Abstract
Objective
Ongoing HIV replication while receiving combination antiretroviral therapy (cART) may reduce survival. Viremia copy-years (VCY) has shown improved mortality risk prediction over single time-point viral load (VL) measures. However, the timing of a patient’s VL history most associated with later mortality has not been studied. Here we determined the optimal duration and temporality of VL history for predicting mortality.
Design
Survival analysis among HIV-positive men who initiated cART in the Multicenter AIDS Cohort Study (1995–2015).
Methods
VCY measures were derived from area-under-the-VL-curve. The overall VCY based upon the complete post-cART VL history was compared to 20 VCYs derived from VLs assessed during different shorter time periods (the most recent 1–10 years and initial 1–10 years following cART initiation) for associations with mortality.
Results
Each 10-fold increase in VCYs based on the most recent 3–8 years was significantly associated with 23–26% decrease in survival times, a magnitude of effect greater than that of the most recent VL (16%). These associations were independent of CD4 cell count and single time-point VLs. In addition, the degree of pre-cART immunodeficiency did not affect the mortality prognostic value of VCY based on VLs in the most recent 3 years. Conversely, the overall VCY and VCYs based on VLs immediately following cART initiation were not independent predictors of mortality.
Conclusion
Among cART-treated men, VCY based upon VLs in the recent 3 years (6 VLs), has a mortality prognostic value greater than that of the overall VCY and single time-point VLs, making the former a more feasible measure for use.
Keywords: Viremia copy-years, Human Immunodeficiency Virus, All-cause mortality, Combination antiretroviral therapy, Men who have sex with men (MSM)
Introduction
Plasma HIV RNA levels (viral load [VL]) are an integral clinical indicator for HIV care practice and research in the current era of combination antiretroviral therapy (cART) [1–4]. Early change in VL levels following cART initiation can predict long-term treatment response [1, 2], and HIV viral replication while on cART increases the risk of disease progression and mortality [5–8]. In clinical and epidemiologic surveillance studies, a recently measured VL is commonly used to assess the prevalence of HIV virologic suppression, a marker of cART effectiveness and HIV transmission risk [9–11]. However, despite being an independent predictor of mortality risk [12], a single, recent VL measure fails to capture an individual’s long-term exposure to viral replication [13].
Interest in measures of cumulative HIV viral burden has increased in recent years with the demonstration of their added value as a prognostic marker of disease progression over single time-point VLs. In particular, viremia copy-years (VCY), a measure of cumulative HIV viral burden, has shown stronger associations with mortality and morbidity compared to the most recent VL and other single time-point VL assessments [14–17]. Yet, the meaningful window of a patient’s VL history that should be included in VCY for optimal prognostic performance remains unclear. No studies have addressed whether a patient’s entire VL history is necessary to accurately predict mortality or morbidity risk, or whether VCY measures over limited time periods of disease history may suffice in predicting these outcomes.
In the present study, we used data from a large, prospective cohort of cART-initiating HIV-positive men who have sex with men (MSM) to assess VCY using alternative windows of VL history, that vary in terms of both the temporal relationship with the outcome assessment and the duration of VL accumulation. We evaluated the prognostic value for mortality risk of these abridged VCY measures, and compared to VCY using the complete VL history since cART initiation, as well as to three single time-point VL measures.
Methods
Study population
The Multicenter AIDS Cohort Study (MACS), as previously described [18–20], is an ongoing prospective cohort study of the natural and treated history of HIV infection. Participants attend semiannual research visits that include standardized interviews and collection of blood specimens. Local institutional review boards approved research protocols. All participants provided written informed consent.
As of March 2015, a total of 7,343 MSM (3,897 HIV-positive) were enrolled at 4 U.S. sites (Baltimore/Washington, Chicago, Pittsburgh, and Los Angeles). Of these, 1,296 met the eligibility criteria: initiated cART after or ≤1 year prior to MACS enrollment between July 1995 and March 2015 (Supplementary Figure 1). The analytic sample consisted of 841 men after excluding those with gaps of ≥2 years between the dates of last report of no cART use and first report of cART use (N=111), ≤1 VL after cART initiation (N=196), cART treatment for >1 year before the first available VL (N=69), or no VL ≤6 months prior to cART initiation (N=79). cART was defined as any regimen of ≥3 antiretroviral agents that included at least one protease inhibitor or one non-nucleoside reverse transcriptase inhibitor or abacavir or tenofovir, or an integrase inhibitor or an entry inhibitor [1]. Individuals were censored if they had no VL measured for >1 year during the follow-up for this study. The date of cART initiation was abstracted from medical records or, when an exact date was not available, estimated as the midpoint between the last reported cART-naïve date and first reported cART-treated date.
Mortality ascertainment
The outcome of interest was all-cause mortality. Mortality data were ascertained through a review of death certificates and use of National Death Index records.
Plasma HIV-1 RNA measures
One of three assays were used for measuring VL: Amplicor HIV-1 monitor test (Roche Diagnostics, lower limit of detection [LLD]=400 copies/mL, Pre-2002: 11%); Amplicor HIV-1 monitor ultrasensitive test (Roche Diagnostics, LLD=50, 2002–2010: 64%); TaqMan HIV-1 test (Roche Diagnostics, LLD=20, 2011–2015: 25%). VLs below the LLD were set to 300, 40 and 10 copies/mL, respectively, per MACS operating protocols. Longitudinal VL data were measured at semiannual study visits. For cART-prevalent individuals, the last VLs within 6 months before cART initiation were abstracted from medical records.
Viral suppression was defined as consistent VL measurements below the LLD allowing one blip <400 copies/mL. Using this definition, proportions of virally suppressed participants were calculated at single time-points (the last pre-cART, first post-cART and most recent visits) and over various time windows (as explained below).
Assessment of overall viremia copy-years
The overall VCY was approximated using the trapezoidal rule [14, 16]. Briefly, we calculated the area-under-the-curve (AUC) of the segment between each 2 consecutive VLs by taking the mean of the 2 VLs (on the natural scale) multiplied by the in-between time interval. If VL was missing at cART initiation, the last available value within the prior 6 months was used. Time-updated VCY was computed by summing the AUC of all segments prior to the current visit.
