Abstract
Stress and tobacco smoking are risk factors for alcoholism, but the underlying neural mechanisms are not well understood. Although stress, nicotine, and alcohol have broad, individual effects in the brain, some of their actions converge onto the same mechanisms and circuits. Stress and nicotine augment alcohol-related behaviors, in part via modulation of alcohol-evoked neuronal plasticity and metaplasticity mechanisms. Stress modulates alcohol-evoked plasticity via the release of signaling molecules that influence synaptic transmission. Nicotine also activates some of the same signaling molecules, cells, and circuits, producing a convergence of both stress and nicotine onto common plasticity mechanisms that influence alcohol self-administration. We describe several forms of alcohol-induced plasticity, including classic Hebbian plasticity at glutamatergic synapses, and we highlight less appreciated forms, such as non-Hebbian and GABAergic synaptic plasticity. Risk factors such as stress and nicotine initiate lasting neural changes that modify subsequent alcohol-induced synaptic plasticity and increase the vulnerability to alcohol addiction.
Keywords: alcohol use disorder, HPA axis, GABA, mesolimbic, nucleus accumbens, KCC2
INTRODUCTION
Alcohol and nicotine (from tobacco) are the two most abused drugs and the most costly drugs to society. Tobacco is the leading cause and alcohol is the fourth leading cause of preventable death in the United States (1, 2). Epidemiological studies consistently find a positive correlation between nicotine and alcohol use, and alcoholism is approximately 10 times more prevalent in smokers than in nonsmokers (3–6). Repetitive tobacco use or excessive drinking promotes the development of addiction. Individually, these drugs are health risks, but alcohol and tobacco in combination dramatically increase the hazards to dependent users (2).
Stress and tobacco smoking promote drinking and represent important risk factors contributing to the development of alcohol addiction (2, 3, 5, 7). Even a single highly stressful day or occasional cigarette serves as a predictor for elevated alcohol drinking in nonaddicted individuals (6, 8). Negative life experiences and tobacco use positively correlate with heavy drinking and increase the incidence of alcohol use disorders (AUDs) (4, 6, 9). Moreover, early life stressors and smoking increase the incidence of AUDs later in life (2, 9).
Animal studies generally support this clinical evidence and human epidemiology: Stress and nicotine potentiate alcohol consumption (10–14), but some results are equivocal (15). In addition to increasing drinking, stress and nicotine facilitate maladaptive behaviors that develop in response to alcohol administration, thereby contributing to the development of addiction (16–19). Typical examples of alcohol-related behaviors in animals include lever pressing for ethanol (operant self-administration), increased hyperactivity following repeated alcohol challenges (locomotor sensitization), and preference for an alcohol-related environment (conditioned place preference).
One influential hypothesis suggests that acquisition and expression of drug-related behaviors involve drug-induced synaptic modifications within the mesocorticolimbic dopamine (DA) system (20–22). Drugs of abuse modify synaptic transmission by usurping mechanisms normally involved in long-term strengthening or weakening of synaptic connections, a phenomenon called drug-evoked synaptic plasticity (21). Although stress, nicotine, and alcohol have broad, individual effects on the brain, some of their actions converge onto the same mechanisms and circuits. We postulate that stress and nicotine enhance alcohol-related behaviors, in part via modulation of alcohol-evoked neuronal plasticity and metaplasticity mechanisms. Stress modulates alcohol-evoked plasticity via the release of signaling molecules that directly and indirectly influence synaptic transmission. Evidence indicates that nicotine activates some of the same signaling molecules, cells, and circuits, producing a partial convergence of both stress and nicotine onto common plasticity mechanisms that influence alcohol self-administration (10, 11, 23). Moreover, stress, nicotine, and alcohol itself converge onto common glutamatergic and GABAergic circuitry within the mesolimbic system. Synaptic mechanisms of plasticity, as well as the broad excitability of circuits, are influenced. For example, stress signaling via glucocorticoids alters the midbrain GABAergic response evoked by alcohol. The stress experience causes specific GABAergic circuit elements that normally broadcast inhibition to become excitatory when responding to alcohol (10, 11). These kinds of plastic circuit changes underlie the increased risk for AUDs. The interplay between stress and drug-evoked synaptic plasticity in mesolimbic circuitry contributes to the formation of habitual alcohol usage behavior, a characteristic feature of AUDs.
SYNAPTIC PLASTICITY, STRESS, NICOTINE, AND ALCOHOL
An organism adapts and optimizes its behavior through learning, which relies on experience-dependent synaptic plasticity in the brain. Synaptic plasticity refers specifically to an activity-dependent change in synaptic strength or efficacy; this and other forms of plasticity underlie the capacity of the brain to transform environmental demands into lasting memories that cue successful behaviors. Synaptic plasticity commonly involves long-term potentiation (LTP) or long-term depression (LTD) of synaptic transmission between neurons.
A typical example of learning through experience is reinforcement learning, in which an animal modifies its behavior to obtain reward or to avoid danger. Drugs of abuse, including nicotine and ethanol, dysregulate this type of learning, leading to addiction, which is a compulsion to obtain the drug despite harmful consequences. Thus, drugs of abuse induce a kind of learning disorder that, on the cellular level, produces long-lasting alterations in synaptic plasticity, called drug-evoked synaptic plasticity (20–22). Drug-evoked synaptic plasticity modifies neuronal circuitry that ultimately participates in drug-associated behaviors (21).
Drug-evoked synaptic plasticity occurring at excitatory and inhibitory synapses within the mesocorticolimbic DA system plays a critical role in the acquisition and expression of drug-associated behaviors (for reviews, see 20, 21). The mesocorticolimbic DA system regulates reward and reinforcement processing, motivation, and goal-directed behaviors. An important DA pathway originates in the ventral tegmental area (VTA) and projects to many brain regions, including the nucleus accumbens (NAc), dorsal striatum, prefrontal cortex (PFC), and amygdala. Ethanol activates midbrain DA neurons, increasing DA levels in the mesocorticolimbic system, as do other drugs of abuse, including nicotine (2, 24). Increased DA levels participate in different forms of ethanol-evoked synaptic plasticity, thereby contributing to the acquisition of various ethanol-related behaviors. Rather than reviewing all forms of ethanol-evoked synaptic plasticity within the mesolimbic system, we focus specifically on ethanol-evoked synaptic plasticity in the VTA and the NAc.
Stress potently modulates synaptic plasticity in many brain areas, including the DA system (25, 26). Growing evidence links stress-signaling molecules to different drug-related mechanisms and behaviors (27, 28). Among numerous stress-related molecules, glucocorticoids play a significant role owing to their ability to regulate various forms of long-term plasticity in the brain. The release of glucocorticoids requires activation of the hypothalamic-pituitary-adrenal (HPA) axis. The activation of the HPA axis involves the production of corticotropin-releasing factor (CRF) in neurons of the paraventricular nucleus of the hypothalamus and release of CRF into the hypothalamo-hypophyseal portal system. CRF then acts on the anterior lobe of the pituitary to stimulate the secretion of adrenocorticotropic hormone (ACTH). ACTH activates the adrenal cortex, promoting the release of glucocorticoids, which influence neuronal activity via both genomic and nongenomic mechanisms.
Nicotine obtained from tobacco binds to nicotinic acetylcholine receptors and activates DA signals in the mesolimbic system as an early step in the addiction process (29). Nicotine and ethanol interact to engage an ensemble of neurons within interconnected brain structures involved in stress and reward processing. These brain structures include the VTA, NAc, dorsomedial PFC, and extended amygdala (30). Nicotine also directly actives the HPA axis and, via that activity, influences the mesoaccumbens DA system (10, 31). Therefore, synaptic plasticity in the mesoaccumbens DA system serves as a locus of interaction between nicotine, stress, and alcohol.
Nicotine has broad interactions throughout the nervous system (29, 32), and acute nicotine activates the stress response and elicits glucocorticoid release (10, 31). The nicotine-mediated increase of glucocorticoids alters ethanol’s pharmacological effects on mesolimbic circuitry and facilitates acquisition of ethanol self-administration (10). Acute pretreatment with nicotine induces an attenuation of ethanol-induced DA signals along the mesoaccumbens pathway that lasts for several days. The decreased ethanol-induced DA release arises from increased GABAergic inhibitory transmission onto VTA DA neurons (10). These nicotine-induced neuroadaptations require a signal that acts significantly within the VTA. Blocking glucocorticoid receptors prior to nicotine exposure prevents the midbrain circuitry changes, the decreased alcohol-induced DA response, and the nicotine-induced increase in alcohol self-administration.
There are several ways that drugs of abuse, such as nicotine, and stress can influence synaptic plasticity. On a mechanistic level, drugs and stress induce various forms of LTP and LTD at excitatory or inhibitory synapses. In addition, drugs and stress trigger lasting changes in neurons and synapses, such that the ability of these neurons and synapses to generate LTP or LTD at later times is altered. This form of modulation establishes a preconditional state and so has been termed metaplasticity. Metaplasticity, therefore, represents a higher-order form of plasticity (i.e., plasticity of synaptic plasticity) (33). Furthermore, chronic exposure to stress and drugs of abuse can result in homeostatic synaptic scaling (34). Homeostatic scaling is a form of plasticity that adjusts the strength of all synapses on a given neuron in response to a prolonged change in activity. Specifically, prolonged inhibition results in potentiation of all synapses on a given neuron, whereas prolonged excitation leads to overall synaptic depression. Homeostatic scaling operates on a much slower timescale than LTP and LTD and may play a role in the transition from drug use to addiction (35, 36).
