050 | 78.8±25 | 95.2±24.8 | 0.029 | 64.4±30.6 | 93.3±23.5 | 0.006 |
| la_index | 18.9±2.3 | 17.9±1.7 | 0.558 | 19.3±4.1 | 18.1±2.1 | 0.353 | 19.6±2.4 | 16.5±1.6 | 0.000 | 18.6±2.4 | 16.9±2.1 | 0.048 |
| ao_index | 19.6±1.6 | 17.9±1.4 | 0.134 | 18.7±2.6 | 17.8±1.3 | 0.678 | 18.1±2.5 | 16.6±1.1 | 0.011 | 18.4±1.9 | 16.6±2.1 | 0.026 |
| TDV_index | 63.9±21.2 | 61.9±16.6 | 0.663 | 50±16.5 | 54.8±11.7 | 0.206 | 56.9±15.1 | 58.9±13 | 0.527 | 50.6±8 | 57.8±15.1 | 0.262 |
| TSV_index | 25.3±11.5 | 25.9±5.7 | 0.919 | 15±5.7 | 13.1±3.3 | 0.553 | 21.1±8 | 15.4±4.7 | 0.029 | 17.4±4.7 | 15.5±6.4 | 0.114 |
| SV_index | 40.6±10.2 | 36.7±15.5 | 0.500 | 35±11.1 | 41.6±10.5 | 0.030 | 35.8±10.3 | 43.7±12.6 | 0.034 | 33.3±6.3 | 42.1±12.8 | 0.067 |
| e/a | 1.6±0.3 | 1.2±0.1 | 0.009 | 1.5±0.3 | 1±0.3 | 0.001 | 1.3±0.3 | 1.2±0.3 | 0.246 | 1.3±0.3 | 1.1±0.3 | 0.255 |
Group 1 – normotensive patients with controlled acromegaly; group 2 –hypertensive patients with controlled acromegaly; group 3- normotensive patients with active acromegaly; group 4 – hypertensive patients with active acromegaly p- value of significance; significant results are bolded; ivstd - end-diastolic interventricular septum thickness; lvidd - left ventricular internal end-diastolic diameter; pwtd – posterior wall thickness in diastole; ivsts - end-systolic interventricular septum thickness; lvids - left ventricular internal end-systolic diameter; pwts – posterior wall thickness in systole; la – left atrium diameter; ao – maximum aortic diameter; ef – ejection fraction; efs – shortening fraction; lvm – left ventricular mass; lvm_index – left ventricular mass index; dte – deceleration time; ivrt - isovolumetric relaxation time; tric e – tricuspidal early filling; tric a – tricuspidal atrial contraction; tric dt – deceleration time of the right ventricle; TDV – telediastolic volume; TSV – telesystolic volume; SV – stroke volume; la_index - left atrium diameter index; ao _index - maximum aortic diameter index; TDV_index - telediastolic volume index; TSV_index - telesystolic volume index; SV_index – stroke volume index; e/a – early filling atrial contraction ratio
Table 3.
Comparison of some echocardiographic markers between the female patient groups and their corresponding control groups
| Females |
Group1 (N=15) |
Control1 (N=15) |
p |
Group2 (N=26) |
Control2 (N=26) |
p |
Group3 (N=18) |
Control3 (N=18) |
p |
Group4 (N=31) |
Control4 (N=31) |
p |
| ivstd | 11.1±2.6 | 11.1±1.3 | 0.816 | 13.1±2.1 | 13.3±0.8 | 0.669 | 10.2±3.4 | 9.6±1.9 | 0.987 | 13.1±2.3 | 13.8±1 | 0.070 |
| lvidd | 44.4±6.5 | 47.2±4.4 | 0.243 | 42.7±8.3 | 45.4±3.5 | 0.175 | 44.7±3.4 | 26.7±3.4 | <0.001 | 45.6±5.7 | 35.3±5.4 | <0.001 |
| pwtd | 10.2±2.2 | 10.9±1.5 | 0.198 | 13.5±6.2 | 12.8±1 | 0.418 | 10.1±2.2 | 9.2±0.9 | 0.095 | 12.4±2.1 | 14.1±0.8 | <0.001 |
| ivsts | 15.6±2.6 | 15.7±2.4 | 0.807 | 17.1±3 | 15.6±1.5 | 0.092 | 15.3±2.7 | 10.6±1.3 | <0.001 | 17.1±2.6 | 19.3±1.9 | 0.001 |
| lvids | 31.7±9.6 | 29.7±2.4 | 0.553 | 26.9±5.7 | 26.9±5.7 | 1.000 | 27.1±3.9 | 38.6±4.9 | <0.001 | 28.2±4.3 | 47.8±4 | <0.001 |
| pwts | 19±3 | 18.8±2.2 | 0.894 | 19.3±3 | 16.5±1.7 | <0.001 | 18.7±4.6 | 11.2±0.8 | <0.001 | 19±2.9 | 18.3±1.5 | 0.220 |
| la | 34.9±6.1 | 34.5±3.1 | 0.597 | 38.8±5.3 | 36.8±3.8 | 0.070 | 35.4±6 | 33.5±2.7 | 0.589 | 38.2±6.4 | 33.7±2.7 | 0.001 |
| ao | 34.8±6.9 | 34.8±6.9 | 1.000 | 34.2±3.5 | 33.5±2.2 | 0.617 | 31.9±2.4 | 33.3±1.8 | 0.029 | 34.4±4.3 | 33±2 | 0.128 |
| ef | 67±10.9 | 70.5±4.7 | 0.803 | 67.8±7.5 | 66.8±5.4 | 0.238 | 71.1±4.1 | 63.3±6.3 | <0.001 | 68.1±5.9 | 64.6±4.2 | 0.017 |