Assessment of VCY using abridged history
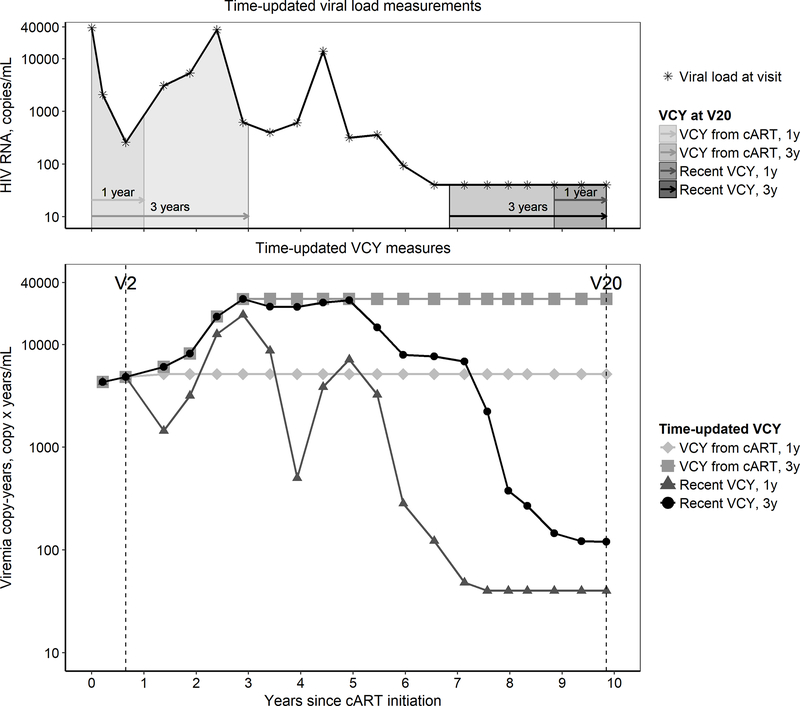
We created 20 VCY measures that estimated HIV viral burden during different post-cART time periods. The first 10 VCYs used VLs measured in the 1 to 10 years immediately following cART initiation. The other 10 VCYs used VLs measured in the most recent 1 to 10 years. Examples of 4 VCYs are illustrated in Figure 1. All VCYs were treated as time-varying: for VCYs following cART initiation, the values were updated up to the end of the targeted exposure window and projected forward using the last available value; for the most recent VCYs, the values were updated as more post-cART history was accumulated and the window of most recent history moved relative to the time of the outcome assessment. If the length of the targeted exposure window exceeded the total post-cART follow-up time, all available VLs during the post-cART period were used in the calculation of VCYs. An example is illustrated in Figure 1, where the total follow-up time at visit 2 (V2) was <1 year, so only the first 3 VLs were used to calculate VCY such that all 4 VCYs had the same value. Missing VLs at the beginning or end of a time period were replaced with a value proportionally interpolated using the two VLs obtained closest in time to the missing measurement [21].
Figure 1.
Examples of 4 VCY measures, each representing the VCY based on VLs measured in the 1 year and 3 years immediately following cART initiation (VCY from cART) and VCY based on VLs measured in the most recent 1 year and 3 years (Recent VCY), respectively, for an individual with VLs at 20 study visits and a follow-up time of 9.8 years following cART initiation.
Top, time-updated VL measurements from study visit 1 to visit 20. Arrows represent the time periods used to assess each of the 4 VCY measures at visit 20 (V20). Shaded areas represent the values of each of the 4 VCY measures at visit 20.
Bottom, time-updated values for the 4 VCY measures from study visit 1 to visit 20.
Abbreviations: VCY, viremia copy-years; VL, viral load; cART, combination antiretroviral therapy
Statistical analysis
To evaluate the association of the various VCY measures with all-cause mortality, we conducted survival analysis with time accrued from cART initiation (considered baseline for the study). Because MACS participants were most often not observed on the exact date of cART initiation, all observations were treated as late entries with entry time defined by date of first post-cART VL assessment. VCYs were log10-transformed and treated as a continuous exposure in analyses. We fit separate conventional lognormal survival models with cluster-robust variance for each VCY measure under the assumption that survival times were proportional [22], and estimated the percent change in survival times for each 10-fold increase in VCYs. To account for other differences in participant characteristics that could affect both VCYs and mortality, models were adjusted for age (per 10 years), race (Caucasian vs. non-Caucasian), MACS study site, cohort enrollment (pre-2001 vs. 2001+), most recent CD4 cell count (per 100 cells/mm3 with a spline at 200), baseline CD4 cell count (per 100 cells/mm3) and history of clinically-defined AIDS diagnosis (Model A). To evaluate the degree to which VCYs provided additional prognostic information beyond that provided by selected specific single time-point VLs, we added the last pre-cART VL, first post-cART VL and most recent VL jointly to Model A (Model B). The Akaike Information Criteria (AIC) statistic was calculated to compare model fit. We further stratified analyses by baseline CD4 cell levels to evaluate whether pre-cART immunodeficiency (<200 cells/mm3) affected the prognostic performance of VCYs [23]. Statistical analyses were performed using Stata/SE version 13.1 (StataCorp, College Station, TX) and R version 3.3.2 (The R Foundation for Statistical Computing, Vienna, Austria). P-values <0.05 guided statistical interpretation. To facilitate future research and clinical activities, we provide the programming codes that allow the computation of VCYs during various time periods on the MACS website (https://statepi.jhsph.edu/software/software.html).
Sensitivity analysis
To evaluate the sensitivity to the handling of undetectable VL, we repeated the analyses with VCYs calculated after substituting values of 0 or 10 (half of the TaqMan assay LLD) for undetectable VLs. We then repeated analysis in a subset of participants with no prior exposure to suboptimal regimens, to enhance the generalizability of our results to treatment-naïve patients currently seen in clinical care. We also conducted an exploratory analysis for cause-specific mortality by limiting to AIDS-related, and then non-AIDS related mortality events, while censoring the other competing event.
Results
Participant characteristics
Table 1 summarizes participant characteristics. Among the 841 cART initiators, 22% were black; 52% initiated cART prior to 2001. At cART initiation, the median age and CD4 cell count were 43 years and 319 cells/mm3; 10% had a clinically-defined AIDS diagnosis; 48% had prior exposure to antiretroviral mono- or dual-therapy.
Table 1.
