Abstract
Tolerance to cannabinoid agonists can develop through desensitization of the cannabinoid receptor 1 (CB1) following prolonged administration. Desensitization results from phosphorylation of CB1 by a G protein-coupled receptor kinase (GRK), and subsequent association of the receptor with arrestin. Mice expressing a mutant form of CB1, in which the serine residues at two putative phosphorylation sites necessary for desensitization have been replaced by non-phosphorylatable alanines (S426A/S430A), display reduced tolerance to Δ9-tetrahydroeannabinol (Δ9-THC). Tolerance to the antinociceptive effects of WIN55,212-2 was delayed in S426A/S430A mutants using the tail-flick and formalin tests. However, tolerance to the antinociceptive effects of once daily CP55,940 injections was not significantly delayed in S426A/S430A mutant mice using either of these tests. Interestingly, the dose response curve shifts for the hypothermic and antinociceptive effects of CP55,940 that were induced by chronic treatment with this agonist in wild-type mice were blocked in S426A/S430A mutant mice. Assessment of mechanical allodynia in mice exhibiting chronic cisplatin-evoked neuropathic pain found that tolerance to the anti-allodynic effects WIN55,212-2 but not CP55,940 was delayed in S426A/S430A mice compared to wild-type littermates. Despite these deficits in tolerance, S426A/S430A mutant mice eventually developed tolerance to both WIN55,212-2 and CP55,940 for all pain assays that were examined, suggesting that other mechanisms likely contribute to tolerance for these cannabinoid agonists. These findings suggest that GRK- and (βarrestin2-mediated desensitization of CB1 may strongly contribute to the rate of tolerance to the antinociceptive effects of WIN55,212-2, and raises the possibility of agonist-specific mechanisms of cannabinoid tolerance.
Keywords: Cannabinoid, Pain, Tolerance, Antinociception, Desensitization, CB1, Mice
1. Introduction
Cannabinoid-based therapeutics have shown efficacy in clinical studies for the treatment of chronic and neuropathic pain (Whiting et al., 2016). Cannabinoid drugs act through cannabinoid receptors including the cannabinoid receptor 1 (CB1) to exert a wide range of effects in the brain. The antinociceptive effects of cannabinoids have been demonstrated in a wide range of preclinical models of acute (Lichtman and Martin, 1991; Morgan et al., 2014), chronic neuropathic (Deng et al., 2015; Fox et al., 2001), and inflammatory pain (Walker et al., 1999). Despite the potential use of cannabinoids as clinical analgesics, there are limitations to their therapeutic use, including dependence (Degenhardt et al., 2013), tolerance (D’Souza et al., 2008), and adverse side effects (Hall, 2015). Therefore, a greater understanding of the mechanisms responsible for these effects is important to facilitate the development of better cannabinoid-based therapies.
The therapeutic actions of cannabinoids are limited, in part, by the development of tolerance during chronic administration (Bedi et al., 2010; D’Souza et al., 2008). Prolonged agonist exposure leads to receptor desensitization and has been implicated as one possible mechanism underlying tolerance (Martin et al., 2004; Morgan et al., 2014; Selley et al., 2004). Phosphorylation of CB1 by G protein-coupled receptor kinases (GRKs) leads to desensitization (Gainetdinov et al., 2004; Sim et al., 2006) and endocytosis (Hsieh et al., 1999; Wu et al., 2008) of the receptor. Agonist-bound CB1 can be phosphorylated at two serine residues (426 and 430) on the intracellular tail of CB1 facilitating interaction with an arrestin protein, such as β-arrestin2 (Breivogel et al., 2008; Daigle et al., 2008; DeWire et al., 2008; Jin et al., 1999). Mutation of these putative phosphorylation sites (S426A/S430A) leads to decreased desensitization of CB1 (Daigle et al., 2008; Delgado-Peraza et al., 2016; Jin et al., 1999; Morgan et al., 2014). We have previously demonstrated that mice expressing this desensitization-resistant form of CB1 are slower to develop tolerance to both the antinociceptive and hypothermic effects of delta-9-tetrahydrocannabinol (Δ9-THC) (Morgan et al., 2014). Similarly, delayed tolerance to Δ9-THC due to disrupted desensitization and down-regulation of CB1 has also been observed in β-arrestin2 knock-out mice (Nguyen et al., 2012).
Cannabinoid agonists exhibit substantial structural diversity (Pertwee, 2008), which may lead to distinct differences in the responses activated by their action at CB1 (Ahn et al., 2013; Georgieva et al., 2008). The cannabinoids R-(+)-[2,3-Dihydro-5-methyl-3-[(morpholinyl-methyl]pyrrolo[1,2,3-de]-1,4-benzoxazinyl]-(1-mapthalenyl)methanone mesylate (WIN55,212-2) and (1α,2β)-R-5-(1,1-dimethylheptyl)-2-[5-hydroxy-2-(3-hydroxypropyl-cyclohexyl]phenyl (CP55,940) are synthetic cannabinoids that are structurally distinct. Cannabinoid receptor agonists, like many other G protein-coupled receptor (GPCR) agonists, are able to selectively activate different intracellular signaling responses, a concept known as biased agonism or functional selectivity (Laprairie et al., 2014; Priestley et al., 2017). Δ9-THC and WIN55,212-2 induce different regional patterns of CB1 desensitization and downregulation in the mouse brain with Δ9-THC treatment generally causing a more robust effect on these neuroadaptations (Sim-Selley and Martin, 2002) raising the possibility of agonist-specific cannabinoid tolerance. The acute antinociceptive effects of CP55,940 were found to be attenuated in β-arrestinl −/− mice, while the effects of Δ9-THC were not altered (Breivogel and Vaghela, 2015). Interestingly, no differences in the development of tolerance to either agonist were observed in β-arrestinl −/− mice. Likewise, the S426A/S430A mutant form of CB1 has been demonstrated to induce biased signaling (Delgado-Peraza et al., 2016).
There is good evidence to support a role for GRK/β-arrestin2-mediated desensitization as a potential mechanism for the development of tolerance to the behavioral effects of cannabinoid agonists. Previous studies have shown that disrupted desensitization and down-regulation of the CB1 receptor in β-arrestin2 knock-out mice (Nguyen et al., 2012) delays cannabinoid tolerance and that GRK3/β-arrestin2 mediated desensitization of CB1 requires phosphorylation of serines 426 and 430 (Jin et al., 1999). However, whether biased agonism through this pathway plays a role in agonist-specific cannabinoid tolerance has not been widely investigated. Therefore, we utilized models of acute nociceptive pain (tail-flick, formalin test) and chronic neuropathic pain to investigate agonist differences in the development of tolerance to the synthetic, high-potency, full cannabinoid agonists WIN55,212-2 and CP55,940 in S426A/S430A mutant mice resistant to GRK/β-arrestin2-mediated desensitization of CB1.
2. Methods
2.1. Animals
This study was performed using 235 experimentally naïve male S426A/S430A mutant (n = 115) and wild-type (n = 120), 8-12-week-old, littermate-controlled mice on a C57BL/6 background. The generation of S426A/S430A mutant mice has been described previously (Morgan et al., 2014). After weaning, mice were group housed, with a standard 12:12h light/dark cycle (lights on at 07:00, lights off at 19:00). Mice were provided with ad libitum access to standard rodent chow (Teklad 2018, Envigo, Indianapolis, IN) and water. All animal care procedures were performed in accordance with the Guidelines of the National Institutes of Health on the Care and Use of Animals and with approval from the Pennsylvania State University College of Medicine Institutional Animal Care and Use Committee (IACUC).
2.2. Drugs
(+)—WIN55,212-2 and (−)—CP55,940 were purchased from Cayman Chemical (Ann Arbor, MI). CP55,940 was prepared in 100% ethanol and subsequently diluted 20-fold in vehicle containing 0.9% saline and 5% Cremaphor EL (Sigma-Aldrich, St Louis, MO) (18:1 vehicle v/v). WIN55,212-2 was prepared in 100% DMSO and diluted in vehicle containing 0.9% saline, 5% Cremaphor EL, and 5% ethanol (18:1:1 vehicle v/v/v) for injection. The total amount of DMSO contained in all WIN55,212-2 injections was 4% v/v. Cisplatin was purchased from Tocris (Minneapolis, MN) and subsequently dissolved in 0.9% saline. Sodium bicarbonate (Fisher Scientific, Pittsburgh, PA) was dissolved in 0.9% saline to prepare a 4% w/v solution for administration. Drugs were administered via intraperitoneal (i.p.) injection (CP55,940, WIN55,212-2, cisplatin) in a single volume of 10ml/kg of body weight or via subcutaneous (s.c.) injection (sodium bicarbonate) in a single volume of 1 ml. Formalin (2.5% v/v) was prepared by diluting formaldehyde (37%, Fisher Scientific) in water.