| efs | 41.1±7.9 | 40.3±6.8 | 0.328 | 37.9±5.3 | 38.3±4.8 | 0.415 | 40.5±3.2 | 36.9±2.5 | 0.001 | 38.6±6.8 | 35.6±2.7 | 0.048 |
| lvm | 204.3±68.1 | 181.7±38.5 | 0.407 | 237±77.6 | 228.8±30.1 | 0.770 | 178.2±78.9 | 111.9±35.3 | 0.006 | 252±107.1 | 272.1±40.6 | 0.025 |
| lvm index | 105.2±25.6 | 100.6±18.5 | 0.570 | 120.7±35.4 | 122.1±16.2 | 0.372 | 96.6±38.7 | 62.8±23.2 | 0.005 | 130±46.9 | 148.6±26.3 | 0.011 |
| dte | 231.3±49.4 | 217.9±39.2 | 0.350 | 255±63.9 | 213.8±40 | 0.005 | 215±54.9 | 182.6±10.9 | 0.003 | 258.4±70.3 | 189.9±14.4 | <0.001 |
| ivrt | 94.8±15.5 | 97.7±12.8 | 0.225 | 119.2±20.1 | 112.7±18.8 | 0.411 | 91.7±17.4 | 75.6±9.7 | 0.010 | 113.9±24.9 | 110±32.7 | 0.344 |
| tric e | 0.5±0.1 | 0.6±0.1 | 0.001 | 0.5±0.1 | 0.5±0.1 | 0.001 | 0.5±0.1 | 0.7±0.1 | <0.001 | 0.4±0.1 | 0.7±0.1 | <0.001 |
| tric a | 0.3±0.1 | 0.4±0.1 | 0.023 | 0.4±0.1 | 0.4±0.1 | 0.101 | 0.4±0.1 | 0.6±0.1 | <0.001 | 0.3±0.1 | 0.6±0.1 | <0.001 |
| tric dt | 237.1±57.3 | 209.2±36.8 | 0.191 | 252.2±61.1 | 182.7±33.4 | <0.001 | 215.1±48.7 | 182.5±17.4 | 0.003 | 238.9±54.7 | 175.4±32.6 | <0.001 |
| TDV | 105.5±24.3 | 107.8±28.1 | 0.819 | 89.6±31.2 | 95.4±22 | 0.317 | 91.8±15.7 | 63.4±24.2 | 0.001 | 98.3±28.3 | 109.9±26.7 | 0.082 |
| TSV | 32.5±9.1 | 28.9±10.8 | 0.092 | 30.5±16.4 | 25.8±15.7 | 0.210 | 26.6±7.4 | 20.5±7.3 | 0.008 | 32.4±11.5 | 37±15.5 | 0.461 |
| SV | 73±17.6 | 78.9±26.2 | 0.406 | 59.2±19 | 68.8±21.9 | 0.055 | 64.2±9.7 | 41.6±19.1 | 0.001 | 65.9±19.3 | 72.9±20.2 | 0.173 |
| la_index | 18.2±1.7 | 18.5±1.3 | 0.678 | 20.5±2.7 | 19.6±2.2 | 0.244 | 19.5±3 | 18.7±2.4 | 0.411 | 20±3.6 | 18.4±1.9 | 0.038 |
| ao_index | 18.3±3.5 | 18.6±3.8 | 0.709 | 18.3±2.3 | 17.9±1.9 | 0.522 | 17.7±1.9 | 18.6±1.8 | 0.235 | 18±2.6 | 18.1±1.8 | 0.838 |
| TDV_index | 55.6±14.2 | 57.7±14.9 | 0.694 | 45.5±14.3 | 50.8±11 | 0.100 | 50.5±8.4 | 35.8±15.1 | 0.006 | 51.4±14.4 | 60.3±15.4 | 0.037 |
| TSV_index | 17.1±4.7 | 15.3±5 | 0.165 | 15.6±8 | 13.7±8.3 | 0.253 | 14.6±3.6 | 11.7±5 | 0.017 | 16.9±5.7 | 20.2±8.2 | 0.273 |
| SV_index | 38.6±10.5 | 42.4±14.3 | 0.372 | 29.9±8.6 | 36.7±11.6 | 0.024 | 35.6±6 | 23.6±11.6 | 0.004 | 34.5±10 | 40.1±12.3 | 0.059 |
| e/a | 1.5±0.4 | 1.7±0.4 | 0.290 | 1.3±0.3 | 1.4±0.4 | 0.516 | 1.4±0.4 | 1.3±0.2 | 0.086 | 1.3±0.3 | 1.2±0.4 | 0.012 |
Group 1 – normotensive patients with controlled acromegaly; group 2 – hypertensive patients with controlled acromegaly; group 3- normotensive patients with active acromegaly; group 4 – hypertensive patients with active acromegaly; p – value of significance; significant results are bolded; ivstd - end-diastolic interventricular septum thickness; lvidd - left ventricular internal end-diastolic diameter; pwtd – posterior wall thickness in diastole; ivsts - end-systolic interventricular septum thickness; lvids - left ventricular internal end-systolic diameter; pwts – posterior wall thickness in systole; la – left atrium diameter; ao – maximum aortic diameter; ef – ejection fraction; efs – shortening fraction; lvm – left ventricular mass; lvm_index – left ventricular mass index; dte – deceleration time; ivrt - isovolumetric relaxation time; tric e – tricuspidal early filling; tric a – tricuspidal atrial contraction; tric dt – deceleration time of the right ventricle; TDV – telediastolic volume; TSV – telesystolic volume; SV – stroke volume; la_index - left atrium diameter index; ao _index - maximum aortic diameter index; TDV_index - telediastolic volume index; TSV_index - telesystolic volume index; SV_index – stroke volume index; e/a – early filling atrial contraction ratio.