Characteristics of 841 HIV+ participants at who initiated combination antiretroviral therapy in the Multicenter AIDS Cohort Study, 1995–2015
| Overall study population (N=841) | Deceased (N=74) | |
|---|---|---|
| Follow-up, years* | 5 (2–12) | 5 (2–8) |
| Age at cART initiation, years | 43 (37–49) | 47 (40–55) |
| Race | ||
| White | 552 (65.6) | 55 (74.3) |
| Black | 184 (21.9) | 15 (20.3) |
| Others | 105 (12.5) | 4 (5.4) |
| cART initiation year | ||
| 1996–2001 | 439 (52.2) | 60 (81.1) |
| 2001–2015 | 402 (47.8) | 14 (18.9) |
| Prior exposure to ART | 409 (48.6) | 56 (75.7) |
| Duration of prior exposure to ART, years | 6 (2–7) | 6 (3–6) |
| Clinically-defined AIDS diagnosis prior to cART initiation | 86 (10.2) | 16 (21.6) |
| Last CD4 cell count within 6 months prior to cART initiation, cells/mm3 | 319 (188–482) | 191 (93–333) |
| First CD4 cell count after cART initiation, cells/mm3 | 417 (260–585) | 292 (122–439) |
| Most recent CD4 cell count, cells/mm3 | 569 (375–761) | 244 (84–424) |
Showing median (IQR) for continuous variables or N (%) for categorical variables
Abbreviations: IQR, interquartile range; cART, combination antiretroviral therapy; ART, suboptimal mono- or dual antiretroviral therapy
Seventy-four deaths (58% attributed to AIDS) occurred over a median (interquartile range [IQR]) follow-up of 5 (2–12) years (6,736 person-years). The median (IQR) time to death was 5 (2–8) years. Among those who died, the median baseline CD4 cell count was 191 cells/mm3; 22% had an AIDS diagnosis.
Virologic data
Table 2 shows the distributions of VCY and VL measures and the proportions of individuals who achieved viral suppression during various time periods following cART initiation. At cART initiation, the median log10(VL) was 4.5 (34438 copies/mL). This had decreased to 1.9 (81 copies/mL) at the first post-cART visit. The median overall log10(VCY) was 4.3 (17944 copy-years/mL). At the most recent visit, 72% of men were virally suppressed. However, when considering all VLs obtained in the most recent 2 and 3 years, the proportion of men with viral suppression decreased to 61% and 55%, respectively (Table 2). Only 33% maintained viral suppression during the entire study follow-up.
Table 2.
The distributions of viremia copy-year and cross-sectional viral load measurements, and the proportions of individuals who maintained virologic suppression during various time periods after cART initiation.
| Time periods | Names of VCY or VL measurements | Log10 median (IQR) of VCY or VL measures* | % Virally suppressed† |
|---|---|---|---|
| Last pre-cART visit | Last pre-cART VL | 4.5 (3.9–5.1) | 5 |
| Most recent visit | Most recent VL | 1.6 (1–2) | 72 |
| First visit after cART | First VL after cART | 1.9 (1.6–3) | 44 |
| Entire follow-up from cART | Overall VCY | 4.3 (3.6–4.9) | 33 |
| First year from cART | VCY from cART, 1y | 3.9 (3.3–4.4) | 55 |
| Initial 2 years from cART | VCY from cART, 2y | 4 (3.4–4.6) | 46 |
| Initial 3 years from cART | VCY from cART, 3y | 4.1 (3.5–4.6) | 42 |
| Initial 4 years from cART | VCY from cART, 4y | 4.1 (3.5–4.7) | 40 |
| Initial 5 years from cART | VCY from cART, 5y | 4.2 (3.5–4.7) | 38 |
| Initial 6 years from cART | VCY from cART, 6y | 4.2 (3.6–4.8) | 37 |
| Initial 7 years from cART | VCY from cART, 7y | 4.2 (3.6–4.8) | 36 |
| Initial 8 years from cART | VCY from cART, 8y | 4.2 (3.6–4.8) | 35 |
| Initial 9 years from cART | VCY from cART, 9y | 4.2 (3.6–4.9) | 34 |
| Initial 10 years from cART | VCY from cART, 10y | 4.2 (3.6–4.9) | 34 |
| Recent 1 year | Recent VCY, 1y | 1.6 (1–3.5) | 69 |
| Recent 2 years | Recent VCY, 2y | 2.2 (1.5–4.1) | 61 |
| Recent 3 years | Recent VCY, 3y | 3.1 (1.8–4.3) | 55 |
| Recent 4 years | Recent VCY, 4y | 3.4 (2.1–4.4) | 50 |
| Recent 5 years | Recent VCY, 5y | 3.6 (2.2–4.5) | 47 |
| Recent 6 years | Recent VCY, 6y | 3.7 (2.4–4.5) | 46 |
| Recent 7 years | Recent VCY, 7y | 3.8 (2.4–4.6) | 44 |
| Recent 8 years | Recent VCY, 8y | 3.8 (2.6–4.6) | 43 |
| Recent 9 years | Recent VCY, 9y | 3.9 (2.8–4.7) | 41 |
| Recent 10 years | Recent VCY, 10y | 4 (3–4.7) | 40 |
Values of the overall VCY, 10 recent VCY measures and the most recent VL were determined at the last follow-up visit in this study.
Viral suppression was defined as consistent VL measurements below the assay-specific level of detection, except for a single blip of <400 copies/mL.
Values of the VCY measures in the first 1 to 10 years following cART were determined at the end of the respective time periods.
Abbreviations: cART, combination antiretroviral therapy; VCY, viremia copy-years; VL, viral load; cART, combination antiretroviral therapy; IQR, interquartile range
Supplementary Figure 2 shows a Spearman’s rank correlation matrix for the 21 VCYs and 3 VLs. To summarize, the overall VCY correlated strongly with the 10 VCYs from cART initiation (0.76–0.99), but to a lesser extent with the 10 recent VCYs (0.31–0.79). Within the 10 recent VCYs, variation started to be observed when the time windows used in VCY calculation differed by ≥4 years (0.71–0.86).
Evaluating various time frames of VCY assessment for predicting mortality
Table 3 shows the associations of mortality risk with 3 single time-point VLs, the overall VCY and the 20 abridged VCYs. After adjusting for demographic covariates and the baseline and most recent CD4 cell count, each 10-fold increase in the most recent VL and the first post-cART VL was significantly associated with 16% and 14% decrease in survival time, respectively. The overall VCY was associated with a comparable magnitude of decrease in survival time (18%), but this was not statistically significant (Model A).