2.3. Procedures
2.3.1. Cumulative dose-responses
Male wild-type and S426A/S430A mutant mice were assessed for antinociceptive and hypothermic responses to vehicle and cumulative doses of 0.3, 1, 3, 10, and 30 mg/kg WIN55,212-2 or 0.01, 0.03, 0.1, 0.3, and 1 mg/kg CP55,940. Mice were administered doses of 0.3, 0.7, 2, 7, and 20 mg/kg WIN55,212-2 or 0.01, 0.02, 0.07, 0.2, and 0.7 mg/kg CP55,940 to achieve final cumulative doses. Following baseline assessments of nociceptive responses, all vehicle and drug measurements were taken 1h apart, or 60 min following each injection of either vehicle or drug. Antinociception was measured using a Columbus Instruments (Columbus, OH) TF-1 tail-flick analgesia meter with the radiant heat source set to an intensity level of 5 as described previously (Nealon et al., 2017). Exposure of the tail to the heat source was limited to 10s to avoid tissue damage. Tail-flick latencies were measured immediately prior to drug administration, and at 60 min after treatment with either vehicle or each dose of drug to calculate the percent maximal possible efficacy (% MPE) where % MPE = [(post drug latency – pre-drug latency)/(10 – pre-drug latency)] * 100. Hypothermia was measured hourly by taking body temperatures with a mouse rectal thermometer (Physitemp Instruments, Clifton, NJ). Body temperature was calculated as percent change in body temperature, where %ΔBT = [(post-drug temperature) – (pre-drug temperature)/(pre-drug temperature)] * 100.
Cumulative dose-response curves to assess tolerance to the antinociceptive and hypothermic effects of WIN55,212-2 and CP55,940 were determined in two groups of mice for each drug. Acute cumulative dose-response curves (pre; Day 0) were determined in drug naïve wild-type and S426A/S430A mice. An additional group of wild-type and S426A/S430A mice were administered either 10 mg/kg WIN55,212-2 or 0.3 mg/kg CP55,940 (i.p.) once-daily for nine consecutive days and assessed for cumulative dose responses (post) on the tenth day as described above to assess changes in drug response following chronic treatment.
2.3.2. WIN55,212-2 and CP55,940 tolerance
To identify any tolerance to repeated drug treatment over time, wild-type and S426A/S430A mutant mice were injected (i.p.) once-daily with either 10 mg/kg WIN55,212-2 for 20 consecutive days or 0.3 mg/kg CP55,940 for 15 consecutive days. Mice were tested for responses to drug effects until nociceptive tail-flick responses reached a plateau of 20–30% MPE in mice of both genotypes, resulting in different durations in the length of treatment for CP55,940 and WIN55,212-2. The doses for each drug selected for chronic administration (10 mg/kg WIN55,212-2 and 0.3 mg/kg CP55,940) were chosen based on the ability to produce antinociceptive responses of approximately 80% MPE in drug naïve mice as assessed in the cumulative dose-response curves (see Fig. 1). Another factor in the selection of 0.3 mg/kg CP55,940 was consistency with previous work from our group (LeFleur et al., 2018) and other laboratories (Deng et al., 2015). Tail-flick antinociception and hypothermia were measured each day immediately prior to and 60 min after drug administration. Drug responses (% MPE and % ΔBT) were calculated as described above.
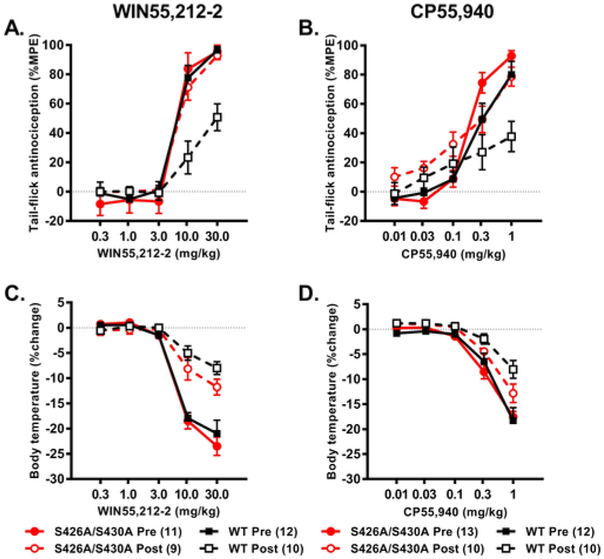
Fig. 1. S426A/S430A mutant mice demonstrate smaller shifts in the antinociceptive and hypothermic dose-response curves following chronic treatment with WIN55,212–2 and CP55,940.
Wild-type and S426A/S430A mutant mice were tested for antinociceptive (A,B) and hypothermic (C,D) responses across a cumulative dose range of either 0.3, 1, 3, 10, and 30 mg/kg WIN55,212-2 or 0.01, 0.03, 0.1, 0.3, and 1 mg/kg CP55,940 as drug naïve (Pre; filled symbols and solid lines) or following nine days of once-daily treatment with either 10 mg/kg WIN55,212-2 or 0.3 mg/kg CP55,940 (Post; open symbols and dashed lines). Means represent [%MPE (A,B) and %ΔBT (C,D)] and error bars represent the standard error of the mean (SEM). Data were analyzed with three-way ANOVA and Bonferroni multiple comparisons test. The number of mice in each group are shown in parentheses.
2.3.3. Formalin test
Wild-type and S426A/S430A mutant mice were assessed for inflammatory pain responses using the formalin test, which produces a characteristic transient, biphasic pattern of pain behavior (Tjølsen et al., 1992). One hour prior to testing, mice were injected (i.p.) with either 3 mg/kg WIN55,212-2, 0.3 mg/kg CP55,940, or the appropriate vehicle. Previous work from our group found that 0.3 but not 0.1 mg/kg CP55,940 was able to attenuate antinociception in the formalin test. In addition to our previous work, other laboratories have demonstrated the ability of 0.3 mg/kg CP55,940 to reverse paclitaxel-evoked neuropathic pain (Deng et al., 2015). The dose of WIN55,212-2 used in the formalin assay (3 mg/kg) was selected as the highest dose that did not preclude the assessment of pain behavior by causing substantial motor effects including catalepsy.
Mice were acclimated in a Plexiglass chamber (5”x5”x5”), on an elevated transparent platform, for 20 min before testing. Prior to and during testing, a mirror placed at a 45° angle underneath the platform was used to view the paws of the mouse, and a high-definition camera (Logitech, Newark, CA) placed underneath the platform was used to record the behavior of the mice in the chamber. Following acclimation (20 min), 10 μl of a 2.5% formalin solution was injected into the plantar surface of a single hind paw, using a 0.5 ml syringe with a 28 ½ gauge needle (Becton Dickson, Franklin Lakes, NJ). Mice were returned to the observation chamber immediately following formalin administration. Nociceptive behavior was measured for 60 min and quantified within twelve 5 min time bins throughout that observation period. Pain behaviors that were quantified included: the injected paw has little weight placed on it (0); the injected paw is held above the surface of the platform (1); or the injected paw is bitten, shaken, or licked (2). The amount of time the mouse spent engaged in each behavior was recorded and weighted using the composite pain score-weighted scores technique (CPS-WST0-2), which produced composite pain scores (CPS) between 0 (no pain behavior) and 2 (continuous pain behavior) for each 5 min bin (Guindon et al., 2011; Marcus et al., 2015; Watson et al., 1997). The area under the curve (AUC) was calculated for the acute phase (0–15 min; Phase 1) and the inflammatory phase (15–60min, Phase II).
Wild-type and S426A/S430A mutant mice were assessed for tolerance to the antinociceptive effects of CP55,940 and WIN 55,212-2 using the formalin test after treatment with once-daily injections of 3 mg/kg WIN55,212-2 and 0.3mg/kg CP55,940 (i.p.) for up to 21 days. Mice were assessed for pain responses in the acute (Phase I) and inflammatory (Phase II) phases following 1, 7, 14, and 21 consecutive days of administration of WIN55,212-2 and following 7, 14, and 21 consecutive days of administration of 0.3 mg/kg CP55,940 (LeFleur et al., 2018). As mice were euthanized immediately after formalin testing, separate groups of mice were used to determine drug responses for each time point. Mice were not assessed for inflammatory pain responses to 0.3 mg/kg CP55,940 on the first day of drug administration due to profound motor effects, including catalepsy, which prevented assessment of pain behaviors.