The hypertensive male patients with active and controlled acromegaly had statistically significant thickening of the end-systolic size of the interventricular septum (IVSTS) compared to the corresponding control groups (Table 2). In the females IVSTS was significantly increased in normo- and hypertensive patients with active acromegaly in comparison to the corresponding controls (Table 3). Due to the low reliability of the statistical analysis because of the low number of male patients with controlled acromegaly without hypertension, we have not discussed the results of this group.
PWTS was significantly increased in the hypertensive male and female patients with controlled acromegaly vs. the corresponding controls (Tables 2, 3). In addition, PWTS was significantly increased in the normotensive female patients with active disease (Table 3).
LVMi was increased, reaching statistical significance in the groups of normotensive male and female patients with active acromegaly, and in the group of hypertensive female patients with active acromegaly (Tables 2, 3; Fig. 1) The frequency of hypertrophy was significantly higher in the normotensive patients with active disease compared to the corresponding controls (Fig. 2). Using cut-offs for LVM_index, patients with disease duration of more than 10 years did not have statistically significant percent distribution of high LVM_index compared to those with less than 10 years of disease duration – 51.5% (34/66 patients) vs. 48.9% (23/47 patients), p=0.85. We performed a multivariate binary logistic regression analysis using LVMI (>136 or 110; ≤136 or 110) as a dependent variable, and sex, age, AH, disease duration, basal GH and IGF-1 as studied factors. Neither of the factors predicted significant hypertrophy (data not shown).
Figure 1.
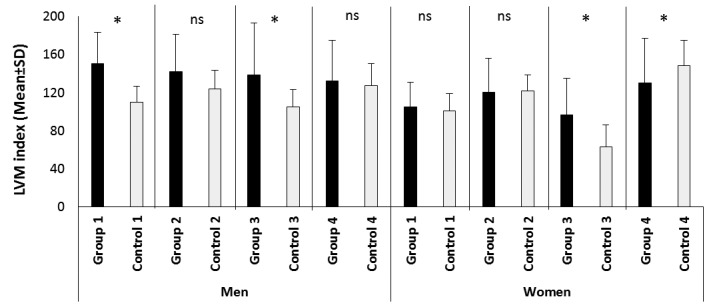
Comparison of LVM index between patients and controls.
LVM index – left ventricular mass index; ns – no significant difference; * - statistically significant difference between patients and their corresponding controls; group 1 – normotensive patients with controlled acromegaly, men n=6, females n=15; group 2 –hypertensive patients with controlled acromegaly, men n=16, females n=26; group 3- normotensive patients with active acromegaly, men n=18, females n=18; group 4 – hypertensive patients with active acromegaly, men n=16, females n=31. All controls match by number, age and presence of arterial hypertension the corresponding patient group.
Figure 2.
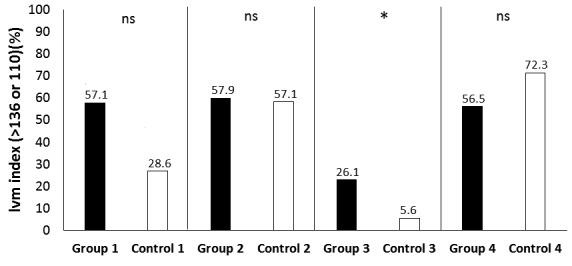
Comparison of LVM index between patients and controls using a cut-off value.
LVM index – left ventricular mass index; ns – no significant difference; * - statistically significant difference between patients and their corresponding controls; group 1 – normotensive patients with controlled acromegaly, n=21; control 1 – n=21; group 2 –hypertensive patients with controlled acromegaly, n=38; control 2 – n=42; group 3- normotensive patients with active acromegaly, n=36; control 3 – n=36; group 4 – hypertensive patients with active acromegaly, n=46; control 4 – n=47. All controls match by age, gender and presence of arterial hypertension the corresponding patient group.
In regard to the size of the left ventricle, LVIDD was statistically increased only in the normo- and hypertensive female patients with active disease, while LVIDS was decreased (Table 3).
Left ventricular volumes presented as indices:
SV_index showed a statistically significant decrease in the groups of hypertensive men and women with controlled disease, and in the group of normotensive men with active acromegaly. However, the group of normotensive females with controlled disease had a statistically significant increase in the latter index (Tables 2, 3).
The results from TDV and TSV were controversial. These indices were significantly increased in normotensive female patients with active acromegaly, with a predominance of TDV, explaining the increased stroke volume in this group. In the group of normotensive men with active disease, however, TSV was decreased, explaining the decreased SV as well. Lower TDV_index was also found in the patient group of normotensive females with active disease in comparison to the corresponding controls (Tables 2, 3).
In regard to the size of the left atrium presented as an index (la_index), it was significantly increased in the groups of normo- and hypertensive men with active disease, and in the group of hypertensive women with active disease (Tables 2, 3).
Ao presented as an index (ao_index) was statistically increased in the groups of normo- and hypertensive males with active disease (Table 2).
Left ventricular function
Parameters for evaluation of diastolic function
We found a statistically significant increase of DTE in all patient groups compared to the corresponding controls, with exception of the group of normotensive females with controlled disease (Tables 2, 3; Fig. 3). IVRT was significantly increased only in the normotensive female patient group with active disease (Tables 2, 3). Using cut-off values, the frequency of prolonged DTE was significantly higher in all patient groups compared to the corresponding controls, except for the normotensive patients with controlled disease (Fig. 4). In regards to IVRT, neither of the groups had higher frequency of increased IVRT in comparison to the corresponding control groups – 47.6% (10/21 patients from group 1) vs. 28.6% (6/21 controls); p=0.204; 82.9% (34/41 patients from group 2) vs. 71.4% (30/42 controls); p=0.297; 39.3% (13/33 patients from group 3) vs. 33.3% (12/36 controls); p=0.625; 73.3% (33/45 patients from group 4) vs. 59.6% (28/47 controls); p=0.190. In a correlation analysis DTE had a significant positive correlation with disease duration in all patients (n=146) (r=0.195; p=0.021), as well as in the patients with active disease (n=83) (r=0.252; p=0.026). Similar finding was demonstrated for IVRT – r=0.175; p=0.039 for all patients, and r=0.262; p=0.021 for patients with active disease. However, in a binary logistic regression analysis disease duration was not an independent predictor of increased DTE and IVRT (Table 4). Both variables were significantly dependent on age, while additional independent predictors of increased IVRT were male gender and the presence of arterial hypertension (Table 4). Levels of GH and IGF-1 significantly correlated with DTE of the left ventricle only in the normotensive group with controlled acromegaly (for GH – r=0.491; p=0.017; for IGF-I – r=0.5; p=0.018). However, no such correlation in the patient groups with active disease could be demonstrated (for GH – r=0.148; p=0.195; for IGF-1 – r=0.065; p=0.569). Similarly, no predictive role of GH and IGF-1 was found in the regression analysis (Table 4).