STRESS, ALCOHOL, AND PLASTICITY AT GLUTAMATERGIC SYNAPSES
AMPA/NMDA Ratios and Mechanisms of Long-Term Potentiation and Long-Term Depression
There are different forms of synaptic plasticity, and drugs of abuse and stress may influence these forms differently. N-methyl-D-aspartate (NMDA) receptor–dependent LTP remains the best-characterized form of long-term synaptic plasticity and is commonly referred to as Hebbian plasticity, after Donald Hebb (37), who predicted that correlations in neuronal activity induce synaptic potentiation (38). Triggering this form of plasticity requires NMDA receptor activation via the coincidence of presynaptic glutamate release with postsynaptic depolarization. The depolarization removes the Mg2+ block of NMDA receptors, which allows Ca2+ to enter postsynaptically (Figure 1a). An increase in intracellular Ca2+, the critical trigger for this and some other forms of synaptic plasticity, activates intracellular signaling cascades that promote the insertion of AMPA receptors into the postsynaptic membrane. This functional increase in postsynaptic strength is maintained over time by increased protein synthesis and is linked with structural modifications of the dendritic spines (39, 40).
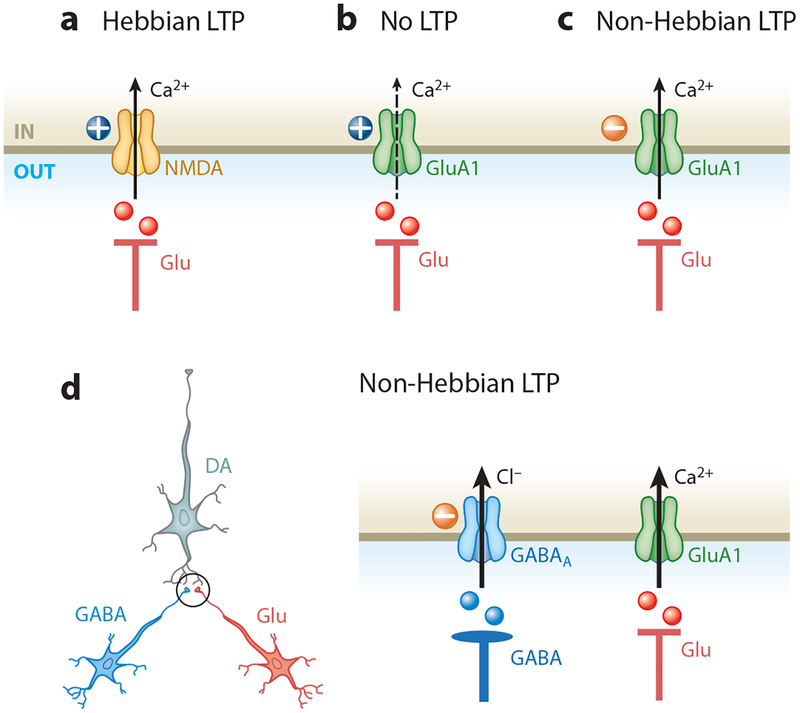
Figure 1.
Hebbian and non-Hebbian long-term potentiation (LTP) in the ventral tegmental area (VTA). (a) Presynaptic glutamate (Glu) release activates postsynaptic AMPA and N-methyl-D-aspartate (NMDA) receptors. AMPA receptor–mediated postsynaptic depolarization (plus sign) relieves the Mg2+ block of NMDA receptors, enabling them to become the main source of the increase in Ca2+ that triggers Hebbian LTP. (b) GluA1 AMPA receptor subunits provide another source of Glu postsynaptic Ca2+, which triggers plasticity independently of NMDA receptors. However, when Glu release is paired with significant postsynaptic depolarization, the decreased Ca2+ entry via GluA1 receptors then decreases the likelihood of LTP. (c) In contrast, pairing Glu release with postsynaptic hyperpolarization (minus sign) permits Ca2+ entry through GluA1 AMPA receptors, thereby increasing the likelihood of GluA1 non-Hebbian LTP (47). (d) In addition to Glu projections, VTA dopamine neurons receive inhibitory inputs from GABAergic neurons. Glu and GABA synapses (encircled on the left) on the dopamine neuron are expanded on the right. Postsynaptic GABA receptor activation prevents membrane depolarization, promoting non-Hebbian LTP.
NMDA receptor activation can also induce LTD of glutamatergic synapses. LTD requires a moderate increase in intracellular Ca2+ via weak NMDA receptor activation. This activity triggers a different Ca2+-dependent intracellular signaling cascade than that required for LTP. NMDA receptor–dependent LTD is mediated by removal of AMPA receptors from the postsynaptic membrane (39). NMDA receptor–dependent LTP and LTD are synapse specific: Only synapses where the NMDA receptors are active may potentially undergo changes in synaptic strength (40).
AMPA receptors are added to or removed from the postsynaptic membrane during Hebbian LTP and LTD, respectively. Therefore, the synaptic strength may be experimentally inferred by calculating the ratio of the postsynaptic current carried by AMPA receptors versus NMDA receptors, called the AMPA/NMDA ratio (20, 41). There are advantages to using the AMPA/NMDA normalization procedure: It is independent of the number of synapses activated and of the intensity of presynaptic stimulation. However, the AMPA/NMDA ratio provides only the relative contribution of AMPA and NMDA receptors, and it is not sufficient to determine whether the change in plasticity is mediated via AMPA receptors, NMDA receptors, or both. In addition, the interpretative value of the AMPA/NMDA ratio is more complex when the plasticity changes the proportion of Ca2+-permeable AMPA receptor subunits. When the AMPA receptor sub-type contains subunits permeable to Ca2+ (i.e., GluA1), it is susceptible to being blocked in a voltage-dependent manner by intracellular polyamines, leading to inward rectification. Therefore, investigators often pair an estimate of the AMPA/NMDA ratio with a measure of the AMPA-mediated currents at both negative and positive membrane potentials. In summary, measuring the AMPA/NMDA ratio remains a relatively simple methodology used to estimate changes in glutamatergic synaptic strength.
Ethanol and Stress Induce Long-Term Potentiation–Like Plasticity in the Ventral Tegmental Area and Nucleus Accumbens
Single, noncontingent (experimenter-administered) in vivo application of ethanol induces LTP-like strengthening of glutamatergic transmission onto VTA DA neurons, observed as an increase of the AMPA/NMDA ratio the next day (41, 42; but see 43). Pretreatment of VTA slices with ethanol or voluntary consumption of ethanol also increases the AMPA/NMDA ratio (44, 45). This form of plasticity has been observed for many drugs of abuse. The potentiation is attributed to an increase of the Ca2+-permeable AMPA receptors, GluA1s, and a simultaneous decrease of NMDA receptor function, likely via the insertion of the quasi-Ca2+-impermeable GluN3A subunit (41, 46–49).
Similar to ethanol, stress potentiates glutamatergic transmission onto DA neurons and increases the AMPA/NMDA ratio. Forced swim stress induces this form of LTP, which requires NMDA receptor activation and upregulation of the AMPA GluA1 subunit (41, 50). Moreover, activation of glucocorticoid receptors is sufficient to increase the AMPA/NMDA ratio, and preventing the activation of glucocorticoid receptors abolishes the effect of acute stress (51). Other forms of stress, including repeated restraint, social defeat, or unpredicted stress, all increase GluA1 levels in the VTA (48, 52).
Although there are no direct results for stress and ethanol, we may generalize from other studies that the induction of plasticity does not affect all VTA DA neurons equally because DA neurons are heterogeneous. That heterogeneity correlates loosely with their topology and projection targets (53, 54). For example, cocaine administration increases the AMPA/NMDA ratio in DA neurons projecting to the NAc shell but not in neurons projecting to the medial PFC (mPFC) (55). In contrast, a painful stimulus (i.e., injection of formalin to the hindpaw) potentiates glutamatergic afferents onto DA neurons projecting to the mPFC but not to those projecting to the NAc shell. Both cocaine and painful stimuli increase the AMPA/NMDA ratio in DA neurons projecting to the lateral shell of the NAc (55). These results suggest that plasticity induction in the VTA has a loose topological granularity.
Acute ethanol and stress potentiate glutamatergic afferents to the NAc, a primary target of midbrain DA neurons. The NAc consists of two major subregions, the core and the shell, which play different roles in drug-related behavior. The NAc shell is often associated with novelty and with the primary reinforcing effects of addictive drugs (56). The core plays a more significant role in behaviors regulated by cues, such as lever pressing for a reward or cue-induced reinstatement of reward seeking (57, 58). The main cell type in the NAc is the GABAergic medium spiny projection neuron (MSN), which usually expresses D1 or D2 DA receptor subtypes. D1 MSNs are thought to mediate positive reward, and D2 MSNs participate in aversion (59). However, this established view has been challenged by recent studies indicating the role of D2 MSNs in motivation-related behavior (60, 61). It is likely that more complexities in the roles of the MSN subtypes will arise with further research.
A single ethanol exposure, or even the first voluntary ethanol drinking session, results in long-lasting potentiation of excitatory transmission onto NAc D1, but not D2, MSNs (62). Ethanol increases the AMPA/NMDA ratio in the NAc shell and promotes a switch in AMPA receptor composition toward more GluA1-containing receptors. This form of ethanol-induced LTP requires activation of D1 receptors and the mechanistic target of rapamycin complex 1 (mTORC1), a kinase responsible for protein synthesis–dependent maintenance of LTP (63). Preventing this form of ethanol-induced plasticity via inhibition of mTORC1 attenuates ethanol consumption during subsequent drinking sessions. Unlike in the shell, potentiation of glutamatergic transmission in the NAc core requires repetitive administration of ethanol (62, 64).