2.3.4. Cisplatin-induced neuropathy and von Frey testing
Chemotherapy-evoked neuropathic pain was induced in male wild-type and S426A/S430A mutant mice following four weeks of once-weekly i.p. injections of 5 mg/kg cisplatin. Mice were co-administered 1 ml 4% sodium bicarbonate via s.c. injection concurrent with cisplatin administration to prevent toxicity due to renal damage (Guindon et al., 2014). Neuropathic pain was assessed using the von Frey test to measure mechanical allodynia. Mice were assayed for mechanical allodynia using an electronic von Frey anesthesiometer equipped with a semi-flexible polypropylene super-tip (IITC Life Science Inc, Woodland Hills, CA). Mice were administered vehicle and assayed for allodynia prior to the first cisplatin injection and also one week following the final cisplatin administration to determine baseline and neuropathic allodynic responses. Anti-allodynic responses to once-daily treatment with 3 mg/kg WIN55,212-2 or 0.3 mg/kg CP55,940 were assessed in male wild-type and S426A/S430A mice 1 h following drug administration on alternating days (1, 3, 5, 7, etc.) until von Frey measurements returned to the pre-drug neuropathic baseline. Mice were administered drug (i.p.) once daily for up to 19 (CP55,940) or 25 (WIN55,212-2) days. These durations of drug treatment and testing were based on the amount of time for the responses of mice to return to their baseline neuropathic values. The doses of WIN55,212-2 (3 mg/kg) and CP55,940 (0.3 mg/kg) used for the formalin test were also selected based on the absence of motor effects to be consistent with previous work from other laboratories that used this dose to evaluate paclitaxel-evoked neuropathic pain (Deng et al., 2015).
2.4. Data analysis
For all dose-response data, three-way mixed ANOVAs were performed with genotype and time as the between-subjects factor and dose as the repeated measure. For all three-way ANOVAs, Mauchley's test of sphericity was determined, and when sphericity was violated, the Greenhouse-Geissler correction was used with degrees of freedom and error rounded off to the nearest whole number. Additionally, nonlinear regression analyses were performed for each dose-response curve to calculate ED50 values which were used to assess shifts in dose-response curves. For all formalin analyses, two-way (genotype and time/number of injections) between subjects ANOVAs were used to assess differences in tolerance across days (excluding vehicle). The half-time to tolerance was calculated using Prism 6 by performing non-linear regression of the hypothermic and antinociceptive effects of WIN55,212-2 and CP55,940 using a single-phase exponential decay curve. For analyses of cisplatin-evoked chronic pain, data were analyzed using mixed two-way ANOVAs where genotype was the between-subjects factor and time/number of injections served as the repeated measure. For both formalin and cisplatin analyses, S426A/S430A and wild-type mice were also assessed against their own vehicle to assess differences in tolerance development. Data were analyzed using Prism 5 (GraphPad, La Jolla, CA) for all two-way ANOVAs and using SPSS version 25.0 (IBM SPSS Statistics, Armonk, New York) for all three-way ANOVAs. For all tests, Bonferroni post-hoc analyses were performed where appropriate, and for all analyses, significance was set at p < 0.05.
3. Results
3.1. WIN55,212-2 and CP55,940 dose-response curve shifts
3.1.1. Tolerance to the antinociceptive effects of WIN55,212-2 and CP55,940
Treatment with WIN55,212-2 dose-dependently increased antinociception in the tail-flick assay (F2,79 = 166.76, p < 0.001; Fig. 1A). There were also main effects of time (F1,38 = 5.39, p = 0.026), genotype (F1,38 = 4.49, p = 0.041), and significant dose x genotype (F2,79 = 4.94, p = 0.009), dose x time (F2 79 = 8.03, p = 0.001), and genotype × time interaction (F1,38 = 7.12, p = 0.011), but no dose x genotype x time (p > 0.05) interaction effect. Post-hoc analyses revealed that S426A/S430A mice were more sensitive to the antinociceptive effects of 10 (p = 0.037) and 30 (p = 0.016) mg/kg WIN55,212-2 compared to wild-type mice. Post-hoc analyses also showed that following nine days of once-daily treatment with 10 mg/kg WIN55,212-2, that wild-type (but not S426A/S430A mutant) mice showed evidence of tolerance due to lower antinociceptive responses to 10 (p = 0.002) and 30 (p < 0.001) mgAg WIN55,212-2 (Fig. 1A). Wild-type (p < 0.0001) but not S426A/S430A (p = 0.8734) mice demonstrated a significant increase in the calculated ED50 values following nine consecutive days of CP55,940 treatment (Table 1), suggesting that only wild-type mice demonstrate tolerance to the antinociceptive effects of WIN55,212-2.
Table 1.
Calculated ED50 values (mg/kg) from tail-flick tests following treatment with WIN55,212-2 and CP55,940. ED50 values were calculated from dose-response curves generated by non-linear regression analysis. Values shown are mean and 95% confidence interval, and 10–12 mice were tested for each group. Potency ratios were calculated as the ratio between the ED50 after chronic drug dosing and the ED50 prior to chronic dosing. Confidence intervals that could not be accurately calculated from curve fitting are listed as not able to determine (ND). Data were analyzed using F tests.
| WIN55,212-2 | CP55,940 | |||
|---|---|---|---|---|
| WT | S426A/S430A | WT | S426A/S430A | |
| Pre drug | 7.668 (4.647–12.65) | 8.624 (ND) | 0.341 (0.251–0.462) | 0.213 (0.177–0.256) |
| Post drug | 28.30 (19.10–41.91) | 7.899 (6.403–9.743) | 2.206 (0.324–15.02) | 0.252 (0.163–0.391) |
| F statistic | 39.29 (p < 0.0001) | 0.22555 (p = 0.8734) | 14.74 (p = 0.0002) | 0.5822 (p = 0.4471) |
| Potency Ratio | 3.70 | 0.92 | 6.47 | 1.18 |
Acute treatment with CP55,940 dose-dependently increased antinociception using the tail-flick assay (F3,104 = 113.78, p < 0.001; Fig. 1B). While there was not an effect of time (p = 0.725) or a dose x genotype × time interaction effect (p = 0.449), there was a main effect of genotype (F1,41 = 5.82, p = 0.020) and significant dose x genotype (F3,104 = 4.09, p = 0.013) and dose x time (F3,104 = 14.57, p < 0.001) interaction effects. Post-hoc analyses showed that the antinociceptive response on day 10 was attenuated following treatment with cumulative doses of 0.3 (p = 0.024), and 1.0 (p = 0.002) mg/kg CP55,940 compared to day 0. However, for both time points, S426A/S430A mice showed a greater antinociceptive response compared to their wild-type littermates at doses of 0.3 (p = 0.022) and 1.0 (p = 0.005) mg/kg of CP55,940. Wild-type (p < 0.0001), but not S426A/S430A (p = 0.3576) mice, demonstrated a significant increase in ED50 following chronic CP55,940 treatment (Table 1), suggesting that only wild-type mice demonstrated tolerance to the antinociceptive effects of CP55,940.
3.1.2. Tolerance to the hypothermic effects of WIN55,212-2 and CP55,940
Dose-dependent decreases in body temperature were observed following treatment with WIN55,212-2 (0.3, 1.0, 3.0, 10.0, and 30.0 mg/kg) (F2,73 = 197.04, p < 0.001; Fig. 1C). There was no effect of genotype (p = 0.183) nor a dose x genotype (p = 0.207) or a dose x time x genotype (p = 0.817) interaction effect. However, there was an effect of time (F1,38 = 38.49, p < 0.001) and a dose x day (F2,73 = 36.49, p < 0.001) interaction effect. Post-hoc analysis of the dose × day interaction effect indicated that mice displayed lower hypothermic responses to 10 (p = 0.003) and 30 (p = 0.001) mg/kg WIN55,212-2 on day 10 compared to day 0. Analysis of the ED50 values for the hypothermic effects of WIN55,212-2 indicate that the shifts in ED50 values were not significant for either wild-type (p = 0.2742) or S426A/S430A mice (p = 0.3339) (Table 2).
Table 2.