Figure 3.
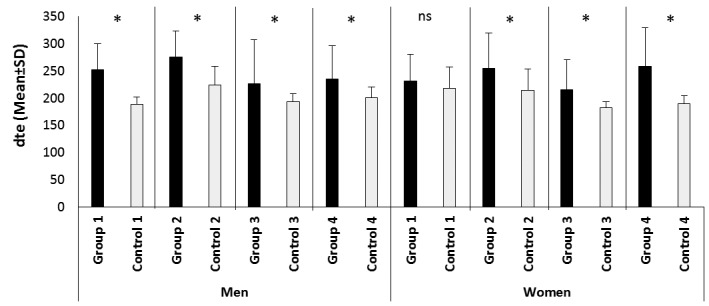
Comparison of dte between patients and controls.
Dte – deceleration time; ns – no significant difference; * - statistically significant difference between patients and their corresponding controls; group 1 – normotensive patients with controlled acromegaly, men n=6, females n=15; group 2 –hypertensive patients with controlled acromegaly, men n=16, females n=26; group 3- normotensive patients with active acromegaly, men n=18, females n=18; group 4 – hypertensive patients with active acromegaly, men n=16, females n=31. All controls match by number, age and presence of arterial hypertension the corresponding patient group.
Figure 4.
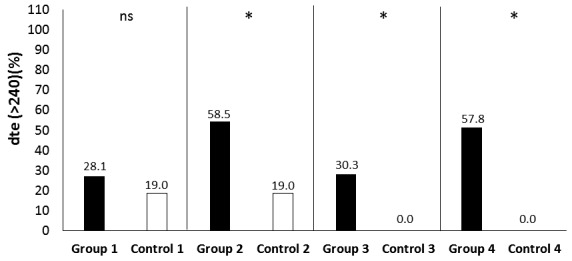
Comparison of dte between patients and controls using a cut-off value.
Dte – deceleration time; ns – no significant difference; * - statistically significant difference between patients and their corresponding controls; group 1 – normotensive patients with controlled acromegaly, n=21; control 1 – n=21; group 2 –hypertensive patients with controlled acromegaly, n=41; control 2 – n=42; group 3- normotensive patients with active acromegaly, n=33; control 3 – n=36; group 4 – hypertensive patients with active acromegaly, n=45; control 4 – n=47. All controls match by age, gender and presence of arterial hypertension the corresponding patient group
Table 4.
Binary logistic regression analysis of factors predicting increased IVRT and DTE
| IVRT(>100; ≤100) | DTE (>240; ≤240) | |||||
| Factor | OR | 95% CI | p | OR | 95% CI | p |
| Sex (Female) | 0.337 | 0.133-0.856 | 0.022 | 0.670 | 0.317-1.434 | 0.306 |
| Age | 1.078 | 1.032-1.125 | 0.001 | 1.037 | 1.002-1.073 | 0.039 |
| AH (yes) | 2.461 | 1.012-5.983 | 0.047 | 0.571 | 0.255-1.279 | 0.173 |
| Disease duration | 1.052 | 0.982-1.127 | 0.151 | 1.055 | 0.997-1.117 | 0.062 |
| Basal GH | 1.002 | 0.981-1.024 | 0.823 | 1.010 | 0.990-1.030 | 0.337 |
| IGF-1 | 0.995 | 0.983-1.008 | 0.434 | 0.998 | 0.986-1.010 | 0.698 |
IVRT - isovolumetric relaxation time; DTE – deceleration time; OR – odds ratio; 95% CI – 95% confidence interval; p – value of significance; AH – arterial hypertension; GH – growth hormone; IGF-1 – insulin-like growth factor 1.
Parameters for evaluation of systolic function
We found a statistically significant increase in the EF and EFS in the normo- and hypertensive female patient groups with active acromegaly in comparison to the corresponding controls (Tables 2, 3). Using cut-off values for normal systolic function, we found significantly higher frequency of patients with decreased EF , in comparison to the corresponding controls – 5.4% (8/146) vs. 0% (0/83); p=0.030 in all patients, and those with active acromegaly – 6.2% (5/83) vs. 0% (0/83); p=0.028. In regard to EFS, neither patients in remission, nor patients with active acromegaly had statistically significant low EFS in comparison to their corresponding control groups. In a binary logistic regression analysis, neither of the factors described above predicted low EF or EFS (data not shown).
Diastolic function of the right heart ventricle
Tric DT was significantly prolonged in the groups of hypertensive women with controlled acromegaly, and normo- and hypertensive females with active disease. Tric e/a ratio was significantly increased in the hypertensive male group of patients with controlled disease and the hypertensive female group of patients with active disease, compared to the corresponding controls (Tables 2, 3).