Exposure to stress potentiates glutamatergic plasticity in the NAc shell and core. Forced swim stress or exposure to exogenous corticosterone increases the AMPA/NMDA ratio in the NAc shell (65). Stress-induced LTP involves an increase in the number or function of GluA2-containing AMPA receptors without any reduction in NMDA receptor signaling. Restraint stress increases the AMPA/NMDA ratio and GluA1 subunit expression in the NAc core for as long as 3 weeks (66, 67). Evidence indicates that the NAc core is important for associating environmental (exteroceptive) cues with rewarding outcomes (68). Glutamatergic afferent transmission in the NAc core participates in the stress-induced augmentation of cocaine self-administration and reinstatement (67, 69). Although direct experimental results are lacking, it is likely that stress-induced synaptic alterations in the NAc core have similar effects on ethanol-related behaviors.
Interestingly, alterations in glutamatergic transmission in the NAc core are also associated with the interoceptive effects of alcohol; interoceptive effects are defined as the subjective sensations experienced following a drug exposure. The interoceptive effects of alcohol are believed to reflect its pharmacological activity in the brain. Similar to exteroceptive cues, such as sounds or specific environments, interoceptive effects, such as feeling cheerful or dizzy, can become associated with the self-administration behavior. Human studies demonstrate that social drinkers exposed to a social stress increase ethanol consumption and report a blunted subjective response to alcohol (70, 71). In that case, an individual may consume more alcohol to achieve the desired interoceptive effects. Importantly, animal studies show that decreased sensitivity to the interoceptive effects of alcohol following corticosterone exposure is linked to neuroadaptations of glutamatergic transmission in the NAc core (72).
Stress and Alcohol Induce Metaplasticity
Exposure to stress and alcohol may induce insertion of GluA1 AMPA receptor subunits into glutamatergic synapses (41, 48–50, 62, 67; but see 65). The GluA1 subunit confers Ca2+ permeability to the AMPA receptors, and at depolarized membrane potentials, the currents are smaller. These properties of the GluA1 subunit may impact the subsequent induction of activity-dependent synaptic plasticity. In naive animals, pairing presynaptic glutamate release with a post-synaptic depolarization induces Hebbian-like LTP via NMDA receptor–dependent calcium entry (Figure 1a). However, after an addictive drug administration increases the AMPA/NMDA ratio, this Hebbian protocol becomes inefficient (Figure 1b) (47). LTP can be induced, however, when an excitatory input is coupled with a hyperpolarization of DA neurons, facilitating Ca2+ entry through calcium-permeable GluA1 AMPA receptors (Figure 1c). This form of synaptic plasticity is non-Hebbian (47). Therefore, in addition to triggering LTP, stress and addictive drugs can alter subsequent plasticity. That is, stress or drugs create a new initial state owing to metaplasticity of VTA glutamatergic afferents.
DA neurons can be hyperpolarized under several conditions. For example, cocaine inhibits the DA transporters and, consequently, may activate D2 autoreceptors that induce a rapid hyperpolarization of DA neurons (73). Ethanol can hyperpolarize DA neurons via increased GABAergic inputs (10, 11, 74). Interestingly, recent findings demonstrate that stress acting via glucocorticoid receptors enhances ethanol-induced GABAA receptor–mediated transmission onto VTA DA neurons (10, 11). Enhanced GABAergic input following stress correlates with a long-lasting (>3 weeks) increase in alcohol self-administration. Ethanol normally increases glutamatergic transmission onto DA neurons, and this effect is not altered following stress (10, 11, 75). By pairing glutamatergic and GABAergic inputs, ethanol dosing, subsequent to stress, may potentiate glutamatergic synapses onto DA neurons via non-Hebbian mechanisms, as didactically illustrated in Figure 1d (47).
Mechanisms of Ethanol- and Stress-Mediated Induction of Long-Term Potentiation
How does ethanol induce LTP in the VTA and the NAc? Several studies point to the central role of DA release: Optogenetic DA neuron stimulation mimics drug-driven AMPA receptor subunit redistribution in the VTA (73), and D1 receptor inhibition prevents ethanol-induced plasticity in the NAc (62). Ethanol-mediated DA release in the VTA and the NAc results from ethanol-induced excitation of DA neurons (24). Interestingly, DA release in the VTA retrogradely activates D1 receptors on presynaptic glutamate terminals and, consequently, promotes glutamatergic release onto DA neurons (75). Increased levels of glutamate activate AMPA receptors on DA neurons, further promoting VTA DA release. This positive feedback loop may significantly contribute to the initiation of ethanol-induced LTP in VTA DA neurons.
Various addictive drugs, including nicotine, cocaine, and benzodiazepines, increase the AMPA/NMDA ratio via activation of NMDA receptors (21, 42). However, the role of NMDA receptors in ethanol-induced LTP remains unclear because numerous studies indicate that ethanol inhibits NMDA receptors (76–78). An obvious contradiction arises between ethanol-mediated inhibition of NMDA receptors and ethanol-induced LTP. One possible explanation for this contradiction is that (depending on the brain region) ethanol inhibition of NMDA receptors decreases following extended (10–15 min) ethanol exposure and reverses to potentiation upon ethanol washout (77, 78). An alternative explanation is offered by a study performed in the mPFC, where NMDA receptor inhibition results in a rapid activation of mTORC1, which upregulates the calcium-permeable GluA1 AMPA receptor subunit (79). As mentioned above, ethanol-induced LTP in the NAc requires activation of mTORC1 (62), counterintuitively suggesting that this form of plasticity may be triggered by NMDA receptor inhibition.
Similar to addictive drugs, stressors, including restraint, foot shock, needle injection, and social defeat, increase DA activity within the mesolimbic system (80–84). This effect on DA activity likely involves stress-induced release of CRF in the VTA, as administration of this hormone increases DA neuron firing (85, 86). In addition to modulating DA neuron activity, CRF potentiates NMDA receptor currents and increases the intracellular Ca2+ concentration in VTA DA neurons (87, 88). Orexin is another neuropeptide that participates in stress-mediated responses. Orexin neurons project from the lateral hypothalamus to many brain areas, including the VTA. Orexin effects on excitatory neurotransmission in the VTA are similar to those of CRF, i.e., potentiation of NMDA currents onto DA neurons (89, 90). Glucocorticoids may also increase glutamatergic release onto DA neurons and potentiate NMDA receptor currents (91, 92).
Effects of Stress and Ethanol on NMDA Receptor Transmission
In addition to acutely modulating NMDA receptor channel function, ethanol promotes synaptic plasticity of NMDA receptors in VTA DA neurons. Evoked synaptic excitation, paired with burst activity of DA neurons, induces LTP of NMDA receptor–mediated excitatory transmission (LTPNMDA) (93). The plasticity requires burst-evoked Ca2+ signals in DA neurons that are amplified by preceding metabotropic glutamate receptor activity (93). Repeated exposure to alcohol (over the course of 7 days) enhances LTPNMDA in the VTA via potentiation of metabotropic (mGluR) receptor-mediated Ca2+ signaling (94). The precise mechanisms for the Ca2+ signal potentiation remain unclear but involve sensitization of the IP3 receptor, which mediates Ca2+ release from internal stores. This potentiation of mGluR-mediated Ca2+ signaling promotes conditioned place preference for addictive drugs, suggesting that this plasticity contributes to associative learning (94–96).
Stress also modulates the plasticity of NMDA receptor–mediated transmission. Exposure to CRF, repeated social defeat, and social isolation in adolescence all enhance LTPNMDA (17, 94,95). The effect of stress is blocked by glucocorticoid receptor antagonist and, like the effect of drugs of abuse, involves sensitization of IP3 receptors. The stressful experiences promote alcohol-, cocaine-, and amphetamine-conditioned place preference in rodents (17, 95), which is consistent with this plasticity underlying associative learning.
Chronic Alcohol- and Stress-Induced Alterations in Glutamatergic Transmission
Acute ethanol increases glutamate release in the NAc (97). The elevated glutamate concentration is short-lasting and is not observed 24 hours after the ethanol exposure (98). In contrast, repetitive ethanol administration, prolonged voluntary self-administration, and chronic intermittent ethanol exposure all increase basal glutamate levels following 24 hours of withdrawal (98–100). Glutamate levels increase in both the NAc core and NAc shell, but this effect does not persist into late withdrawal (98, 101). However, after ethanol dependence is induced using chronic intermittent ethanol vapor, elevated basal glutamate concentrations persist for >7 days (100). In a similar result, one session of restraint stress boosts the basal glutamate concentration in the NAc core but not in the shell (67). The glutamate levels remain elevated for at least 3 weeks. Although the exact mechanisms underlying these long-lived increases in glutamate are not known, it is likely that glutamate reuptake is decreased (66, 98, 99). These enduring elevations of glutamate can induce homeostatic synaptic scaling in the NAc. This scaling arises from postsynaptic AMPA and NMDA receptor changes that then modulate subsequent synaptic plasticity (34).
Low-frequency stimulation of NAc glutamatergic afferents triggers NMDA receptor– dependent LTD specifically in D1 MSNs. After repetitive intermittent ethanol exposure induces metaplasticity at the NAc glutamatergic synapses, low-frequency stimulation produces LTP in D1 MSNs for a day (102, 103). Protracted withdrawal from ethanol disrupts both LTD and LTP in the NAc (64, 103), and evidence suggests that persistent disruption of NAc synaptic plasticity is indicative of a drug-dependent state (104). NMDA receptor expression and function parallel the ethanol-mediated changes in synaptic plasticity: NMDA signaling increases early in withdrawal and decreases during protracted withdrawal (64, 102). Opposing plasticity occurs in D2 MSNs in the NAc. In naive animals, low-frequency stimulation has no effect on putative D2 MSNs, but similar stimulation induces LTD after repetitive exposure to ethanol, suggesting potentiation of glutamatergic inputs onto these neurons during withdrawal (102).