Calculated ED50 values (mg/kg) from body temperature measures following treatment with WIN55,212-2 and CP55,940. ED50 values were calculated from dose-response curves generated by non-linear regression analysis. Values shown are the means with the 95% confidence intervals in parentheses. Potency ratios were calculated as the ratio between the ED50 after chronic drug dosing and the ED50 prior to chronic dosing. A sample size of 10–13 mice were tested for each group. Confidence intervals that could not be accurately calculated from curve fitting are listed as not able to determine (ND). Data were analyzed using F tests.
| WIN55,212-2 | CP55,940 | |||
|---|---|---|---|---|
| WT | S426A/S430A | WT | S426A/S430A | |
| Pre drug | 5.878 (4.148–8.330) | 6.396 (5.041–8.115) | 0.267 (0.045–1.598) | 0.341 (0.218–0.534) |
| Post drug | 9.643 (ND) | 9.577 (ND) | 1.221 (0.031–47.73) | 0.386 (0.179–0.893) |
| F statistic | 0.1209 (p = 0.2742) | 0.9436 (p = 0.3339) | 4.464 (p = 0.037) | 0.0855 (p = 0.7706) |
| Potency Ratio | 1.64 | 1.59 | 4.57 | 1.13 |
Dose-dependent hypothermia was observed following treatment with CP55,940 (0.01, 0.03, 0.1, 0.3 and 1mg/kg) (F2,73 = 184.68, p < 0.001; Fig. 1D). There was not an effect of genotype (p = 0.308) or either a dose x genotype (p = 0.151) or a dose x time x genotype (p = 0.230) interaction effect. There was an effect of time (F1,41 = 18.73, p < 0.001) as well as a dose x time (F2,73 = 8.72, p = 0.001) interaction effect. Post-hoc analyses revealed that mice display less hypothermia to 0.01 (p = 0.002), 0.03 (p = 0.012), 0.1 (p = 0.005), 0.3 (p = 0.003), and 1.0 (p < 0.001) mg/kg CP55,940 on day 10 compared to day 0. The ED50 values for the hypothermic effect of CP55,940 differed between drug naive and chronically treated wildtype (p = 0.0260) but not naïve and chronically treated S426A/S430A mice (p = 0.7678) (Table 2). These results suggest that wild-type mice develop tolerance to the hypothermic effects of WIN55,212-2 and CP55,940, while tolerance to these drugs is not complete in S426A/S430A mutants.
3.2. Tolerance to once-daily injections of WIN55,212-2 and CP55,940
3.2.1. Tolerance to the antinociceptive effects of WIN55,212-2 and CP55,940
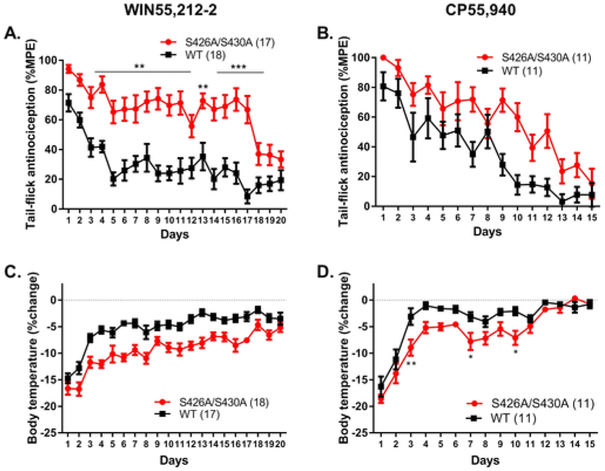
Tolerance to once-daily treatment with either 10 mg/kg WIN55,212-2 or 0.3 mg/kg CP55,940 was also assessed in wild-type and S426A/S430A mutant mice (Fig. 2). There was an effect of time (number of WIN55,212-2 injections) (F19,627 = 10.03, p < 0.0001), genotype (F1,33 = 95.89, p < 0.0001), and a significant time × genotype interaction effect (F19,627 = 1.73, p = 0.0275). Post-hoc analyses revealed that tolerance to the antinociceptive effects of 10 mg/kg WIN55,212-2 was altered in S426A/S420A mutant mice compared to wild-type littermates after 3–11 (3, 7, 8, p < 0.01; 4, 5, 6, 9, 10, 11, p < 0.001) and 13–17 (13, p < 0.01; 14–17, p < 0.001) days of WIN55,212-2 treatment (Fig. 2A). The half-time for tolerance to the antinociceptive effects of 10 mg/kg WIN55,212-2 was longer (16.62 d, 95%CI 4.5954–27.83 d) in S426A/S430A mutant mice compared to wild-type controls (1.746 d, 95%CI 1.187–3.301 d).
Fig. 2. Antinociceptive tolerance to WIN55,212–2 but not CP55,940 is significantly delayed in S426A/S430A mice.
Wild-type and S426A/S430A mice were assessed for the development of tolerance to the antinociceptive (A,B) and hypothermic (C,D) effects of once-daily 10 mg/kg WIN55,212-2 (A,C) or 0.3 mg/kg CP55,940 (B,D) in wild-type (black lines, squares) and desensitization-resistant S426A/S430A (red lines, circles) mice. Means represent [(%MPE (A,B) and %ΔBT (C,D)] and error bars represent the standard error of the mean (SEM). Data were analyzed with two-way ANOVA and Bonferroni multiple comparisons test. The number of mice in each group are shown in parentheses. *p < 0.05, **p < 0.01, ***p < 0.001. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Analysis of antinociception in mice treated daily with 0.3 mg/kg CP55,940 resulted in an effect of time (number of CP55,940 injections) (F14,280 = 15.93, p < 0.0001) and genotype (F1,20 = 23.07, p = 0.0001), but not a time × genotype interaction (p = 0.0517) effect. While the half-time for tolerance to antinociceptive effect of 0.3 mg/kg CP55,940 in wild-type mice was 12.32 days (95%CI 4.246-infinity), the half-time for tolerance in S426A/S430A mice could not be calculated using our curve fitting analysis. Therefore, although S426A/S430A mutants were more sensitive to the antinociceptive effects of CP55,940 compared to their wild-type littermates, there was no evidence of a significant difference in tolerance to 0.3 mg/kg CP55,940 (Fig. 2B).
3.2.2. Tolerance to the hypothermic effects to WIN55,212-2 and CP55,940
Analysis of hypothermia in mice treated once-daily with 10 mg/kg WIN55,212-2 indicated an effect of time (number of WIN55,212-2 injections) (F19,627 = 27.60, p < 0.0001) and genotype (F1,33 = 58.23, p < 0.0001) but not a significant time × genotype interaction effect (p = 0.2267) (Fig. 2C). This data analysis indicates that although S426A/S430A mutant mice were more sensitive to the hypothermic effects of WIN55,212-2 than their wild-type littermates, there was no difference in tolerance to the hypothermic effects for this agonist. In contrast, analysis of hypothermia in mice treated once-daily with 0.3 mg/kg CP55,940 indicated an effect of time (number of CP55,940 injections) (F14,280 = 40.46, p < 0.0001), genotype (F1,20 = 16.18, p = 0.0007), and a time × genotype interaction effect (F14,280 = 1.98, p = 0.0191) (Fig. 2D). Post-hoc analyses revealed that tolerance to the hypothermic effects of 0.3 mg/kg CP55,940 was different in S426A/S430A mutants compared to wild-type littermates on days 3 (p < 0.01), 7 (p < 0.05), and 10 (p < 0.05) of treatment.
3.3. Antinociceptive effects of WIN55,212-2 and CP55,940 in the formalin test
3.3.1. Acute phase (phase I) of the formalin test
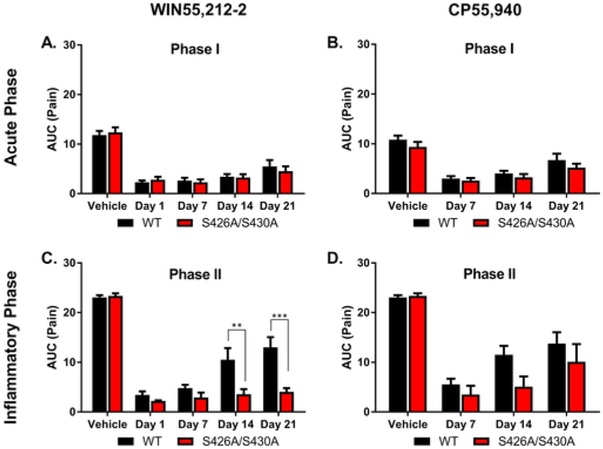
Tolerance to the antinociceptive effects of WIN55,212-2 and CP55,940 in the acute and inflammatory phases of the formalin test was also assessed in wild-type and S426A/S430A mutant mice. Analysis of antinociception in mice treated once-daily with 3 mg/kg WIN55,212-2 found an effect of time (number of WIN55,212-2 injections) (F4,31 = 52.25, p < 0.0001) in the acute phase of the formalin test (Fig. 3A). The main effect of time was driven by the differences in pain responses following vehicle compared to WIN55,212-2 as pre-treatment with 3 mg/kg WIN55,212-2 significantly attenuated pain responses on all days tested (1, 7, 14, and 21; p < 0.0001) compared to vehicle. There was not a main effect of genotype (p = 0.8740) or a genotype × time interaction (p = 0.8716), suggesting that the S426A/S430A mutation did not affect either tolerance or the acute antinociceptive response to WIN55,212-2 in phase I of the formalin test.