Table 3.
Adjusted percent changes in survival times for viremia copy-years and cross-sectional viral load measurements among 841 cART-initiating HIV-positive MACS study participants.
| Model A* | Model B† | |||
|---|---|---|---|---|
| % Change in survival time‡ (95% CI) | AIC | % Change in survival time‡ (95% CI) | AIC | |
| Last pre-cART VL | −7 (−26, 12) | 458.1 | NA | NA |
| Most recent VL | −16 (−28, −4) | 453.8 | NA | NA |
| First post-cART VL | −14 (−27, −1) | 455.8 | NA | NA |
| Overall VCY | −18 (−39, 4) | 456.2 | −4 (−36, 27) | 458.5 |
| VCY from cART, 1y | −12 (−32, 8) | 457.5 | 11 (−44, 66) | 458.4 |
| VCY from cART, 2y | −15 (−36, 5) | 456.8 | 1 (−36, 37) | 458.6 |
| VCY from cART, 3y | −19 (−39, 1) | 455.8 | −9 (−40, 22) | 458.4 |
| VCY from cART, 4y | −20 (−40, 0) | 455.5 | −11 (−42, 19) | 458.2 |
| VCY from cART, 5y | −20 (−40, 1) | 455.7 | −9 (−40, 22) | 458.3 |
| VCY from cART, 6y | −18 (−39, 3) | 456.0 | −6 (−37, 26) | 458.5 |
| VCY from cART, 7y | −17 (−38, 5) | 456.5 | −1 (−33, 31) | 458.6 |
| VCY from cART, 8y | −17 (−38, 5) | 456.5 | −1 (−32, 31) | 458.6 |
| VCY from cART, 9y | −17 (−38, 5) | 456.6 | −1 (−32, 31) | 458.6 |
| VCY from cART, 10y | −17 (−38, 5) | 456.5 | −1 (−33, 30) | 458.6 |
| Recent VCY, 1y | −17 (−29, −5) | 453.7 | −9 (−29, 11) | 458.0 |
| Recent VCY, 2y | −20 (−32, −7) | 451.9 | −15 (−32, 2) | 456.6 |
| Recent VCY, 3y | −24 (−36, −12) | 449.1 | −21 (−37, −6) | 454.1 |
| Recent VCY, 4y | −23 (−37, −10) | 450.5 | −20 (−36, −4) | 455.0 |
| Recent VCY, 5y | −24 (−39, −10) | 450.2 | −22 (−39, −5) | 454.5 |
| Recent VCY, 6y | −26 (−41, −11) | 449.6 | −25 (−43, −6) | 453.8 |
| Recent VCY, 7y | −26 (−42, −10) | 450.5 | −24 (−43, −5) | 454.6 |
| Recent VCY, 8y | −25 (−42, −8) | 451.6 | −22 (−43, −2) | 455.4 |
| Recent VCY, 9y | −22 (−40, −5) | 453.4 | −17 (−40, 5) | 456.9 |
| Recent VCY, 10y | −22 (−41, −4) | 453.8 | −17 (−41, 6) | 457.1 |
Bold indicates statistical significance
Model A was adjusted for age (per 10-year increase), non-Caucasian race, most recent CD4+ T cell count (per 100 cells/mm3 increase with linear spline at 200 cells/mm3), baseline CD4+ T cell count (per 100 cells/mm3 increase), AIDS diagnosis prior to cART initiation, MACS study site and cohort enrollment
Model B evaluated each VCY measure jointly with the three cross-sectional VLs (last pre-cART VL, first VL after cART and the most recent VL) after adjusting for all demographic and clinical factors included in Model A. Among the three cross-sectional VLs, adjusted percent change in survival times are only shown for the most recent VL.
Percent change in survival times was estimated for each 10-fold increase in the 21 VCY measures (copy-years/mL) and 3 cross-sectional VLs (copies/mL)
Abbreviations: VCY, viremia copy-years; VL, viral load; cART, combination antiretroviral therapy; MACS, Multicenter AIDS Cohort Study; CI, confidence interval; AIC, Akaike Information Criteria
Among the 20 abridged VCYs, only the 10 measures using recent VL history were significantly associated with mortality independent of CD4 cell count (Model A). For each 10-fold increase in these recent VCYs, the decrease in survival times varied between 17 to 26%, with the strongest prognostic value observed for VCYs based on VLs obtained during the most recent 3 to 8 years (23 to 26%). In contrast, the 10 VCYs based on VLs in the first 1 to 10 years following cART initiation were not significantly associated with mortality (Model A).
When each of the 21 VCYs was evaluated in a combined VL model with the 3 single time-point VLs (Model B), each 10-fold increase in VCYs based on VLs in the most recent 3 to 8 years remained associated with a 20 to 25% decrease in survival time. By contrast, the overall VCY and the VCYs from period following cART initiation were not predictive of mortality. None of the 3 single time-point VLs was significantly associated with mortality when they were evaluated in the same model with the most recent VCYs (Supplementary Table 1). Assessments of model fit (AIC) indicated better fits to the data using the recent VCYs measures; the model with VCY using the most recent 3 year period alone had the lowest AIC value (Table 3).
Mortality by baseline CD4 cell levels
Table 4 displays the results of analyses stratified by baseline CD4 cell count. The follow-up time was comparable in the two CD4 groups (p=0.69). Men with <200 cells/mm3 (27%) accounted for 1,793 person-years and 39 deaths. Among all VCY and single time-point VL measures, the VCY based on VLs in the most recent 3 years was the only one that was independently associated with mortality risk in both baseline CD4 groups. Among men with CD4 cell count ≥200 cells/mm3, a stronger association with mortality was generally observed for VCYs based on VLs that were accumulated over longer time periods.
Table 4.