Different chronic stress protocols depotentiate glutamatergic synapses in the NAc and reduce LTD selectively in D1-expressing MSNs, an effect associated with anhedonia (105, 106). In contrast to NAc D1 neurons, chronic social defeat increases excitatory synaptic transmission onto D2 MSNs, and optogenetic stimulation of D2 MSNs promotes the susceptibility to social defeat without inducing anhedonia (107). The effect of chronic stress on synaptic transmission in the NAc is input specific. Chronic social defeat increases glutamatergic pathways selectively from the ventral hippocampus to D1 MSNs but decreases glutamatergic synaptic transmission from the mPFC (108). Thus, the interaction between alcohol- and stress-induced synaptic plasticity has specificity at different pathways in the NAc. Although results are not yet available for alcohol, a recent study compares synaptic strength at the single-spine level for cocaine-induced and stress-induced neuroadaptations. The results suggest that stress and cocaine induce divergent changes in synaptic function in the NAc (109).
STRESS, ALCOHOL, AND PLASTICITY AT INHIBITORY SYNAPSES
Most addiction studies focus on glutamatergic synaptic plasticity, but drugs of abuse and stress also modulate inhibitory synaptic transmission (110, 111). Stress-induced changes to inhibitory VTA synapses influence various drug-related behaviors (10, 11, 112, 113). Although inhibitory synapses exhibit multiple forms of long-term synaptic plasticity, their modulation by stress or alcohol has not been extensively studied. As it does at glutamatergic synapses, high-frequency glutamatergic stimulation induces LTP at GABAA synapses onto VTA DA neurons (LTPGABA) (114). Inducing this form of LTP requires a NMDA receptor–dependent increase in postsynaptic Ca2+. Therefore, LTPGABA is heterosynaptic: It requires glutamatergic NMDA receptor activity, but GABAergic synaptic transmission is potentiated. Ca2+ entry through NMDA receptors activates the release of nitric oxide (NO), a diffusible signal produced by VTA DA neurons. NO potentiates inhibitory synapses by increasing GABA release from presynaptic terminals (114).
An alternative way to induce synaptic plasticity is to stimulate the presynaptic neuron shortly before or shortly after stimulating the postsynaptic neuron. This form of synaptic plasticity depends on the temporal correlation between the action potentials of pre- and postsynaptic neurons, called spike timing–dependent synaptic plasticity (STDP). Compared to other methods that induce synaptic plasticity, STDP protocols often more closely mimic the natural neuronal activity that induces plasticity (38). STDP is also often bidirectional; i.e., it can result in either LTP or LTD depending on the relative timing between pre- and postsynaptic action potentials. For example, GABAergic synapses onto VTA DA neurons undergo spike timing–dependent LTP when the presynaptic glutamatergic spike arrives shortly (i.e., in the millisecond range) before the post-synaptic action potential, whereas the same synapses exhibit spike timing–dependent LTD when the postsynaptic action potential fires shortly before the presynaptic spike (115). This form of LTPGABA is also heterosynaptic because it requires NMDA receptor activation. However, STDP in the VTA does not alter presynaptic GABA release, suggesting that postsynaptic changes mediate this form of plasticity (115).
Another form of GABAergic synaptic plasticity is mediated by shifts in the GABAA receptor reversal potential. The GABAA receptor reversal potential is the membrane potential at which anionic current through GABAA receptor channels changes direction from inward to outward. The current through GABAA receptors is carried by chloride (Cl−) and bicarbonate (HCO3−) ions. Because the permeability for Cl− is higher than that for HCO3− and the extracellular Cl− concentration is high, when GABAA channels open, the membrane potential moves toward the Cl− reversal potential. The GABAA reversal potential varies depending primarily on the intracellular Cl− concentration because the extracellular Cl− concentration (as well as the HCO3− concentration) is relatively constant. In adult neurons, the intracellular Cl− concentration is low, keeping the GABAA reversal more negative than the neuron’s resting potential. Low intracellular Cl− creates the driving force for Cl− ions to flow into the cell through the GABAA receptor (Figure 2a). This Cl− gradient underlies the normally hyperpolarizing, inhibitory action of GABAA receptor activity (116). In developing neurons and under several pathological conditions, the intracellular Cl− concentration becomes higher, shifting the GABAA reversal potential in the depolarized direction (117, 118). A depolarized GABAA reversal potential indicates a decreased Cl− gradient and a decrease in the strength of synaptic inhibition (Figure 2b) (119, 120). Although the shifts in the GABAA reversal potential were originally discovered during development and pathology, accumulating evidence demonstrates that they are triggered in the adult brain during very high physiological GABAergic activity (11, 121, 122).
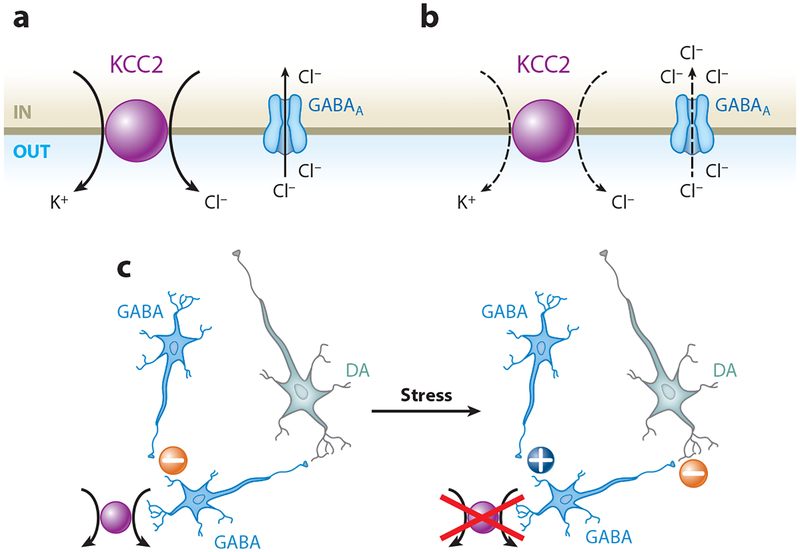
Figure 2.
Functional expression of the K+-Cl− cotransporter (KCC2) regulates neural circuitry in the ventral tegmental area (VTA). (a) KCC2 mediates Cl− extrusion from neurons, maintaining the concentration gradient that favors Cl− entry through the GABAA receptor. (b) Decreased KCC2 function leads to intracellular Cl− accumulation, resulting in an impaired Cl− gradient and decreased synaptic GABAA receptor inhibition. (c) KCC2 mediates normal GABAergic inhibition of VTA GABA neurons projecting onto dopamine (DA) neurons (left minus sign). Exposure to stress downregulates KCC2 and shifts GABAA receptor signaling toward excitation of VTA GABA neurons (plus sign). Excitation of VTA GABA neurons promotes inhibition of DA neurons (right minus sign) (11).
Shifts in the GABAA reversal potential are often attributed to functional changes in the anion transporters, which establish and maintain the Cl− gradient in neurons (123). Some of these transporters load Cl− into the cell, and others extrude Cl−. The Na+-K+-Cl− cotransporter, NKCC1, and the Cl−-HCO3− exchanger, AE3, mainly accumulate Cl− into neurons. The K+-Cl− cotransporter, KCC2, is the main mechanism to extrude Cl− ions, but the Na+-dependent Cl−-HCO3− exchanger, NDAE, also performs that function in neurons. Not all neurons express all of these mechanisms. It is more common that a subset of these transporters (possibly along with other mechanisms) controls the Cl− gradient of individual cells.
KCC2 is expressed specifically in neurons, where it moves Cl− out of the cell against its gradient, maintaining low intracellular Cl− (Figure 2a). Several studies report activity-induced and Ca2+-dependent downregulation of KCC2 function, resulting in impaired Cl− extrusion and weakened GABAergic inhibition (121, 122, 124). When KCC2 efficacy is decreased, strong GABAA receptor activity leads to a smaller Cl− gradient (Figure 2b). The increased intracellular Cl− moves the reversal potential for GABAA in the depolarized direction. Thus, the normally inhibitory GABAA synapse moves toward excitatory depolarization (125). In contrast, enhancing KCC2 activity increases the strength of inhibition owing to very low intracellular Cl−, resulting in a more negative reversal potential (11, 124, 126). It seems reasonable to hypothesize that modulation of transporters and exchangers that control the Cl− gradient produces variations in the strength of GABAA inhibition. This process may serve as a kind of plasticity that regulates the response of circuits on both a fine and a broad scale.
Ethanol- and Stress-Mediated Plasticity at GABAergic Synapses in the Ventral Tegmental Area
A single in vivo exposure to ethanol enhances GABAergic release onto VTA DA neurons (11,74). Ethanol-induced synaptic potentiation is long lasting (>1 week) and correlates with increased ethanol self-administration. In addition, ex vivo administration of ethanol in midbrain slices increases GABAergic release onto DA neurons (10, 11, 127; but see 128).
A single pre-exposure to restraint stress (10–15 hours before ethanol exposure) increases ethanol-induced midbrain GABAergic transmission onto DA neurons and ethanol self-administration (11). The stress alone does not potentiate GABA transmission onto DA neurons. Rather, stress increases the ethanol-induced excitation of VTA GABA neurons, which arises because excitatory GABAergic inputs drive other GABA neurons. The Cl− gradient collapse in specific GABA neurons of the circuitry results in ethanol-induced GABAergic excitation of GABA neurons that, in turn, project to DA neurons (Figure 2c) (11). The shift in the GABAA reversal potential arises owing to glucocorticoid-mediated downregulation of KCC2 function. Importantly, in the VTA and substantia nigra (SN), KCC2 is expressed exclusively in non-DA neurons, indicating the presence of another chloride extrusion mechanism in DA neurons (11, 129, 130). The stress treatment enables the ethanol-induced transition to excitatory GABAA receptor signaling in some VTA GABA neurons, but not in DA neurons (11). Because nicotine activates some aspects of the stress axis, nicotine may act, at least in part, similarly to stress and produce comparable effects on midbrain circuitry and alcohol self-administration in rats (10).