Fig. 3. The S426A/S430A mutation delays the rate of antinociceptive tolerance development to WIN55,212–2 (but not CP55,940) in a model of acute inflammatory pain.
Wild-type (black bars; n = 4–5/dose) and S426A/S430A mutants (red bars; n = 4–6/dose) were assessed for tolerance to the antinociceptive effects of either 3 mg/kg WIN55,212 (A,C) or 0.3 mg/kg CP55,940 (B,D) in the acute (phase I; A,B) and inflammatory (phase II; C,D) phases of the formalin test. Means represent the AUC of the composite pain score and error bars represent the standard error of the mean (SEM). Data were analyzed with two-way ANOVA and Bonferroni multiple comparisons test. *p < 0.05, **p < 0.01, ***p < 0.001. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Analysis of antinociception in mice treated once-daily with 0.3 mg/kg CP55,940 did not reveal either a main effect of genotype (p = 0.0584) or a time × genotype interaction effect (p = 0.8610), but did indicate a main effect of time (number of CP55,940 injections) (F3,27 = 37.61, p < 0.0001) in the acute phase (Phase I; Fig. 3B) of the formalin test. This main effect was due to differences in baseline pain following pre-treatment with vehicle versus pre-treatment with 0.3 mg/kg CP55,940 on days 7 (p < 0.0001), 14 (p < 0.0001), and 21 (p < 0.01) and the onset of partial tolerance following 21 days of once-daily CP55,940 treatment compared to 7 days of once-daily treatment (p < 0.05; Fig. 3B).
3.3.2. Inflammatory phase (phase II) of the formalin test
Tolerance to the antinociceptive effects of once-daily 3 mg/kg WIN55,212-2 and 0.3 mg/kg CP55,940 were also assessed in the inflammatory phase (Phase II) of the formalin test (Fig. 3C and D). For the inflammatory phase of the formalin test, there was an effect of time (number of WIN55,212-2 injections) (F4,30 = 70.66, p < 0.0001), genotype (F1,31 = 18.46, p = 0.0002), and a time × genotype interaction effect (F4,31 = 4.20, p = 0.0081; Fig. 3C). Post-hoc analyses revealed that wild-type mice were quicker to develop tolerance to WIN 55,212-2 than S426A/S430A mice. Wild-type mice showed significantly greater pain responses following 14 (p < 0.01) and 21 (p < 0.001) days of once-daily treatment with WIN55,212-2 than S426A/S430A mice.
There was also an effect of time (F3,3027 = 32.63, p < 0.0001) and genotype (F1,27 = 4.39, p = 0.0457) but not a time × genotype interaction (p = 0.4102) on the antinociceptive effects of once-daily injections of 0.3 mg/kg CP55,940 in the inflammatory phase of the formalin test. While the lack of a significant interaction indicates there was no difference in tolerance between wild-type and S426A/S430A mice, overall, wild-type mice were less sensitive to the anti-inflammatory effects of CP55,940 compared to S426A/S430A mice. Taken together, these data suggest that the S426A/S430A mutation might selectively alter tolerance to the antinociceptive effects of WIN55,212-2 specifically in the inflammatory phase of the formalin test.
3.4. Tolerance to WIN55,212-2 and CP55,940 in cisplatin-evoked chronic neuropathic pain
Tolerance to the anti-allodynic effects of 3 mg/kg WIN55,212-2 and 0.3 mg/kg CP55,940 was examined in wild-type and S426A/S430A mutant mice with cisplatin-induced neuropathic pain. We found no difference in baseline paw withdrawal thresholds between untreated wild-type and S426A/S430A mutant mice (p = 0.8094). Neuropathic pain, as evidenced by a decrease in von Frey paw pressure thresholds, was induced by four weeks of cisplatin treatment [t(2) = 6.478, p = 0.0230] and this effect was reversed by 3 mg/kg WIN55,212-2 [t(2) =4.549, p = 0.0451; Fig. 4A]. Mice developed tolerance to the anti-allodynic effects of WIN55,212-2 (F12,252 = 14.97, p < 0.0001) across 25 days of once-daily treatment. There was not an effect of genotype (p = 0.1225) but there was a significant genotype × time interaction effect (F12,252 = 4.60, p < 0.0001) indicating that tolerance to WIN55,212-2 was different between mutant and wild-type mice. Post-hoc analysis of this interaction effect indicates that tolerance to the anti-allodynic effects of WIN55,212-2 was altered in S426A/S430A mutants compared to wild-type mice following 15 (p < 0.05), 17 (p < 0.05), and 19 (p < 0.01) days of WIN55,212-2 injections. Tolerance was confirmed by determining when the anti-allodynic effects of WIN55,212-2 were no longer different from their neuropathic pain baseline. Wild-type mice displayed tolerance to WIN55,212-2 by day 15 (p > 0.05) while S426A/S430A mutants exhibited tolerance by day 21 (p > 0.05) (Fig. 4A), providing further evidence that tolerance for the anti-allodynic effects of WIN55,21202 were disrupted in S426A/S430A mutants compared to wild-type littermates.
Fig. 4. The S426A/S430A mutation delays tolerance to the anti-allodynic effects of WIN55,212–2 (but not CP55,940) in a model of chronic neuropathic pain.
The von Frey test was used to assess the anti-allodynic effects of once-daily treatment with either 3mg/kg WIN55,212-2 (A) or 0.3 mg/kg CP55,940 (B) in wild-type (black lines, squares) and desensitization-resistant S426A/S430A (red lines, circles) mice. Baseline (BL) and cisplatin-induced neuropathic (Cis) allodynic responses and anti-allodynic responses to 3 mg/kg WIN55,212-2 (Days 1–25) or 0.3 mg/kg CP55,940 (Days 1–19) are shown. The mean represents the amount of force (g) required for paw withdrawal from a noxious mechanical stimulus (A,B) and error bars represent the standard error of the mean (SEM). The number of mice in each group is indicated within the parentheses. Data were analyzed with two-way RM ANOVA and Bonferroni multiple comparisons test. *p < 0.05, **p < 0.01. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Tolerance to the anti-allodynic effects of 0.3 mg/kg CP55,940 in mice with cisplatin-induced neuropathic pain was also assessed in wild-type and S426A/S430A mutant mice (Fig. 4B). Mice demonstrated significant neuropathic pain as evidenced by a decrease in von Frey thresholds following cisplatin treatment [t(2) = 10.25, p = 0.0094] that was reversed by treatment with 0.3 mg/kg CP55,940 [t(2) = 18.19, p = 0.0030]. Tolerance to the anti-allodynic effects of CP55,940 (F9,225 = 16.57, p < 0.0001) developed across 19 consecutive days of treatment. Subsequent analyses indicated that wild-type and S426A/S430A mutant mice developed tolerance to the anti-allodynic effects after 15 days of once-daily 0.3 mg/kg CP55,940 treatment (no difference from neuropathic baseline; p > 0.05). Interestingly, S426A/S430A mice did show a slight difference from neuropathic baseline following day 19 (p < 0.05). There was no effect of genotype (p = 0.1637) nor a genotype × time interaction effect (F9,225 = 1.07, p = 0.3878), suggesting that tolerance to the anti-allodynic effects of CP55,940 was not altered in S426A/S430A mutants.