DISCUSSION
Cardiovascular disease is a major cause of increased mortality in acromegaly (1, 3, 5, 7, 10, 26). Growth hormone, mediated mainly through IGF-1, induces myocyte overgrowth. On the other hand, IGF-1 could contribute directly to the cardiac hypertrophy, independently of GH. A local increase of IGF-1 and subsequent RNA expression in the cardiac muscle has been described in response to increased cardiac pre- and afterload (27). It could be considered as one of the possible mechanisms of compensatory cardiac hypertrophy. In addition to its function as a growth factor, IGF-1 is known to increase myocardial contractility (28).
Arterial hypertension in acromegaly leads to increased cardiac afterload and further aggravation of myocardial hypertrophy, explaining the more pronounced hypertrophy in hypertensive in comparison to normotensive patients with acromegaly (29). In order to eliminate the influence of arterial hypertension, we have divided our studied subjects into normo- and hypertensive groups. Vascular resistance and circular volume are additional factors of myocardial hypertrophy, increasing pre- and afterload. In acromegaly, however, despite the increased circular volume, the end-diastolic pressure (the preload) is normal, excluding cases with heart failure. A possible explanation could be found in the increased vascular compliance. Furthermore, decreased peripheral vascular resistance could be observed (30). The exact mechanism of this vascular effect of GH is unknown.
Growth hormone and/or IGF-1 could possibly influence the cardiovascular system by indirect mechanisms. One of these is insulin resistance, and the majority of patients with active, uncomplicated acromegaly has insulin resistance and compensatory hyperinsulinemia (31-34). Induction of myocardial hypertrophy is probably related to the structural similarity of insulin to IGF-1 and its ability to stimulate the IGF-1 receptors (35).
A number of echocardiographic studies show an increase of the left ventricular mass with a relatively normal size of the heart (11, 12, 29, 36, 37), indicating the presence of concentric left ventricular hypertrophy. Right ventricular hypertrophy has also been described (29, 36, 38, 39). Using the cardiac mass/body surface index as a criterion, true cardiomegaly, disproportionate to the general visceromegaly, is found in most of the patients with uncomplicated acromegaly (29). Similar results are described in autopsy studies. Cardiac weight significantly exceeds the expected one for given body weight and height in 90% of the patients with disease duration of more than 10 years (14). Cardiac dilation is present in the late stages of cardiac disease, usually in cases of apparent heart failure, and is considered a bad prognostic factor.
In our study, we found thickening of the interventricular septum in several patient groups (hypertensive males with active and controlled disease and in normo - and hypertensive women with active acromegaly). Thickening of the posterior left ventricular wall was observed as well in hypertensive patients of both sexes with controlled disease, and in normotensive women with controlled disease (Tables 2, 3). The left ventricular mass was increased in absolute values in several patient groups - normotensive males and females with active disease and hypertensive females with active disease (Tables 2, 3). This observation was confirmed using cut-off values for hypertrophy – the frequency of cardiac hypertrophy was higher in the patients with acromegaly compared to their corresponding controls (Fig. 2). Our conclusions are not definitive as such statistically significant differences could not be demonstrated in all patient groups compared to their corresponding controls. No correlation between hypertrophy and disease duration was found, either as absolute values or using a cut-off of more than 10 years of active acromegaly duration. Furthermore, disease duration, as well as other factors (sex, age, AH, basal GH and IGF-1) did not seem to predict left ventricular hypertrophy in a binary logistic regression analysis. In agreement, other studies do not show a statistically significant correlation between cardiac hypertrophy and levels of GH and IGF-1 (29). In contrast to our study, other groups report a significant correlation between cardiac hypertrophy and disease duration (20, 21, 29).
We found a statistically significant difference in LVMI in normotensive patients with remission of acromegaly vs. the corresponding control group (Fig.1), which suggests that the control of GH hypersecretion does not always lead to a reversal of the structural changes of the heart. Similar observations are reported by other authors (40). Such disadvantageous tendency is observed in 50% of the patients with biochemical disease control and is more prominent in middle-aged subjects with long disease duration (41), as in our cohort.
Our study could not find significant dilation of the ventricles. It is in agreement with the statement that, currently, the prevalence of heart failure and dilative cardiomyopathy in acromegaly patients is lower than in the past, most probably due to the improved treatment outcomes (1, 42).
We found left atrial dilation in several groups of patients, predominantly in those with active disease and presence of AH (Tables 2, 3). This observation could be most probably explained with the left ventricular diastolic dysfunction.
A number of echocardiographic studies show impaired diastolic dysfunction, even at rest (11, 21, 37, 39, 43). According to Doppler echocardiography, the key defect is the impaired diastolic filling of the left chamber (29). We found prolonged deceleration time in almost all patient groups, as well as increased isovolumetric relaxation time in part of the patients (Tables 2, 3). This observation was confirmed by comparing the percent distribution of diastolic dysfunction between patients and their corresponding controls. Such a tendency was stronger in regard to the DTE than to the IVRT. Both markers (DTE and IVRT) correlated with disease duration. However, disease duration did not prove to be an independent predictor of diastolic dysfunction in the regression analysis. (Table 4). Although DTE and IVRT correlated with the levels of GH and IGF-1 in normotensive patients with biochemically controlled acromegaly, regression analysis did not prove a predictive role of these factors (Table 4). Our results display that the changes in the left ventricular diastolic function are the most prevalent pathological finding.
Markers of systolic function at rest are not disrupted in some studies (11, 29, 38), in contrast to others (21). However, impaired left ventricular ejection fraction response to exercise is described - EF increases in healthy controls while in patients with acromegaly it remains unchanged (38). In patients with persistent disease activity (untreated patients or those with suboptimal treatment efficiency), cardiac function progressively declines to congestive heart failure. At the last stage, cardiac output also decreases (5, 44). In the present study we found increased EF and SF (using absolute values) in some of the patient groups - normo- and hypertensive females with controlled disease (Table 3). On the other hand, using cut-off values, the frequency of systolic dysfunction (estimated by EF) was higher in patients with active disease vs. the corresponding controls. These results show that the effect of hypersomatotropism on cardiac contractility is divergent – from increased to decreased. A possible explanation is that contractility increases or decreases in the different phases of acromegaly. However, this hypothesis could not be confirmed as disease duration did not predict a decline in EF and EFS using regression analysis. Obviously, myocardiac contractility is a complex issue dependent on various factors, some of which remain to be elucidated.