GABA neurons compose approximately one-third of VTA neurons and play an important role in various drug-related behaviors (11, 113). VTA GABA neurons provide local inhibition and project to other brain areas (113). Synaptic inputs onto VTA GABA neurons also include projections from the NAc, lateral hypothalamus, and bed nucleus of the stria terminalis (113, 131, 132). In VTA GABA neurons, the KCC2-dependent transition toward depolarizing or excitatory GABA signaling occurs in response to morphine (129, 133). In the neonatal brain, KCC2 expression is low, and activation of GABAA receptors induces depolarization, which activates NMDA receptors or voltage-gated calcium channels, triggering Ca2+ entry and, consequently, LTP or LTD at GABAergic synapses (134, 135). In addition to its Cl− extrusion function, KCC2 plays a critical role in the development and plasticity of glutamatergic synapses. Although the precise mechanisms are still unclear, KCC2 interacts with the cytoskeleton, contributing to the formation of spines and to the delivery of AMPA receptors to the membrane (136, 137). Therefore, stress-induced changes in KCC2 function and in the GABAA reversal potential could represent a form of metaplasticity that modifies GABAergic and glutamatergic plasticity in VTA GABA neurons. This metaplasticity then alters the circuit’s response to subsequent ethanol exposures.
Stress and Ethanol Modulate Stimulus-Induced GABA Plasticity in the Ventral Tegmental Area
A single in vivo or ex vivo exposure to ethanol inhibits LTPGABA induced by high-frequency stimulation (138). Ethanol inhibits LTPGABA via mu-opioid receptor-dependent intracellular mechanisms downstream of NO signaling (114, 138).
Exposure to acute forced swim stress also blocks LTPGABA in the VTA (112). The effect of stress on LTPGABA involves activation of glucocorticoid and kappa-opioid receptors (112). It is noteworthy that kappa-opioid receptors do not mediate the stress-induced increase of the AMPA/NMDA ratio at the excitatory synapses (112), indicating that stress induces distinct forms of metaplasticity at GABAergic and glutamatergic synapses.
Early life stressors, such as maternal deprivation, also modify inhibitory neurotransmission in the VTA (139). Maternal deprivation attenuates GABAergic signaling onto VTA DA neurons when this signaling is measured several days later. In addition, maternal deprivation impairs the ability of GABAergic synapses to exhibit normal bidirectional STDP. Early life stress also induces abnormalities in GABAergic signaling through epigenetic modifications in VTA DA neurons (139). Future experiments must determine how VTA GABAergic changes induced by maternal deprivation influence alcohol drinking behavior later in life (140).
TOPOGRAPHICAL CONSIDERATIONS
Stress, nicotine, and ethanol trigger various forms of plasticity at multiple synapses within the VTA and the NAc. As observed at both excitatory and inhibitory synapses, stress, ethanol, and other drugs of addiction often converge in potentiating or depressing similar pathways, but the mechanisms can be different. It is important to understand how these pathways contribute to the acquisition and expression of various alcohol-related behaviors and, eventually, induce addiction. The anatomical layout of the midbrain DA system offers clues to the function of the mesolimbic pathways.
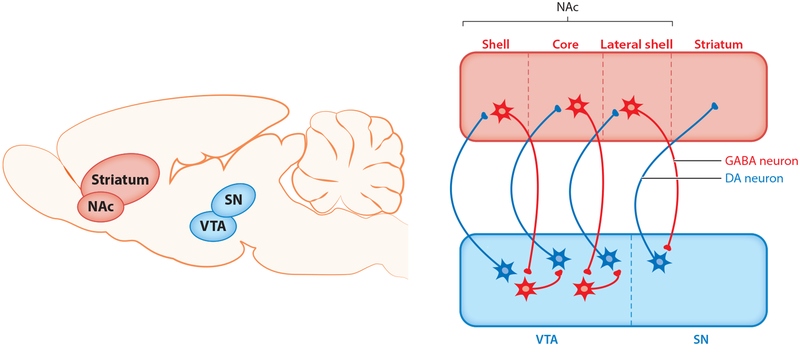
Anatomical studies indicate that the midbrain (VTA and SN) and the striatum (NAc and dorsal striatum) are connected in a spiraling manner (Figure 3) (141, 142). Many medial DA neurons in the VTA project to the medial shell of the NAc (54). D1 MSNs from the medial shell project back, via interneurons, to DA neurons in the more lateral part of the VTA (132). Those lateral DA neurons, in turn, project to the more lateral core of the NAc. MSNs in the core connect to DA neurons in the most lateral parts of the VTA and SN (143). DA neurons in the SN project to the dorsal striatum (Figure 3). It has been suggested that the gradual recruitment of more lateral parts of the VTA and SN complex, together with more dorsal areas of the striatum, mediates the transition from goal-directed actions to habitual behaviors during the progression of the addiction process (144, 145).
Figure 3.
A schematic representation of the connectivity between the ventral tegmental area (VTA) and the nucleus accumbens (NAc). Connections between the midbrain and the striatum form a spiral that emanates from medial parts of the VTA and NAc, moving toward lateral parts of the substantia nigra (SN) and the striatum. Dopamine (DA) neurons from the VTA project to GABAergic medium spiny projection neurons (MSNs) in the NAc. D1 MSNs from the NAc project back to VTA GABA and DA neurons. During early phases of addiction, exposure to drugs of abuse, including alcohol, potentiates synaptic connectivity in the most medial parts of the spiral. Repeated drug administration progressively activates more lateral parts of the spiral in the SN, eventually recruiting the dorsal striatum, a brain region implicated in habitual drug seeking. We hypothesize that stress promotes habitual drinking, in part via modulation of alcohol-evoked synaptic plasticity within the spiral (147).
One influential hypothesis is that the initial synaptic plasticity in the medial part of the spiral amplifies the connectivity within the spiral. This potentiation makes it easier to activate more lateral parts of the spiral, thus promoting habitual behavior during the later stages of addiction (146, 147). A progressive shift toward habitual ethanol self-administration has been reported in human and animal studies (for a review, see 148). Similarly, exposure to acute and chronic stress promotes habitual behaviors in humans and animals (149–151). Future studies may determine whether the stress-induced escalation in drinking is mediated through facilitation of habitual behaviors.
CONCLUSIONS
During the early stages of addiction, stress, nicotine, and alcohol often engage synaptic plasticity in comparable areas of the brain that contribute to the addiction process. However, few studies provide mechanistic insights into how stress- or nicotine-induced plasticity modifies subsequent ethanol-induced plasticity. Among the many actions of nicotine is its ability to activate, at least in part, the stress signaling axis, producing some similarities to stress’s influences over mesolimbic circuitry. It will be important to determine input specificity, i.e., whether individual synapses at DA neurons and MSNs are regulated similarly by stress and alcohol. Given the different mechanisms involved in stress- and alcohol-induced synaptic plasticity, it is unlikely that stress and alcohol act at identical synapses via identical mechanisms. However, despite the possible difference in input specificity, shared downstream mechanisms may contribute to the phenomenon of comorbidity between stress (or nicotine) and alcohol. Although the synaptic alterations described in this review often correlate with several alcohol-related behaviors, future studies should provide the direct causal link between changes in synaptic transmission and certain behaviors driven by alcohol. For now, we can only speculate that stress produces its most powerful and long-lasting effects on alcohol-related behaviors via modulation of alcohol-evoked synaptic plasticity and metaplasticity of circuit elements.