Adjusted percent changes in survival times for viremia copy-year and cross-sectional viral load measurements among 841 cART-initiating HIV-positive MACS study participants, stratified by CD4+ T cell levels at cART initiation
| Model A*: Baseline CD4<200 cells/mm3 (N=223) | Model A*: Baseline CD4≥200 cells/mm3 (N=618) | |
|---|---|---|
| % Change in survival time† (95% CI) | % Change in survival time (95% CI) | |
| Last pre-cART VL | 10 (−19, 38) | −18 (−41, 4) |
| Most recent VL | −16 (−29, −4) | −14 (−34, 6) |
| First post-cART VL | −14 (−29, 1) | −13 (−35, 9) |
| Overall VCY | −6 (−34, 23) | −28 (−58, 2) |
| VCY from cART, 1y | −10 (−36, 15) | −10 (−38, 18) |
| VCY from cART, 2y | −11 (−38, 16) | −14 (−42, 14) |
| VCY from cART, 3y | −11 (−38, 16) | −21 (−48, 6) |
| VCY from cART, 4y | −9 (−37, 19) | −26 (−52, 1) |
| VCY from cART, 5y | −8 (−36, 20) | −26 (−53, 1) |
| VCY from cART, 6y | −7 (−36, 21) | −26 (−54, 3) |
| VCY from cART, 7y | −7 (−35, 22) | −24 (−53, 5) |
| VCY from cART, 8y | −7 (−36, 21) | −23 (−53, 6) |
| VCY from cART, 9y | −7 (−35, 22) | −24 (−53, 5) |
| VCY from cART, 10y | −6 (−35, 22) | −25 (−54, 4) |
| Recent VCY, 1y | −16 (−31, −2) | −16 (−35, 4) |
| Recent VCY, 2y | −20 (−35, −5) | −17 (−37, 3) |
| Recent VCY, 3y | −21 (−36, −5) | −24 (−43, −6) |
| Recent VCY, 4y | −17 (−34, 1) | −27 (−47, −7) |
| Recent VCY, 5y | −17 (−35, 1) | −30 (−51, −8) |
| Recent VCY, 6y | −19 (−38, 1) | −31 (−54, −8) |
| Recent VCY, 7y | −17 (−38, 4) | −32 (−56, −9) |
| Recent VCY, 8y | −14 (−37, 8) | −34 (−58, −11) |
| Recent VCY, 9y | −11 (−35, 13) | −32 (−58, −7) |
| Recent VCY, 10y | −12 (−37, 12) | −31 (−58, −5) |
Bold indicates statistical significance
Model A was adjusted for age (per 10-year increase), non-Caucasian race, most recent CD4+ T cell count (per 100 cells/mm3 increase with linear spline at 200 cells/mm3), baseline CD4+ T cell count (per 100 cells/mm3 increase), AIDS diagnosis prior to cART initiation, MACS study site and cohort enrollment
Percent change in survival times was estimated for each 10-fold increase in the 21 VCY measurements (copy-years/mL) and 3 cross-sectional VLs (copies/mL)
Abbreviations: VCY, viremia copy-years; VL, viral load; cART, combination antiretroviral therapy; MACS, Multicenter AIDS Cohort Study; CI, confidence interval
Sensitivity analyses
Substituting undetectable VLs with values of 0 or 10 had minimal impact on the observed association between the overall VCY and mortality risk (Supplementary Table 2). When limited to the 432 treatment-naïve participants (18 deaths; 2,549 person-years), survival times were significantly shortened by 22–31% per 10-fold increase in VCY based on VLs in the recent 3 to 8 years – results similar to the main analysis (Supplementary Table 3). When stratified by the type of mortality events, VCYs seemed to have better prognostic value for AIDS-related over non-AIDS-related deaths. However, the difference in prognostic power appeared to diminish when longer recent VL history was used in VCY calculation (Supplementary Table 4).
Discussion
To our knowledge, this is the first study that has systemically evaluated the mortality prognostic value of VCYs derived from varying windows of VL history. We showed that all 10 recent VCYs were significantly associated with mortality after adjusting for the baseline and most recent CD4 cell count. In addition, after taking into account single time-point VLs, VCYs using VLs measured during the most recent 3 to 8 years remained significantly predictive of mortality risk. By contrast, the overall VCY since cART initiation, or VCYs based on VLs measured immediately following cART initiation were not independent predictors of mortality risk. After stratifying by baseline CD4 cell count (as a proxy for pre-cART immunodeficiency), only the VCY based on VLs in the most recent 3 years showed consistent associations with mortality. These findings have potentially important implications for both HIV research and care practice.
A number of recent observational studies examined the prognostic values of VCY measures. While some did not show improved prognostic performance of VCY over that of the most recent VL [23–25], others have demonstrated VCY as an independent predictor for AIDS and non-AIDS clinical events, including mortality [15, 16, 21, 26–30]. Although there’s a growing interest in the potential use of VCY in clinical research, it is unclear whether knowing the entire VL history provides improved prognosis for mortality risk that could justify the greater challenge of assessing VCY. We demonstrated that among cART-treated individuals, recent trends in VL seem to be more predictive of mortality risk compared to high VL occurring earlier in a patient’s history. These findings highlight the importance of recent VL history in mortality risk prognosis among cART-treated individuals. The lack of independent relevance of temporally distant VLs may be explained by the fact that treatment-induced viral suppression can partially reverse some of the pathological consequences of HIV infection, such as opportunistic infections and high levels of inflammatory markers [31–34].
However, despite the apparent relevance of recent VLs, a single VL assessment at the most recent time point can still lead to misclassification of HIV viral burden [13]. In our study, the proportion of virally suppressed participants decreased from 72% on the most recent visit to 55% over the past 3 years, indicating that a single most recent VL underestimated viral non-suppression in the past 3 years by approximately 17%. Furthermore, when considering the VL history in these past 3 years, VCY showed a stronger association with mortality than the most recent VL (24% vs. 16% reduced survival time per 10-fold increase), and this association remained significant after accounting for the last pre-cART, first post-cART and most recent VLs. These data support the notion that VCYs based on recent VLs are better than a single VL assessment at capturing recent viral non-suppression as a result of virologic failure or treatment nonadherence, which may have important clinical implications for the development of adverse outcomes such as mortality.