4. Discussion
Tolerance to the antinociceptive effects of cannabinoid agonists has been described in both rodents (Morgan et al., 2014; Sim-Selley and Martin, 2002) and humans (Clark et al., 1981). However, the molecular mechanisms underlying tolerance to cannabinoids are still being elucidated. Previous work demonstrated that phosphorylation of two serines at residues 426 and 430 by GRK3 and subsequent association with β-arrestin2 (Daigle et al., 2008; Jin et al., 1999) was sufficient for desensitization of cannabinoid receptor 1 (CB1) in vitro. Previous work has shown that mice expressing a mutant, desensitization-resistant form of CB1 (S426A/S430A) develop tolerance to Δ9-tetrahydrocannabinol (Δ9-THC) more slowly than wild-type littermates (Morgan et al., 2014). There is growing evidence to suggest that some cannabinoid agonists, including WIN55,212-2, can induce biased signaling through β-arrestin pathways (Flores-Otero et al., 2014). Evidence from cell culture models also suggests that the S426A/S430A mutant form of CB1 exhibits biased signaling through β-arrestin1 versus G-protein-mediated signaling mechanisms (Delgado-Peraza et al., 2016). Evidence of biased signaling at CB1 highlights the need to test whether cannabinoid tolerance occurs through agonist-specific mechanisms. The current study evaluates this possibility by assessing the role of GRK/β-arrestin2-mediated desensitization of CB1 in tolerance to CP55,940, and WIN55,212-2, two structurally diverse synthetic cannabinoid agonists with known signaling biases.
Tolerance to the effects of a drug often develops following prolonged administration, where a constant dose of drug produces a reduced effect (Martin et al., 2004; Taylor and Fleming, 2001). In this study, we used two models of acute nociceptive pain as well as a model of chronic neuropathic pain to assess tolerance to the antinociceptive, hypothermic, and anti-allodynic effects of WIN55,212-2 and CP55,950 in desensitization-deficient S426A/S430A mutant mice. We found that tolerance to the antinociceptive and anti-allodynic effects of WIN55,212-2 was attenuated in S426A/S430A mutant mice consistent with previous studies examining tolerance to Δ9-THC (Morgan et al., 2014). S426A/S430A mutant mice demonstrated a longer half-time to tolerance for WIN55,212-2 than wild-type littermates. Tolerance to the antinociceptive effects of WIN55,212-2 was also disrupted in S426A/S430A mice using the formalin test. Tolerance to the antinociceptive effects of once-daily injections of CP55,940 was not disrupted in S426A/S430A mutants while tolerance to the hypothermic effects of these injections was modestly reduced in S426A/S430A mutants. However, the dose-response curve shifts for the antinociceptive and hypothermic effects of CP55,940 following 10 days of once daily CP55,940 administration was absent in S426A/S430A mutant mice. These results suggest that CP55,940 tolerance is mediated through complex mechanisms that are likely dose and response dependent and not well-understood. Collectively, our current results, as well as those from previous work, demonstrate that disruption of β-arrestin2-mediated desensitization, either through removal of the protein or its binding sites on CB1, might alter tolerance to cannabinoid agonists.
In this study, tolerance to specific cannabinoid agonists was differentially affected by blockade of GRK/β-arrestin2-mediated CB1 desensitization. For example, tolerance to the antinociceptive and anti-allodynic effects of WIN55,212-2 was significantly disrupted and delayed in S426A/S430A mutant mice. In contrast, tolerance to CP55,940 was altered in S426A/S430A mutant mice under certain experimental conditions but not others. Previous in vitro studies demonstrated that CP55,940 and Δ9-THC binding induced greater association of β-arrestin2 with CB1 compared to WIN55,212-2 (Laprairie et al., 2014), suggesting that interfering with β-arrestin2-mediated desensitization might have a greater effect on tolerance to CP55,940 and Δ9-THC. Instead, we observed an opposite effect on tolerance to the antinociceptive effects of these agonists in vivo, highlighting the necessity of investigating agonist biases in whole-animal models. Further studies are needed to fully elucidate the other pathways that may contribute to tolerance for the effects of these agonists.
The results we observed for tolerance to these agonists in acute pain assays did not fully manifest in our assay for chronic chemotherapy-evoked neuropathic pain. We found that disruption of GRK/β-arrestin-mediated CB1 desensitization produced only a modest effect on tolerance to the anti-allodynic effects of WIN55,212-2 in mice experiencing chronic cisplatin-induced neuropathy. The effect of the S426A/S430A mutation in CB1 on cannabinoid tolerance may be masked in pathological chronic pain conditions where CB2 signaling pathways play a more important role in modulating antinociception. This possibility is supported by an upregulation of CB2 expression that occurs in chronic pain states following peripheral nerve injury (Wotherspoon et al., 2005; Zhang et al., 2003). WIN55,212-2 and CP55,940 are agonists for both CB1 and CB2 (Howlett, 2002) and multiple studies demonstrate a lack of tolerance for CB2 agonists (Deng et al., 2015; Yuill et al., 2017). Therefore, it is possible that upregulation of CB2 in cisplatin-treated mice leads to a sufficient level of CB2-mediated nociceptive signaling to mask the effects of the S426A/S430A mutation on cannabinoid tolerance. Future studies could investigate this possibility by using a CB1selective agonist such as arachidonyl-2-chloroethlamide (ACEA) (Hillard et al., 1999) or by co-administering these mixed agonists to S426A/ S430A double mutant mice that lack CB2 to isolate the specific contribution of the S426A/S430A mutation to tolerance for these agonists in this chronic pain model. Further, the use of single doses of WIN55,212-2 and CP55,940 for evaluating the effect of the S426A/S430A mutation on cannabinoid tolerance is a limitation to our study, and it is possible that more pronounced genotype effects might be detected using other doses. Nonetheless, our findings suggest that GRK/β-arrestin-mediated desensitization plays a more profound role in tolerance to WIN55,212-2, while this pathway plays only a minor role in tolerance to CP55,940 and Δ9-THC (Morgan et al., 2014).
We report that tolerance to the antinociceptive effect of WIN55,212-2 was profoundly delayed but not entirely blocked in S426/S430A mutant mice. One advantage of this study is the relatively long duration of daily drug administration. This allowed us to look at the development of robust tolerance and to better attempt to determine the half-time to tolerance for each drug and assess differences in those half-times as a function of the mutation. Mice lacking GPCR-associated sorting protein 1 (GASP-1), a protein involved in CB1 trafficking and degradation, exhibit delayed tolerance to the antinociceptive but not the hypothermic effects of WIN55,212-2 (Martini et al., 2010). GASP-1 does not appear to interact with CB1 in the distal regions of the C-terminal tail responsible for internalization (Hsieh et al., 1999; Tappe-Theodor et al., 2007), but it is unknown if GASP-1 interacts with CB1 in the separate more proximal arrestin-binding regions responsible for CB1 desensitization. Additionally, mice lacking protein kinase C epsilon (PKCε) demonstrate accelerated tolerance to WIN55,212-2 (Wallace et al., 2009). Similar to our findings, an agonist bias was observed for the effect of PKCε in cannabinoid tolerance as tolerance to CP55,940 was not affected by PKCε deletion. Either one of these proteins could be involved in delayed tolerance to WIN55,212-2 in the S426A/S430A mutant mice through some unknown interaction or mechanism. Therefore, a better understanding of how these two proteins might interact with the arrestin-binding region that is mutated in the S426A/S430A mice would provide valuable insight into this possibility.
Previous work from our laboratory has demonstrated enhanced acute antinociceptive and hypothermic responses to Δ9-THC, N-arachidonoylethanolamine (AEA) in combination with the fatty acid amide hydrolase (FAAH) inhibitor URB597, and the dual monoacylglycerol lipase (MAGL)/FAAH inhibitor JZL195 (Morgan et al., 2014). These findings demonstrate an increased acute response to endocannabinoid and cannabinoid agonists in S426A/S430A mutant mice that is also supported by the current work showing enhanced responses to WIN55,212-2 and CP55,940. Since most of the previous work assessed the acute response to partial cannabinoid agonists such as Δ9-THC and AEA, the current work showing that this trend is maintained for potent full agonists, such as WIN55,212-2 and CP55,940, augments this important effect of the S426A/S430A mutation. Taken together, our current data and previously published work demonstrate the crucial role of GRK/β-arrestin2-mediated CB1 desensitization in regulating the magnitude of acute responses to a diverse range of cannabinoid agonists.