The right ventricle could also be engaged in acromegalic cardiomyopathy. The main complication is impaired diastolic ventricular filling (29). In agreement, we found increased deceleration time in the normo- and hypertensive female patient groups with active disease, hypertensive female patients with controlled disease, and decreased e/a tric ratio in the hypertensive male patient group with disease control and hypertensive females with active disease (Tables 2, 3).
The lack of correlation between GH, IGF-1 and the studied echocardiographic markers, with exception of left ventricular diastolic dysfunction in the normotensive patient groups with controlled disease, is confirmed by other studies (29, 42, 45). A possible explanation is that cardiac impairments could persist even after controlling hypersomatotropism, probably due to indirect mechanisms (arterial hypertension, hyperinsulinism, vascular resistance, and others). Another explanation could be the long period between disease manifestation and control of hypersomatotropism (either due to long disease duration before diagnosis, or difficulties in treatment).
We found a significant correlation between disease duration and markers of left ventricular diastolic dysfunction in agreement with other studies (5, 17, 18, 20, 21, 46). However, regression analysis did not confirm the predictive role of disease duration in regards to diastolic dysfunction (Table 4). No such association was demonstrated also in regard to the morphological changes of the heart (hypertrophy), unlike other research groups (20, 21). It could be due to some factors already described above. On the other hand, in cases with longer disease duration, age and arterial hypertension could play an additional significant role. However, after excluding those two factors (by having control groups corresponding to age and arterial hypertension), the tendency of hypertrophy was not distinct.
In conclusion, based on our results, we could conclude that acromegaly is associated with high prevalence of morphological and functional changes of the heart. Cardiomegaly, predominantly of the left ventricle, is the most frequent morphological change. Diastolic dysfunction, which is the main functional impairment, is most probably a direct consequence of concentric left ventricular hypertrophy. Diastolic dysfunction is dependent on age and disease duration. Changes in the systolic function are divergent, covering the two ends of the spectrum. Right ventricular diastolic dysfunction is also present. Prompt diagnosis and treatment of acromegaly and its complications (arterial hypertension, hyperinsulinism) could probably prevent consequent cardiovascular changes and mortality.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Courville C, Mason VR. The heart in acromegaly. Arch Intern Med. 1998:704–713. [Google Scholar]
- 2.Wright AD, Hill DM, Lowy C, Fraser TR. Mortality in acromegaly. Q J Med. 1970;39:1–16. [PubMed] [Google Scholar]
- 3.Melmed S, Colao A, Barkan A, Molitch M, Grossman AB, Kleinberg D, Clemmons D, Chanson P, Laws E, Schlechte J, Vance ML, Ho K, Giustina A. Guidelines for acromegaly management: an update. J Clin Endocrinol Metab. 2009;94:1509–1517. doi: 10.1210/jc.2008-2421. [DOI] [PubMed] [Google Scholar]
- 4.Clayton RN. Cardiovascular function in acromegaly. Endocr Rev. 2003;24:272–277. doi: 10.1210/er.2003-0009. [DOI] [PubMed] [Google Scholar]
- 5.Colao A, Ferone D, Marzullo P, Lombardi G. Systemic complications of acromegaly: epidemiology, pathogenesis, and management. Endocr Rev. 2004;25:102–152. doi: 10.1210/er.2002-0022. [DOI] [PubMed] [Google Scholar]
- 6.Holdaway IM, Rajasoorya RC, Gamble GD. Factors influencing mortality in acromegaly. J Clin Endocrinol Metab. 2004;89:667–674. doi: 10.1210/jc.2003-031199. [DOI] [PubMed] [Google Scholar]
- 7.Holdaway IM, Bolland MJ, Gamble GD. A meta-analysis of the effect of lowering serum levels of GH and IGF-I on mortality in acromegaly. Eur J Endocrinol. 2008;159:89–95. doi: 10.1530/EJE-08-0267. [DOI] [PubMed] [Google Scholar]
- 8.Ritvonen E, Loyttyniemi E, Jaatinen P, Ebeling T, Moilanen L, Nuutila P, Kauppinen-Makelin R, Schalin-Jantti C. Mortality in acromegaly: a 20-year follow-up study. Endocr Relat Cancer. 2016;23:469–480. doi: 10.1530/ERC-16-0106. [DOI] [PubMed] [Google Scholar]
- 9.Mercado M, Gonzalez B, Vargas G, Ramirez C, de los Monteros AL, Sosa E, Jervis P, Roldan P, Mendoza V, Lopez-Felix B, Guinto G. Successful mortality reduction and control of comorbidities in patients with acromegaly followed at a highly specialized multidisciplinary clinic. J Clin Endocrinol Metab. 2014;99:4438–4446. doi: 10.1210/jc.2014-2670. [DOI] [PubMed] [Google Scholar]
- 10.Melmed S. Medical progress: Acromegaly. The New England journal of medicine. 2006;355:2558–2573. doi: 10.1056/NEJMra062453. [DOI] [PubMed] [Google Scholar]