Stress: threatening and harmful condition that culminates in the release of stress-signaling molecules, including glucocorticoids, catecholamines, and neuropeptides
Metaplasticity: activity-dependent changes in neurons and synapses that modulate subsequent synaptic plasticity; often referred as the plasticity of synaptic plasticity
ACKNOWLEDGMENTS
We thank Blake A. Kimmey, Alyse M. Thomas, and Shannon Wolfman for helpful comments and discussions. The authors were supported by grants from the US National Institutes of Health (NINDS NS021229 and NIDA DA009411 and DA036572).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. 2004. Actual causes of death in the United States, 2000. JAMA 291:1238–45 [DOI] [PubMed] [Google Scholar]
- 2.Dani JA, Harris RA. 2005. Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nat. Neurosci 8:1465–70 [DOI] [PubMed] [Google Scholar]
- 3.Batel P, Pessione F, Maitre C, Rueff B. 1995. Relationship between alcohol and tobacco dependencies among alcoholics who smoke. Addiction 90:977–80 [DOI] [PubMed] [Google Scholar]
- 4.Weitzman ER, Chen YY. 2005. The co-occurrence of smoking and drinking among young adults in college: national survey results from the United States. Drug Alcohol Depend 80:377–86 [DOI] [PubMed] [Google Scholar]
- 5.Barrett SP, Tichauer M, Leyton M, Pihl RO. 2006. Nicotine increases alcohol self-administration in non-dependent male smokers. Drug Alcohol Depend 81:197–204 [DOI] [PubMed] [Google Scholar]
- 6.Harrison EL, Desai RA, McKee SA. 2008. Nondaily smoking and alcohol use, hazardous drinking, and alcohol diagnoses among young adults: findings from the NESARC. Alcohol. Clin. Exp. Res 32:2081–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uhart M, Wand GS. 2009. Stress, alcohol and drug interaction: an update of human research. Addict. Biol 14:43–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ayer LA, Harder VS, Rose GL, Helzer JE. 2011. Drinking and stress: an examination of sex and stressor differences using IVR-based daily data. Drug Alcohol Depend 115:205–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keyes KM, Hatzenbuehler ML, Grant BF, Hasin DS. 2012. Stress and alcohol: epidemiologic evidence. Alcohol Res 34:391–400 [PMC free article] [PubMed] [Google Scholar]
- 10.Doyon WM, Dong Y, Ostroumov A, Thomas AM, Zhang TA, Dani JA. 2013. Nicotine decreases ethanol-induced dopamine signaling and increases self-administration via stress hormones. Neuron 79:530–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ostroumov A, Thomas AM, Kimmey BA, Karsch JS, Doyon WM, Dani JA. 2016. Stress increases ethanol self-administration via a shift toward excitatory GABA signaling in the ventral tegmental area. Neuron 92:493–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Becker HC, Lopez MF, Doremus-Fitzwater TL. 2011. Effects of stress on alcohol drinking: a review of animal studies. Psychopharmacology 218:131–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blomqvist O, Ericson M, Johnson DH, Engel JA, Soderpalm B. 1996. Voluntary ethanol intake in the rat: effects of nicotinic acetylcholine receptor blockade or subchronic nicotine treatment. Eur. J. Pharmacol 314:257–67 [DOI] [PubMed] [Google Scholar]
- 14.Skelly MJ, Chappell AE, Carter E, Weiner JL. 2015. Adolescent social isolation increases anxiety-like behavior and ethanol intake and impairs fear extinction in adulthood: possible role of disrupted noradrenergic signaling. Neuropharmacology 97:149–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noori HR, Helinski S, Spanagel R. 2014. Cluster and meta-analyses on factors influencing stress-induced alcohol drinking and relapse in rodents. Addict. Biol 19:225–32 [DOI] [PubMed] [Google Scholar]
- 16.Phillips TJ, Roberts AJ, Lessov CN. 1997. Behavioral sensitization to ethanol: genetics and the effects of stress. Pharmacol. Biochem. Behav 57:487–93 [DOI] [PubMed] [Google Scholar]
- 17.Whitaker LR, Degoulet M, Morikawa H. 2013. Social deprivation enhances VTA synaptic plasticity and drug-induced contextual learning. Neuron 77:335–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gubner NR, Phillips TJ. 2015. Effects of nicotine on ethanol-induced locomotor sensitization: a model of neuroadaptation. Behav. Brain Res 288:26–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maddux JN, Chaudhri N. 2017. Nicotine-induced enhancement of Pavlovian alcohol-seeking behavior in rats. Psychopharmacology 234:727–38 [DOI] [PubMed] [Google Scholar]
- 20.Kauer JA, Malenka RC. 2007. Synaptic plasticity and addiction. Nat. Rev. Neurosci 8:844–58 [DOI] [PubMed] [Google Scholar]
- 21.Luscher C 2016. The emergence of a circuit model for addiction. Annu. Rev. Neurosci 39:257–76 [DOI] [PubMed] [Google Scholar]
- 22.Hyman SE, Malenka RC, Nestler EJ. 2006. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu. Rev. Neurosci 29:565–98 [DOI] [PubMed] [Google Scholar]
- 23.Armario A 2010. Activation of the hypothalamic-pituitary-adrenal axis by addictive drugs: different pathways, common outcome. Trends Pharmacol. Sci 31:318–25 [DOI] [PubMed] [Google Scholar]
- 24.Doyon WM, Thomas AM, Ostroumov A, Dong Y, Dani JA. 2013. Potential substrates for nicotine and alcohol interactions: a focus on the mesocorticolimbic dopamine system. Biochem. Pharmacol 86:1181–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polter AM, Kauer JA. 2014. Stress and VTA synapses: implications for addiction and depression. Eur.J. Neurosci 39:1179–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Francis TC, Lobo MK. 2017. Emerging role for nucleus accumbens medium spiny neuron subtypes in depression. Biol. Psychiatry 81:645–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marinelli M, Piazza PV. 2002. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur. J. Neurosci 16:387–94 [DOI] [PubMed] [Google Scholar]
- 28.Pastor R, Reed C, Meyer PJ, McKinnon C, Ryabinin AE, Phillips TJ. 2012. Role of corticotropin-releasing factor and corticosterone in behavioral sensitization to ethanol. J. Pharmacol. Exp. Ther 341:455–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dani JA, Bertrand D. 2007. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu. Rev. Pharmacol. Toxicol 47:699–729 [DOI] [PubMed] [Google Scholar]
- 30.Leao RM, Cruz FC, Vendruscolo LF, de Guglielmo G, Logrip ML, et al. 2015. Chronic nicotine activates stress/reward-related brain regions and facilitates the transition to compulsive alcohol drinking.J. Neurosci 35:6241–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Porcu P, Sogliano C, Cinus M, Purdy RH, Biggio G, Concas A. 2003. Nicotine-induced changes in cerebrocortical neuroactive steroids and plasma corticosterone concentrations in the rat. Pharmacol. Biochem. Behav 74:683–90 [DOI] [PubMed] [Google Scholar]
- 32.De Biasi M, Dani JA. 2011. Reward, addiction, withdrawal to nicotine. Annu. Rev. Neurosci 34:105–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abraham WC. 2008. Metaplasticity: tuning synapses and networks for plasticity. Nat. Rev. Neurosci 9:387. [DOI] [PubMed] [Google Scholar]
- 34.Turrigiano GG. 2008. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell 135:422–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, et al. 2004. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci. Biobehav. Rev 27:739–49 [DOI] [PubMed] [Google Scholar]
- 36.DiFranza JR, Wellman RJ. 2005. A sensitization-homeostasis model of nicotine craving, withdrawal, and tolerance: integrating the clinical and basic science literature. Nicot. Tob. Res 7:9–26 [DOI] [PubMed] [Google Scholar]
- 37.Hebb DO. 1949. The Organization of Behavior: A Neuropsychological Theory. New York: Wiley [Google Scholar]
- 38.Caporale N, Dan Y. 2008. Spike timing-dependent plasticity: a Hebbian learning rule. Annu. Rev. Neurosci 31:25–46 [DOI] [PubMed] [Google Scholar]
- 39.Citri A, Malenka RC. 2008. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology 33:18–41 [DOI] [PubMed] [Google Scholar]
- 40.Luscher C, Malenka RC. 2012. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb. Perspect. Biol 4:a005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saal D, Dong Y, Bonci A, Malenka RC. 2003. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron 37:577–82 [DOI] [PubMed] [Google Scholar]
- 42.Heikkinen AE, Moykkynen TP, Korpi ER. 2009. Long-lasting modulation of glutamatergic transmission in VTA dopamine neurons after a single dose of benzodiazepine agonists. Neuropsychopharmacology 34:290–98 [DOI] [PubMed] [Google Scholar]
- 43.Wanat MJ, Sparta DR, Hopf FW, Bowers MS, Melis M, Bonci A. 2009. Strain specific synaptic modifications on ventral tegmental area dopamine neurons after ethanol exposure. Biol. Psychiatry 65:646–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stuber GD, Hopf FW, Hahn J, Cho SL, Guillory A, Bonci A. 2008. Voluntary ethanol intake enhances excitatory synaptic strength in the ventral tegmental area. Alcohol. Clin. Exp. Res 32:1714–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Engle SE, McIntosh JM, Drenan RM. 2015. Nicotine and ethanol cooperate to enhance ventral tegmental area AMPA receptor function via α6-containing nicotinic receptors. Neuropharmacology 91:13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuan T, Mameli M, O’Connor EC, Dey PN, Verpelli C, et al. 2013. Expression of cocaine-evoked synaptic plasticity by GluN3A-containing NMDA receptors. Neuron 80:1025–38 [DOI] [PubMed] [Google Scholar]
- 47.Mameli M, Bellone C, Brown MT, Luscher C. 2011. Cocaine inverts rules for synaptic plasticity of glutamate transmission in the ventral tegmental area. Nat. Neurosci 14:414–16 [DOI] [PubMed] [Google Scholar]
- 48.Fitzgerald LW, Ortiz J, Hamedani AG, Nestler EJ. 1996. Drugs of abuse and stress increase the expression of GluR1 and NMDAR1 glutamate receptor subunits in the rat ventral tegmental area: common adaptations among cross-sensitizing agents. J. Neurosci 16:274–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ortiz J, Fitzgerald LW, Charlton M, Lane S, Trevisan L, et al. 1995. Biochemical actions of chronic ethanol exposure in the mesolimbic dopamine system. Synapse 21:289–98 [DOI] [PubMed] [Google Scholar]