Although we observed enhanced acute antinociceptive responses to WIN55,212-2 and CP55,940 in S426A/S430A mutants, we did not observe similar changes in the acute hypothermic response to these agonists. Such an enhancement of the acute antinociceptive but not hypothermic response to these high potency full agonists represents a significant difference compared to what we have previously observed for the partial agonists, Δ9-THC and AEA. This result raises the possibility of response and/or tissue specific differences in the effect of the S426/S430A mutation on the enhancement of specific cannabinoid-induced responses. Another possible explanation for these full agonists is the loss of body temperature in excess of 20% represents a basement or floor effect where further enhancement of cannabinoid-induced hypothermia is not possible.
S426A/S430A mutant mice demonstrated delayed tolerance to the hypothermic effects of CP55,940 but not WIN55,212-2, suggesting that the effects of interference with GRK/β-arrestin2-mediated desensitization may modulate tolerance in ways that are also response and/or tissue-specific. This possibility is supported by previous work showing that tolerance develops more slowly for the motor effects of cannabinoids due to resistance of the neuroadaptations responsible for CB1 desensitization and down-regulation in brain regions, such as the striatum that are involved in these motor responses (Breivogel et al., 1999; McKinney et al., 2008). Further work is needed to understand the potential signaling and regional mechanisms involved in tolerance to specific cannabinoid responses.
In summary, the findings from our study suggest that tolerance to WIN55,212-2 and CP55,940 may develop through different mechanisms, where tolerance to WIN55,212-2 is more highly dependent on a signaling mechanism involving GRK/β-arrestin-mediated desensitization of CB1. However, disruption of GRK/β-arrestin-mediated CB1 desensitization had a limited effect on tolerance to CP55,940. S426A/S430A mutants displayed an enhanced anti-nociceptive response to both WIN55,212-2 and CP55,940. This study provides behavioral and physiological evidence that the mechanisms involved in tolerance may differ for specific agonists. However, additional future work is needed using additional doses of these agonists to examine tolerance and also neuroadaptations at CB1 that mediate the effects on cannabinoid tolerance.
Acknowledgments:
The authors wish to thank Dr. Diane McCloskey for critical review of this manuscript and Diana Sepulveda and Michael Zee for assistance with genotyping and animal husbandry. This work has been supported by NIH grants DA036385 (DJM), DA037355 (DJM), DA044999 (DJM) and is also funded, in part, by grant 4100057673 from the Pennsylvania Department of Health using Tobacco CURE Funds (DJM). The authors declare no competing financial interests.
Abbreviations:
- AUC
area under the curve
- CB1
cannabinoid receptor 1
- CP55,940
(1α,2β)-R-5-(1,1-dimethylheptyl)-2-[5-hydroxy-2-(3-hydroxypropyl-cyclohexyl]phenyl
- CPS
composite pain score
- GASP-1
G protein coupled receptor associated sorting protein 1
- GPCR
G protein-coupled receptor
- GRK
G protein-related kinase
- i.p.
intraperitoneal
- %MPE
percent maximal possible effect
- PKCε
epsilon isoform of protein kinase c
- s.c.
subcutaneous
- Δ;9-THC
delta-9-tetrahydrocannabinol
- WIN55,212-2
R-(+)-[2,3-Dihydro-5-methyl-3-[(morpholinyl-methyl]pyrrolo[1,2,3-de]-1,4-benzoxazinyl]-(1-mapthalenyl)methanone mesylate (WIN55,212-2).
References
- Ahn KH, Mahmoud MM, Shim JY, Kendall DA, 2013. Distinct roles of β-arrestin 1 and β-arrestin 2 in ORG27569-induced biased signaling and internalization of the cannabinoid receptor 1 (CB1). J. Biol. Chem 288, 9790–9800. 10.1074/jbc.M112.438804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi G, Foltin RW, Gunderson EW, Rabkin J, Hart CL, Comer SD, Vosburg SK, Haney M, 2010. Efficacy and tolerability of high-dose dronabinol maintenance in HIV-positive marijuana smokers: a controlled laboratory study. Psychopharmacology (Berlin) 212, 675–686. 10.1007/s00213-010-1995-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breivogel CS, Vaghela MS, 2015. The effects of beta-arrestin1 deletion on acute cannabinoid activity, brain cannabinoid receptors and tolerance to cannabinoids in mice. J. Recept. Signal Transduct 35, 98–106. 10.3109/10799893.2014.1003659. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Vogt LJ, Sim-Selley LJ, 1999. Chronic delta9-tetrahydrocannabinol treatment produces a time-dependent loss of cannabinoid receptors and cannabinoid receptor-activated G proteins in rat brain. J. Neurochem 73, 2447–2459. 10.1046/j.1471-4159.1999.0732447x. [DOI] [PubMed] [Google Scholar]
- Clark WC, Janal MN, Zeidenberg P, Nahas GG, 1981. Effects of moderate and high doses of marihuana on thermal pain: a sensory decision theory analysis. J. Clin. Pharmacol 21, 299S–310S. 10.1002/j.1552-4604.1981.tb02608.x. [DOI] [PubMed] [Google Scholar]
- Daigle T, Kearn C, Mackie K, 2008. Rapid CB 1 cannabinoid receptor desensitization defines the time course of ERK1/2 MAP kinase signaling. Neuropharmacology 54, 36–44. 10.1016/j.neuropharm.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Ferrari AJ, Calabria B, Hall WD, Norman RE, McGrath J, Flaxman AD, Engell RE, Freedman GD, Whiteford HA, Vos T, 2013. The global epidemiology and contribution of cannabis use and dependence to the global burden of disease: results from the GBD 2010 study. PLoS One 8, 1–13. 10.1371/journal.pone.0076635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Peraza F, Ahn KH, Nogueras-Ortiz C, Mungrue IN, Mackie K, Kendall DA, Yudowski GA, 2016. Mechanisms of biased β-arrestin-mediated signaling downstream from the cannabinoid 1 receptor. Mol. Pharmacol 89, 618–629. 10.1124/mol.115.103176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Guindon J, Cornett BL, Makriyannis A, Mackie K, Hohmann AG, 2015. Archival report chronic cannabinoid receptor 2 activation reverses paclitaxel neuropathy without tolerance or cannabinoid receptor 1 – dependent withdrawal. Biol. Psychiatry 77, 475–487. 10.1016/j.biopsych.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWire SM, Kim J, Whalen EJ, Ahn S, Chen M, Lefkowitz RJ, 2008. β-Arrestin-Mediated signaling regulates protein synthesis. J. Biol. Chem 283, 10611–10620. 10.1074/jbc.M710515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza DC, Ranganathan M, Braley G, Gueorguieva R, Zimolo Z, Cooper T, Perry E, Krystal J, 2008. Blunted psychotomimetic and amnestic effects of Δ-9- tetrahydrocannabinol in frequent users of cannabis. Neuropsychopharmacology 33, 2505–2516. 10.1038/sj.npp.1301643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Otero J, Ahn KH, Delgado-Peraza F, Mackie K, Kendall DA, Yudowski GA, 2014. Ligand-specific endocytic dwell times control functional selectivity of the cannabinoid receptor 1. Nat. Commun 5, 1–11. 10.1038/ncomms5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A, Kesingland A, Gentry C, McNair K, Patel S, Urban L, James I, 2001. The role of central and peripheral Cannabinoid 1 receptors in the antihyperalgesic activity of cannabinoids in a model of neuropathic pain. Pain 92, 91–100. 10.1016/S0304-3959(00)00474-7. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG, 2004. Desensitization of G protein-coupled receptors and neuronal functions. Annu. Rev. Neurosci 27, 107–144. 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- Georgieva T, Devanathan S, Stropova D, Park CK, Salamon Z, Tollin G, Hruby VJ, Roeske WR, Yamamura HI, Varga E, 2008. Unique agonist-bound cannabinoid CB1 receptor conformations indicate agonist specificity in signaling. Eur. J. Pharmacol 581, 19–29. 10.1016/j.ejphar.2007.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon J, Guijarro A, Piomelli D, Hohmann AG, 2011. Peripheral antinociceptive effects of inhibitors of monoacylglycerol lipase in a rat model of inflammatory pain. Br. J. Pharmacol 163, 1464–1478. 10.1111/j.1476-5381.2010.01192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon J, Deng L, Fan B, Wager-Miller J, Hohmann AG, 2014. Optimization of a cisplatin model of chemotherapy-induced peripheral neuropathy in mice: use of vitamin C and sodium bicarbonate pretreatments to reduce nephrotoxicity and improve animal health status. Mol. Pain 10, 1 10.1186/1744-8069-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W, 2015. What has research over the past two decades revealed about the adverse health effects of recreational cannabis use?. Addiction 110, 19–35. 10.1111/add.12703. [DOI] [PubMed] [Google Scholar]