We also observed that the mortality prognostic value of VCY measures may vary by the degree of immunological suppression at cART initiation – an important consideration when assessing VCYs. While almost all recent VCYs were strongly associated with mortality risk in individuals with baseline CD4 cell levels ≥200 cells/mm3, only VCYs based upon VLs over the most recent 1 to 3 years significantly predicted death in individuals with <200 cells/mm3. This observation may be explained by differences in clinical disease pathogenesis in individuals with varying degrees of immunodeficiency. While prolonged viremic periods with attendant increases in systemic inflammation and immune activation may be a driving force behind mortality risk regardless of the baseline CD4 cell counts [35, 36], pre-cART immunodeficiency can represent a primary risk for AIDS-related deaths [37, 38]. Thus, the prognostic value of VCY measures, which may serve as a proxy of elevated levels of chronic inflammation and immune activation, can be diminished in patients with more advanced immunodeficiency, though we cannot rule out the possibility of unstable estimation due to smaller sample sizes in the stratified analysis. Apart from the degree of immunologic suppression, we demonstrated that the prognostic value of recent VCYs is robust to prior exposure to suboptimal mono- or dual antiretroviral therapy and changing assumptions for handling unobserved or undetectable VLs. Accumulating VLs on the natural or log scale may also affect the prognostic value of VCYs [23]. In well-treated HIV-positive individuals, high VL values as a result of virologic failure have important implications for mortality risk as compared to low detectable VLs [1, 39]. Therefore, calculating VCYs from VLs on the natural scale, which assigns greater weight to high VL values, is appropriate in this study setting.
Plasma HIV RNA levels are important indicators of treatment response and can inform clinical decision making in the management of HIV-positive patients [1, 12]. Although there is no consensus on the routine use of VCY measures in HIV care practice, our findings serve as a proof of concept that such measures may be feasible in clinical settings and may improve clinicians’ ability to identify patients who are at increased risk of clinical progression. Better discrimination of high-risk patients can facilitate the development of individualized disease monitoring and treatment strategies. For maximal clinical utility of VCY measures based on recent VL data, future work should validate their predictive accuracy and precision for individual mortality risk classification [40]. In addition, further effort should be dedicated to formally assess the prognostic power of VCY measures for cause-specific mortality. In our exploratory analysis, VCY measures in general appeared to have better prognostic performance for AIDS-related mortality. However, this difference in prognostic power seemed to be diminishing for recent VCYs based on longer recent VL history, suggesting that recent VCYs might be a reliable clinical predictor for both types of mortality.
This study benefited from the extensive participant follow-up and careful ascertainment of mortality, as well as longitudinal VL data, allowing us to assess VCY over various time periods. We focused on cART-treated participants, as routine VL assessments often only occur after entry into HIV care and cART initiation [41]. In prior studies evaluating VCY as a primary exposure, Cox proportional hazards regression models have been the standard method of analysis [14, 16, 17]. However, we found that relative hazards were not proportional throughout follow-up. A possible violation of this key assumption would lead to model misspecification and its inferential consequences. Here we used the lognormal accelerated failure time models to avoid proportionality assumptions.
This study has several limitations. First, as in all observational studies, we cannot infer causality as there may be uncontrolled confounders such as behavioral factors that could lead to both poor cART adherence and higher mortality risk. Second, due to the inconsistent association between VCY and mortality across studies published to date, the generalizability of our results to all HIV-positive persons needs to be confirmed in larger studies that include participants more representative of the overall HIV-positive population. Third, we used a simple deterministic method to handle VLs below the limit of detection, which will underrepresent variability in low-level viremia. This could have influenced results, as low-level viremia may represent an important mechanism of the persistent immune active state among suppressed HIV-positive populations [34]. Finally, larger studies with longer follow-up are warranted to assess the prognostic performance of VCY measures among individuals with baseline CD4 cell count >500 cells/mm3, and also to discriminate between VCYs based on VL history longer than 5 years due to the strong positive correlations between these VCY measures.
In summary, our results suggest that, among cART-initiating HIV-positive men, VCY based upon VL information in the recent 3 years predicts mortality risk better than single time-point VLs and the overall VCY and is less influenced by the degree of immunological suppression. Therefore, it may represent a strong indicator of mortality risk that can be feasibly calculated in the analysis of observational data and in care management of HIV-positive patients. A clinical online tool to calculate VCY automatically from medical records could be developed or potentially integrated it into existing mortality risk scoring systems, such as the Veterans Aging Cohort Study (VACS) index [42], to facilitate use in clinical practice.
Supplementary Material
Acknowledgement
Data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS) with centers at Baltimore (U01-AI35042): The Johns Hopkins University Bloomberg School of Public Health: Joseph B. Margolick (PI), Todd Brown (PI), Jay Bream, Adrian Dobs, Michelle Estrella, W. David Hardy, Lisette Johnson-Hill, Sean Leng, Anne Monroe, Cynthia Munro, Michael W. Plankey, Wendy Post, Ned Sacktor, Jennifer Schrack, Chloe Thio; Chicago (U01-AI35039): Feinberg School of Medicine, Northwestern University, and Cook County Bureau of Health Services: Steven M. Wolinsky (PI), Sheila Badri, Dana Gabuzda, Frank J. Palella, Jr., Sudhir Penugonda, John P. Phair, Susheel Reddy, Matthew Stephens, Linda Teplin; Los Angeles (U01-AI35040): University of California, UCLA Schools of Public Health and Medicine: Roger Detels (PI), Otoniel Martínez-Maza (PI), Otto Yang (Co-PI), Peter Anton, Robert Bolan, Elizabeth Breen, Anthony Butch, Shehnaz Hussain, Beth Jamieson, John Oishi, Harry Vinters, Dorothy Wiley, Mallory Witt, Stephen Young, Zuo Feng Zhang; Pittsburgh (U01-AI35041): University of Pittsburgh, Graduate School of Public Health: Charles R. Rinaldo (PI), Lawrence A. Kingsley (PI), Jeremy J. Martinson (PI), James T. Becker, Phalguni Gupta, Kenneth Ho, Susan Koletar, John W. Mellors, Anthony J. Silvestre, Ronald D. Stall; Data Coordinating Center (UM1-AI35043): The Johns Hopkins University Bloomberg School of Public Health: Lisa P. Jacobson (PI), Gypsyamber D’Souza (PI), Alison Abraham, Keri Althoff, Michael Collaco, Priya Duggal, Sabina Haberlen, Eithne Keelaghan, Heather McKay, Alvaro Muñoz, Derek Ng, Anne Rostich, Eric C. Seaberg, Sol Su, Pamela Surkan, Nicholas Wada. Institute of Allergy and Infectious Diseases: Robin E. Huebner; National Cancer Institute: Geraldina Dominguez. The MACS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute of Mental Health (NIMH). Targeted supplemental funding for specific projects was also provided by the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute on Deafness and Communication Disorders (NIDCD). MACS data collection is also supported by UL1-TR001079 (JHU ICTR) from the National Center for Advancing Translational Sciences (NCATS) a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH), Johns Hopkins ICTR, or NCATS. The MACS website is located at http://aidscohortstudy.org/.