- 11.Popielarz-Grygalewicz A, Gasior JS, Konwicka A, Grygalewicz P, Stelmachowska-Banas M, Zgliczynski W, Dabrowski M. Heart in Acromegaly: The echocardiographic characteristics of patients diagnosed with acromegaly in various stages of the disease. Int J Endocrinol. 2018:6935054. doi: 10.1155/2018/6935054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nascimento GC, de Oliveira MT, Carvalho VC, Lopes MH, Sa AM, Souza MT, Ferreira Ade S, Ferreira PA, Faria Mdos S. Acromegalic cardiomyopathy in an extensively admixed population: is there a role for GH/IGF-I axis? Clin Endocrinol (Oxf) 2013;78:94–101. doi: 10.1111/j.1365-2265.2012.04472.x. [DOI] [PubMed] [Google Scholar]
- 13.Mizera L, Elbaum M, Daroszewski J, Bolanowski M. Cardiovascular complications of acromegaly. Acta Endocrinologica (Buc) 2018;XIV:365–374. doi: 10.4183/aeb.2018.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lie JT, Grossman SJ. Pathology of the heart in acromegaly: anatomic findings in 27 autopsied patients. American Heart Journal. 1980:41–52. doi: 10.1016/0002-8703(80)90277-x. [DOI] [PubMed] [Google Scholar]
- 15.Colao A, Baldelli R, Marzullo P, Ferretti E, Ferone D, Gargiulo P, Petretta M, Tamburrano G, Lombardi G, Liuzzi A. Systemic hypertension and impaired glucose tolerance are independently correlated to the severity of the acromegalic cardiomyopathy. J Clin Endocrinol Metab. 2000;85:193–199. doi: 10.1210/jcem.85.1.6318. [DOI] [PubMed] [Google Scholar]
- 16.Natchev E, Kirilov G, Matrozova J, Andreeva I, Kalinov K, Zacharieva S. Prevalence of hypertension in acromegaly and humoral factors, playing role in its pathogenesis. Endocrinologia. 2009:156–167. [Google Scholar]
- 17.Colao A, Spinelli L, Cuocolo A, Spiezia S, Pivonello R, di Somma C, Bonaduce D, Salvatore M, Lombardi G. Cardiovascular consequences of early-onset growth hormone excess. J Clin Endocrinol Metab. 2002;87:3097–3104. doi: 10.1210/jcem.87.7.8573. [DOI] [PubMed] [Google Scholar]
- 18.Minniti G, Jaffrain-Rea ML, Moroni C, Baldelli R, Ferretti E, Cassone R, Gulino A, Tamburrano G. Echocardiographic evidence for a direct effect of GH/IGF-I hypersecretion on cardiac mass and function in young acromegalics. Clin Endocrinol (Oxf) 1998;49:101–106. doi: 10.1046/j.1365-2265.1998.00493.x. [DOI] [PubMed] [Google Scholar]
- 19.Resmini E, Minuto F, Colao A, Ferone D. Secondary diabetes associated with principal endocrinopathies: the impact of new treatment modalities. Acta Diabetol. 2009;46:85–95. doi: 10.1007/s00592-009-0112-9. [DOI] [PubMed] [Google Scholar]
- 20.Jayasena CN, Comninos AN, Clarke H, Donaldson M, Meeran K, Dhillo WS. The effects of long-term growth hormone and insulin-like growth factor-1 exposure on the development of cardiovascular, cerebrovascular and metabolic co-morbidities in treated patients with acromegaly. Clin Endocrinol (Oxf) 2011;75:220–225. doi: 10.1111/j.1365-2265.2011.04019.x. [DOI] [PubMed] [Google Scholar]
- 21.Colao A, Pivonello R, Grasso LF, Auriemma RS, Galdiero M, Savastano S, Lombardi G. Determinants of cardiac disease in newly diagnosed patients with acromegaly: results of a 10 year survey study. Eur J Endocrinol. 2011;165:713–721. doi: 10.1530/EJE-11-0408. [DOI] [PubMed] [Google Scholar]
- 22.Melmed S, Casanueva FF, Cavagnini F, Chanson P, Frohman L, Grossman A, Ho K, Kleinberg D, Lamberts S, Laws E, Lombardi G, Vance ML, Werder KV, Wass J, Giustina A. Guidelines for acromegaly management. J Clin Endocrinol Metab. 2002;87:4054–4058. doi: 10.1210/jc.2002-011841. [DOI] [PubMed] [Google Scholar]
- 23.Devereux RB. Detection of left ventricular hypertrophy by M-mode echocardiography. Anatomic validation, standardization, and comparison to other methods. Hypertension. 1987;9:II19–26. doi: 10.1161/01.hyp.9.2_pt_2.ii19. [DOI] [PubMed] [Google Scholar]
- 24.Krumholz HM, Larson M, Levy D. Prognosis of left ventricular geometric patterns in the Framingham Heart Study. J Am Coll Cardiol. 1995;25:879–884. doi: 10.1016/0735-1097(94)00473-4. [DOI] [PubMed] [Google Scholar]
- 25.Pye MP, Pringle SD, Cobbe SM. Reference values and reproducibility of Doppler echocardiography in the assessment of the tricuspid valve and right ventricular diastolic function in normal subjects. Am J Cardiol. 1991;67:269–273. doi: 10.1016/0002-9149(91)90558-3. [DOI] [PubMed] [Google Scholar]
- 26.Giustina A, Boni E, Romanelli G, Grassi V, Giustina G. Cardiopulmonary performance during exercise in acromegaly, and the effects of acute suppression of growth hormone hypersecretion with octreotide. Am J Cardiol. 1995;75:1042–1047. doi: 10.1016/s0002-9149(99)80721-8. [DOI] [PubMed] [Google Scholar]
- 27.Ito H, Hiroe M, Hirata Y, Tsujino M, Adachi S, Shichiri M, Koike A, Nogami A, Marumo F. Insulin-like growth factor-I induces hypertrophy with enhanced expression of muscle specific genes in cultured rat cardiomyocytes. Circulation. 1993;87:1715–1721. doi: 10.1161/01.cir.87.5.1715. [DOI] [PubMed] [Google Scholar]