- 50.Dong Y, Saal D, Thomas M, Faust R, Bonci A, et al. 2004. Cocaine-induced potentiation of synaptic strength in dopamine neurons: behavioral correlates in GluRA−/− mice. PNAS 101:14282–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daftary SS, Panksepp J, Dong Y, Saal DB. 2009. Stress-induced, glucocorticoid-dependent strengthening of glutamatergic synaptic transmission in midbrain dopamine neurons. Neurosci. Lett 452:273–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Covington HE III, Tropea TF, Rajadhyaksha AM, Kosofsky BE, Miczek KA. 2008. NMDA receptors in the rat VTA: a critical site for social stress to intensify cocaine taking. Psychopharmacology 197:203–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ford CP, Mark GP, Williams JT. 2006. Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. J. Neurosci 26:2788–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lammel S, Hetzel A, Hackel O, Jones I, Liss B, Roeper J. 2008. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron 57:760–73 [DOI] [PubMed] [Google Scholar]
- 55.Lammel S, Ion DI, Roeper J, Malenka RC. 2011. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron 70:855–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, et al. 2004. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology 47(Suppl. 1):227–41 [DOI] [PubMed] [Google Scholar]
- 57.Robbins TW, Ersche KD, Everitt BJ. 2008. Drug addiction and the memory systems of the brain. Ann. N. Y. Acad. Sci 1141:1–21 [DOI] [PubMed] [Google Scholar]
- 58.Kalivas PW, McFarland K. 2003. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology 168:44–56 [DOI] [PubMed] [Google Scholar]
- 59.Lobo MK, Covington HE III, Chaudhury D, Friedman AK, Sun H, et al. 2010. Cell type–specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science 330:385–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Soares-Cunha C, Coimbra B, David-Pereira A, Borges S, Pinto L, et al. 2016. Activation of D2 dopamine receptor-expressing neurons in the nucleus accumbens increases motivation. Nat. Commun 7:11829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trifilieff P, Feng B, Urizar E, Winiger V, Ward RD, et al. 2013. Increasing dopamine D2 receptor expression in the adult nucleus accumbens enhances motivation. Mol. Psychiatry 18:1025–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beckley JT, Laguesse S, Phamluong K, Morisot N, Wegner SA, Ron D. 2016. The first alcohol drink triggers mTORC1-dependent synaptic plasticity in nucleus accumbens dopamine D1 receptor neurons. J. Neurosci 36:701–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buffington SA, Huang W, Costa-Mattioli M. 2014. Translational control in synaptic plasticity and cognitive dysfunction. Annu. Rev. Neurosci 37:17–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abrahao KP, Ariwodola OJ, Butler TR, Rau AR, Skelly MJ, et al. 2013. Locomotor sensitization to ethanol impairs NMDA receptor-dependent synaptic plasticity in the nucleus accumbens and increases ethanol self-administration. J. Neurosci 33:4834–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Campioni MR, Xu M, McGehee DS. 2009. Stress-induced changes in nucleus accumbens glutamate synaptic plasticity. J. Neurophysiol 101:3192–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garcia-Keller C, Kupchik YM, Gipson CD, Brown RM, Spencer S, et al. 2016. Glutamatergic mechanisms of comorbidity between acute stress and cocaine self-administration. Mol. Psychiatry 21:1063–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garcia-Keller C, Martinez SA, Esparza MA, Bollati F, Kalivas PW, Cancela LM. 2013. Cross-sensitization between cocaine and acute restraint stress is associated with sensitized dopamine but not glutamate release in the nucleus accumbens. Eur. J. Neurosci 37:982–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Day JJ, Carelli RM. 2007. The nucleus accumbens and Pavlovian reward learning. Neuroscientist 13: 148–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.De Giovanni LN, Guzman AS, Virgolini MB, Cancela LM. 2016. NMDA antagonist MK 801 in nucleus accumbens core but not shell disrupts the restraint stress-induced reinstatement of extinguished cocaine-conditioned place preference in rats. Behav. Brain Res 315:150–59 [DOI] [PubMed] [Google Scholar]
- 70.Childs E, O’Connor S, de Wit H. 2011. Bidirectional interactions between acute psychosocial stress and acute intravenous alcohol in healthy men. Alcohol. Clin. Exp. Res 35:1794–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de Wit H, Soderpalm AH, Nikolayev L, Young E. 2003. Effects of acute social stress on alcohol consumption in healthy subjects. Alcohol. Clin. Exp. Res 27:1270–77 [DOI] [PubMed] [Google Scholar]
- 72.Besheer J, Fisher KR, Jaramillo AA, Frisbee S, Cannady R. 2014. Stress hormone exposure reduces mGluR5 expression in the nucleus accumbens: functional implications for interoceptive sensitivity to alcohol. Neuropsychopharmacology 39:2376–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brown MT, Bellone C, Mameli M, Labouebe G, Bocklisch C, et al. 2010. Drug-driven AMPA receptor redistribution mimicked by selective dopamine neuron stimulation. PLOS ONE 5:e15870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Melis M, Camarini R, Ungless MA, Bonci A. 2002. Long-lasting potentiation of GABAergic synapses in dopamine neurons after a single in vivo ethanol exposure. J. Neurosci 22:2074–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xiao C, Shao XM, Olive MF, Griffin WC III, Li KY, et al. 2009. Ethanol facilitates glutamatergic transmission to dopamine neurons in the ventral tegmental area. Neuropsychopharmacology 34:307–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lovinger DM, White G, Weight FF. 1989. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science 243:1721–24 [DOI] [PubMed] [Google Scholar]
- 77.Yaka R, Phamluong K, Ron D. 2003. Scaffolding of Fyn kinase to the NMDA receptor determines brain region sensitivity to ethanol. J. Neurosci 23:3623–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ron D, Wang J. 2009. The NMDA receptor and alcohol addiction In Biology of the NMDA Receptor, ed. Van Dongen AM, pp. 59–78. Boca Raton, FL: CRC Press; [PubMed] [Google Scholar]
- 79.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, et al. 2010. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329:959–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brischoux F, Chakraborty S, Brierley DI, Ungless MA. 2009. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. PNAS 106:4894–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Imperato A, Puglisi-Allegra S, Casolini P, Zocchi A, Angelucci L. 1989. Stress-induced enhancement of dopamine and acetylcholine release in limbic structures: role of corticosterone. Eur. J. Pharmacol 165:337–38 [DOI] [PubMed] [Google Scholar]
- 82.Anstrom KK, Woodward DJ. 2005. Restraint increases dopaminergic burst firing in awake rats. Neuropsychopharmacology 30:1832–40 [DOI] [PubMed] [Google Scholar]
- 83.Tidey JW, Miczek KA. 1996. Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Res 721:140–49 [DOI] [PubMed] [Google Scholar]
- 84.Dong Y, Zhang T, Li W, Doyon WM, Dani JA. 2010. Route of nicotine administration influences in vivo dopamine neuron activity: habituation, needle injection, and cannula infusion. J. Mol. Neurosci 40:164–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Korotkova TM, Brown RE, Sergeeva OA, Ponomarenko AA, Haas HL. 2006. Effects of arousal- and feeding-related neuropeptides on dopaminergic and GABAergic neurons in the ventral tegmental area of the rat. Eur. J. Neurosci 23:2677–85 [DOI] [PubMed] [Google Scholar]
- 86.Wanat MJ, Hopf FW, Stuber GD, Phillips PEM, Bonci A. 2008. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J. Physiol 586:2157–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Riegel AC, Williams JT. 2008. CRF facilitates calcium release from intracellular stores in midbrain dopamine neurons. Neuron 57:559–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. 2003. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron 39:401–7 [DOI] [PubMed] [Google Scholar]
- 89.Bonci A, Borgland S. 2009. Role of orexin/hypocretin and CRF in the formation of drug-dependent synaptic plasticity in the mesolimbic system. Neuropharmacology 56(Suppl. 1):107–11 [DOI] [PubMed] [Google Scholar]
- 90.Berridge CW, Espana RA, Vittoz NM. 2010. Hypocretin/orexin in arousal and stress. Brain Res 1314: 91–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Overton PG, Tong ZY, Brain PF, Clark D. 1996. Preferential occupation of mineralocorticoid receptors by corticosterone enhances glutamate-induced burst firing in rat midbrain dopaminergic neurons. Brain Res 737:146–54 [DOI] [PubMed] [Google Scholar]
- 92.Cho K, Little HJ. 1999. Effects of corticosterone on excitatory amino acid responses in dopamine-sensitive neurons in the ventral tegmental area. Neuroscience 88:837–45 [DOI] [PubMed] [Google Scholar]
- 93.Harnett MT, Bernier BE, Ahn KC, Morikawa H. 2009. Burst-timing-dependent plasticity of NMDA receptor-mediated transmission in midbrain dopamine neurons. Neuron 62:826–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bernier BE, Whitaker LR, Morikawa H. 2011. Previous ethanol experience enhances synaptic plasticity of NMDA receptors in the ventral tegmental area. J. Neurosci 31:5205–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stelly CE, Pomrenze MB, Cook JB, Morikawa H. 2016. Repeated social defeat stress enhances glutamatergic synaptic plasticity in the VTA and cocaine place conditioning. eLife 5:e15448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ahn KC, Bernier BE, Harnett MT, Morikawa H. 2010. IP3 receptor sensitization during in vivo amphetamine experience enhances NMDA receptor plasticity in dopamine neurons of the ventral tegmental area. J. Neurosci 30:6689–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moghaddam B, Bolinao ML. 1994. Biphasic effect of ethanol on extracellular accumulation of glutamate in the hippocampus and the nucleus accumbens. Neurosci. Lett 178:99–102 [DOI] [PubMed] [Google Scholar]
- 98.Melendez RI, Hicks MP, Cagle SS, Kalivas PW. 2005. Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcohol. Clin. Exp. Res 29:326–33 [DOI] [PubMed] [Google Scholar]