Funding
This work was supported by grants U01-AI35039, U01-AI35040, U01-AI35041, U01-AI35042, UM1-AI35043 from the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute of Mental Health (NIMH). Targeted supplemental funding for specific projects in the MACS was provided by the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute on Deafness and Communication Disorders (NIDCD). MACS data collection is also supported by UL1-TR001079 (JHU ICTR) from the National Center for Advancing Translational Sciences (NCATS) a component of the National Institutes of Health (NIH).
Footnotes
Conflicts of Interest
None of the authors have a commercial or other association that might pose a conflict of interest.
References
- 1.Panel on Antiretroviral Guidelines for Adults and Adolescents Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents 2018. Department of Health and Human Services; Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Accessed March 27, 2018. [Google Scholar]
- 2.Chene G, Sterne JA, May M, Costagliola D, Ledergerber B, Phillips AN, et al. Prognostic importance of initial response in HIV-1 infected patients starting potent antiretroviral therapy: analysis of prospective studies. Lancet 2003; 362(9385):679–686. [DOI] [PubMed] [Google Scholar]
- 3.HIV Surrogate Marker Collaborative Group. Human immunodeficiency virus type 1 RNA level and CD4 count as prognostic markers and surrogate end points: a meta-analysis. HIV Surrogate Marker Collaborative Group. AIDS Res Hum Retroviruses 2000; 16(12):1123–1133. [DOI] [PubMed] [Google Scholar]
- 4.Mellors JW, Munoz A, Giorgi JV, Margolick JB, Tassoni CJ, Gupta P, et al. Plasma viral load and CD4(+) lymphocytes as prognostic markers of HIV-1 infection. Annals of Internal Medicine 1997; 126(12):946–954. [DOI] [PubMed] [Google Scholar]
- 5.Egger M, May M, Chene G, Phillips AN, Ledergerber B, Dabis F, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet 2002; 360(9327):119–129. [DOI] [PubMed] [Google Scholar]
- 6.Marschner IC, Collier AC, Coombs RW, D’Aquila RT, DeGruttola V, Fischl MA, et al. Use of changes in plasma levels of human immunodeficiency virus type 1 RNA to assess the clinical benefit of antiretroviral therapy. J Infect Dis 1998; 177(1):40–47. [DOI] [PubMed] [Google Scholar]
- 7.Murray JS, Elashoff MR, Iacono-Connors LC, Cvetkovich TA, Struble KA. The use of plasma HIV RNA as a study endpoint in efficacy trials of antiretroviral drugs. AIDS 1999; 13(7):797–804. [DOI] [PubMed] [Google Scholar]
- 8.Thiebaut R, Morlat P, Jacqmin-Gadda H, Neau D, Mercie P, Dabis F, et al. Clinical progression of HIV-1 infection according to the viral response during the first year of antiretroviral treatment. Groupe d’Epidemiologie du SIDA en Aquitaine (GECSA). AIDS 2000; 14(8):971–978. [DOI] [PubMed] [Google Scholar]
- 9.Doshi RK, Milberg J, Isenberg D, Matthews T, Malitz F, Matosky M, et al. High rates of retention and viral suppression in the US HIV safety net system: HIV care continuum in the Ryan White HIV/AIDS Program, 2011. Clin Infect Dis 2015; 60(1):117–125. [DOI] [PubMed] [Google Scholar]
- 10.Althoff KN, Buchacz K, Hall HI, Zhang J, Hanna DB, Rebeiro P, et al. U.S. trends in antiretroviral therapy use, HIV RNA plasma viral loads, and CD4 T-lymphocyte cell counts among HIV-infected persons, 2000 to 2008. Ann Intern Med 2012; 157(5):325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradley H, Hall HI, Wolitski RJ, Van Handel MM, Stone AE, LaFlam M, et al. Vital Signs: HIV diagnosis, care, and treatment among persons living with HIV--United States, 2011. MMWR Morb Mortal Wkly Rep 2014; 63(47):1113–1117. [PMC free article] [PubMed] [Google Scholar]
- 12.Olsen CH, Gatell J, Ledergerber B, Katlama C, Friis-Moller N, Weber J, et al. Risk of AIDS and death at given HIV-RNA and CD4 cell count, in relation to specific antiretroviral drugs in the regimen. AIDS 2005; 19(3):319–330. [PubMed] [Google Scholar]
- 13.Marks G, Patel U, Stirratt MJ, Mugavero MJ, Mathews WC, Giordano TP, et al. Single Viral Load Measurements Overestimate Stable Viral Suppression among HIV Patients in Care: Clinical and Public Health Implications. J Acquir Immune Defic Syndr 2016; 73(2):205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cole SR, Napravnik S, Mugavero MJ, Lau B, Eron JJ Jr., Saag MS. Copy-years viremia as a measure of cumulative human immunodeficiency virus viral burden. Am J Epidemiol 2010; 171(2):198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kowalkowski MA, Day RS, Du XL, Chan W, Chiao EY. Cumulative HIV viremia and non-AIDS-defining malignancies among a sample of HIV-infected male veterans. J Acquir Immune Defic Syndr 2014; 67(2):204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mugavero MJ, Napravnik S, Cole SR, Eron JJ, Lau B, Crane HM, et al. Viremia copy-years predicts mortality among treatment-naive HIV-infected patients initiating antiretroviral therapy. Clin Infect Dis 2011; 53(9):927–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zoufaly A, Stellbrink HJ, Heiden MA, Kollan C, Hoffmann C, van Lunzen J, et al. Cumulative HIV viremia during highly active antiretroviral therapy is a strong predictor of AIDS-related lymphoma. J Infect Dis 2009; 200(1):79–87. [DOI] [PubMed] [Google Scholar]
- 18.Detels R, Jacobson L, Margolick J, Martinez-Maza O, Munoz A, Phair J, et al. The multicenter AIDS Cohort Study, 1983 to. Public Health 2012; 126(3):196–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dudley J, Jin S, Hoover D, Metz S, Thackeray R, Chmiel J. The Multicenter AIDS Cohort Study: retention after 9 ½ years. Am J Epidemiol 1995; 142(3):323–330. [DOI] [PubMed] [Google Scholar]