- Hillard CJ, Manna S, Greenberg MJ, DiCamelli R, Ross RA, Stevenson LA, Murphy V, Pertwee RG, Campbell WB, 1999. Synthesis and characterization of potent and selective agonists of the neuronal cannabinoid receptor (CB1). J. Pharmacol. Exp. Therapeut 289, 1427–1433. [PubMed] [Google Scholar]
- Howlett AC, 2002. International union of pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev 54, 161–202. 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Hsieh C, Brown S, Derleth C, Mackie K, 1999. Internalization and recycling of the CB1 cannabinoid receptor. J. Neurochem 73, 493–501. 10.1046/j.1471-4159.1999.0730493.x. [DOI] [PubMed] [Google Scholar]
- Jin W, Brown S, Roche JP, Hsieh C, Celver JP, Kovoor a, Chavkin C, Mackie K, 1999. Distinct domains of the CB1 cannabinoid receptor mediate desensitization and internalization. J. Neurosci 19, 3773–3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprairie RB, Bagher AM, Kelly MEM, Dupre DJ, Denovan-wright EM, 2014. Type 1 cannabinoid receptor ligands display functional selectivity in a cell culture model of striatal medium spiny projection neurons. J. Biol. Chem 289, 24845–24862. 10.1074/jbc.M114.557025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFleur RA, Wilson RP, Morgan DJ, Henderson-Redmond AN, 2018. Sex differences in the development of tolerance to delta-9-tetrahydrocannabinol (Δ9-THC) and CP55,940 in a mouse model of inflammatory pain. Neuroreport [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Martin BR, 1991. Spinal and supraspinal components of cannabinoid-induced antinociception. J. Pharmacol. Exp. Therapeut 258, 517–523. [PubMed] [Google Scholar]
- Marcus DJ, Zee M, Hughes A, Yuill MB, Hohmann AG, Mackie K, Guindon J, Morgan DJ, 2015. Tolerance to the antinociceptive effects of chronic morphine requires c-Jun N-terminal kinase. Mol. Pain 11, 34 10.1186/s12990-015-0031-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BR, Sim-Selley LJ, Selley DE, 2004. Signaling pathways involved in the development of cannabinoid tolerance. Trends Pharmacol. Sci 25, 325–330. 10.1016/j.tips.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Martini L, Thompson D, Kharazia V, Whistler JL, 2010. Differential regulation of behavioral tolerance to WIN55,212-2 by GASP1. Neuropsychopharmacology 35, 1363–1373. 10.1038/npp.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney DL, Cassidy MP, Collier LM, Martin BR, Wiley JW, Selley DE, Sim-Selley LJ, 2008. Dose-related differences in the regional pattern of cannabinoid receptor adaptation and in vivo tolerance development to Δ9-tetrahydrocannabinol. J. Pharmacol. Exp. Therapeut 324, 664–673. 10.1124/jpet.107.130328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DJ, Davis BJ, Kearn CS, Marcus D, Cook AJ, Wager-Miller J, Straiker A, Myoga MH, Karduck J, Leishman E, Sim-Selley LJ, Czyzyk TA, Bradshaw HB, Selley DE, Mackie K, 2014. Mutation of putative GRK phosphorylation sites in the cannabinoid receptor 1 (CB1R) confers resistance to cannabinoid tolerance and hypersensitivity to cannabinoids in mice. J. Neurosci 34,5152–5163. 10.1523/JNEUROSCI.3445-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealon CM, Patel C, Worley BL, Henderson-Redmond AN, Morgan DJ, Czyzyk TA, 2017. Alterations in nociception and morphine antinociception in mice fed a high-fat diet. Brain Res. Bull 10.1016/j.brainresbull.2017.06.019. [DOI] [PubMed] [Google Scholar]
- Nguyen PT, Schmid CL, Raehal KM, Selley DE, Bohn LM, Sim-Selley LJ, 2012. β-arrestin2 regulates cannabinoid CB1 receptor signaling and adaptation in a central nervous system region-dependent manner. Bio Psychiatry 71, 714–724. 10.1016/j.biopsych.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, 2008. Ligands that target cannabinoid receptors in the brain: from THC to anandamide and beyond. Addict. Biol 13, 147–159. 10.1111/j.1369-1600.2008.00108.x. [DOI] [PubMed] [Google Scholar]
- Priestley R, Glass M, Kendall D, 2017. Functional selectivity at cannabinoid receptors In: Advances in Pharmacology, first ed. Elsevier Inc; 10.1016/bs.apha.2017.03.005. [DOI] [PubMed] [Google Scholar]
- Selley LJ, Cassidy MP, Martin BR, Sim-Selley LJ, 2004. Long-term administration of Δ9-tetrahydrocannabinol desensitizes CB1, adenosineA1, and GABAB-mediated inhibition of adenylyl cylase in mouse cerebellum. Mol. Pharmacol 66, 1275–1284 10.1124/mol.104.000604. [DOI] [PubMed] [Google Scholar]
- Sim-Selley LJ, Martin BR, 2002. Effect of chronic administration of R-( + )-[2,3dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-l,4- benzoxazinyl]-(1-naphthalenyl)methanone mesylate (WIN55,212-2) or delta-9-tetrahydrocannabinol on cannabinoid receptor adaptation in mice. J. Pharmacol. Exp. Therapeut 303, 36–44. 10.1124/jpet.102.035618. [DOI] [PubMed] [Google Scholar]
- Tappe-Theodor A, Agarwal N, Katona I, Rubino T, Martini L, Swiercz J, Mackie K, Monyer H, Parolaro D, Whistler J, Kuner T, Kuner R, 2007. A molecular basis of analgesic tolerance to cannabinoids. J. Neurosci 27, 4165–4177. 10.1523/JNEUROSCI.5648-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DA, Fleming WW, 2001. Unifying perspectives of the mechanisms underlying the development of tolerance and physical dependence to opioids. J. Pharmacol. Exp. Therapeut 297, 11–18. [PubMed] [Google Scholar]
- Tjølsen A, Berge OG, Hunskaar S, Rosland JH, Hole K, 1992. The formalin test: an evaluation of the method. Pain 51, 5–17. 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- Walker JM, Huang SM, Strangman NM, Tsou K, Sañudo-Peña MC, 1999. Pain modulation by release of the endogenous cannabinoid anandamide. Proc. Natl. Acad. Sci. U. S. A 96, 12198–12203. 10.1073/pnas.96.21.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MJ, Newton PM, Mcmahon T, Connolly J, Huibers A, Whistler J, Messing RO, 2009. PKCe regulates behavioral sensitivity, binding and tolerance to the CB1 receptor agonist WIN55,212-2. Nature 34, 1733–1742. 10.1038/npp.2008.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson G, Sufka K, Coderre T, 1997. Optimal scoring strategies and weights for the formalin test in rats. Pain 70, 53–58. [DOI] [PubMed] [Google Scholar]
- Whiting PF, Wolff RF, Deshpande S, Nisio M.Di, Duffy S, Hernandez AV, Keurentjes JC, Lang S, Misso K, 2016. Cannabinoids for Medical Use A Systematic Review and Meta-analysis. JAMA 313, 2456–2473. 10.1001/jama.2015.6358. [DOI] [PubMed] [Google Scholar]
- Wotherspoon G, Fox A, Mcintyre P, Colley S, Bevan S, Winter J, 2005. Protein expression in rat sensory neurons. J. Neurosci 135, 235–245. 10.1016/j.neuroscience.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Wu D-F, Yang L-Q, Goschke A, Stumm R, Brandenburg L-O, Liang Y-J, Höllt V, Koch T, 2008. Role of receptor internalization in the agonist-induced desensitization of cannabinoid type 1 receptors. J. Neurochem 104, 1132–1143. 10.1111/j.1471-4159.2007.05063.x. [DOI] [PubMed] [Google Scholar]
- Yuill MB, Hale DE, Guindon J, Morgan DJ, 2017. Anti-nociceptive interactions between opioids and a cannabinoid receptor 2 agonist in inflammatory pain. Mol. Pain 13, 1–15. 10.1177/1744806917728227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Hoffert C, Vu HK, Groblewski T, Ahmad S, O'Donnell D, 2003. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. Eur. J. Neurosci 17, 2750–2754. 10.1046/j.1460-9568.2003.02704.x. [DOI] [PubMed] [Google Scholar]