- 28.Mayoux E, Ventura-Clapier R, Timsit J, Behar-Cohen F, Hoffmann C, Mercadier JJ. Mechanical properties of rat cardiac skinned fibers are altered by chronic growth hormone hypersecretion. Circ Res. 1993;72:57–64. doi: 10.1161/01.res.72.1.57. [DOI] [PubMed] [Google Scholar]
- 29.Fazio S, Cittadini A, Sabatini D, Merola B, Colao AM, Biondi B, Lombardi G, Sacca L. Evidence for biventricular involvement in acromegaly: a Doppler echocardiographic study. Eur Heart J. 1993;14:26–33. doi: 10.1093/eurheartj/14.1.26. [DOI] [PubMed] [Google Scholar]
- 30.Hasdai D, Rizza RA, Holmes DR, Jr., Richardson DM, Cohen P, Lerman A. Insulin and insulin-like growth factor-I cause coronary vasorelaxation in vitro. Hypertension. 1998;32:228–234. doi: 10.1161/01.hyp.32.2.228. [DOI] [PubMed] [Google Scholar]
- 31.Colao A, Marzullo P, Di Somma C, Lombardi G. Growth hormone and the heart. Clin Endocrinol (Oxf) 2001;54:137–154. doi: 10.1046/j.1365-2265.2001.01218.x. [DOI] [PubMed] [Google Scholar]
- 32.Isgaard J, Tivesten A, Friberg P, Bengtsson BA. The role of the GH/IGF-I axis for cardiac function and structure. Horm Metab Res. 1999;31:50–54. doi: 10.1055/s-2007-978698. [DOI] [PubMed] [Google Scholar]
- 33.Niculescu DA, Purice M, Lichiardopol R, Coculescu M. Both insulin resistance and insulin secretion are involved in the pre-diabetes of acromegaly. Acta Endocrinologica (Buc) 2010;6:35–42. [Google Scholar]
- 34.Celebi OO, Celebi S, Canbay A, Gokaslan S, Diker E. Impaired Heart Rate Recovery in Patients with Impaired Glucose Tolerance. Acta Endo (Buc) 2014;10:76–83. [Google Scholar]
- 35.Straus DS. Growth-stimulatory actions of insulin in vitro and in vivo. Endocr Rev. 1984;5:356–369. doi: 10.1210/edrv-5-2-356. [DOI] [PubMed] [Google Scholar]
- 36.Zlatareva N, Zacharieva S, Natchev E. Acromegalic cadiomyopathy. Endocrinologia. 2004:73–80. [Google Scholar]
- 37.Bogazzi F, Lombardi M, Strata E, Aquaro G, Di Bello V, Cosci C, Sardella C, Talini E, Martino E. High prevalence of cardiac hypertophy without detectable signs of fibrosis in patients with untreated active acromegaly: an in vivo study using magnetic resonance imaging. Clin Endocrinol (Oxf) 2008;68:361–368. doi: 10.1111/j.1365-2265.2007.03047.x. [DOI] [PubMed] [Google Scholar]
- 38.Fazio S, Cittadini A, Cuocolo A, Merola B, Sabatini D, Colao A, Biondi B, Lombardi G, Sacca L. Impaired cardiac performance is a distinct feature of uncomplicated acromegaly. J Clin Endocrinol Metab. 1994;79:441–446. doi: 10.1210/jcem.79.2.8045960. [DOI] [PubMed] [Google Scholar]
- 39.Guo X, Gao L, Zhang S, Li Y, Wu Y, Fang L, Deng K, Yao Y, Lian W, Wang R, Xing B. Cardiovascular system changes and related risk factors in acromegaly patients: a case-control study. Int J Endocrinol. 2015:573643. doi: 10.1155/2015/573643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colao A, Pivonello R, Galderisi M, Cappabianca P, Auriemma RS, Galdiero M, Cavallo LM, Esposito F, Lombardi G. Impact of treating acromegaly first with surgery or somatostatin analogs on cardiomyopathy. J Clin Endocrinol Metab. 2008;93:2639–2646. doi: 10.1210/jc.2008-0299. [DOI] [PubMed] [Google Scholar]
- 41.Colao A, Marzullo P, Cuocolo A, Spinelli L, Pivonello R, Bonaduce D, Salvatore M, Lombardi F. Reversal of acromegalic cardiomyopathy in young but not in middle-aged patients after 12 months of treatment with the depot long-acting somatostatin analogue octreotide. Clin Endocrinol (Oxf) 2003;58:169–176. doi: 10.1046/j.1365-2265.2003.01689.x. [DOI] [PubMed] [Google Scholar]
- 42.McGuffin WL, Jr., Sherman BM, Roth F, Gorden P, Kahn CR, Roberts WC, Frommer PL. Acromegaly and cardiovascular disorders. A prospective study. Ann Intern Med. 1974;81:11–18. doi: 10.7326/0003-4819-81-1-11. [DOI] [PubMed] [Google Scholar]
- 43.Bertoni PD, Morandi G. Impaired left ventricular diastolic function in acromegaly: an echocardiographic study. Acta Cardiol. 1987;42:1–10. [PubMed] [Google Scholar]
- 44.Chanson P, Timsit J, Masquet C, Warnet A, Guillausseau PJ, Birman P, Harris AG, Lubetzki J. Cardiovascular effects of the somatostatin analog octreotide in acromegaly. Ann Intern Med. 1990;113:921–925. doi: 10.7326/0003-4819-113-12-921. [DOI] [PubMed] [Google Scholar]
- 45.Hayward RP, Emanuel RW, Nabarro JD. Acromegalic heart disease: influence of treatment of the acromegaly on the heart. Q J Med. 1987;62:41–58. [PubMed] [Google Scholar]
- 46.Colao A, Cuocolo A, Marzullo P, Nicolai E, Ferone D, Della Morte AM, Petretta M, Salvatore M, Lombardi G. Impact of patient’s age and disease duration on cardiac performance in acromegaly: a radionuclide angiography study. J Clin Endocrinol Metab. 1999;84:1518–1523. doi: 10.1210/jcem.84.5.5674. [DOI] [PubMed] [Google Scholar]