- 20.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR Jr. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol 1987; 126(2):310–318. [DOI] [PubMed] [Google Scholar]
- 21.Dubrow R, Qin L, Lin H, Hernandez-Ramirez RU, Neugebauer RS, Leyden W, et al. Associations of CD4+ T-cell count, HIV-1 RNA viral load, and antiretroviral therapy with Kaposi sarcoma risk among HIV-infected persons in the United States and Canada. J Acquir Immune Defic Syndr 2017; 75(4):382–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cox C, Chu H, Schneider MF, Munoz A. Parametric survival analysis and taxonomy of hazard functions for the generalized gamma distribution. Stat Med 2007; 26(23):4352–4374. [DOI] [PubMed] [Google Scholar]
- 23.Sempa JB, Dushoff J, Daniels MJ, Castelnuovo B, Kiragga AN, Nieuwoudt M, et al. Reevaluating Cumulative HIV-1 Viral Load as a Prognostic Predictor: Predicting Opportunistic Infection Incidence and Mortality in a Ugandan Cohort. Am J Epidemiol 2016; 184(1):67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laut K, Shepherd LC, Pedersen C, Rockstroh JK, Sambatakou H, Paduta D, et al. Associations between HIV-RNA-based indicators and virological and clinical outcomes. AIDS 2016; 30(12):1961–1972. [DOI] [PubMed] [Google Scholar]
- 25.Chirouze C, Journot V, Le Moing V, Raffi F, Piroth L, Reigadas S, et al. Viremia copy-years as a predictive marker of all-cause mortality in HIV-1-infected patients initiating a protease inhibitor-containing antiretroviral treatment. J Acquir Immune Defic Syndr 2015; 68(2):204–208. [DOI] [PubMed] [Google Scholar]
- 26.Gonciulea A, Wang R, Althoff KN, Palella FJ, Lake J, Kingsley LA, et al. An Increased Rate of Fracture Occurs a Decade Earlier in HIV+ Compared to HIV- men in the Multicenter AIDS Cohort Study (MACS). AIDS 2017; 31(10):1435–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quiros-Roldan E, Raffetti E, Castelli F, Foca E, Castelnuovo F, Di Pietro M, et al. Low-level viraemia, measured as viraemia copy-years, as a prognostic factor for medium-long-term all-cause mortality: a MASTER cohort study. J Antimicrob Chemother 2016; 71(12):3519–3527. [DOI] [PubMed] [Google Scholar]
- 28.Salinas JL, Rentsch C, Marconi VC, Tate J, Budoff M, Butt AA, et al. Baseline, Time-Updated, and Cumulative HIV Care Metrics for Predicting Acute Myocardial Infarction and All-Cause Mortality. Clin Infect Dis 2016; 63(11):1423–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schrack JA, Jacobson LP, Althoff KN, Erlandson KM, Jamieson BD, Koletar SL, et al. Effect of HIV-infection and cumulative viral load on age-related decline in grip strength. AIDS 2016; 30(17):2645–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright ST, Hoy J, Mulhall B, O’Connor CC, Petoumenos K, Read T, et al. Determinants of viremia copy-years in people with HIV/AIDS after initiation of antiretroviral therapy. J Acquir Immune Defic Syndr 2014; 66(1):55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El-Sadr WM, Burman WJ, Grant LB, Matts JP, Hafner R, Crane L, et al. Discontinuation of prophylaxis against Mycobacterium avium complex disease in HIV-infected patients who have a response to antiretroviral therapy. Terry Beirn Community Programs for Clinical Research on AIDS. N Engl J Med 2000; 342(15):1085–1092. [DOI] [PubMed] [Google Scholar]
- 32.Furrer H, Egger M, Opravil M, Bernasconi E, Hirschel B, Battegay M, et al. Discontinuation of primary prophylaxis against Pneumocystis carinii pneumonia in HIV-1-infected adults treated with combination antiretroviral therapy. Swiss HIV Cohort Study. N Engl J Med 1999; 340(17):1301–1306. [DOI] [PubMed] [Google Scholar]
- 33.Weverling GJ, Mocroft A, Ledergerber B, Kirk O, Gonzales-Lahoz J, d’Arminio Monforte A, et al. Discontinuation of Pneumocystis carinii pneumonia prophylaxis after start of highly active antiretroviral therapy in HIV-1 infection. EuroSIDA Study Group. Lancet 1999; 353(9161):1293–1298. [DOI] [PubMed] [Google Scholar]
- 34.Wada NI, Jacobson LP, Margolick JB, Breen EC, Macatangay B, Penugonda S, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS 2015; 29(4):463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008; 5(10):e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wada NI, Bream JH, Martinez-Maza O, Macatangay B, Galvin SR, Margolick JB, et al. Inflammatory Biomarkers and Mortality Risk Among HIV-Suppressed Men: A Multisite Prospective Cohort Study. Clin Infect Dis 2016; 63(7):984–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365(6):493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Severe P, Juste MA, Ambroise A, Eliacin L, Marchand C, Apollon S, et al. Early versus standard antiretroviral therapy for HIV-infected adults in Haiti. N Engl J Med 2010; 363(3):257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laprise C, de Pokomandy A, Baril JG, Dufresne S, Trottier H. Virologic failure following persistent low-level viremia in a cohort of HIV-positive patients: results from 12 years of observation. Clin Infect Dis 2013; 57(10):1489–1496. [DOI] [PubMed] [Google Scholar]
- 40.Whittemore AS. Evaluating health risk models. Stat Med 2010; 29(23):2438–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention. Monitoring Selected National HIV Prevention and Care Objectives by Using HIV Surveillance Data - United States and 6 Dependent Areas, 2015. In: HIV Surveillance Supplemental Report 2017; 22(No2). [Google Scholar]
- 42.Justice AC, Modur SP, Tate JP, Althoff KN, Jacobson LP, Gebo KA, et al. Predictive accuracy of the Veterans Aging Cohort Study index for mortality with HIV infection: a North American cross cohort analysis. J Acquir Immune Defic Syndr 2013; 62(2):149–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.