- 99.Pati D, Kelly K, Stennett B, Frazier CJ, Knackstedt LA. 2016. Alcohol consumption increases basal extra-cellular glutamate in the nucleus accumbens core of Sprague-Dawley rats without increasing spontaneous glutamate release. Eur. J. Neurosci 44:1896–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Griffin WC III, Haun HL, Hazelbaker CL, Ramachandra VS, Becker HC. 2014. Increased extracellular glutamate in the nucleus accumbens promotes excessive ethanol drinking in ethanol dependent mice. Neuropsychopharmacology 39:707–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ding ZM, Rodd ZA, Engleman EA, Bailey JA, Lahiri DK, McBride WJ. 2013. Alcohol drinking and deprivation alter basal extracellular glutamate concentrations and clearance in the mesolimbic system of alcohol-preferring (P) rats. Addict. Biol 18:297–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Renteria R, Maier EY, Buske TR, Morrisett RA. 2017. Selective alterations of NMDAR function and plasticity in D1 and D2 medium spiny neurons in the nucleus accumbens shell following chronic intermittent ethanol exposure. Neuropharmacology 112:164–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jeanes ZM, Buske TR, Morrisett RA. 2011. In vivo chronic intermittent ethanol exposure reverses the polarity of synaptic plasticity in the nucleus accumbens shell. J. Pharmacol. Exp. Ther 336:155–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kasanetz F, Deroche-Gamonet V, Berson N, Balado E, Lafourcade M, et al. 2010. Transition to addiction is associated with a persistent impairment in synaptic plasticity. Science 328:1709–12 [DOI] [PubMed] [Google Scholar]
- 105.Jiang B, Wang W, Wang F, Hu ZL, Xiao JL, et al. 2013. The stability of NR2B in the nucleus accumbens controls behavioral and synaptic adaptations to chronic stress. Biol. Psychiatry 74:145–55 [DOI] [PubMed] [Google Scholar]
- 106.Lim BK, Huang KW, Grueter BA, Rothwell PE, Malenka RC. 2012. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature 487:183–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Francis TC, Chandra R, Friend DM, Finkel E, Dayrit G, et al. 2015. Nucleus accumbens medium spiny neuron subtypes mediate depression-related outcomes to social defeat stress. Biol. Psychiatry 77:212–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bagot RC, Parise EM, Pena CJ, Zhang HX, Maze I, et al. 2015. Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat. Commun 6:7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Khibnik LA, Beaumont M, Doyle M, Heshmati M, Slesinger PA, et al. 2016. Stress and cocaine trigger divergent and cell type-specific regulation of synaptic transmission at single spines in nucleus accumbens. Biol. Psychiatry 79:898–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xin W, Edwards N, Bonci A. 2016. VTA dopamine neuron plasticity: the unusual suspects. Eur. J. Neurosci 44:2975–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maguire J 2014. Stress-induced plasticity of GABAergic inhibition. Front. Cell Neurosci 8:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Graziane NM, Polter AM, Briand LA, Pierce RC, Kauer JA. 2013. Kappa opioid receptors regulate stress-induced cocaine seeking and synaptic plasticity. Neuron 77:942–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Creed MC, Ntamati NR, Tan KR. 2014. VTA GABA neurons modulate specific learning behaviors through the control of dopamine and cholinergic systems. Front. Behav. Neurosci 8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nugent FS, Penick EC, Kauer JA. 2007. Opioids block long-term potentiation of inhibitory synapses. Nature 446:1086–90 [DOI] [PubMed] [Google Scholar]
- 115.Kodangattil JN, Dacher M, Authement ME, Nugent FS. 2013. Spike timing-dependent plasticity at GABAergic synapses in the ventral tegmental area. J. Physiol 591:4699–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, et al. 1999. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 397:251–55 [DOI] [PubMed] [Google Scholar]
- 117.De Koninck Y 2007. Altered chloride homeostasis in neurological disorders: a new target. Curr. Opin. Pharmacol 7:93–99 [DOI] [PubMed] [Google Scholar]
- 118.Ben-Ari Y 2002. Excitatory actions of GABA during development: the nature of the nurture. Nat. Rev. Neurosci 3:728–39 [DOI] [PubMed] [Google Scholar]
- 119.Hewitt SA, Wamsteeker JI, Kurz EU, Bains JS. 2009. Altered chloride homeostasis removes synaptic inhibitory constraint of the stress axis. Nat. Neurosci 12:438–43 [DOI] [PubMed] [Google Scholar]
- 120.Coull JAM, Boudreau D, Bachand K, Prescott SA, Nault F, et al. 2003. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature 424:938–42 [DOI] [PubMed] [Google Scholar]
- 121.Woodin MA, Ganguly K, Poo MM. 2003. Coincident pre- and postsynaptic activity modifies GABAergic synapses by postsynaptic changes in Cl− transporter activity. Neuron 39:807–20 [DOI] [PubMed] [Google Scholar]
- 122.Fiumelli H, Cancedda L, Poo MM. 2005. Modulation of GABAergic transmission by activity via post-synaptic Ca2+-dependent regulation of KCC2 function. Neuron 48:773–86 [DOI] [PubMed] [Google Scholar]
- 123.Hubner CA, Holthoff K. 2013. Anion transport and GABA signaling. Front. Cell Neurosci 7:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lee HH, Deeb TZ, Walker JA, Davies PA, Moss SJ. 2011. NMDA receptor activity downregulates KCC2 resulting in depolarizing GABAA receptor-mediated currents. Nat. Neurosci 14:736–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Staley KJ, Soldo BL, Proctor WR. 1995. Ionic mechanisms of neuronal excitation by inhibitory GABAA receptors. Science 269:977–81 [DOI] [PubMed] [Google Scholar]
- 126.Gagnon M, Bergeron MJ, Lavertu G, Castonguay A, Tripathy S, et al. 2013. Chloride extrusion enhancers as novel therapeutics for neurological diseases. Nat. Med 19:1524–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Theile JW, Morikawa H, Gonzales RA, Morrisett RA. 2008. Ethanol enhances GABAergic transmission onto dopamine neurons in the ventral tegmental area of the rat. Alcohol. Clin. Exp. Res 32:1040–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xiao C, Ye JH. 2008. Ethanol dually modulates GABAergic synaptic transmission onto dopaminergic neurons in ventral tegmental area: role of μ-opioid receptors. Neuroscience 153:240–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Taylor AM, Castonguay A, Ghogha A, Vayssiere P, Pradhan AA, et al. 2016. Neuroimmune regulation of GABAergic neurons within the ventral tegmental area during withdrawal from chronic morphine. Neuropsychopharmacology 41:949–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gulacsi A, Lee CR, Sik A, Viitanen T, Kaila K, et al. 2003. Cell type-specific differences in chloride-regulatory mechanisms and GABAA receptor-mediated inhibition in rat substantia nigra. J. Neurosci 23:8237–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nieh EH, Vander Weele CM, Matthews GA, Presbrey KN, Wichmann R, et al. 2016. Inhibitory input from the lateral hypothalamus to the ventral tegmental area disinhibits dopamine neurons and promotes behavioral activation. Neuron 90:1286–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xia Y, Driscoll JR, Wilbrecht L, Margolis EB, Fields HL, Hjelmstad GO. 2011. Nucleus accumbens medium spiny neurons target non-dopaminergic neurons in the ventral tegmental area. J. Neurosci 31:7811–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Laviolette SR, Gallegos RA, Henriksen SJ, van der Kooy D. 2004. Opiate state controls bi-directional reward signaling via GABAA receptors in the ventral tegmental area. Nat. Neurosci 7:160–69 [DOI] [PubMed] [Google Scholar]
- 134.Gubellini P, Ben-Ari Y, Gaiarsa JL. 2001. Activity- and age-dependent GABAergic synaptic plasticity in the developing rat hippocampus. Eur. J. Neurosci 14:1937–46 [DOI] [PubMed] [Google Scholar]
- 135.McLean HA, Caillard O, Ben-Ari Y, Gaiarsa JL. 1996. Bidirectional plasticity expressed by GABAergic synapses in the neonatal rat hippocampus. J. Physiol 496(2):471–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chevy Q, Heubl M, Goutierre M, Backer S, Moutkine I, et al. 2015. KCC2 gates activity-driven AMPA receptor traffic through cofilin phosphorylation. J. Neurosci 35:15772–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li H, Khirug S, Cai C, Ludwig A, Blaesse P, et al. 2007. KCC2 interacts with the dendritic cytoskeleton to promote spine development. Neuron 56:1019–33 [DOI] [PubMed] [Google Scholar]
- 138.Guan YZ, Ye JH. 2010. Ethanol blocks long-term potentiation of GABAergic synapses in the ventral tegmental area involving μ-opioid receptors. Neuropsychopharmacology 35:1841–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Authement ME, Langlois LD, Kassis H, Gouty S, Dacher M, et al. 2016. Morphine-induced synaptic plasticity in the VTA is reversed by HDAC inhibition. J. Neurophysiol 116:1093–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nylander I, Roman E. 2013. Is the rodent maternal separation model a valid and effective model for studies on the early-life impact on ethanol consumption? Psychopharmacology 229:555–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ikemoto S 2007. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res. Rev 56:27–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Haber SN, Fudge JL, McFarland NR. 2000. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J. Neurosci 20:2369–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Edwards NJ, Tejeda HA, Pignatelli M, Zhang S, McDevitt RA, et al. 2017. Circuit specificity in the inhibitory architecture of the VTA regulates cocaine-induced behavior. Nat. Neurosci 20:438–48 [DOI] [PubMed] [Google Scholar]
- 144.Belin D, Everitt BJ. 2008. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron 57:432–41 [DOI] [PubMed] [Google Scholar]
- 145.Everitt BJ, Robbins TW. 2005. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci 8:1481–89 [DOI] [PubMed] [Google Scholar]
- 146.Grueter BA, Rothwell PE, Malenka RC. 2012. Integrating synaptic plasticity and striatal circuit function in addiction. Curr. Opin. Neurobiol 22:545–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Luscher C, Bellone C. 2008. Cocaine-evoked synaptic plasticity: a key to addiction? Nat. Neurosci 11:737–38 [DOI] [PubMed] [Google Scholar]
- 148.Barker JM, Taylor JR. 2014. Habitual alcohol seeking: modeling the transition from casual drinking to addiction. Neurosci. Biobehav. Rev 47:281–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gourley SL, Swanson AM, Jacobs AM, Howell JL, Mo M, et al. 2012. Action control is mediated by prefrontal BDNF and glucocorticoid receptor binding. PNAS 109:20714–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, et al. 2009. Chronic stress causes frontostriatal reorganization and affects decision-making. Science 325:621–25 [DOI] [PubMed] [Google Scholar]
- 151.Schwabe L, Wolf OT. 2009. Stress prompts habit behavior in humans. J. Neurosci 29:7191–98 [DOI] [PMC free article] [PubMed] [Google Scholar]